95579c7106a707ca4e74ce35d37e85f2.ppt
- Количество слайдов: 26
 Types of Chemical Reactions
Types of Chemical Reactions
 Types of Chemical Reactions There are only five (5) different types of chemical reactions: 1) Double Replacement 2) Single Replacement 3) Combustion 4) Synthesis 5) Decomposition
Types of Chemical Reactions There are only five (5) different types of chemical reactions: 1) Double Replacement 2) Single Replacement 3) Combustion 4) Synthesis 5) Decomposition
 Reaction Type 1 – Double Replacement - Example
Reaction Type 1 – Double Replacement - Example
 Reaction Type 1 – Double Replacement Occur between two ionic compounds Does not usually happen with covalent compounds Involves an exchange of cations The cation of one compound trades places with the cation of another compound to form two new compounds
Reaction Type 1 – Double Replacement Occur between two ionic compounds Does not usually happen with covalent compounds Involves an exchange of cations The cation of one compound trades places with the cation of another compound to form two new compounds
 Double Replacement Characteristics occur in solution when the compounds are in an aqueous state (aq) reactants are either aqueous to begin with, or solid compounds dissolved in water to form an aqueous solution of ions in order to drive the reaction, one of the products must be removed from the aqueous solution this can occur via one of three ways:
Double Replacement Characteristics occur in solution when the compounds are in an aqueous state (aq) reactants are either aqueous to begin with, or solid compounds dissolved in water to form an aqueous solution of ions in order to drive the reaction, one of the products must be removed from the aqueous solution this can occur via one of three ways:
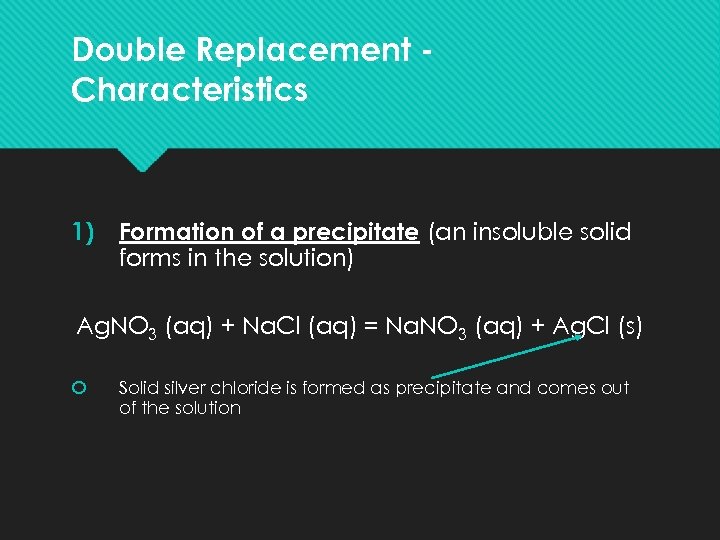 Double Replacement Characteristics 1) Formation of a precipitate (an insoluble solid forms in the solution) Ag. NO 3 (aq) + Na. Cl (aq) = Na. NO 3 (aq) + Ag. Cl (s) Solid silver chloride is formed as precipitate and comes out of the solution
Double Replacement Characteristics 1) Formation of a precipitate (an insoluble solid forms in the solution) Ag. NO 3 (aq) + Na. Cl (aq) = Na. NO 3 (aq) + Ag. Cl (s) Solid silver chloride is formed as precipitate and comes out of the solution
