a4822d0b644d931522f293bd7ce27b3b.ppt
- Количество слайдов: 35
 TSH SECRETING TUMORS: AN UPDATE AND THE ISRAELI EXPERIENCE Rosane Abramof Ness Sapir Medical Center
TSH SECRETING TUMORS: AN UPDATE AND THE ISRAELI EXPERIENCE Rosane Abramof Ness Sapir Medical Center
 TSH-Secreting Pituitary Adenomas Rare cause of hyperthyroidism n Originate from pituitary thyrotrophs just 2 ectopic case (nasopharynx) reported. n First case documented in 1960 (TSH measured by bioassay) n Hamilton et al reported the first case of TSH -oma proved by measuring RIA in 1970. n
TSH-Secreting Pituitary Adenomas Rare cause of hyperthyroidism n Originate from pituitary thyrotrophs just 2 ectopic case (nasopharynx) reported. n First case documented in 1960 (TSH measured by bioassay) n Hamilton et al reported the first case of TSH -oma proved by measuring RIA in 1970. n
 Epidemiology n n n Prevalence: 1/1, 000 0. 5 -1% of all pituitary tumors. 336 cases published (7/2004). Since 1990 the number of reported cases has tripled. TSH-omas are equally frequent in men and women. Familial cases have been reported only as part of the multiple neoplasia type 1 syndrome (MEN 1)
Epidemiology n n n Prevalence: 1/1, 000 0. 5 -1% of all pituitary tumors. 336 cases published (7/2004). Since 1990 the number of reported cases has tripled. TSH-omas are equally frequent in men and women. Familial cases have been reported only as part of the multiple neoplasia type 1 syndrome (MEN 1)
 Pathology n n The majority of TSH-secreting adenomas (75%) secrete TSH alone, often accompanied by unbalanced hypersecretion of its alpha-subunit (a. GSU) Mixed adenomas: with concomitant hypersecretion of other pituitary hormones are found in 25% of cases. The most frequent are cosecretion of GH and PRL with its respective syndromes. The somatotroph and lactotroph cells share with thyrot common transcription factors such as Prop-1 and Pit-1. n Rare cases of mixed TSH/gonadotropin adenomas
Pathology n n The majority of TSH-secreting adenomas (75%) secrete TSH alone, often accompanied by unbalanced hypersecretion of its alpha-subunit (a. GSU) Mixed adenomas: with concomitant hypersecretion of other pituitary hormones are found in 25% of cases. The most frequent are cosecretion of GH and PRL with its respective syndromes. The somatotroph and lactotroph cells share with thyrot common transcription factors such as Prop-1 and Pit-1. n Rare cases of mixed TSH/gonadotropin adenomas
 TSH-oma n n Mostly macroadenomas that show invasiveness into the surrounding structure. Extrasellar extension in the supra- and/or parasellar area is present in the majority of cases. The occurrence of invasive macroadenomas is particularly high among patients with previous thyroid ablation by surgery or radioiodine. Microadenomas < 1 cm reported in less than 15% although they are increasingly recognized. 1974 -1986: 1/11 (9%) 1987 -2001: 8/32 (25%) Valdes Socin H et al. European Journal of Endocrinology 2003; 148: 433 -442.
TSH-oma n n Mostly macroadenomas that show invasiveness into the surrounding structure. Extrasellar extension in the supra- and/or parasellar area is present in the majority of cases. The occurrence of invasive macroadenomas is particularly high among patients with previous thyroid ablation by surgery or radioiodine. Microadenomas < 1 cm reported in less than 15% although they are increasingly recognized. 1974 -1986: 1/11 (9%) 1987 -2001: 8/32 (25%) Valdes Socin H et al. European Journal of Endocrinology 2003; 148: 433 -442.
