1e10e429131eeaa6757b70c31cfd3d3b.ppt
- Количество слайдов: 51
 Trost’s Palladium Catalysed Asymmetric Allylic Alkylation (Pd-AAA) Literature Meeting Charette’s group Miguel St-Onge October 9 th, 2007 1
Trost’s Palladium Catalysed Asymmetric Allylic Alkylation (Pd-AAA) Literature Meeting Charette’s group Miguel St-Onge October 9 th, 2007 1
 Presentation 1. Trost and Palladium 2. -allyl complexes i. iii. iv. 3. 4. 5. 6. 7. 8. 9. Stereochemistry of oxidative addition and nucleophilic attack Counter anion effects Syn vs anti complexes Nucleophilic approach on allyl terminus Ligands and cartoon model Classes of enantiodiscrimination processes Types of nucleophiles and their application to total synthesis Exceptions to the model AAA with other metals Total synthesis of Tipranavir Conclusion 2
Presentation 1. Trost and Palladium 2. -allyl complexes i. iii. iv. 3. 4. 5. 6. 7. 8. 9. Stereochemistry of oxidative addition and nucleophilic attack Counter anion effects Syn vs anti complexes Nucleophilic approach on allyl terminus Ligands and cartoon model Classes of enantiodiscrimination processes Types of nucleophiles and their application to total synthesis Exceptions to the model AAA with other metals Total synthesis of Tipranavir Conclusion 2
 Pr. Barry M. Trost Ø Born in 1941 in Philadelphia Ø Received B. A. From University of Pennsylvania (1962) Ø Received Ph. D. at MIT under H. O. House’s supervision (1965) Ø Professor of chemistry at University of Wisconsin (1969) Vilas research professor of chemistry (1982) Ø Professor of chemistry at Standford University (1987) Takami professor of humanities and sciences (1990) Ø 803 publications (2006) Ø 38 honors and awards Ø 14 Patents Barry Trost web page at www. stanford. edu/group/bmtrost 3
Pr. Barry M. Trost Ø Born in 1941 in Philadelphia Ø Received B. A. From University of Pennsylvania (1962) Ø Received Ph. D. at MIT under H. O. House’s supervision (1965) Ø Professor of chemistry at University of Wisconsin (1969) Vilas research professor of chemistry (1982) Ø Professor of chemistry at Standford University (1987) Takami professor of humanities and sciences (1990) Ø 803 publications (2006) Ø 38 honors and awards Ø 14 Patents Barry Trost web page at www. stanford. edu/group/bmtrost 3
 Palladium • Discovered in 1803 by William Hyde Wollaston • Isolated from (NH 4)2 Pt. Cl 6 • Name comes from Greek goddess of wisdom, Pallas or Palladion • Atomic number 46 • [Kr] 4 d 10 • Pd 0 = 18 e square planar complexes • Pd(II) = 14 e square planar complexes 4
Palladium • Discovered in 1803 by William Hyde Wollaston • Isolated from (NH 4)2 Pt. Cl 6 • Name comes from Greek goddess of wisdom, Pallas or Palladion • Atomic number 46 • [Kr] 4 d 10 • Pd 0 = 18 e square planar complexes • Pd(II) = 14 e square planar complexes 4
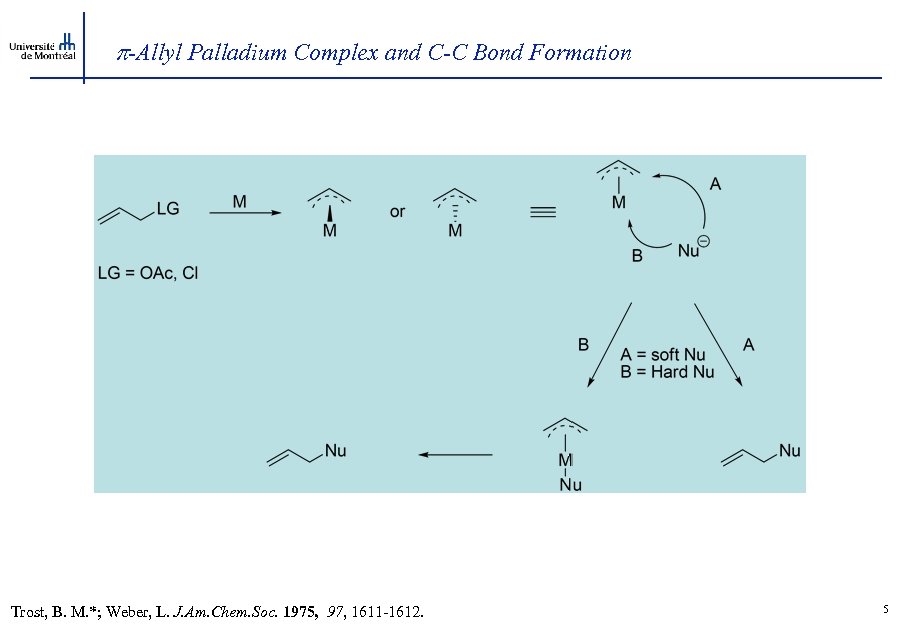 -Allyl Palladium Complex and C-C Bond Formation Trost, B. M. *; Weber, L. J. Am. Chem. Soc. 1975, 97, 1611 -1612. 5
-Allyl Palladium Complex and C-C Bond Formation Trost, B. M. *; Weber, L. J. Am. Chem. Soc. 1975, 97, 1611 -1612. 5
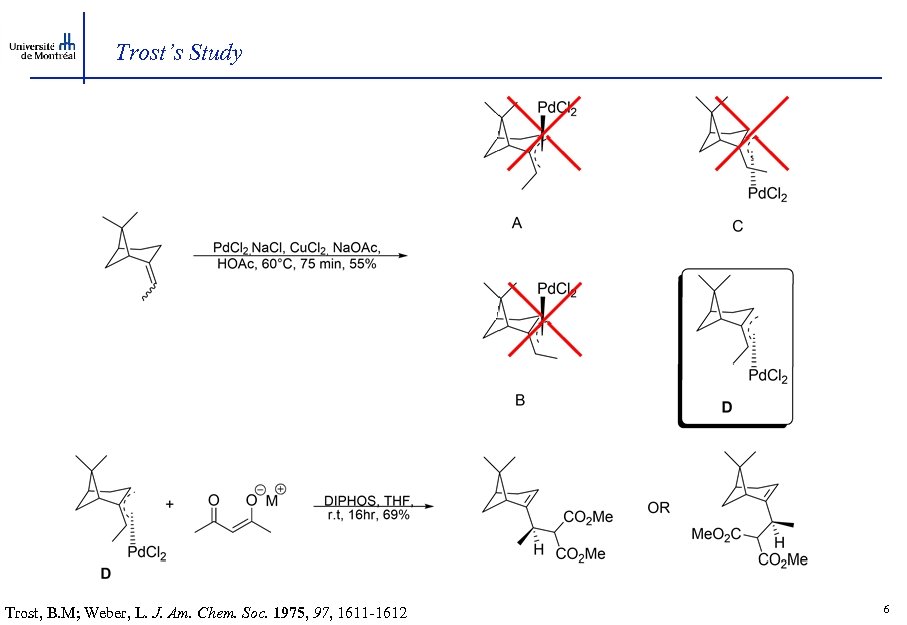 Trost’s Study Trost, B. M; Weber, L. J. Am. Chem. Soc. 1975, 97, 1611 -1612 6
Trost’s Study Trost, B. M; Weber, L. J. Am. Chem. Soc. 1975, 97, 1611 -1612 6