 Double Replacement Characteristics 2) Formation of a gas Fe. S (aq) + 2 HCl (aq) = H 2 S (g) + Fe. Cl 2 (aq) Hydrogen sulfide gas is formed and comes out of the solution
Double Replacement Characteristics 2) Formation of a gas Fe. S (aq) + 2 HCl (aq) = H 2 S (g) + Fe. Cl 2 (aq) Hydrogen sulfide gas is formed and comes out of the solution
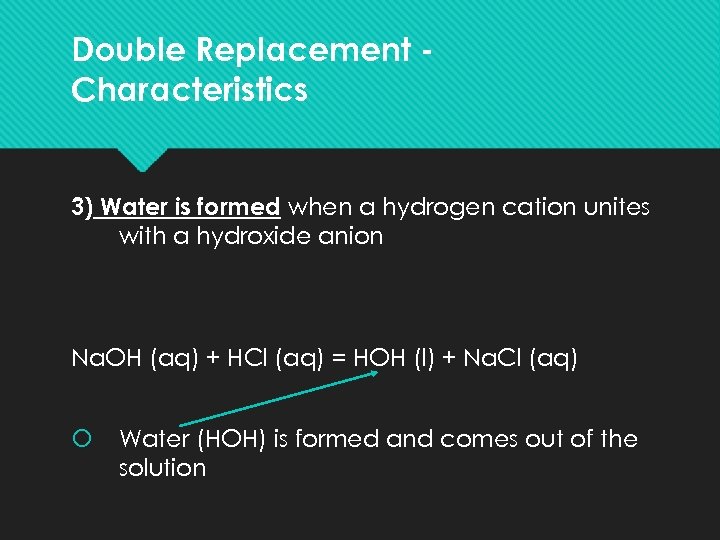 Double Replacement Characteristics 3) Water is formed when a hydrogen cation unites with a hydroxide anion Na. OH (aq) + HCl (aq) = HOH (l) + Na. Cl (aq) Water (HOH) is formed and comes out of the solution
Double Replacement Characteristics 3) Water is formed when a hydrogen cation unites with a hydroxide anion Na. OH (aq) + HCl (aq) = HOH (l) + Na. Cl (aq) Water (HOH) is formed and comes out of the solution
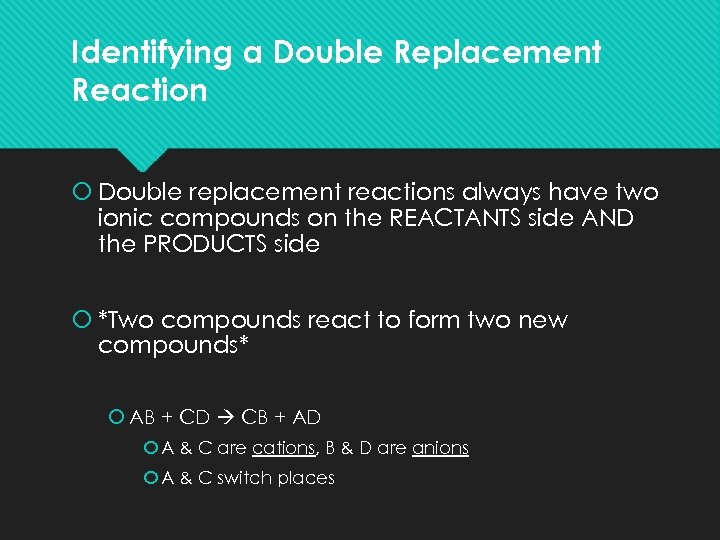 Identifying a Double Replacement Reaction Double replacement reactions always have two ionic compounds on the REACTANTS side AND the PRODUCTS side *Two compounds react to form two new compounds* AB + CD CB + AD A & C are cations, B & D are anions A & C switch places
Identifying a Double Replacement Reaction Double replacement reactions always have two ionic compounds on the REACTANTS side AND the PRODUCTS side *Two compounds react to form two new compounds* AB + CD CB + AD A & C are cations, B & D are anions A & C switch places
 Single Replacement Reactions
Single Replacement Reactions
 Single Replacement Reactions: Characteristics: Atom (s) of a lone element replace the atom (s) of an element in a compound Metals replace metals (or cations replace cations) Non-metals replace non-metals (or anions replace anions)
Single Replacement Reactions: Characteristics: Atom (s) of a lone element replace the atom (s) of an element in a compound Metals replace metals (or cations replace cations) Non-metals replace non-metals (or anions replace anions)
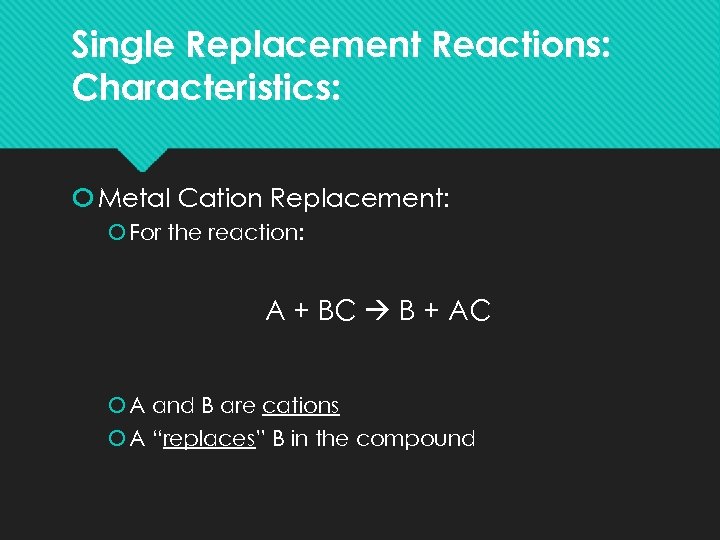 Single Replacement Reactions: Characteristics: Metal Cation Replacement: For the reaction: A + BC B + AC A and B are cations A “replaces” B in the compound