 Etiology n n n Molecular mechanisms leading to TSH-oma are presently unknown. Derive from the clonal expansion of a single initially transformed cell. Candidate genes: Ras, gsp, mutation in TRH receptor gene, dopamine D 2 receptor gene- NEGATIVE Pit-1 mutations: NEGATIVE Loss of function of antioncogenes: p 53 (found in 1 tumor), MENIN- NEGATIVE Somatic mutations of thyroid hormone receptor beta may be responsible for the defect in negative regulation of TSH secretion in some TSH-omas (few cases and not confirmed by all studies)
Etiology n n n Molecular mechanisms leading to TSH-oma are presently unknown. Derive from the clonal expansion of a single initially transformed cell. Candidate genes: Ras, gsp, mutation in TRH receptor gene, dopamine D 2 receptor gene- NEGATIVE Pit-1 mutations: NEGATIVE Loss of function of antioncogenes: p 53 (found in 1 tumor), MENIN- NEGATIVE Somatic mutations of thyroid hormone receptor beta may be responsible for the defect in negative regulation of TSH secretion in some TSH-omas (few cases and not confirmed by all studies)
 Cell Cultures Somatostatin (SRIH): almost all TSH-omas express a variable number of SRIH receptor. n Highest SRIH-binding site density found in mixed GH/TSH adenomas. n Dopamine receptors: large heterogeneity of TSH response to dopamine agonists. n
Cell Cultures Somatostatin (SRIH): almost all TSH-omas express a variable number of SRIH receptor. n Highest SRIH-binding site density found in mixed GH/TSH adenomas. n Dopamine receptors: large heterogeneity of TSH response to dopamine agonists. n
 Clinical Findings Hyperthyroidism (TSH: N- , FT 4 , FT 3 ) n Neurologic symptoms associated to pressure effects of the pituitary adenoma (visual field defects, headaches) n Symptoms due to associated hypersecretion. n Loss of anterior pituitary function n
Clinical Findings Hyperthyroidism (TSH: N- , FT 4 , FT 3 ) n Neurologic symptoms associated to pressure effects of the pituitary adenoma (visual field defects, headaches) n Symptoms due to associated hypersecretion. n Loss of anterior pituitary function n
 Thyrotoxicosis with Inappropriately high TSH levels n Mouse ab interfering with TSH assay n Central hyperthyroidism: q Pituitary tumor: TSH secreting. q Resistance to thyroid hormone (RTH)
Thyrotoxicosis with Inappropriately high TSH levels n Mouse ab interfering with TSH assay n Central hyperthyroidism: q Pituitary tumor: TSH secreting. q Resistance to thyroid hormone (RTH)
 Resistance to Thyroid Hormone Autosomal dominant disorder characterized by reduced responsiveness of target tissues to thyroid hormone due to a mutation in the thyroid hormone receptor beta. n First reported in 1967. n Variable severity of hormonal resistance in different tissues. n