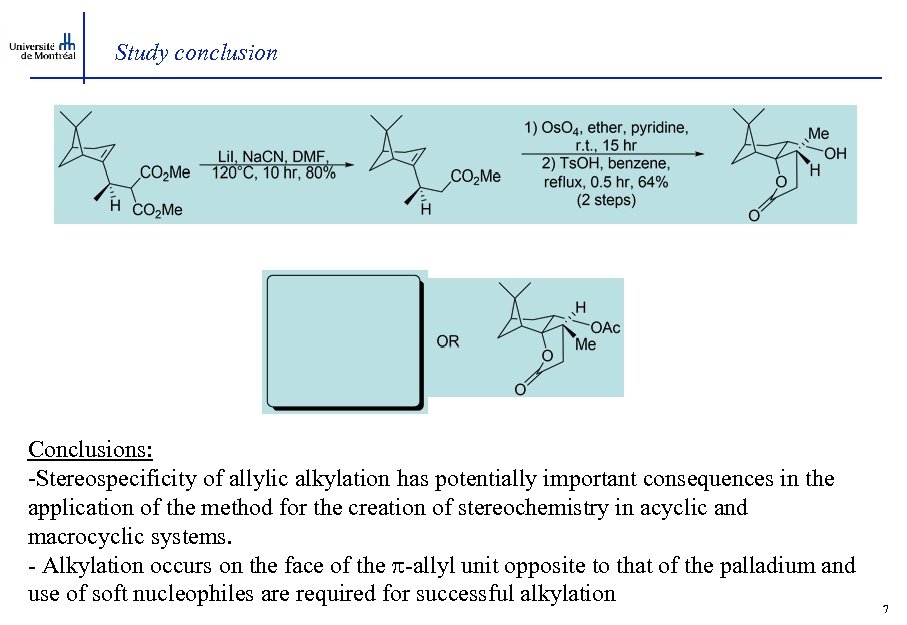 Study conclusion Conclusions: -Stereospecificity of allylic alkylation has potentially important consequences in the application of the method for the creation of stereochemistry in acyclic and macrocyclic systems. - Alkylation occurs on the face of the -allyl unit opposite to that of the palladium and use of soft nucleophiles are required for successful alkylation 7
Study conclusion Conclusions: -Stereospecificity of allylic alkylation has potentially important consequences in the application of the method for the creation of stereochemistry in acyclic and macrocyclic systems. - Alkylation occurs on the face of the -allyl unit opposite to that of the palladium and use of soft nucleophiles are required for successful alkylation 7
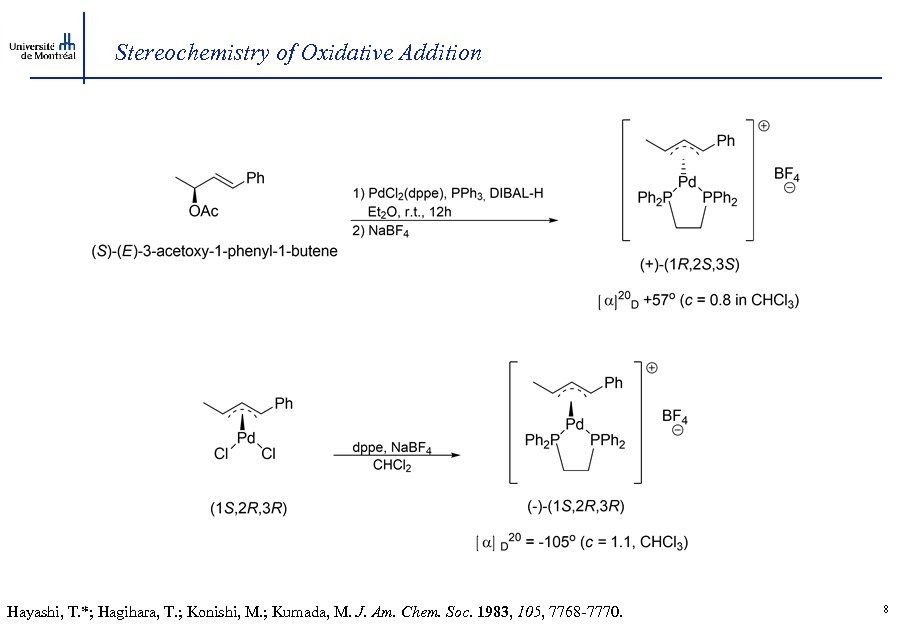 Stereochemistry of Oxidative Addition Hayashi, T. *; Hagihara, T. ; Konishi, M. ; Kumada, M. J. Am. Chem. Soc. 1983, 105, 7768 -7770. 8
Stereochemistry of Oxidative Addition Hayashi, T. *; Hagihara, T. ; Konishi, M. ; Kumada, M. J. Am. Chem. Soc. 1983, 105, 7768 -7770. 8
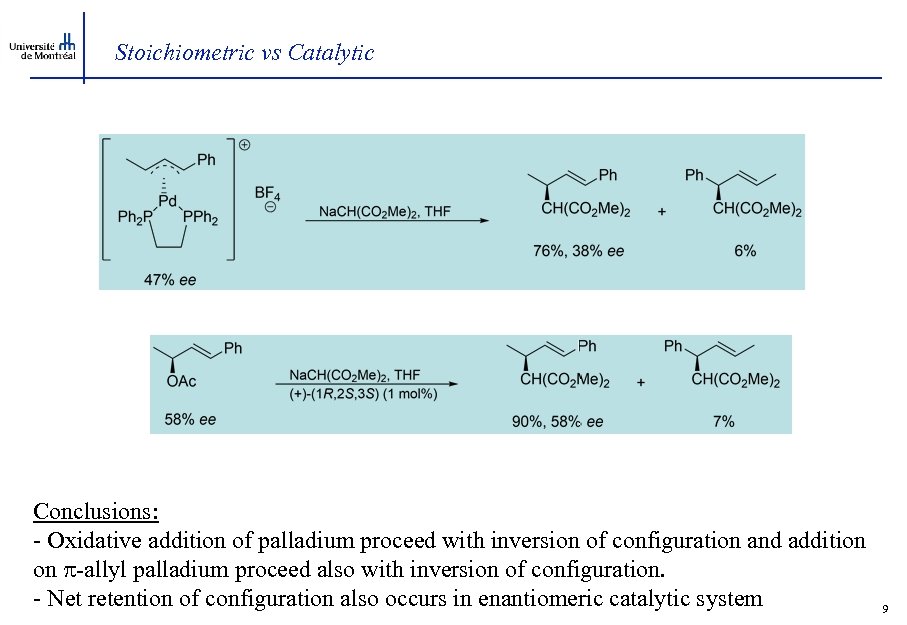 Stoichiometric vs Catalytic Conclusions: - Oxidative addition of palladium proceed with inversion of configuration and addition on -allyl palladium proceed also with inversion of configuration. - Net retention of configuration also occurs in enantiomeric catalytic system 9
Stoichiometric vs Catalytic Conclusions: - Oxidative addition of palladium proceed with inversion of configuration and addition on -allyl palladium proceed also with inversion of configuration. - Net retention of configuration also occurs in enantiomeric catalytic system 9
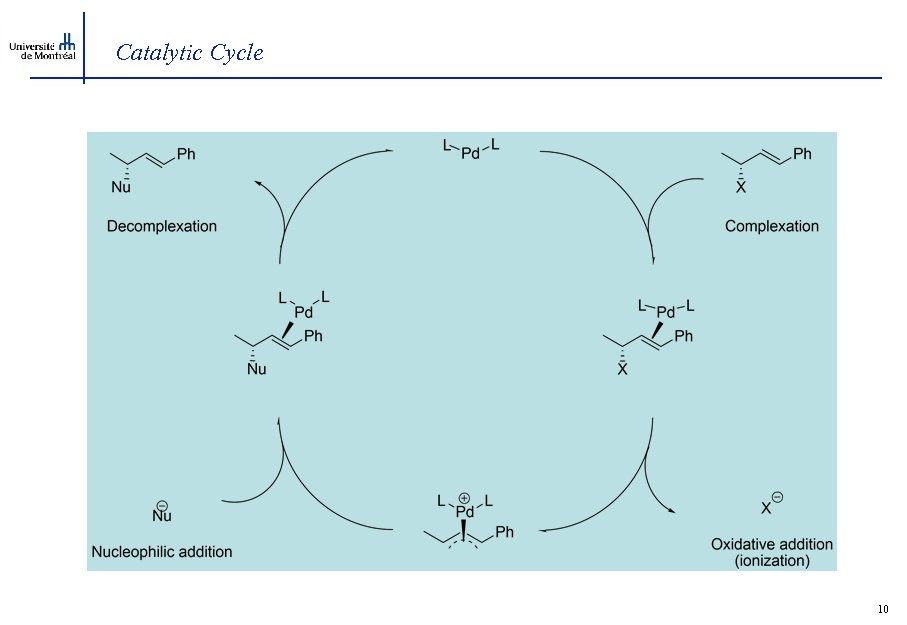 Catalytic Cycle 10
Catalytic Cycle 10
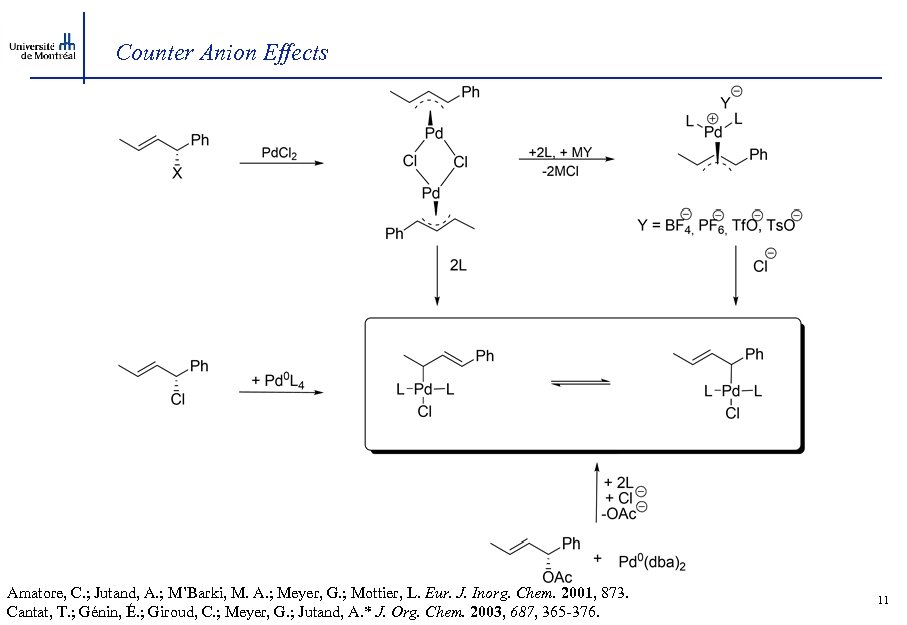 Counter Anion Effects Amatore, C. ; Jutand, A. ; M’Barki, M. A. ; Meyer, G. ; Mottier, L. Eur. J. Inorg. Chem. 2001, 873. Cantat, T. ; Génin, É. ; Giroud, C. ; Meyer, G. ; Jutand, A. * J. Org. Chem. 2003, 687, 365 -376. 11
Counter Anion Effects Amatore, C. ; Jutand, A. ; M’Barki, M. A. ; Meyer, G. ; Mottier, L. Eur. J. Inorg. Chem. 2001, 873. Cantat, T. ; Génin, É. ; Giroud, C. ; Meyer, G. ; Jutand, A. * J. Org. Chem. 2003, 687, 365 -376. 11
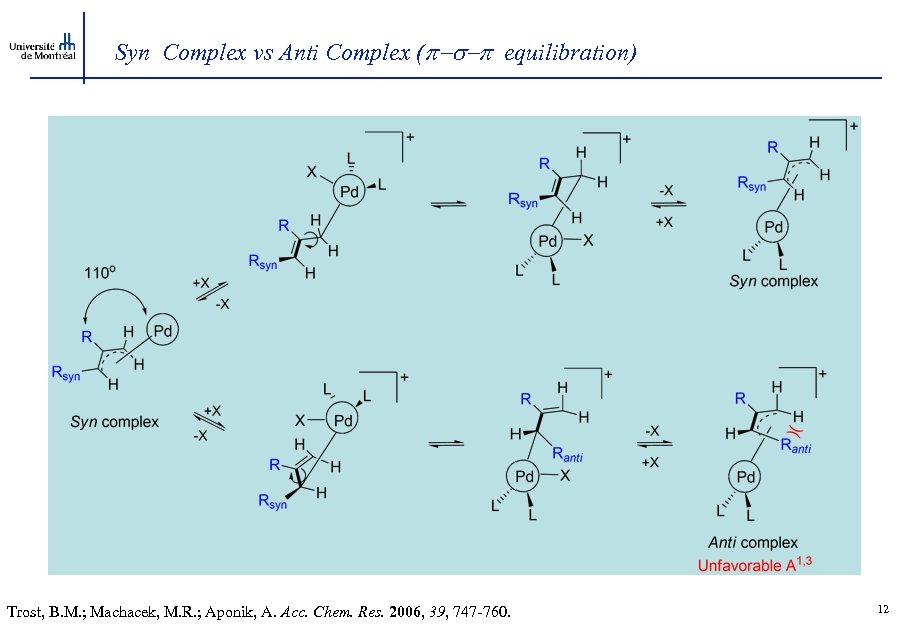 Syn Complex vs Anti Complex ( -s- equilibration) Trost, B. M. ; Machacek, M. R. ; Aponik, A. Acc. Chem. Res. 2006, 39, 747 -760. 12
Syn Complex vs Anti Complex ( -s- equilibration) Trost, B. M. ; Machacek, M. R. ; Aponik, A. Acc. Chem. Res. 2006, 39, 747 -760. 12
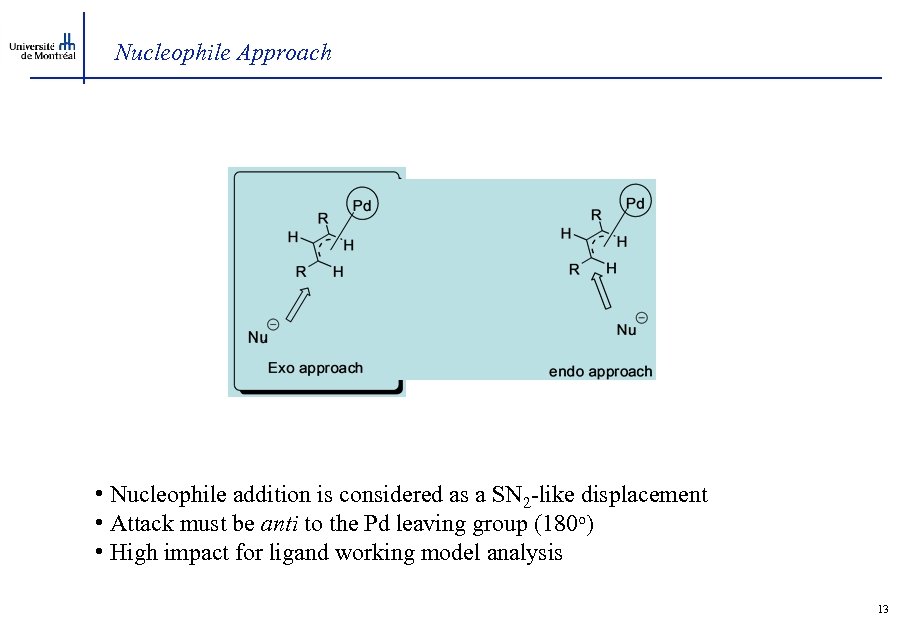 Nucleophile Approach • Nucleophile addition is considered as a SN 2 -like displacement • Attack must be anti to the Pd leaving group (180 o) • High impact for ligand working model analysis 13
Nucleophile Approach • Nucleophile addition is considered as a SN 2 -like displacement • Attack must be anti to the Pd leaving group (180 o) • High impact for ligand working model analysis 13
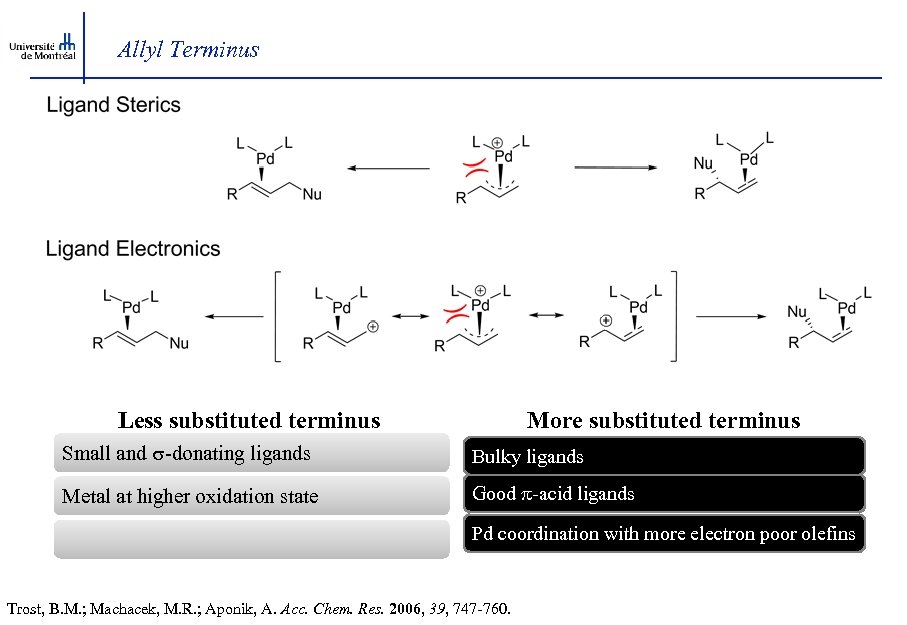 Allyl Terminus Less substituted terminus More substituted terminus Small and s-donating ligands Bulky ligands Metal at higher oxidation state Good -acid ligands Pd coordination with more electron poor olefins Trost, B. M. ; Machacek, M. R. ; Aponik, A. Acc. Chem. Res. 2006, 39, 747 -760.
Allyl Terminus Less substituted terminus More substituted terminus Small and s-donating ligands Bulky ligands Metal at higher oxidation state Good -acid ligands Pd coordination with more electron poor olefins Trost, B. M. ; Machacek, M. R. ; Aponik, A. Acc. Chem. Res. 2006, 39, 747 -760.