Single Replacement Reactions: Characteristics: Metal Cation Replacement: For the reaction: A + BC B + AC A and B are cations A “replaces” B in the compound
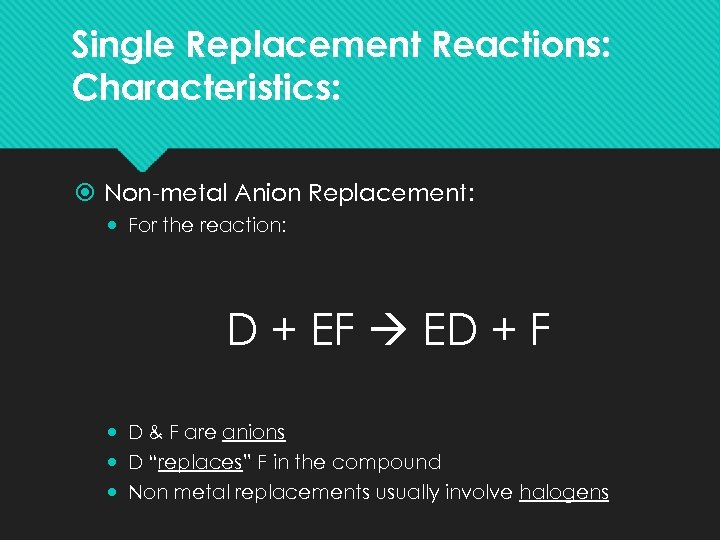 Single Replacement Reactions: Characteristics: Non-metal Anion Replacement: For the reaction: D + EF ED + F D & F are anions D “replaces” F in the compound Non metal replacements usually involve halogens
Single Replacement Reactions: Characteristics: Non-metal Anion Replacement: For the reaction: D + EF ED + F D & F are anions D “replaces” F in the compound Non metal replacements usually involve halogens
 Restrictions on Single Replacement Reactions Activity Series: A characteristic of metals and halogens referring to their reactivity Determines whether or not a single replacement reaction will occur or not Metals: on the handout provided there is an activity series of metals arranged in order of decreasing activity Non-metals (Halogens) : as you move down the group on the periodic table activity decreases
Restrictions on Single Replacement Reactions Activity Series: A characteristic of metals and halogens referring to their reactivity Determines whether or not a single replacement reaction will occur or not Metals: on the handout provided there is an activity series of metals arranged in order of decreasing activity Non-metals (Halogens) : as you move down the group on the periodic table activity decreases
 Restrictions on Single Replacement Reactions A single replacement reaction WILL NOT OCCUR if the reactivity of the pure element reactant is less than that of the compound reactant i. e. : Sn (s) + Na. NO 3 (aq) no reaction b/c tin is less reactive than sodium
Restrictions on Single Replacement Reactions A single replacement reaction WILL NOT OCCUR if the reactivity of the pure element reactant is less than that of the compound reactant i. e. : Sn (s) + Na. NO 3 (aq) no reaction b/c tin is less reactive than sodium
 Restrictions on Single Replacement Reactions A single replacement reaction WILL OCCUR if the reactivity of the pure element reactant is greater than that of the compound reactant i. e. : Zn (s) + H 2 SO 4 (aq) Zn. SO 4 (aq) + H 2 (g) Reaction occurs because the reactivity of zinc is higher than hydrogen