Resistance to Thyroid Hormone Autosomal dominant disorder characterized by reduced responsiveness of target tissues to thyroid hormone due to a mutation in the thyroid hormone receptor beta. n First reported in 1967. n Variable severity of hormonal resistance in different tissues. n
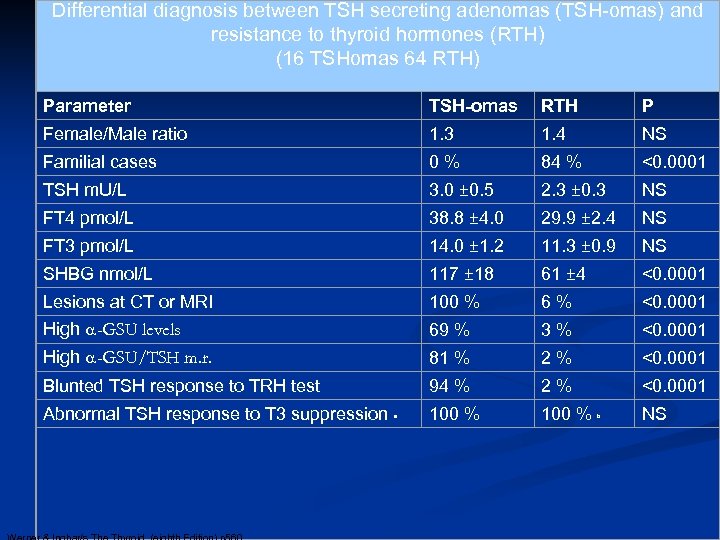 Differential diagnosis between TSH secreting adenomas (TSH-omas) and resistance to thyroid hormones (RTH) (16 TSHomas 64 RTH) Parameter TSH-omas RTH P Female/Male ratio 1. 3 1. 4 NS Familial cases 0 % 84 % <0. 0001 TSH m. U/L 3. 0 ± 0. 5 2. 3 ± 0. 3 NS FT 4 pmol/L 38. 8 ± 4. 0 29. 9 ± 2. 4 NS FT 3 pmol/L 14. 0 ± 1. 2 11. 3 ± 0. 9 NS SHBG nmol/L 117 ± 18 61 ± 4 <0. 0001 Lesions at CT or MRI 100 % 6 % <0. 0001 High a-GSU levels 69 % 3 % <0. 0001 High a-GSU/TSH m. r. 81 % 2 % <0. 0001 Blunted TSH response to TRH test 94 % 2 % <0. 0001 100 % Abnormal TSH response to T 3 suppression a b NS
Differential diagnosis between TSH secreting adenomas (TSH-omas) and resistance to thyroid hormones (RTH) (16 TSHomas 64 RTH) Parameter TSH-omas RTH P Female/Male ratio 1. 3 1. 4 NS Familial cases 0 % 84 % <0. 0001 TSH m. U/L 3. 0 ± 0. 5 2. 3 ± 0. 3 NS FT 4 pmol/L 38. 8 ± 4. 0 29. 9 ± 2. 4 NS FT 3 pmol/L 14. 0 ± 1. 2 11. 3 ± 0. 9 NS SHBG nmol/L 117 ± 18 61 ± 4 <0. 0001 Lesions at CT or MRI 100 % 6 % <0. 0001 High a-GSU levels 69 % 3 % <0. 0001 High a-GSU/TSH m. r. 81 % 2 % <0. 0001 Blunted TSH response to TRH test 94 % 2 % <0. 0001 100 % Abnormal TSH response to T 3 suppression a b NS
 A Pituitary Tumor in a Patient with Thyroid Hormone Resistance: A Diagnostic Dilemma Safer et al Thyroid 2001
A Pituitary Tumor in a Patient with Thyroid Hormone Resistance: A Diagnostic Dilemma Safer et al Thyroid 2001
 Localization n MRI Pituitary scintigraphy with radiolabeled octreotide (octreoscan) n PET : (11)C-Methionine PET n Petrosal sinus sampling (PSS) n
Localization n MRI Pituitary scintigraphy with radiolabeled octreotide (octreoscan) n PET : (11)C-Methionine PET n Petrosal sinus sampling (PSS) n
 Treatment Surgery n Radiation therapy n Somatostatin analogues n Dopamine agonists n
Treatment Surgery n Radiation therapy n Somatostatin analogues n Dopamine agonists n
 Surgical Treatment n First therapeutic approach n Normalization of TFT’s and disappearance of tumor in 33 -44% of patients. n Normalization of TFT’s in 25% n Unsuccessful in 25%
Surgical Treatment n First therapeutic approach n Normalization of TFT’s and disappearance of tumor in 33 -44% of patients. n Normalization of TFT’s in 25% n Unsuccessful in 25%
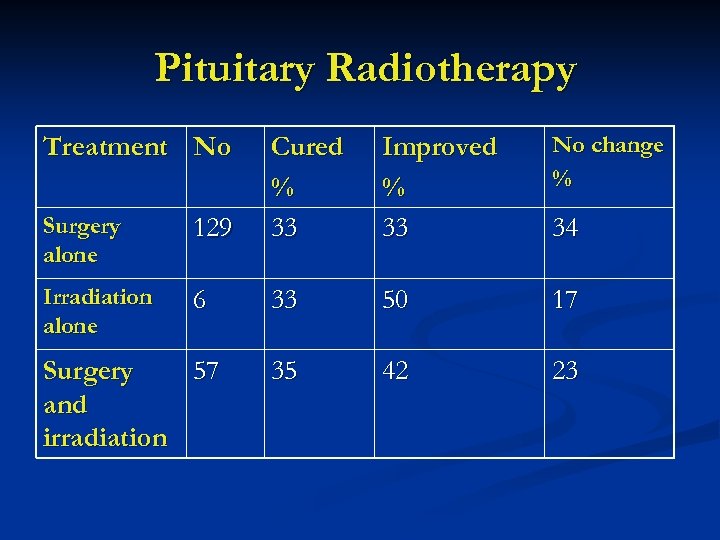 Pituitary Radiotherapy Treatment No Improved % 33 No change % Surgery alone 129 Cured % 33 Irradiation alone 6 33 50 17 35 42 23 Surgery 57 and irradiation 34
Pituitary Radiotherapy Treatment No Improved % 33 No change % Surgery alone 129 Cured % 33 Irradiation alone 6 33 50 17 35 42 23 Surgery 57 and irradiation 34