 Redesign Catalytic Cycle 15
Redesign Catalytic Cycle 15
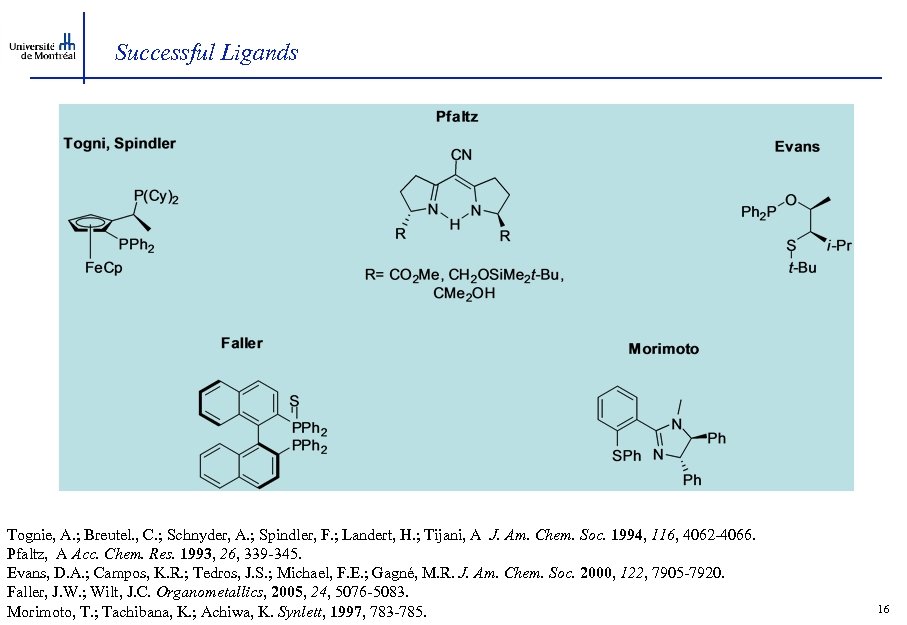 Successful Ligands Tognie, A. ; Breutel. , C. ; Schnyder, A. ; Spindler, F. ; Landert, H. ; Tijani, A J. Am. Chem. Soc. 1994, 116, 4062 -4066. Pfaltz, A Acc. Chem. Res. 1993, 26, 339 -345. Evans, D. A. ; Campos, K. R. ; Tedros, J. S. ; Michael, F. E. ; Gagné, M. R. J. Am. Chem. Soc. 2000, 122, 7905 -7920. Faller, J. W. ; Wilt, J. C. Organometallics, 2005, 24, 5076 -5083. Morimoto, T. ; Tachibana, K. ; Achiwa, K. Synlett, 1997, 783 -785. 16
Successful Ligands Tognie, A. ; Breutel. , C. ; Schnyder, A. ; Spindler, F. ; Landert, H. ; Tijani, A J. Am. Chem. Soc. 1994, 116, 4062 -4066. Pfaltz, A Acc. Chem. Res. 1993, 26, 339 -345. Evans, D. A. ; Campos, K. R. ; Tedros, J. S. ; Michael, F. E. ; Gagné, M. R. J. Am. Chem. Soc. 2000, 122, 7905 -7920. Faller, J. W. ; Wilt, J. C. Organometallics, 2005, 24, 5076 -5083. Morimoto, T. ; Tachibana, K. ; Achiwa, K. Synlett, 1997, 783 -785. 16
 Trost’s Classic Ligands 17
Trost’s Classic Ligands 17
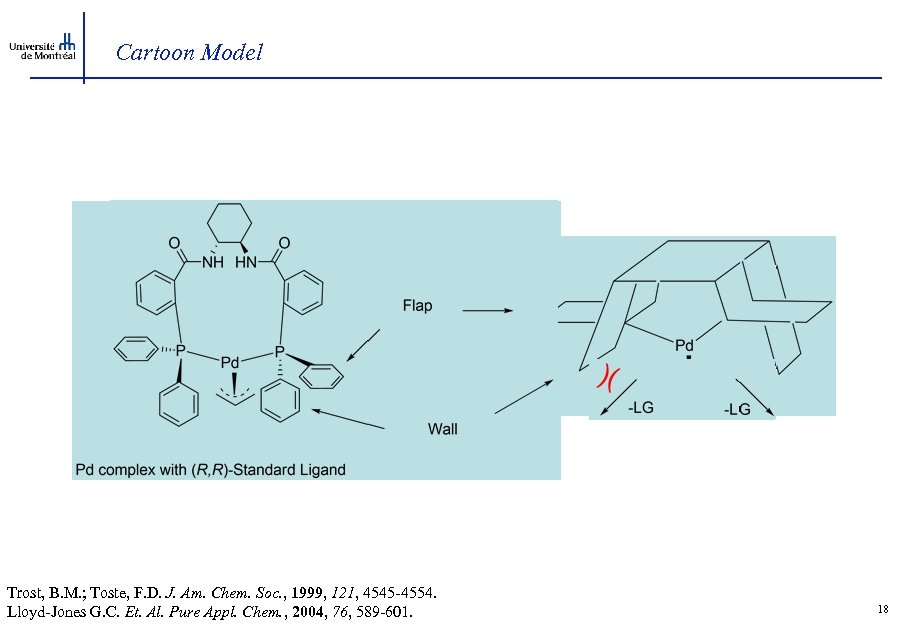 Cartoon Model Trost, B. M. ; Toste, F. D. J. Am. Chem. Soc. , 1999, 121, 4545 -4554. Lloyd-Jones G. C. Et. Al. Pure Appl. Chem. , 2004, 76, 589 -601. 18
Cartoon Model Trost, B. M. ; Toste, F. D. J. Am. Chem. Soc. , 1999, 121, 4545 -4554. Lloyd-Jones G. C. Et. Al. Pure Appl. Chem. , 2004, 76, 589 -601. 18
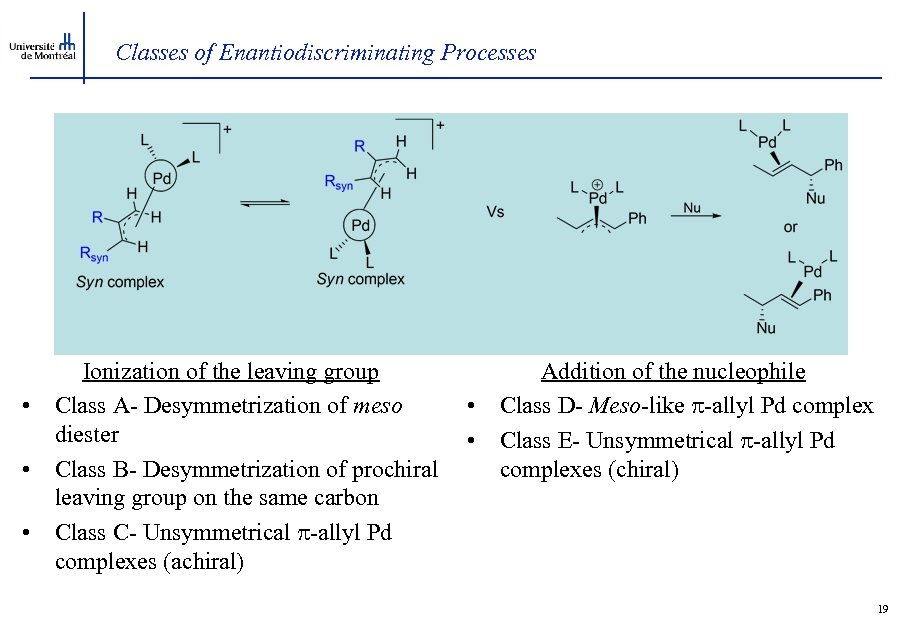 Classes of Enantiodiscriminating Processes • • • Ionization of the leaving group Class A- Desymmetrization of meso diester Class B- Desymmetrization of prochiral leaving group on the same carbon Class C- Unsymmetrical -allyl Pd complexes (achiral) • • Addition of the nucleophile Class D- Meso-like -allyl Pd complex Class E- Unsymmetrical -allyl Pd complexes (chiral) 19
Classes of Enantiodiscriminating Processes • • • Ionization of the leaving group Class A- Desymmetrization of meso diester Class B- Desymmetrization of prochiral leaving group on the same carbon Class C- Unsymmetrical -allyl Pd complexes (achiral) • • Addition of the nucleophile Class D- Meso-like -allyl Pd complex Class E- Unsymmetrical -allyl Pd complexes (chiral) 19
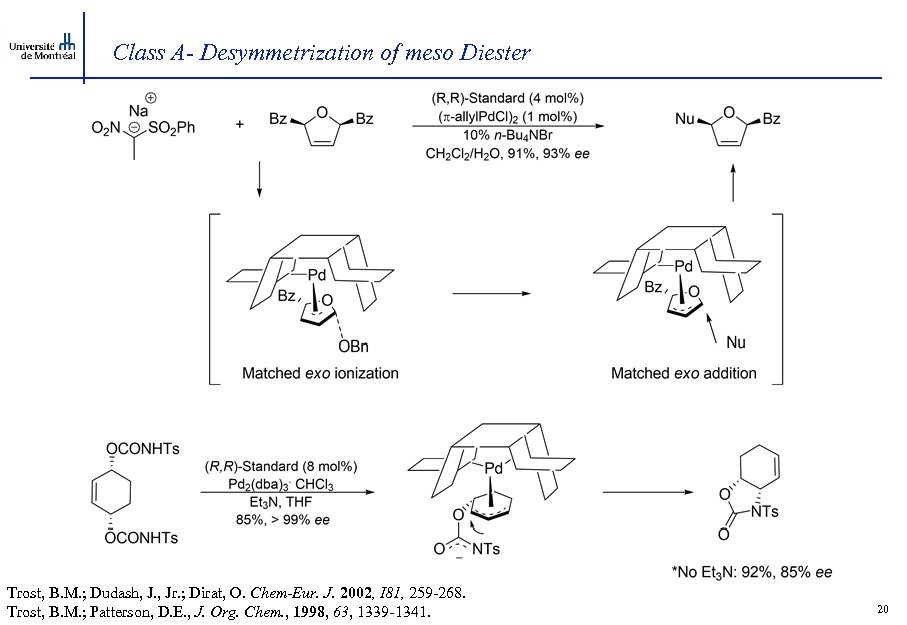 Class A- Desymmetrization of meso Diester Trost, B. M. ; Dudash, J. , Jr. ; Dirat, O. Chem-Eur. J. 2002, I 81, 259 -268. Trost, B. M. ; Patterson, D. E. , J. Org. Chem. , 1998, 63, 1339 -1341. 20
Class A- Desymmetrization of meso Diester Trost, B. M. ; Dudash, J. , Jr. ; Dirat, O. Chem-Eur. J. 2002, I 81, 259 -268. Trost, B. M. ; Patterson, D. E. , J. Org. Chem. , 1998, 63, 1339 -1341. 20
