7f13b75af750898a889e4e3182e6a392.ppt
- Количество слайдов: 2
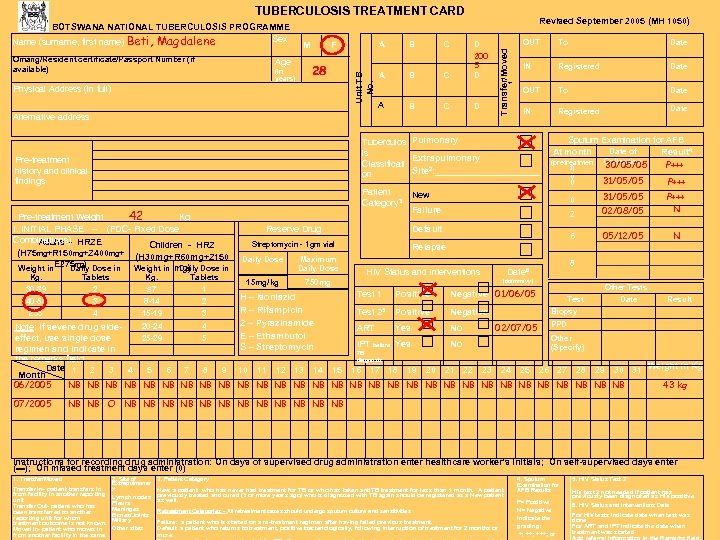
TUBERCULOSIS TREATMENT CARD (in years) 28 Physical Address (in full) B C A B C D 200 5 D A B C D Alternative address To Date IN Registered Date OUT To Date IN Registered Date Tuberculos Pulmonary is Extrapulmonary Classificati Site 2: ___________ on Pre-treatment history and clinical findings Pre-treatment Weight_____42_______Kg I. INITIAL PHASE – (FDC- Fixed Dose Combinations) HRZE Adults Children - HRZ (H 75 mg+R 150 mg+Z 400 mg+ (H 30 mg+R 60 mg+Z 150 Weight in. E 275 mg) Dose in Daily Weight in mg) Dose in Daily Kg. 30 -39 Tablets 2 Kg. ≤ 7 Tablets 1 40 -54 3 8 -14 2 ≥ 55 OUT 1 Age A F Revised September 2005 (MH 1050) Transfer/Moved Omang/Resident certificate/Passport Number (if available) M Unit TB No. BOTSWANA NATIONAL TUBERCULOSIS PROGRAMME Sex Name (surname, first name) Beti, Magdalene 4 15 -19 3 Patient Category 3 Sputum Examination for AFB Date of At month Result 4 (pretreatmen Collection P+++ 30/05/05 t) 0 31/05/05 0 P+++ New 2 Reserve Drug P+++ N 6 Default Streptomycin - 1 gm vial 31/05/05 02/08/05 05/12/05 N 0 Failure Relapse Daily Dose 15 mg/kg Maximum Daily Dose HIV Status and Interventions 750 mg H – Isoniazid R – Rifampicin Z – Pyrazinamide E – Ethambutol S – Streptomycin 8 Date 6 (dd/mm/yy) Test 1 Positive Test 25 Positive Negative Other Tests Negative 01/06/05 Test Date Result Biopsy 20 -24 4 Note: If severe drug side 02/07/05 PPD ART Yes No Other effect, use single dose 25 -29 5 IPT before Yes No (Specify) regimen and indicate in TB diagnosis the remarks field Date 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 Weight in Kg Month 06/2005 NB NB NB NB NB NB NB NB 43 kg 07/2005 NB NB O NB NB NB Instructions for recording drug administration: On days of supervised drug administration enter healthcare worker’s initials; On self-supervised days enter (▬); On missed treatment days enter (0) 1. Transfer/Moved Transfer In– patient transfers in from facility in another reporting unit Transfer Out- patient who has been transferred to another reporting unit for whom treatment outcome is not known. Moved In- patient who moves in from another facility in the same 2. Site of Extrapulmonar y Lymph nodes Pleura Meninges Bones/Joints Miliary Other sites 3. Patient Category New: a patient who has never had treatment for TB or who has taken anti. TB treatment for less than 1 month. A patient previously treated and cured (3 or more years ago) who is diagnosed with TB again should be registered as a New patient as well. Retreatment Categories - All retreatment cases should undergo sputum culture and sensitivities Failure: a patient who is started on a re-treatment regimen after having failed previous treatment. Default: a patient who returns to treatment, positive bacteriologically, following interruption of treatment for 2 months or more. 4. Sputum Examination for AFB Results P= Positive N= Negative Indicate the grading: +; +++; or 5. HIV Status Test 2 HIV test 2 not needed if patient has previously been diagnosed as HIV positive 6. HIV Status and Interventions Date For HIV tests indicate date when test was done For ART and IPT indicate the date when treatment was started Add referral information in the Remarks field.