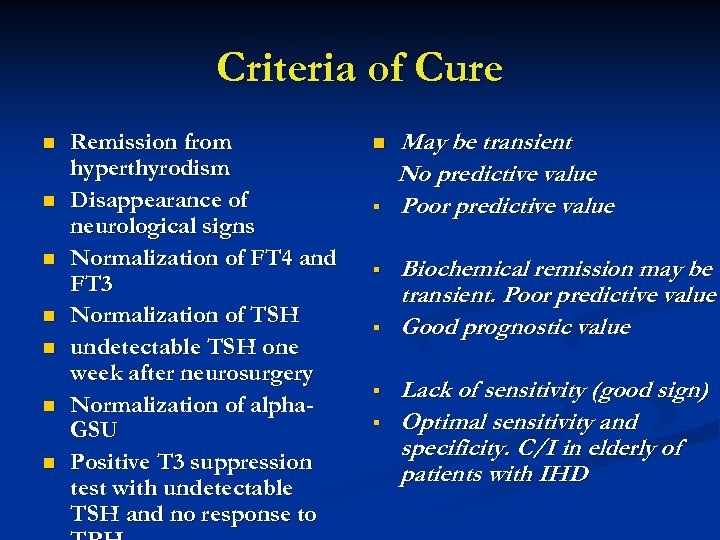 Criteria of Cure n n n n Remission from hyperthyrodism Disappearance of neurological signs Normalization of FT 4 and FT 3 Normalization of TSH undetectable TSH one week after neurosurgery Normalization of alpha. GSU Positive T 3 suppression test with undetectable TSH and no response to n § § § May be transient No predictive value Poor predictive value Biochemical remission may be transient. Poor predictive value Good prognostic value Lack of sensitivity (good sign) Optimal sensitivity and specificity. C/I in elderly of patients with IHD
Criteria of Cure n n n n Remission from hyperthyrodism Disappearance of neurological signs Normalization of FT 4 and FT 3 Normalization of TSH undetectable TSH one week after neurosurgery Normalization of alpha. GSU Positive T 3 suppression test with undetectable TSH and no response to n § § § May be transient No predictive value Poor predictive value Biochemical remission may be transient. Poor predictive value Good prognostic value Lack of sensitivity (good sign) Optimal sensitivity and specificity. C/I in elderly of patients with IHD
 Medical Therapy n Somatostatin analogues: TSH reduction (> 50%) 90% Alpha-GSU reduction 93% Thyroid hormone normalization 96% Goiter size reduction 20% Tumor mass shrinkage (>20%) 45% Resistance 4% Discontinuation of therapy (s/e) 7% Beck- eccoz Persani. Medical Management of Peccoz and. Thyrotropin -Secreting Pituitary Adenomas. P Pituitary 2002 : 83 -88
Medical Therapy n Somatostatin analogues: TSH reduction (> 50%) 90% Alpha-GSU reduction 93% Thyroid hormone normalization 96% Goiter size reduction 20% Tumor mass shrinkage (>20%) 45% Resistance 4% Discontinuation of therapy (s/e) 7% Beck- eccoz Persani. Medical Management of Peccoz and. Thyrotropin -Secreting Pituitary Adenomas. P Pituitary 2002 : 83 -88
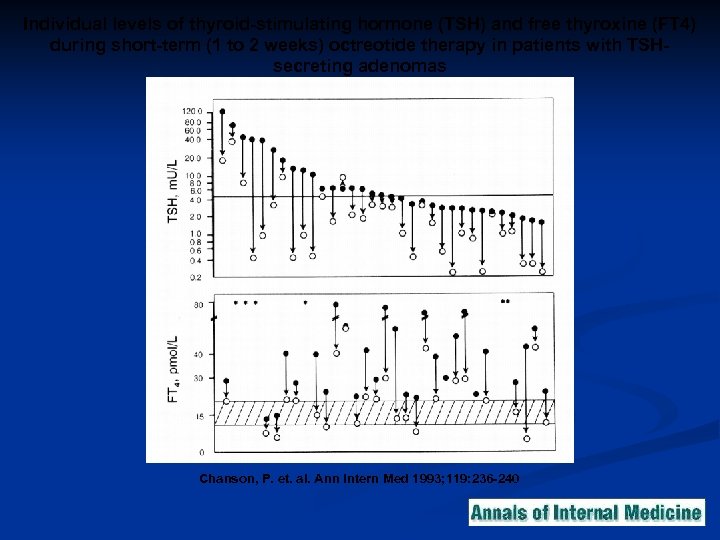 Individual levels of thyroid-stimulating hormone (TSH) and free thyroxine (FT 4) during short-term (1 to 2 weeks) octreotide therapy in patients with TSHsecreting adenomas Chanson, P. et. al. Ann Intern Med 1993; 119: 236 -240
Individual levels of thyroid-stimulating hormone (TSH) and free thyroxine (FT 4) during short-term (1 to 2 weeks) octreotide therapy in patients with TSHsecreting adenomas Chanson, P. et. al. Ann Intern Med 1993; 119: 236 -240
 Dopamine Agonists No long term effect in obtaining normalization of TFT’s or tumor shrinkage n Effective in cases of TSH-PRL co secretion n
Dopamine Agonists No long term effect in obtaining normalization of TFT’s or tumor shrinkage n Effective in cases of TSH-PRL co secretion n
 TSH-secreting tumors: The Israeli Experience Ness-Abramof R, Ishay A, Greenman Y, Harel G and Shimon I.