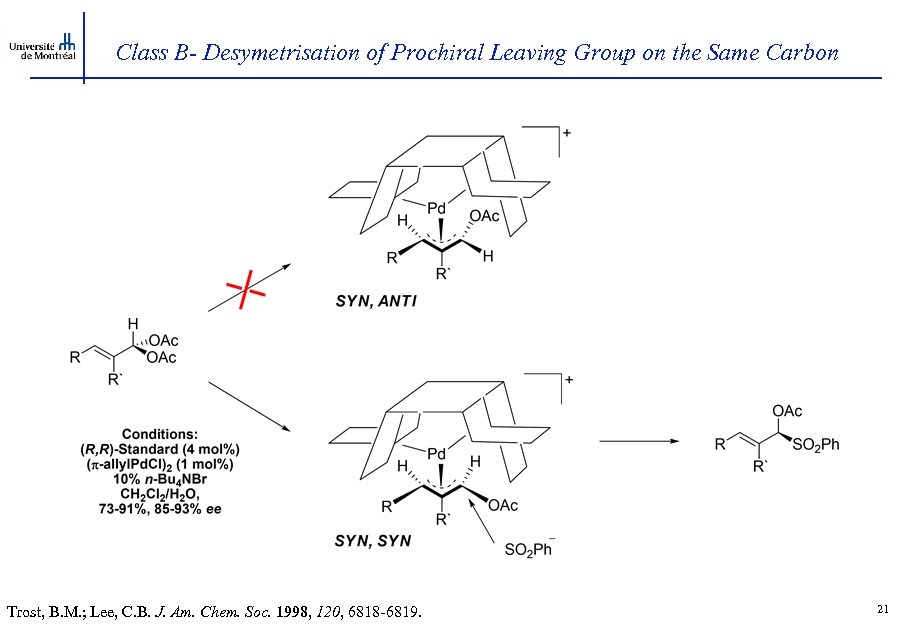 Class B- Desymetrisation of Prochiral Leaving Group on the Same Carbon Trost, B. M. ; Lee, C. B. J. Am. Chem. Soc. 1998, 120, 6818 -6819. 21
Class B- Desymetrisation of Prochiral Leaving Group on the Same Carbon Trost, B. M. ; Lee, C. B. J. Am. Chem. Soc. 1998, 120, 6818 -6819. 21
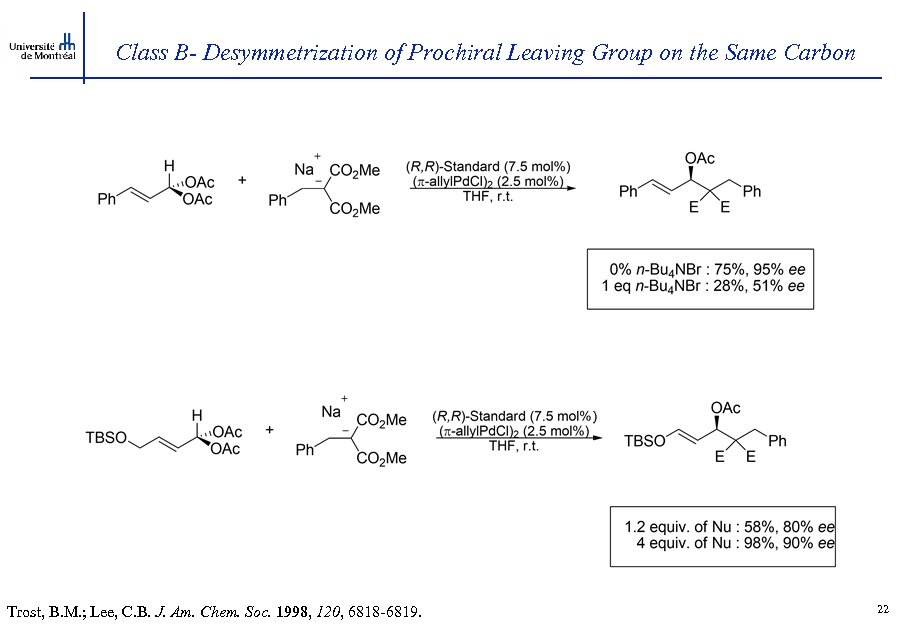 Class B- Desymmetrization of Prochiral Leaving Group on the Same Carbon Trost, B. M. ; Lee, C. B. J. Am. Chem. Soc. 1998, 120, 6818 -6819. 22
Class B- Desymmetrization of Prochiral Leaving Group on the Same Carbon Trost, B. M. ; Lee, C. B. J. Am. Chem. Soc. 1998, 120, 6818 -6819. 22
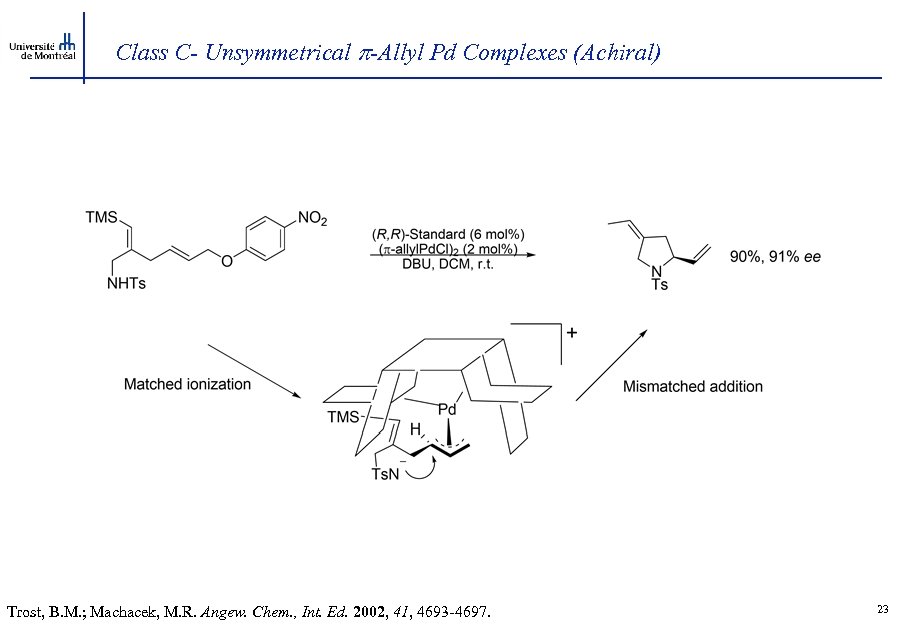 Class C- Unsymmetrical -Allyl Pd Complexes (Achiral) Trost, B. M. ; Machacek, M. R. Angew. Chem. , Int. Ed. 2002, 41, 4693 -4697. 23
Class C- Unsymmetrical -Allyl Pd Complexes (Achiral) Trost, B. M. ; Machacek, M. R. Angew. Chem. , Int. Ed. 2002, 41, 4693 -4697. 23
 Class C- Unsymmetrical -Allyl Pd Complexes (Achiral) Trost, B. M. ; Machacek, M. R. Angew. Chem. , Int. Ed. 2002, 41, 4693 -4697. 24
Class C- Unsymmetrical -Allyl Pd Complexes (Achiral) Trost, B. M. ; Machacek, M. R. Angew. Chem. , Int. Ed. 2002, 41, 4693 -4697. 24
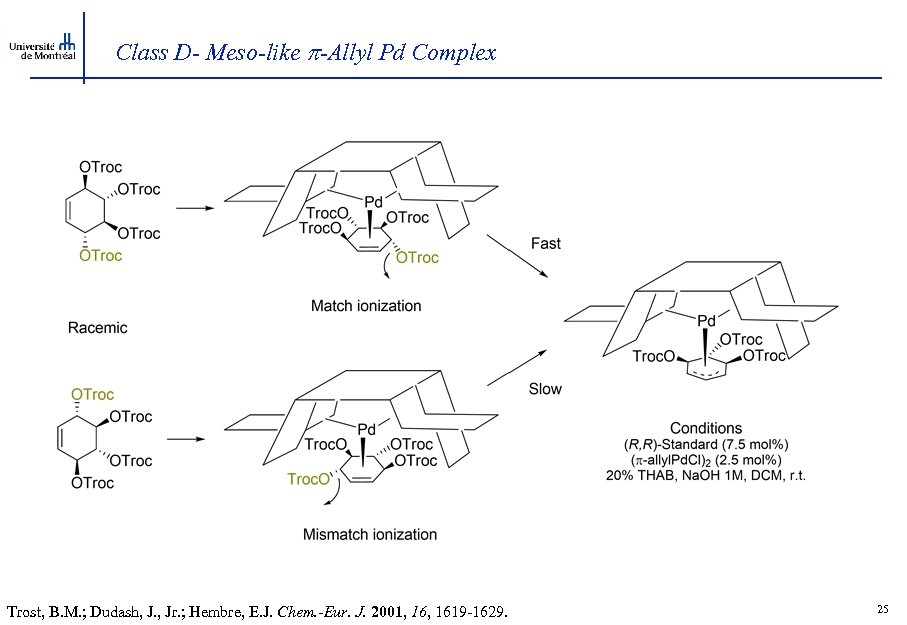 Class D- Meso-like -Allyl Pd Complex Trost, B. M. ; Dudash, J. , Jr. ; Hembre, E. J. Chem. -Eur. J. 2001, 1619 -1629. 25
Class D- Meso-like -Allyl Pd Complex Trost, B. M. ; Dudash, J. , Jr. ; Hembre, E. J. Chem. -Eur. J. 2001, 1619 -1629. 25
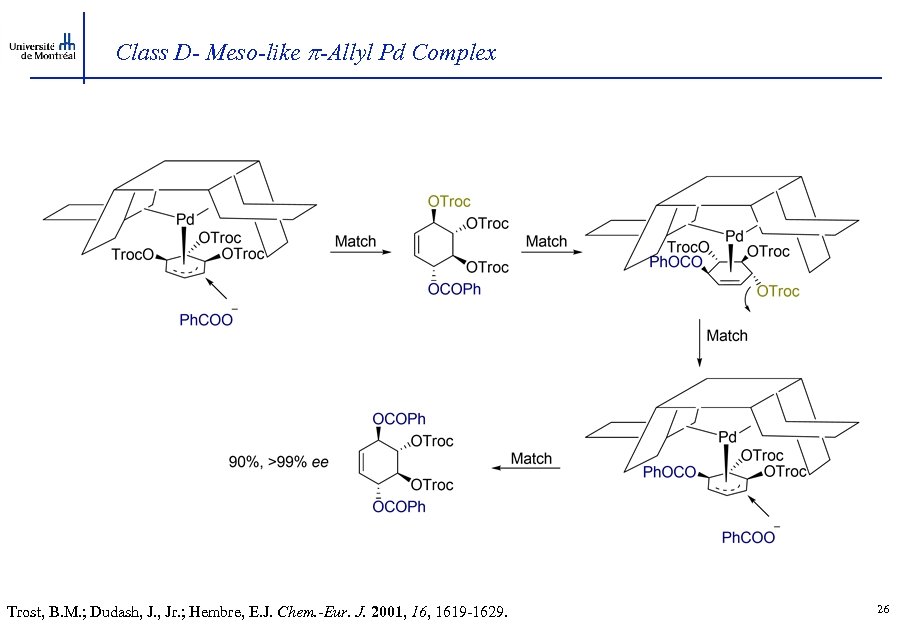 Class D- Meso-like -Allyl Pd Complex Trost, B. M. ; Dudash, J. , Jr. ; Hembre, E. J. Chem. -Eur. J. 2001, 1619 -1629. 26
Class D- Meso-like -Allyl Pd Complex Trost, B. M. ; Dudash, J. , Jr. ; Hembre, E. J. Chem. -Eur. J. 2001, 1619 -1629. 26
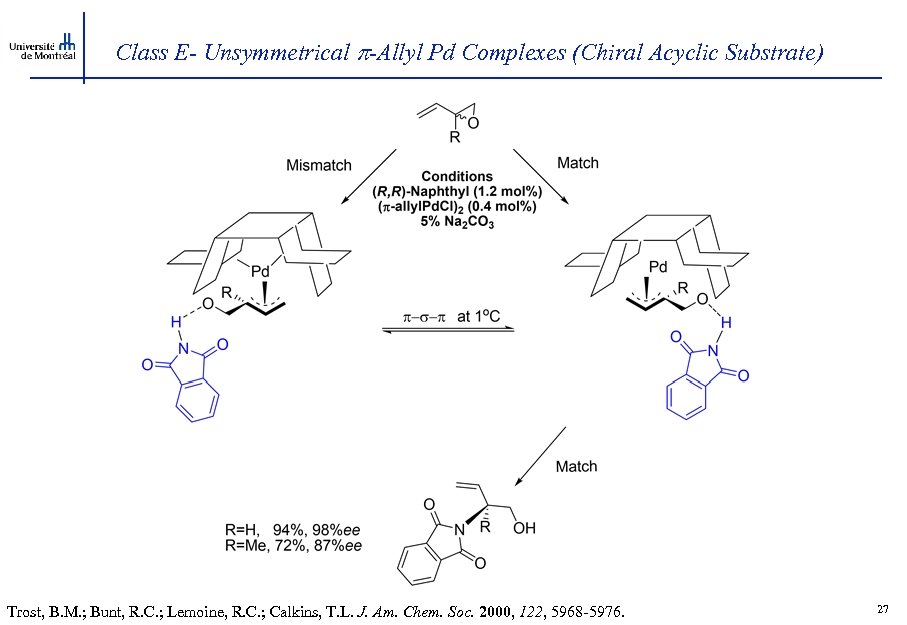 Class E- Unsymmetrical -Allyl Pd Complexes (Chiral Acyclic Substrate) Trost, B. M. ; Bunt, R. C. ; Lemoine, R. C. ; Calkins, T. L. J. Am. Chem. Soc. 2000, 122, 5968 -5976. 27
Class E- Unsymmetrical -Allyl Pd Complexes (Chiral Acyclic Substrate) Trost, B. M. ; Bunt, R. C. ; Lemoine, R. C. ; Calkins, T. L. J. Am. Chem. Soc. 2000, 122, 5968 -5976. 27
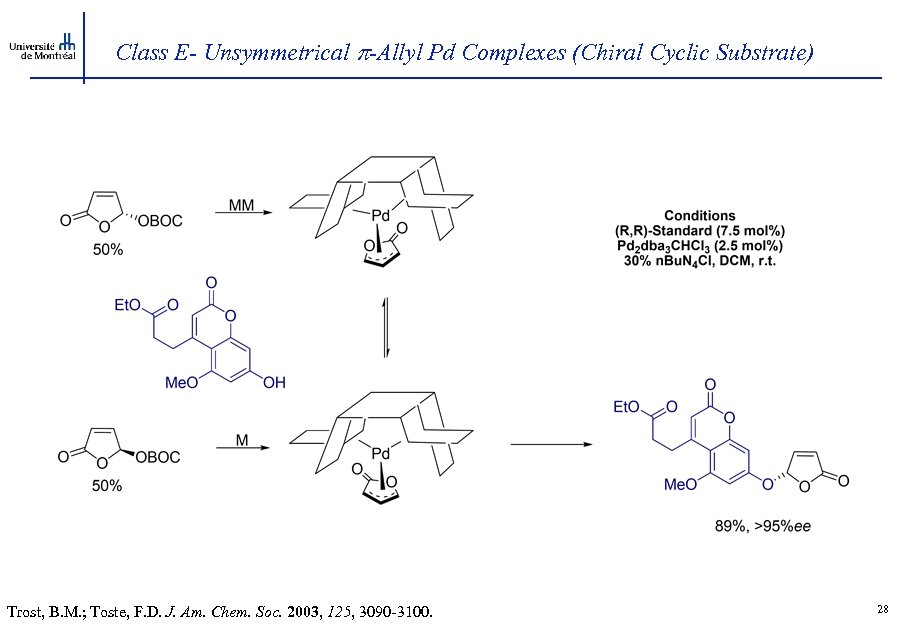 Class E- Unsymmetrical -Allyl Pd Complexes (Chiral Cyclic Substrate) Trost, B. M. ; Toste, F. D. J. Am. Chem. Soc. 2003, 125, 3090 -3100. 28
Class E- Unsymmetrical -Allyl Pd Complexes (Chiral Cyclic Substrate) Trost, B. M. ; Toste, F. D. J. Am. Chem. Soc. 2003, 125, 3090 -3100. 28
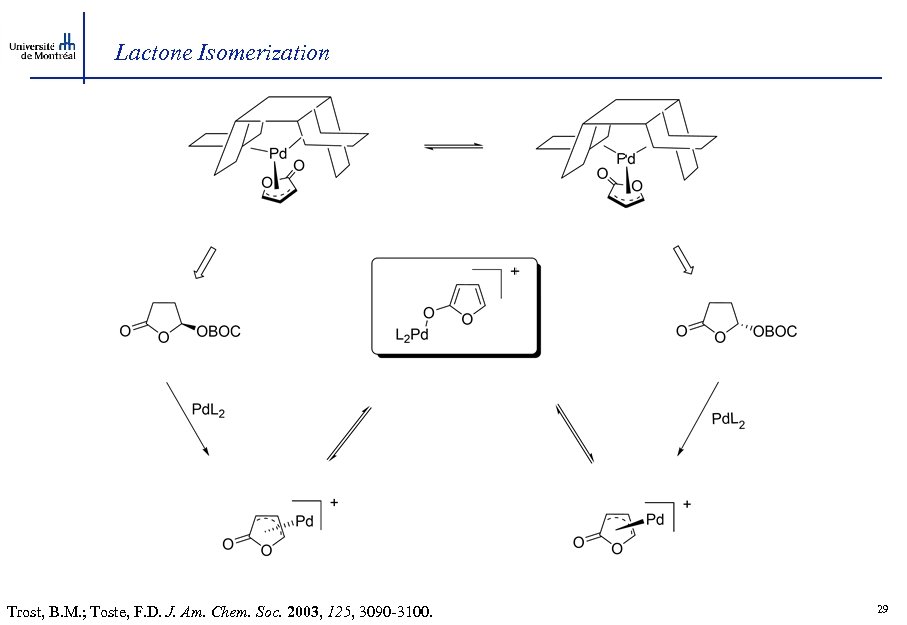 Lactone Isomerization Trost, B. M. ; Toste, F. D. J. Am. Chem. Soc. 2003, 125, 3090 -3100. 29
Lactone Isomerization Trost, B. M. ; Toste, F. D. J. Am. Chem. Soc. 2003, 125, 3090 -3100. 29