Restrictions on Single Replacement Reactions A single replacement reaction WILL OCCUR if the reactivity of the pure element reactant is greater than that of the compound reactant i. e. : Zn (s) + H 2 SO 4 (aq) Zn. SO 4 (aq) + H 2 (g) Reaction occurs because the reactivity of zinc is higher than hydrogen
 Identifying Single Replacement Reactions Single replacement reactions ALWAYS have 1 lone element and 1 compound on the reactants side and the products side Reactants will always be: 1 lone element + 1 compound Products will always be: 1 lone element + 1 compound
Identifying Single Replacement Reactions Single replacement reactions ALWAYS have 1 lone element and 1 compound on the reactants side and the products side Reactants will always be: 1 lone element + 1 compound Products will always be: 1 lone element + 1 compound
 Reaction Type #3 - Combustion Also known as burning or explosions Hydrocarbon Compound composed of only carbon and hydrogen and sometimes oxygen Reactants will always be: Hydrocarbon + oxygen Products will always be: Carbon dioxide + water i. e. : C 6 H 6 + O 2 CO 2 + H 2 O
Reaction Type #3 - Combustion Also known as burning or explosions Hydrocarbon Compound composed of only carbon and hydrogen and sometimes oxygen Reactants will always be: Hydrocarbon + oxygen Products will always be: Carbon dioxide + water i. e. : C 6 H 6 + O 2 CO 2 + H 2 O
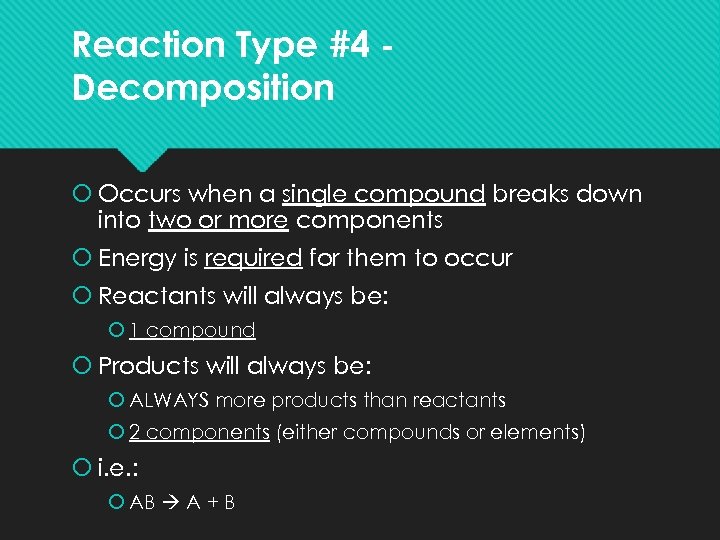 Reaction Type #4 Decomposition Occurs when a single compound breaks down into two or more components Energy is required for them to occur Reactants will always be: 1 compound Products will always be: ALWAYS more products than reactants 2 components (either compounds or elements) i. e. : AB A + B
Reaction Type #4 Decomposition Occurs when a single compound breaks down into two or more components Energy is required for them to occur Reactants will always be: 1 compound Products will always be: ALWAYS more products than reactants 2 components (either compounds or elements) i. e. : AB A + B
 Decomposition Example: One unit breaks down into its component parts i. e. – Remember N’Sync? They split up leaving the resulting in Justin Timberlake and four guys we don’t remember.