TSH-secreting tumors: The Israeli Experience Ness-Abramof R, Ishay A, Greenman Y, Harel G and Shimon I.
 Patient’s Characteristics n n n § No : 9 Sex: 4 M/ 5 F Age 44 ± 18 years (range: 18 -80 y) Goiter: 5/7 pts Symptoms of TX: 5/9 (tremor, PAF) Duration of symptoms before diagnosis: 2. 5 y ± 1. 7 ( range 0. 5 - 4 years) Duration of follow up: 9. 4 ± 5. 5 years (range 1. 5 -16 )
Patient’s Characteristics n n n § No : 9 Sex: 4 M/ 5 F Age 44 ± 18 years (range: 18 -80 y) Goiter: 5/7 pts Symptoms of TX: 5/9 (tremor, PAF) Duration of symptoms before diagnosis: 2. 5 y ± 1. 7 ( range 0. 5 - 4 years) Duration of follow up: 9. 4 ± 5. 5 years (range 1. 5 -16 )
 Laboratory tests n TSH 5. 0 m. U/L ± 1. 5 (nl: 0. 3 -4) (range: 3 -8. 6 m. U/L) n FT 4 45. 7 pmol/L ± 17. 5 (nl: 10 -20) (range 24. 3 - > 77 pmol/L) (6/9 pts) n TT 4 228 ± 23. 3 nmol/L (nl: 53 -143) ( 2/9 pts) n T 3 T 5. 49 nmol/L ± 3. 8 (nl: 1 -2. 8) (range 3. 1 - 13 nmol/L) (6/9 pts)
Laboratory tests n TSH 5. 0 m. U/L ± 1. 5 (nl: 0. 3 -4) (range: 3 -8. 6 m. U/L) n FT 4 45. 7 pmol/L ± 17. 5 (nl: 10 -20) (range 24. 3 - > 77 pmol/L) (6/9 pts) n TT 4 228 ± 23. 3 nmol/L (nl: 53 -143) ( 2/9 pts) n T 3 T 5. 49 nmol/L ± 3. 8 (nl: 1 -2. 8) (range 3. 1 - 13 nmol/L) (6/9 pts)
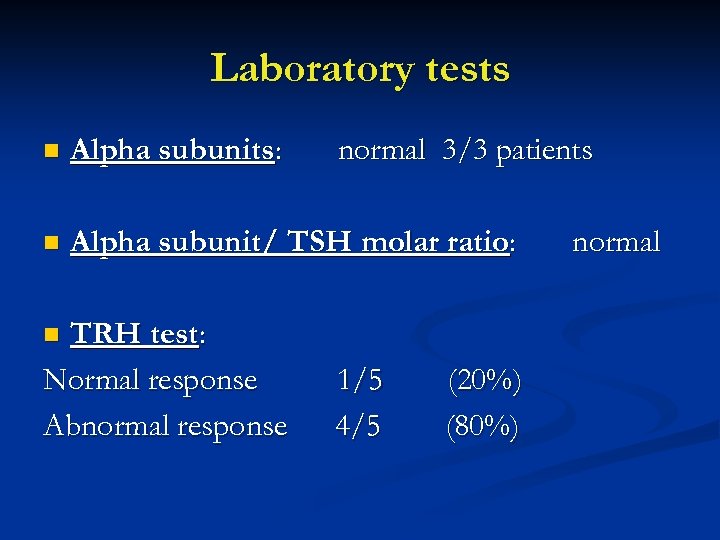 Laboratory tests n Alpha subunits: n Alpha subunit/ TSH molar ratio: TRH test: Normal response Abnormal response normal 3/3 patients n 1/5 4/5 (20%) (80%) normal
Laboratory tests n Alpha subunits: n Alpha subunit/ TSH molar ratio: TRH test: Normal response Abnormal response normal 3/3 patients n 1/5 4/5 (20%) (80%) normal
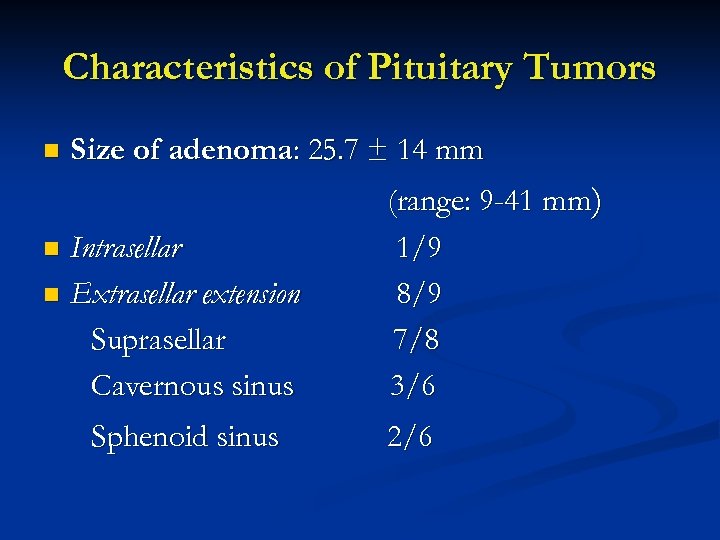 Characteristics of Pituitary Tumors n Size of adenoma: 25. 7 ± 14 mm Intrasellar n Extrasellar extension Suprasellar Cavernous sinus n Sphenoid sinus (range: 9 -41 mm) 1/9 8/9 7/8 3/6 2/6