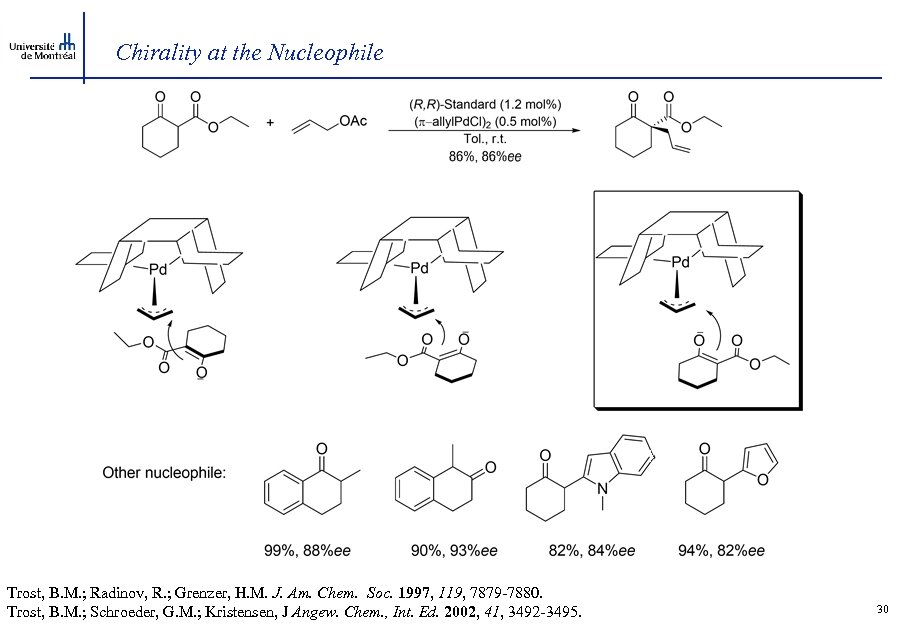 Chirality at the Nucleophile Trost, B. M. ; Radinov, R. ; Grenzer, H. M. J. Am. Chem. Soc. 1997, 119, 7879 -7880. Trost, B. M. ; Schroeder, G. M. ; Kristensen, J Angew. Chem. , Int. Ed. 2002, 41, 3492 -3495. 30
Chirality at the Nucleophile Trost, B. M. ; Radinov, R. ; Grenzer, H. M. J. Am. Chem. Soc. 1997, 119, 7879 -7880. Trost, B. M. ; Schroeder, G. M. ; Kristensen, J Angew. Chem. , Int. Ed. 2002, 41, 3492 -3495. 30
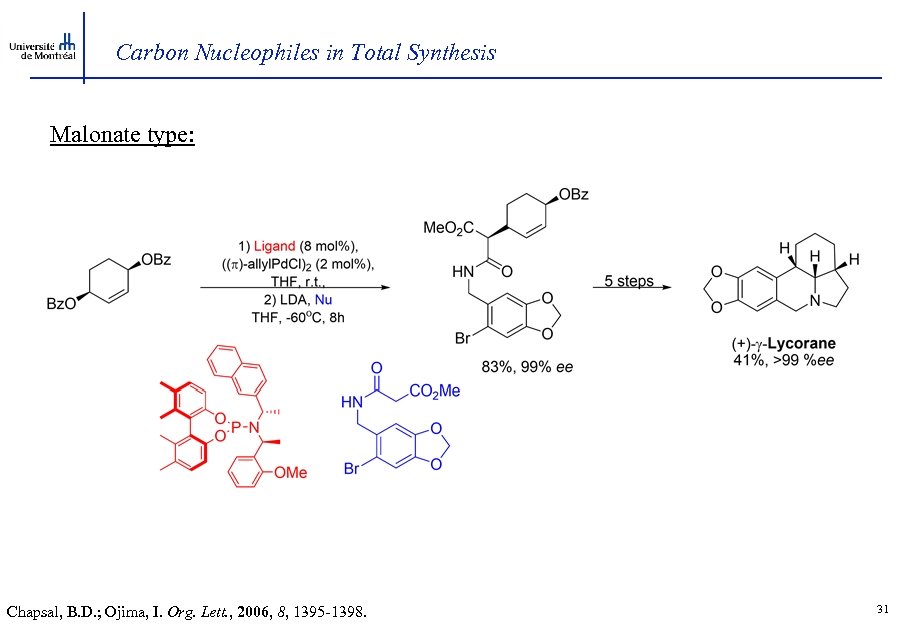 Carbon Nucleophiles in Total Synthesis Malonate type: Chapsal, B. D. ; Ojima, I. Org. Lett. , 2006, 8, 1395 -1398. 31
Carbon Nucleophiles in Total Synthesis Malonate type: Chapsal, B. D. ; Ojima, I. Org. Lett. , 2006, 8, 1395 -1398. 31
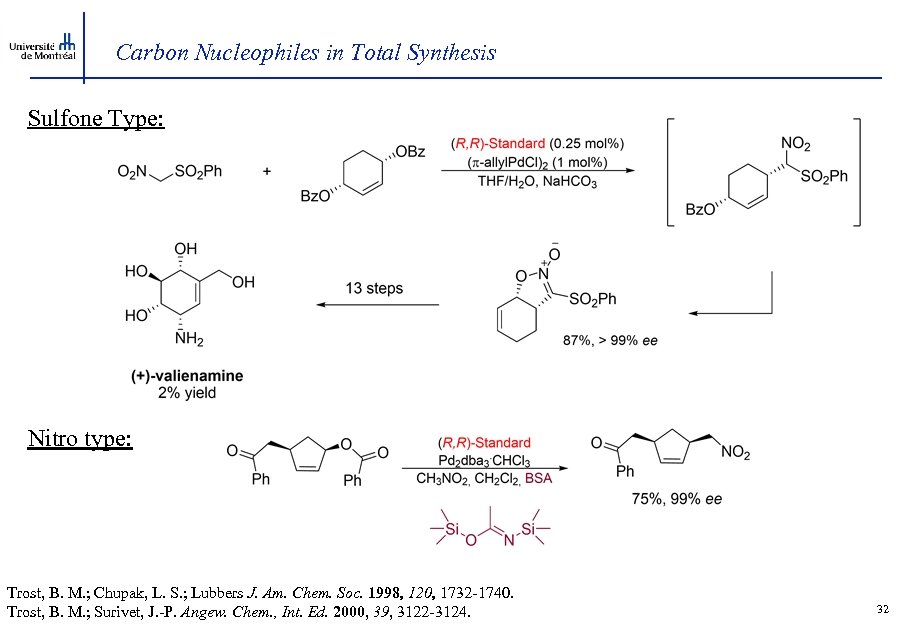 Carbon Nucleophiles in Total Synthesis Sulfone Type: Nitro type: Trost, B. M. ; Chupak, L. S. ; Lubbers J. Am. Chem. Soc. 1998, 120, 1732 -1740. Trost, B. M. ; Surivet, J. -P. Angew. Chem. , Int. Ed. 2000, 39, 3122 -3124. 32
Carbon Nucleophiles in Total Synthesis Sulfone Type: Nitro type: Trost, B. M. ; Chupak, L. S. ; Lubbers J. Am. Chem. Soc. 1998, 120, 1732 -1740. Trost, B. M. ; Surivet, J. -P. Angew. Chem. , Int. Ed. 2000, 39, 3122 -3124. 32
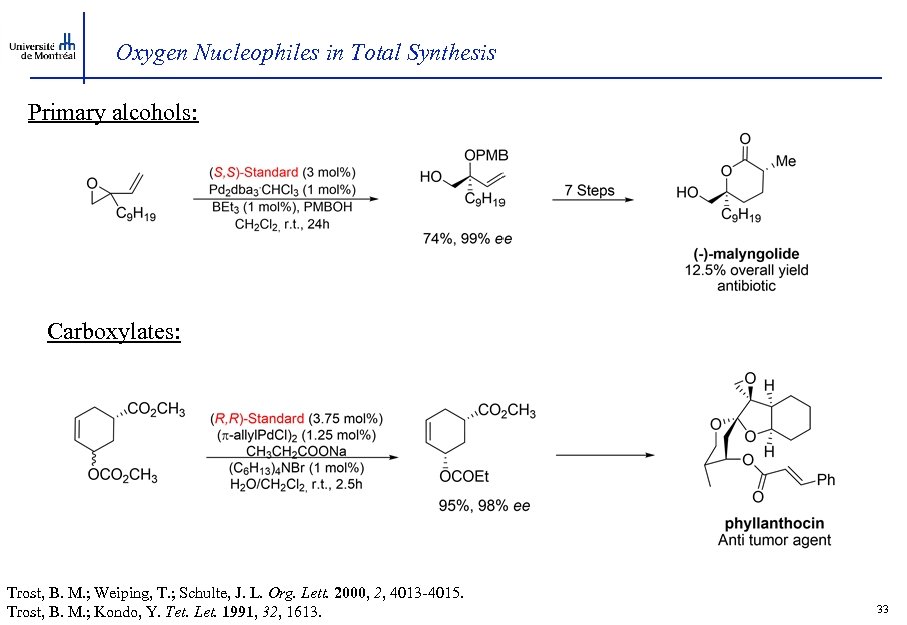 Oxygen Nucleophiles in Total Synthesis Primary alcohols: Carboxylates: Trost, B. M. ; Weiping, T. ; Schulte, J. L. Org. Lett. 2000, 2, 4013 -4015. Trost, B. M. ; Kondo, Y. Tet. Let. 1991, 32, 1613. 33
Oxygen Nucleophiles in Total Synthesis Primary alcohols: Carboxylates: Trost, B. M. ; Weiping, T. ; Schulte, J. L. Org. Lett. 2000, 2, 4013 -4015. Trost, B. M. ; Kondo, Y. Tet. Let. 1991, 32, 1613. 33
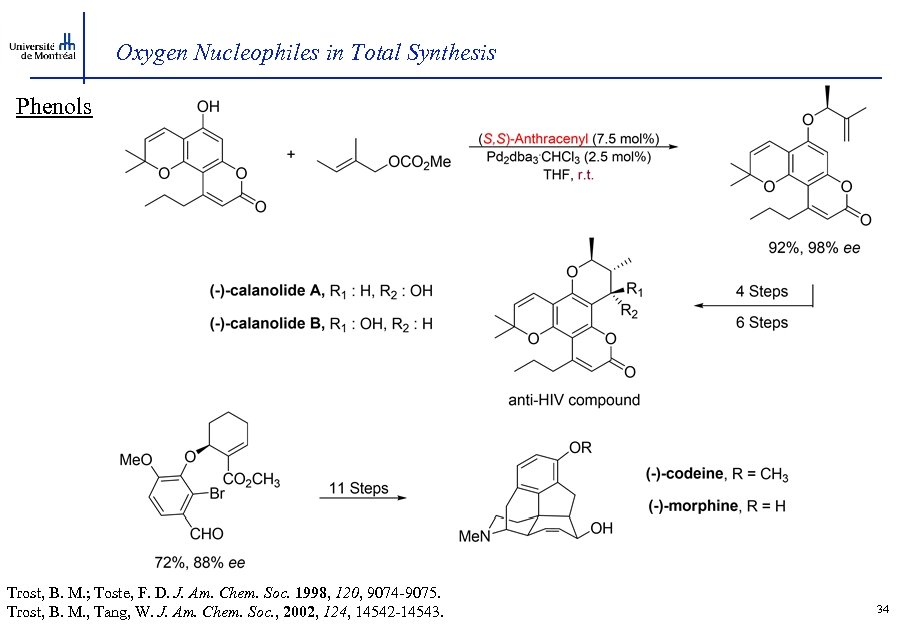 Oxygen Nucleophiles in Total Synthesis Phenols Trost, B. M. ; Toste, F. D. J. Am. Chem. Soc. 1998, 120, 9074 -9075. Trost, B. M. , Tang, W. J. Am. Chem. Soc. , 2002, 124, 14542 -14543. 34
Oxygen Nucleophiles in Total Synthesis Phenols Trost, B. M. ; Toste, F. D. J. Am. Chem. Soc. 1998, 120, 9074 -9075. Trost, B. M. , Tang, W. J. Am. Chem. Soc. , 2002, 124, 14542 -14543. 34
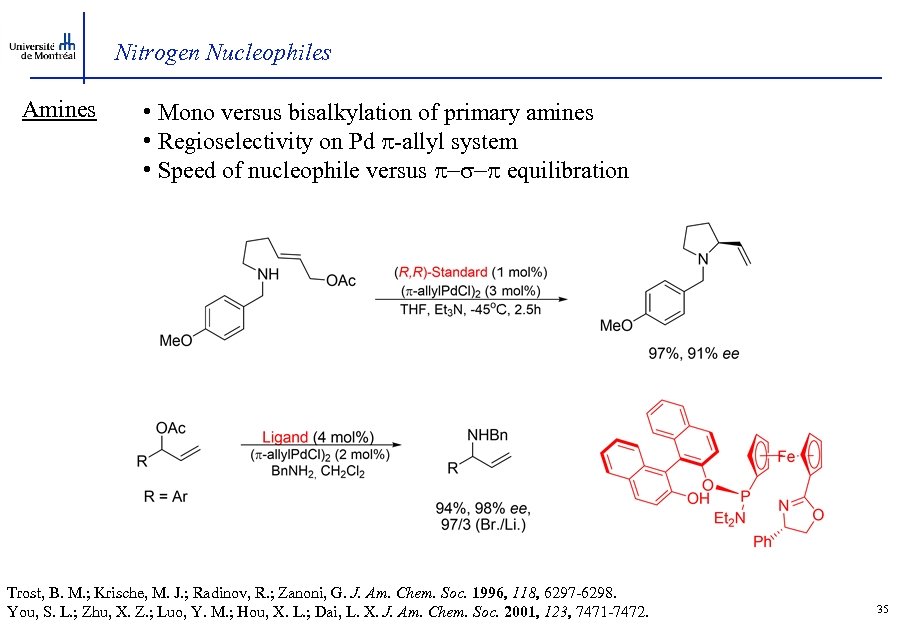 Nitrogen Nucleophiles Amines • Mono versus bisalkylation of primary amines • Regioselectivity on Pd -allyl system • Speed of nucleophile versus -s- equilibration Trost, B. M. ; Krische, M. J. ; Radinov, R. ; Zanoni, G. J. Am. Chem. Soc. 1996, 118, 6297 -6298. You, S. L. ; Zhu, X. Z. ; Luo, Y. M. ; Hou, X. L. ; Dai, L. X. J. Am. Chem. Soc. 2001, 123, 7471 -7472. 35
Nitrogen Nucleophiles Amines • Mono versus bisalkylation of primary amines • Regioselectivity on Pd -allyl system • Speed of nucleophile versus -s- equilibration Trost, B. M. ; Krische, M. J. ; Radinov, R. ; Zanoni, G. J. Am. Chem. Soc. 1996, 118, 6297 -6298. You, S. L. ; Zhu, X. Z. ; Luo, Y. M. ; Hou, X. L. ; Dai, L. X. J. Am. Chem. Soc. 2001, 123, 7471 -7472. 35