Decomposition Example: One unit breaks down into its component parts i. e. – Remember N’Sync? They split up leaving the resulting in Justin Timberlake and four guys we don’t remember.
 Decomposition Example 2 H 2 O 2 (aq) → 2 H 2 O (l) + O 2 (g) Hydrogen peroxide naturally decomposes into water and oxygen gas If you have old hydrogen peroxide in your house, it may have turned into a bottle of water Don’t try and drink it though. Obviously.
Decomposition Example 2 H 2 O 2 (aq) → 2 H 2 O (l) + O 2 (g) Hydrogen peroxide naturally decomposes into water and oxygen gas If you have old hydrogen peroxide in your house, it may have turned into a bottle of water Don’t try and drink it though. Obviously.
 Reaction Type #5 – Synthesis Occurs when two or more components come together to form one compound Energy is released when these reactions occur Reactants will always be: ALWAYS more reactants than products! 2 or more components (either elements or compounds) Products will always be: 1 single compound i. e. : A + B AB
Reaction Type #5 – Synthesis Occurs when two or more components come together to form one compound Energy is released when these reactions occur Reactants will always be: ALWAYS more reactants than products! 2 or more components (either elements or compounds) Products will always be: 1 single compound i. e. : A + B AB
 Synthesis Example Two individual components coming together to form one unit
Synthesis Example Two individual components coming together to form one unit
 Special Cases for Decomposition & Synthesis Reactions Decomposition and Synthesis Reactions are opposite processes Special Case #1: A non metal oxide + water combine to form an acid i. e. : SO 3 + H 2 O H 2 SO 4 AND an acid will decompose to form a non-metal oxide + water i. e. : H 2 SO 4 SO 3 + H 2 O
Special Cases for Decomposition & Synthesis Reactions Decomposition and Synthesis Reactions are opposite processes Special Case #1: A non metal oxide + water combine to form an acid i. e. : SO 3 + H 2 O H 2 SO 4 AND an acid will decompose to form a non-metal oxide + water i. e. : H 2 SO 4 SO 3 + H 2 O
 Special Cases for Decomposition & Synthesis/Formation Reactions Special Case #2 A metal oxide + water combine to form a metal hydroxide i. e. : Ca. O + H 2 O Ca(OH)2 A metal hydroxide will decompose into a metal oxide + water i. e. : Ca(OH)2 Ca. O + H 2 O
Special Cases for Decomposition & Synthesis/Formation Reactions Special Case #2 A metal oxide + water combine to form a metal hydroxide i. e. : Ca. O + H 2 O Ca(OH)2 A metal hydroxide will decompose into a metal oxide + water i. e. : Ca(OH)2 Ca. O + H 2 O
 Special Cases for Decomposition & Synthesis/Formation Reactions Special Case #3: All carbonates decompose into carbon dioxide and an oxide i. e. : Ca. CO 3 Ca. O + CO 2 Vice versa for synthesis/formation reactions Special Case #4: All chlorates decompose into oxygen and a binary salt i. e. : KCl. O 3 KCl + O 2 Vice versa for synthesis/formation reactions
Special Cases for Decomposition & Synthesis/Formation Reactions Special Case #3: All carbonates decompose into carbon dioxide and an oxide i. e. : Ca. CO 3 Ca. O + CO 2 Vice versa for synthesis/formation reactions Special Case #4: All chlorates decompose into oxygen and a binary salt i. e. : KCl. O 3 KCl + O 2 Vice versa for synthesis/formation reactions