Characteristics of Pituitary Tumors n Size of adenoma: 25. 7 ± 14 mm Intrasellar n Extrasellar extension Suprasellar Cavernous sinus n Sphenoid sinus (range: 9 -41 mm) 1/9 8/9 7/8 3/6 2/6
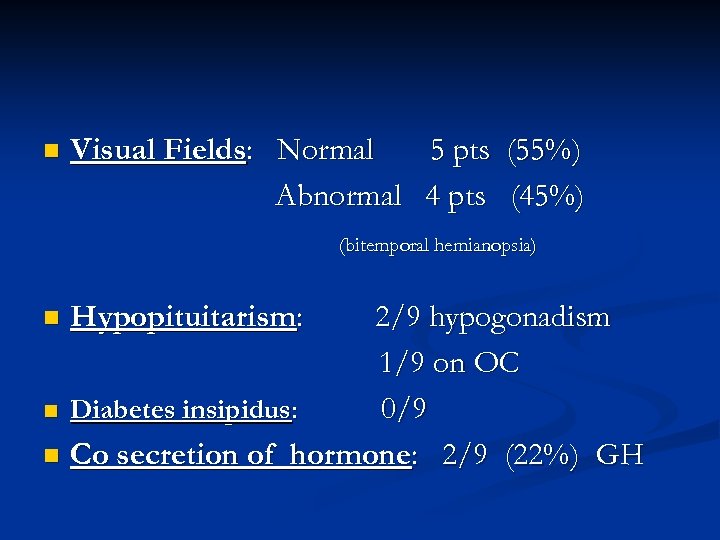 n Visual Fields: Normal 5 pts (55%) Abnormal 4 pts (45%) (bitemporal hemianopsia) 2/9 hypogonadism 1/9 on OC n Diabetes insipidus: 0/9 n Co secretion of hormone: 2/9 (22%) GH n Hypopituitarism:
n Visual Fields: Normal 5 pts (55%) Abnormal 4 pts (45%) (bitemporal hemianopsia) 2/9 hypogonadism 1/9 on OC n Diabetes insipidus: 0/9 n Co secretion of hormone: 2/9 (22%) GH n Hypopituitarism:
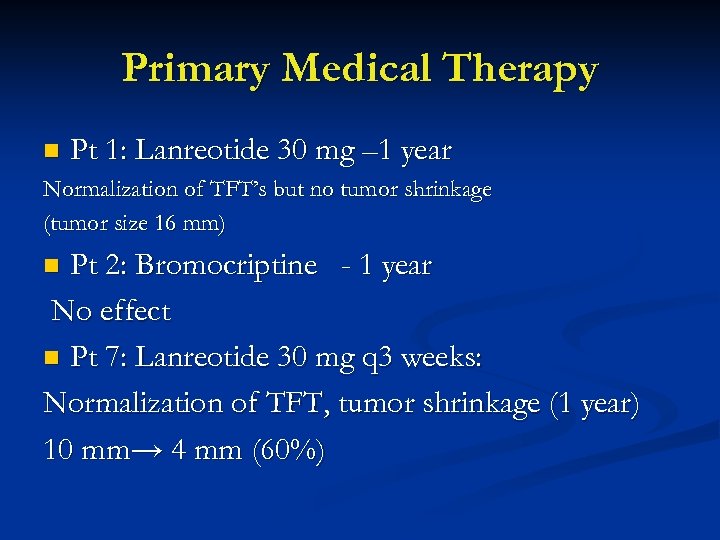 Primary Medical Therapy n Pt 1: Lanreotide 30 mg – 1 year Normalization of TFT’s but no tumor shrinkage (tumor size 16 mm) Pt 2: Bromocriptine - 1 year No effect n Pt 7: Lanreotide 30 mg q 3 weeks: Normalization of TFT, tumor shrinkage (1 year) 10 mm→ 4 mm (60%) n
Primary Medical Therapy n Pt 1: Lanreotide 30 mg – 1 year Normalization of TFT’s but no tumor shrinkage (tumor size 16 mm) Pt 2: Bromocriptine - 1 year No effect n Pt 7: Lanreotide 30 mg q 3 weeks: Normalization of TFT, tumor shrinkage (1 year) 10 mm→ 4 mm (60%) n
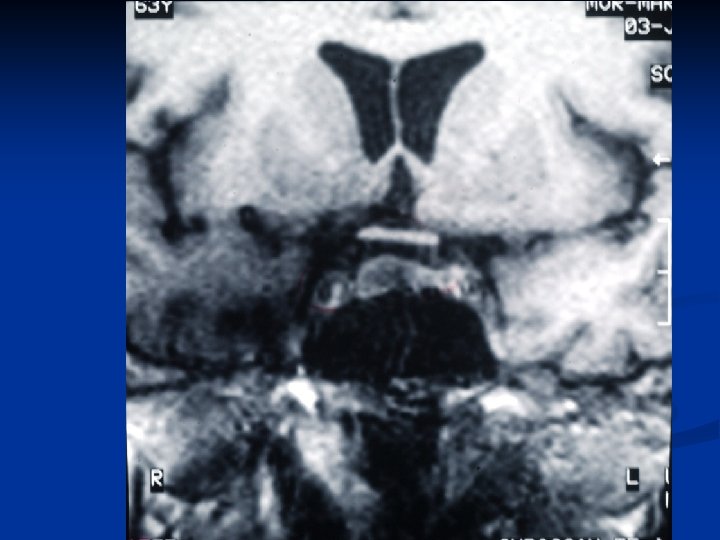
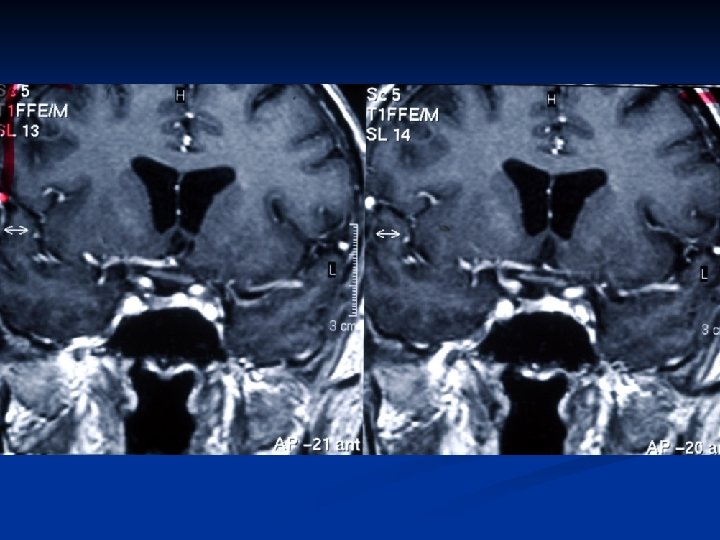
 Surgical Therapy n 8 patients ( 2/8 had 2 surgeries) n Normalization of TFT’s: 1 pt (transient) n Abnormal TFT’s : 5 pts ( The patient with a microadenoma didn’t have surgery) n Approach: Transphenoidal: Transfrontal: • 7/8 1/8 Hypopituitarism : 3/7 Hypogonadism : 2/7 Adrenal insufficiency : 1/7 Diabetes insipidus: 0/7 (2 patients were treated perioperatively) n Residual tumor: 8 pts