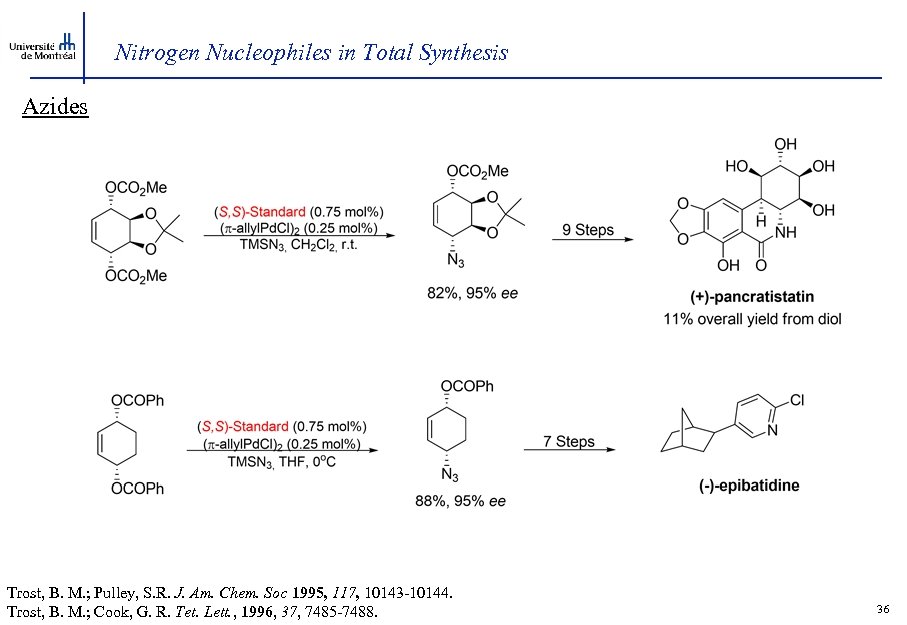 Nitrogen Nucleophiles in Total Synthesis Azides Trost, B. M. ; Pulley, S. R. J. Am. Chem. Soc 1995, 117, 10143 -10144. Trost, B. M. ; Cook, G. R. Tet. Lett. , 1996, 37, 7485 -7488. 36
Nitrogen Nucleophiles in Total Synthesis Azides Trost, B. M. ; Pulley, S. R. J. Am. Chem. Soc 1995, 117, 10143 -10144. Trost, B. M. ; Cook, G. R. Tet. Lett. , 1996, 37, 7485 -7488. 36
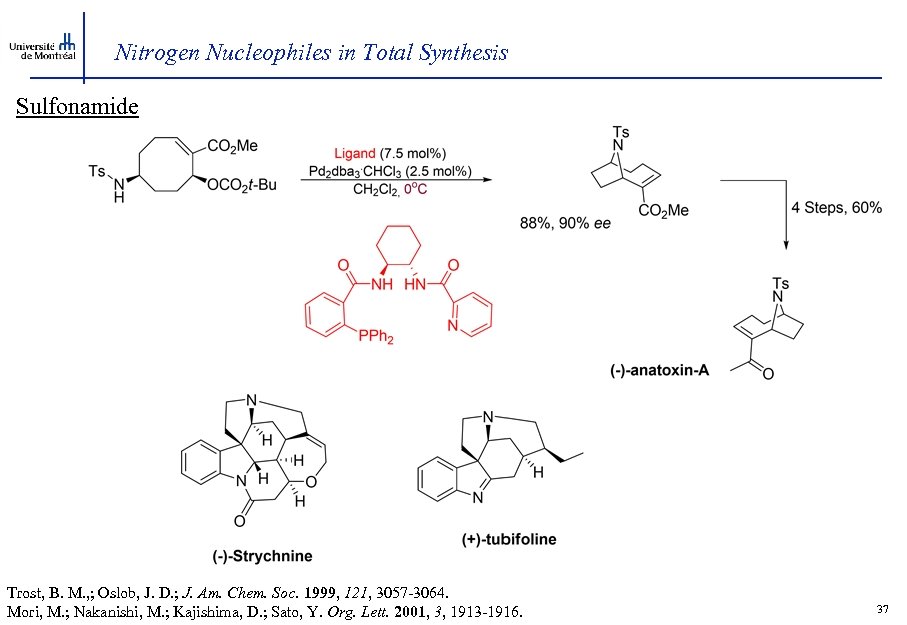 Nitrogen Nucleophiles in Total Synthesis Sulfonamide Trost, B. M. , ; Oslob, J. D. ; J. Am. Chem. Soc. 1999, 121, 3057 -3064. Mori, M. ; Nakanishi, M. ; Kajishima, D. ; Sato, Y. Org. Lett. 2001, 3, 1913 -1916. 37
Nitrogen Nucleophiles in Total Synthesis Sulfonamide Trost, B. M. , ; Oslob, J. D. ; J. Am. Chem. Soc. 1999, 121, 3057 -3064. Mori, M. ; Nakanishi, M. ; Kajishima, D. ; Sato, Y. Org. Lett. 2001, 3, 1913 -1916. 37
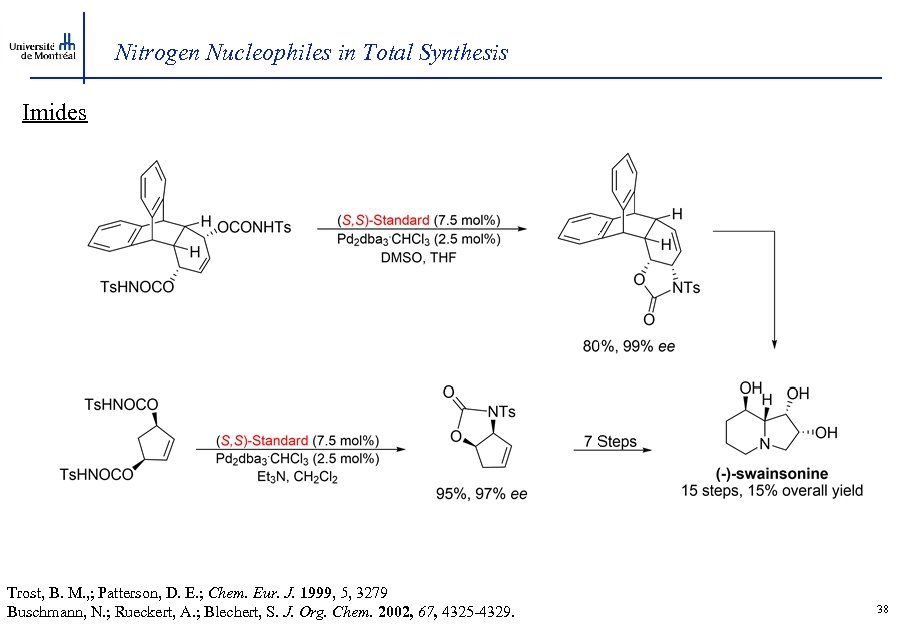 Nitrogen Nucleophiles in Total Synthesis Imides Trost, B. M. , ; Patterson, D. E. ; Chem. Eur. J. 1999, 5, 3279 Buschmann, N. ; Rueckert, A. ; Blechert, S. J. Org. Chem. 2002, 67, 4325 -4329. 38
Nitrogen Nucleophiles in Total Synthesis Imides Trost, B. M. , ; Patterson, D. E. ; Chem. Eur. J. 1999, 5, 3279 Buschmann, N. ; Rueckert, A. ; Blechert, S. J. Org. Chem. 2002, 67, 4325 -4329. 38
 Nitrogen Nucleophiles in Total Synthesis Trost, B. M. ; Shi, Z. J. Am. Chem. Soc. 1996, 118, 3037 -3038. Trost, B. M. ; Madsen, R. ; Guile, S. D. ; Tet. Lett. , 1997, 38, 1707 -1710. 39
Nitrogen Nucleophiles in Total Synthesis Trost, B. M. ; Shi, Z. J. Am. Chem. Soc. 1996, 118, 3037 -3038. Trost, B. M. ; Madsen, R. ; Guile, S. D. ; Tet. Lett. , 1997, 38, 1707 -1710. 39
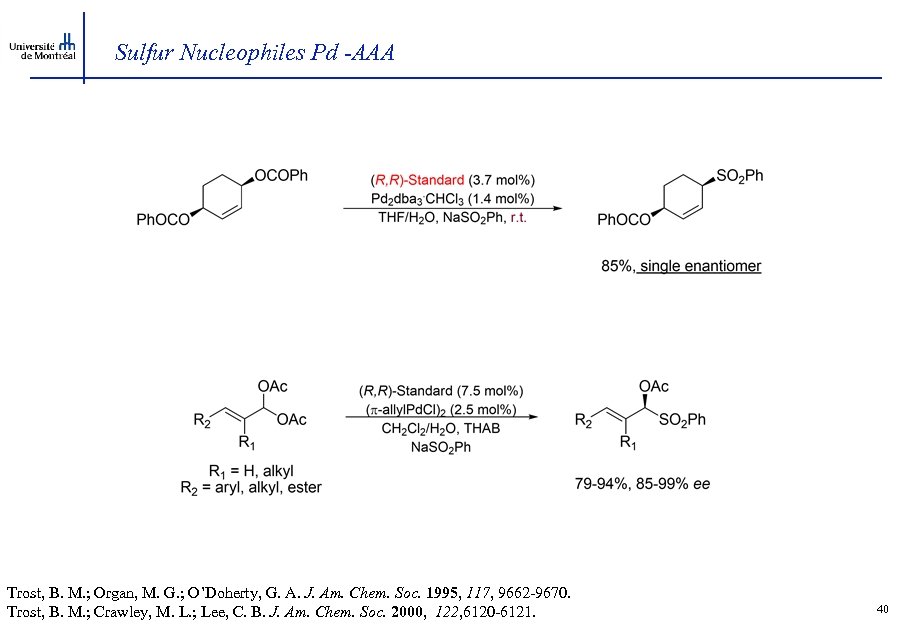 Sulfur Nucleophiles Pd -AAA Trost, B. M. ; Organ, M. G. ; O’Doherty, G. A. J. Am. Chem. Soc. 1995, 117, 9662 -9670. Trost, B. M. ; Crawley, M. L. ; Lee, C. B. J. Am. Chem. Soc. 2000, 122, 6120 -6121. 40
Sulfur Nucleophiles Pd -AAA Trost, B. M. ; Organ, M. G. ; O’Doherty, G. A. J. Am. Chem. Soc. 1995, 117, 9662 -9670. Trost, B. M. ; Crawley, M. L. ; Lee, C. B. J. Am. Chem. Soc. 2000, 122, 6120 -6121. 40
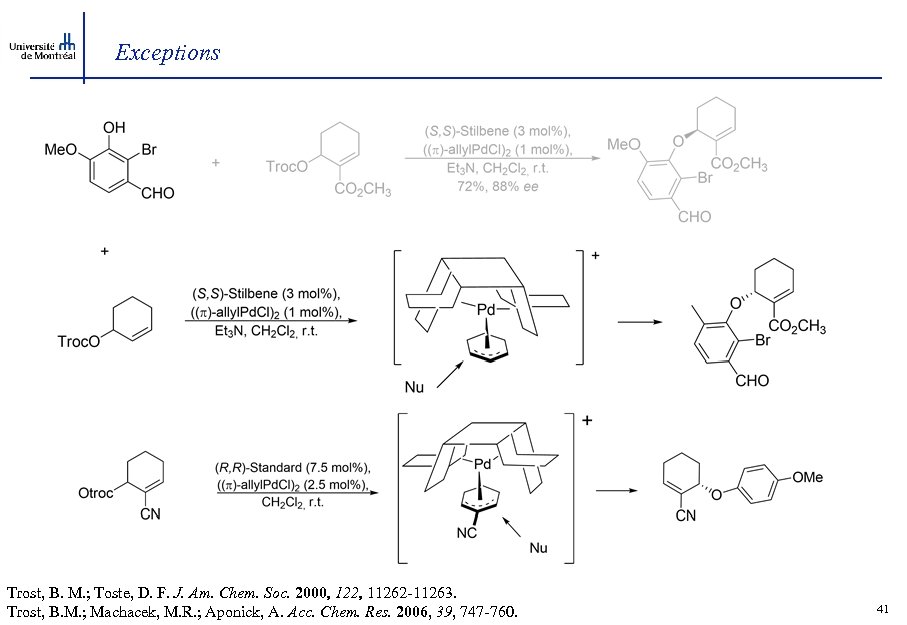 Exceptions Trost, B. M. ; Toste, D. F. J. Am. Chem. Soc. 2000, 122, 11262 -11263. Trost, B. M. ; Machacek, M. R. ; Aponick, A. Acc. Chem. Res. 2006, 39, 747 -760. 41
Exceptions Trost, B. M. ; Toste, D. F. J. Am. Chem. Soc. 2000, 122, 11262 -11263. Trost, B. M. ; Machacek, M. R. ; Aponick, A. Acc. Chem. Res. 2006, 39, 747 -760. 41