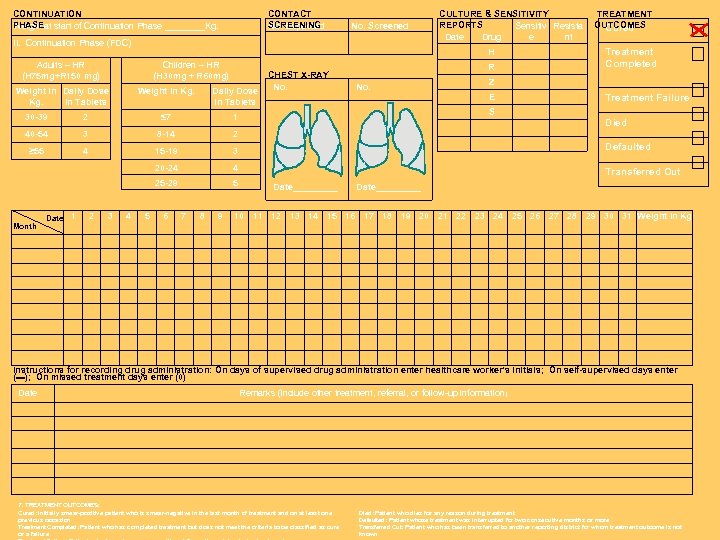
CONTINUATION PHASEat start of Continuation Phase ____Kg. Weight CONTACT SCREENING No. Expected No. Screened II. Continuation Phase (FDC) H Adults – HR (H 75 mg+R 150 mg) Children – HR (H 30 mg + R 60 mg) Weight in Daily Dose Kg. in Tablets CULTURE & SENSITIVITY REPORTS Sensitiv Resista Date Drug e nt Weight in Kg. Daily Dose in Tablets CHEST X-RAY No. R No. TREATMENT OUTCOMES Cured Treatment Completed Z E S Treatment Failure 30 -39 2 ≤ 7 1 40 -54 3 8 -14 2 ≥ 55 4 15 -19 3 Defaulted 20 -24 4 25 -29 5 Transferred Out Month Date 1 2 3 4 5 6 7 8 9 Date_____ Died Date_____ 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 Weight in Kg Instructions for recording drug administration: On days of supervised drug administration enter healthcare worker’s initials; On self-supervised days enter (▬); On missed treatment days enter (0) Date Remarks (include other treatment, referral, or follow-up information) 7. TREATMENT OUTCOMES: Cured: Initially smear-positive patient who is smear-negative in the last month of treatment and on at least one previous occasion Treatment Completed: Patient who has completed treatment but does not meet the criteria to be classified as cure or a failure Died: Patient who dies for any reason during treatment Defaulted: Patient whose treatment was interrupted for two consecutive months or more Transferred Out: Patient who has been transferred to another reporting district for whom treatment outcome is not known
7f13b75af750898a889e4e3182e6a392.ppt