Surgical Therapy n 8 patients ( 2/8 had 2 surgeries) n Normalization of TFT’s: 1 pt (transient) n Abnormal TFT’s : 5 pts ( The patient with a microadenoma didn’t have surgery) n Approach: Transphenoidal: Transfrontal: • 7/8 1/8 Hypopituitarism : 3/7 Hypogonadism : 2/7 Adrenal insufficiency : 1/7 Diabetes insipidus: 0/7 (2 patients were treated perioperatively) n Residual tumor: 8 pts
 Post Operative Therapy n Radiation therapy: 3 patients (2 due to residual tumor and 1 tumor regrowth) Medical therapy (7 pts) Lanreotide: 3 pts (Somatuline 30 mg, Autogel 60 mg) Octreotide: LAR: 3 pts (dose 30 mg) s. c: 2 pts (dose 100 mic/day) Dopamine agonists: 2 pts (1 s/e, 1 ineffective) n (1 patients lost to f/u)
Post Operative Therapy n Radiation therapy: 3 patients (2 due to residual tumor and 1 tumor regrowth) Medical therapy (7 pts) Lanreotide: 3 pts (Somatuline 30 mg, Autogel 60 mg) Octreotide: LAR: 3 pts (dose 30 mg) s. c: 2 pts (dose 100 mic/day) Dopamine agonists: 2 pts (1 s/e, 1 ineffective) n (1 patients lost to f/u)
 Chronic Somatostatin Analogue Therapy (post surgical and primary therapy) Duration of therapy: 4. 6 ± 4. 3 years (range: 1. 5 -14 years) § Normalization of TFT’s: 8/8 patients n Tumor shrinkage: 2/8 patients (10 mm→ 4 mm) (12 mm → 9 mm) n Tumor growth : 0/8 patients n Central hypothyroidism: 2/8 patients n Side effects: cholelithiasis (1) abdominal pain: (4) n
Chronic Somatostatin Analogue Therapy (post surgical and primary therapy) Duration of therapy: 4. 6 ± 4. 3 years (range: 1. 5 -14 years) § Normalization of TFT’s: 8/8 patients n Tumor shrinkage: 2/8 patients (10 mm→ 4 mm) (12 mm → 9 mm) n Tumor growth : 0/8 patients n Central hypothyroidism: 2/8 patients n Side effects: cholelithiasis (1) abdominal pain: (4) n