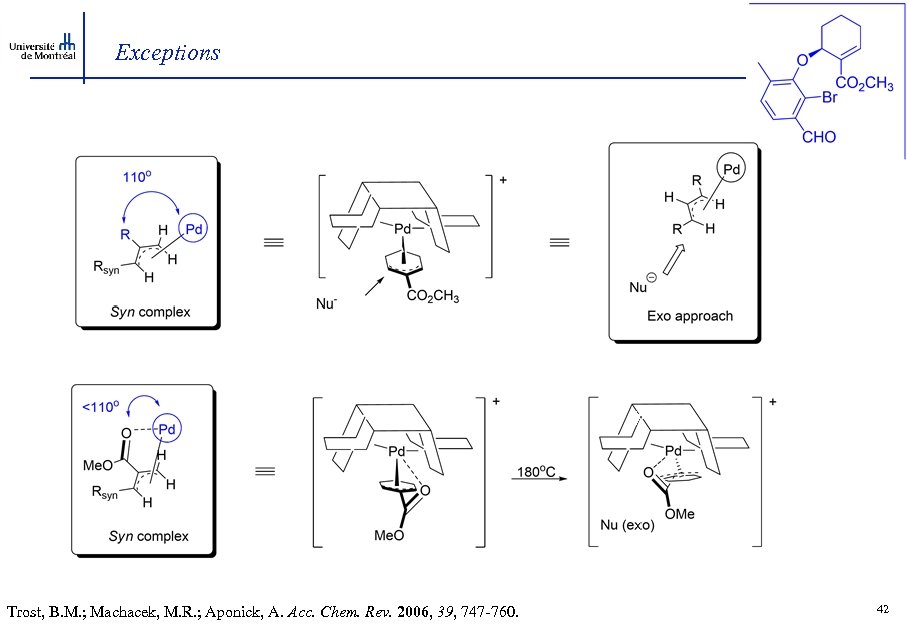 Exceptions Trost, B. M. ; Machacek, M. R. ; Aponick, A. Acc. Chem. Rev. 2006, 39, 747 -760. 42
Exceptions Trost, B. M. ; Machacek, M. R. ; Aponick, A. Acc. Chem. Rev. 2006, 39, 747 -760. 42
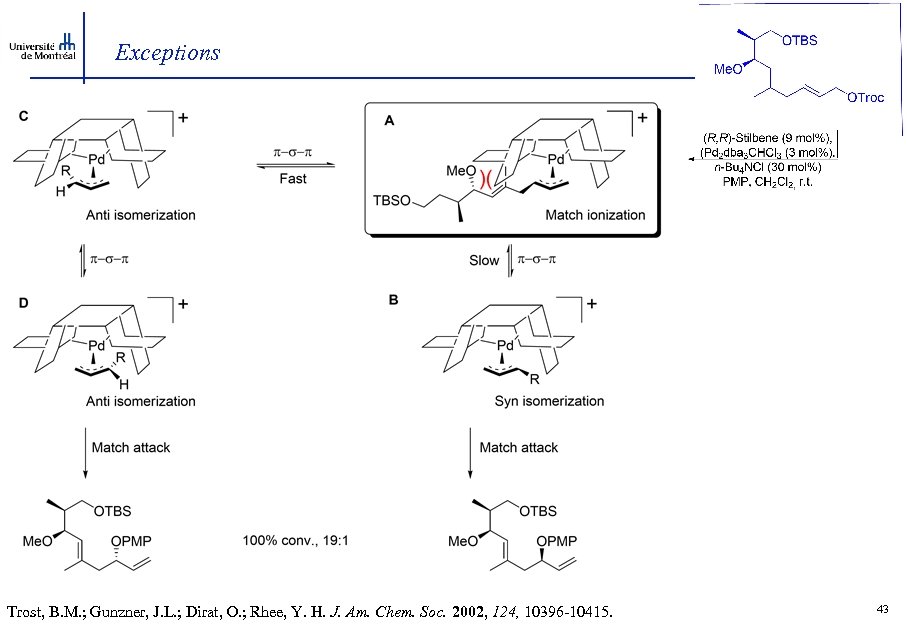 Exceptions Trost, B. M. ; Gunzner, J. L. ; Dirat, O. ; Rhee, Y. H. J. Am. Chem. Soc. 2002, 124, 10396 -10415. 43
Exceptions Trost, B. M. ; Gunzner, J. L. ; Dirat, O. ; Rhee, Y. H. J. Am. Chem. Soc. 2002, 124, 10396 -10415. 43
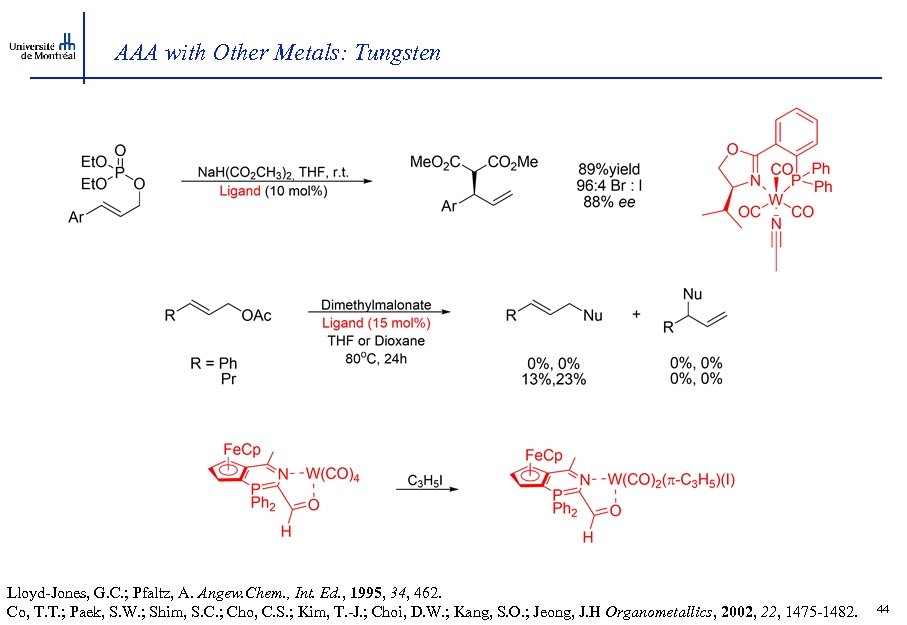 AAA with Other Metals: Tungsten Lloyd-Jones, G. C. ; Pfaltz, A. Angew. Chem. , Int. Ed. , 1995, 34, 462. Co, T. T. ; Paek, S. W. ; Shim, S. C. ; Cho, C. S. ; Kim, T. -J. ; Choi, D. W. ; Kang, S. O. ; Jeong, J. H Organometallics, 2002, 22, 1475 -1482. 44
AAA with Other Metals: Tungsten Lloyd-Jones, G. C. ; Pfaltz, A. Angew. Chem. , Int. Ed. , 1995, 34, 462. Co, T. T. ; Paek, S. W. ; Shim, S. C. ; Cho, C. S. ; Kim, T. -J. ; Choi, D. W. ; Kang, S. O. ; Jeong, J. H Organometallics, 2002, 22, 1475 -1482. 44
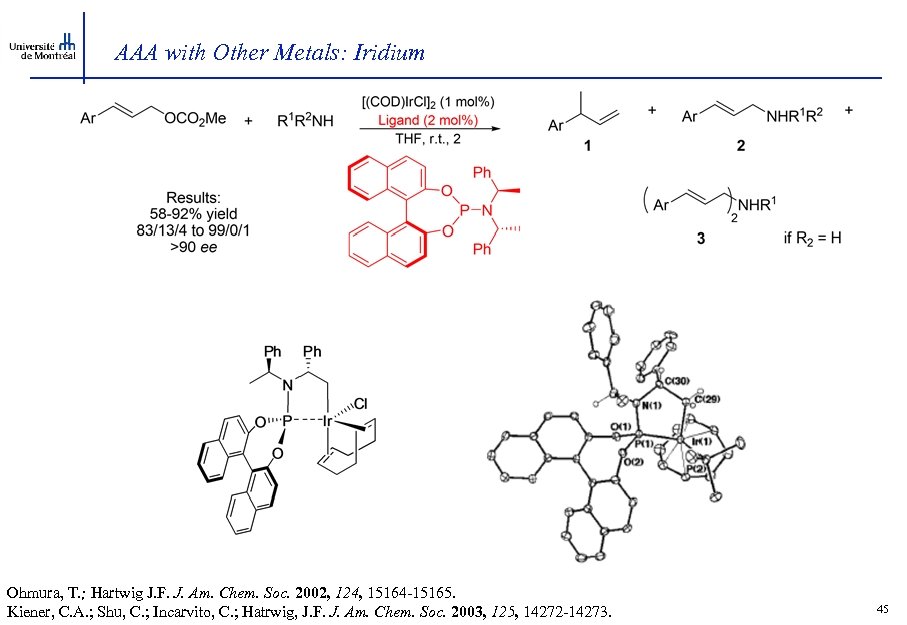 AAA with Other Metals: Iridium Ohmura, T. ; Hartwig J. F. J. Am. Chem. Soc. 2002, 124, 15164 -15165. Kiener, C. A. ; Shu, C. ; Incarvito, C. ; Hatrwig, J. F. J. Am. Chem. Soc. 2003, 125, 14272 -14273. 45
AAA with Other Metals: Iridium Ohmura, T. ; Hartwig J. F. J. Am. Chem. Soc. 2002, 124, 15164 -15165. Kiener, C. A. ; Shu, C. ; Incarvito, C. ; Hatrwig, J. F. J. Am. Chem. Soc. 2003, 125, 14272 -14273. 45
 Novel Iridium Utilisation • Preparation of -Substituted Allylboronates by Chemoselective Iridium. Catalyzed Asymmetric Allylic Alkylation of 1 -Propenylboronates - Peng, F. ; Hall*, D. G. Tet. Lett. 2007, 18, 3305 -3309 • Salt-Free Iridium-Catalyzed Asymmetric Allylic Aminations with N, NDiacylamines and ortho-Nosylamide as Ammonia Equivalents - Weihofen, R. ; Tverskoy, O. ; Helmchen, G. ; Angew. Chem. , Int. Ed. 2006, 33, 5546 -5549 • Very Efficient Phosphoramidite Ligand for Asymmetric Iridium-Catalyzed Allylic Alkylation - Alexakis*, A. ; Polet, D. ; Org. Lett. 2004, 20, 3529 -3532 • Regio- and Enantioselective Iridium-Catalyzed Allylic Alkylation with In Situ Activated P, C-Chelate Complexes - Lipowsky, G. ; Miller, N. ; Helmchen, G. Angew. Chem. , Int. Ed. 2004, 43, 4595 – 4597 46
Novel Iridium Utilisation • Preparation of -Substituted Allylboronates by Chemoselective Iridium. Catalyzed Asymmetric Allylic Alkylation of 1 -Propenylboronates - Peng, F. ; Hall*, D. G. Tet. Lett. 2007, 18, 3305 -3309 • Salt-Free Iridium-Catalyzed Asymmetric Allylic Aminations with N, NDiacylamines and ortho-Nosylamide as Ammonia Equivalents - Weihofen, R. ; Tverskoy, O. ; Helmchen, G. ; Angew. Chem. , Int. Ed. 2006, 33, 5546 -5549 • Very Efficient Phosphoramidite Ligand for Asymmetric Iridium-Catalyzed Allylic Alkylation - Alexakis*, A. ; Polet, D. ; Org. Lett. 2004, 20, 3529 -3532 • Regio- and Enantioselective Iridium-Catalyzed Allylic Alkylation with In Situ Activated P, C-Chelate Complexes - Lipowsky, G. ; Miller, N. ; Helmchen, G. Angew. Chem. , Int. Ed. 2004, 43, 4595 – 4597 46
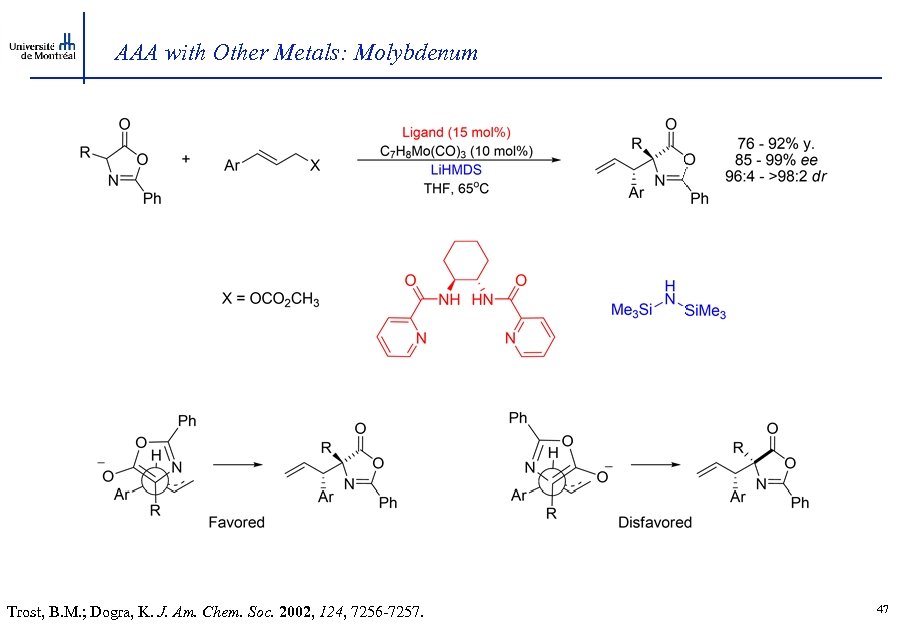 AAA with Other Metals: Molybdenum Trost, B. M. ; Dogra, K. J. Am. Chem. Soc. 2002, 124, 7256 -7257. 47
AAA with Other Metals: Molybdenum Trost, B. M. ; Dogra, K. J. Am. Chem. Soc. 2002, 124, 7256 -7257. 47
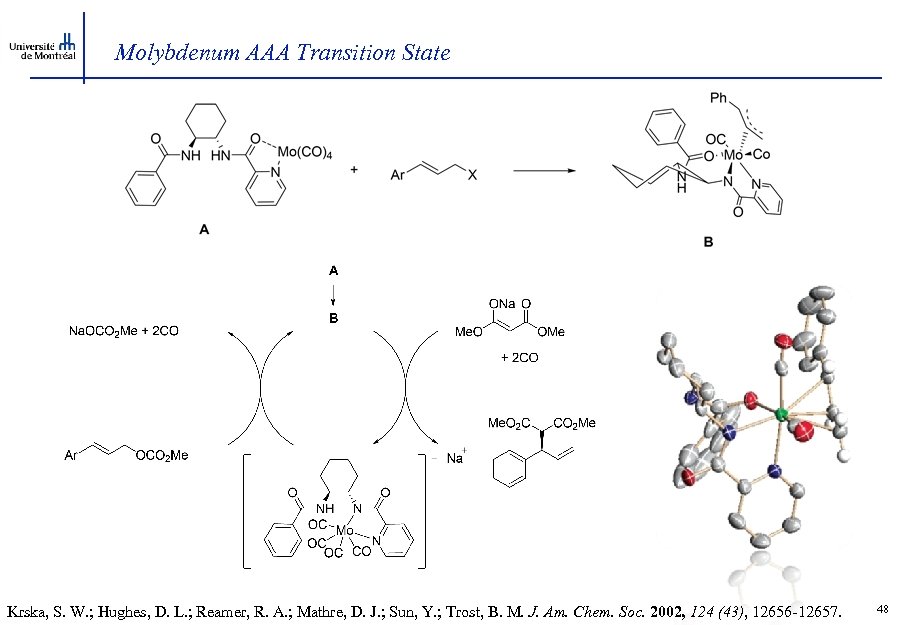 Molybdenum AAA Transition State Krska, S. W. ; Hughes, D. L. ; Reamer, R. A. ; Mathre, D. J. ; Sun, Y. ; Trost, B. M. J. Am. Chem. Soc. 2002, 124 (43), 12656 -12657. 48
Molybdenum AAA Transition State Krska, S. W. ; Hughes, D. L. ; Reamer, R. A. ; Mathre, D. J. ; Sun, Y. ; Trost, B. M. J. Am. Chem. Soc. 2002, 124 (43), 12656 -12657. 48
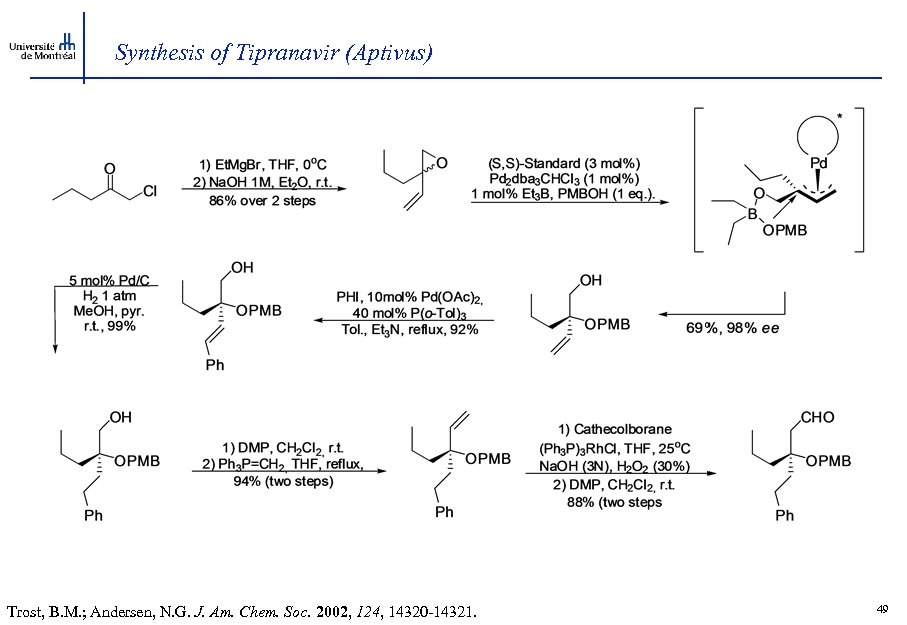 Synthesis of Tipranavir (Aptivus) Trost, B. M. ; Andersen, N. G. J. Am. Chem. Soc. 2002, 124, 14320 -14321. 49
Synthesis of Tipranavir (Aptivus) Trost, B. M. ; Andersen, N. G. J. Am. Chem. Soc. 2002, 124, 14320 -14321. 49
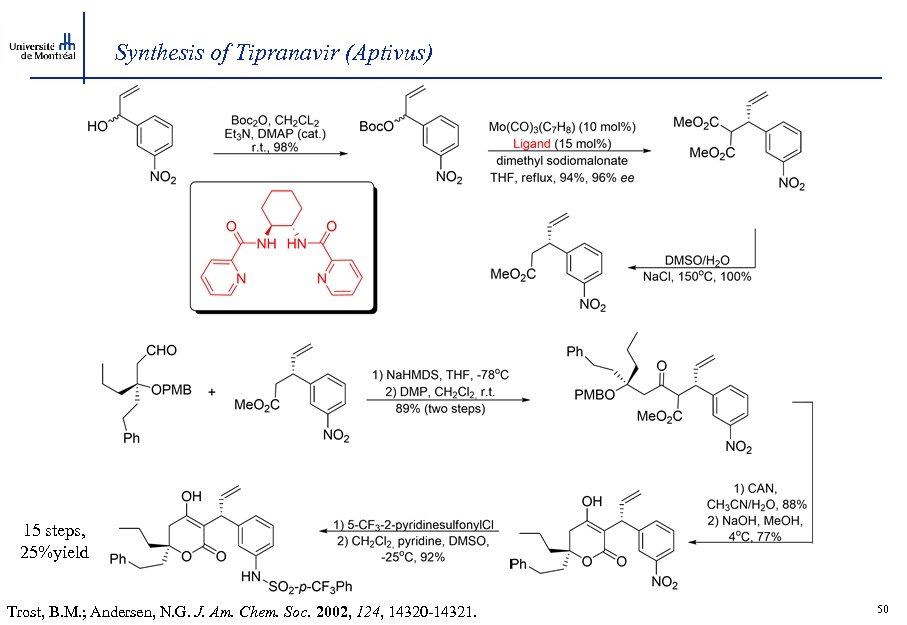 Synthesis of Tipranavir (Aptivus) 15 steps, 25%yield Trost, B. M. ; Andersen, N. G. J. Am. Chem. Soc. 2002, 124, 14320 -14321. 50
Synthesis of Tipranavir (Aptivus) 15 steps, 25%yield Trost, B. M. ; Andersen, N. G. J. Am. Chem. Soc. 2002, 124, 14320 -14321. 50
 Conclusion • • • High yields and enantioselectivities are obtain 5 mechanisms for enantiodiscrimination Diversity of bond type (C-C, C-O, C-N, C-S) Chirality can be set at substrates, nucleophiles or both AAA react with sp 3 instead of sp 2 centers Transforms achiral, prochiral and more importantly chiral racemic substrates into enantiopure compounds (through DYKAT) Cartoon model developped to predict final stereochemistry (almost no exceptions) Versatile method using mild conditions Usefull central strategy for total synthesis Scope have been expanded to other metals 51
Conclusion • • • High yields and enantioselectivities are obtain 5 mechanisms for enantiodiscrimination Diversity of bond type (C-C, C-O, C-N, C-S) Chirality can be set at substrates, nucleophiles or both AAA react with sp 3 instead of sp 2 centers Transforms achiral, prochiral and more importantly chiral racemic substrates into enantiopure compounds (through DYKAT) Cartoon model developped to predict final stereochemistry (almost no exceptions) Versatile method using mild conditions Usefull central strategy for total synthesis Scope have been expanded to other metals 51