 Conclusions n n Surgical therapy was not curative. Somatostatin analogues were highly effective in normalizing thyroid function tests (100%) No tumor growth during somatostatin analogue therapy was observed. The role of somatostatin analogues as primary therapy for TSH secreting tumors, particularly microadenomas needs to be further evaluated.
Conclusions n n Surgical therapy was not curative. Somatostatin analogues were highly effective in normalizing thyroid function tests (100%) No tumor growth during somatostatin analogue therapy was observed. The role of somatostatin analogues as primary therapy for TSH secreting tumors, particularly microadenomas needs to be further evaluated.
 THANK YOU
THANK YOU
 The changing spectrum of TSH-secreting pituitary adenomas: diagnosis and management in 43 patients Proportion of microadenoma X macroadenoma 1974 -1986: 1/11 (9%) 1987 -2001: 8/32 (25%) v Medical therapy with somatostatin analogues was the first line therapy in 26 patients (19 had surgery) v TSH levels were reduced by more than 50% in 23/26 patients (normalization of TFT 22/26 – 85%) v Tumor shrinkage of more than 20% was observed in 5/13 cases (36%). v Valdes Socin H et al. European Journal of Endocrinology 2003; 148: 433 -442.
The changing spectrum of TSH-secreting pituitary adenomas: diagnosis and management in 43 patients Proportion of microadenoma X macroadenoma 1974 -1986: 1/11 (9%) 1987 -2001: 8/32 (25%) v Medical therapy with somatostatin analogues was the first line therapy in 26 patients (19 had surgery) v TSH levels were reduced by more than 50% in 23/26 patients (normalization of TFT 22/26 – 85%) v Tumor shrinkage of more than 20% was observed in 5/13 cases (36%). v Valdes Socin H et al. European Journal of Endocrinology 2003; 148: 433 -442.


