384db50f7fabb76d108d6b28bd6e1cac.ppt
- Количество слайдов: 1
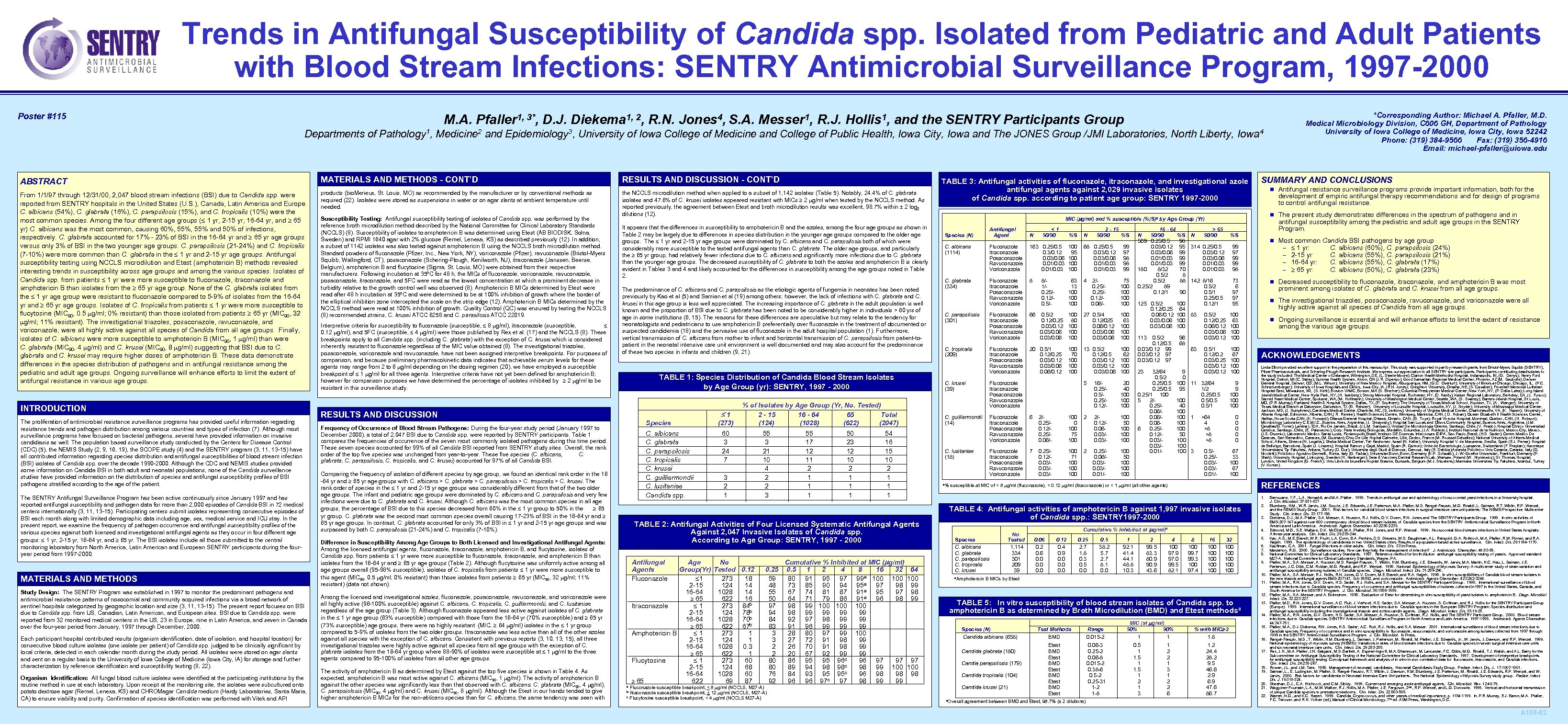
Trends in Antifungal Susceptibility of Candida spp. Isolated from Pediatric and Adult Patients with Blood Stream Infections: SENTRY Antimicrobial Surveillance Program, 1997 -2000 Poster #115 M. A. Pfaller 1, 3*, D. J. Diekema 1, 2, R. N. Jones 4, S. A. Messer 1, R. J. Hollis 1, and the SENTRY Participants Group Departments of Pathology 1, Medicine 2 and Epidemiology 3, University of Iowa College of Medicine and College of Public Health, Iowa City, Iowa and The JONES Group /JMI Laboratories, North Liberty, Iowa 4 ABSTRACT MATERIALS AND METHODS - CONT’D RESULTS AND DISCUSSION - CONT’D From 1/1/97 through 12/31/00, 2, 047 blood stream infections (BSI) due to Candida spp. were reported from SENTRY hospitals in the United States (U. S. ), Canada, Latin America and Europe. C. albicans (54%), C. glabrata (16%), C. parapsilosis (15%), and C. tropicalis (10%) were the most common species. Among the four different age groups ( 1 yr, 2 -15 yr, 16 -64 yr, and 65 yr) C. albicans was the most common, causing 60%, 55% and 50% of infections, respectively. C. glabrata accounted for 17% - 23% of BSI in the 16 -64 yr and 65 yr age groups versus only 3% of BSI in the two younger age groups. C. parapsilosis (21 -24%) and C. tropicalis (7 -10%) were more common than C. glabrata in the 1 yr and 2 -15 yr age groups. Antifungal susceptibility testing using NCCLS microdilution and Etest (amphotericin B) methods revealed interesting trends in susceptibility across age groups and among the various species. Isolates of Candida spp. from patients 1 yr were more susceptible to fluconazole, itraconazole and amphotericin B than isolates from the 65 yr age group. None of the C. glabrata isolates from the 1 yr age group were resistant to fluconazole compared to 5 -9% of isolates from the 16 -64 yr and 65 yr age groups. Isolates of C. tropicalis from patients 1 yr were more susceptible to flucytosine (MIC 90, 0. 5 g/ml; 0% resistant) than those isolated from patients 65 yr (MIC 90, 32 g/ml; 11% resistant). The investigational triazoles, posaconazole, ravuconazole, and voriconazole, were all highly active against all species of Candida from all age groups. Finally, isolates of C. albicans were more susceptible to amphotericin B (MIC 90, 1 g/ml) than were C. glabrata (MIC 90, 4 g/ml) and C. krusei (MIC 90, 8 g/ml) suggesting that BSI due to C. glabrata and C. krusei may require higher doses of amphotericin B. These data demonstrate differences in the species distribution of pathogens and in antifungal resistance among the pediatric and adult age groups. Ongoing surveillance will enhance efforts to limit the extent of antifungal resistance in various age groups. products (bio. Merieux, St. Louis, MO) as recommended by the manufacturer or by conventional methods as required (22). Isolates were stored as suspensions in water or on agar slants at ambient temperature until needed. the NCCLS microdilution method when applied to a subset of 1, 142 isolates (Table 5). Notably, 24. 4% of C. glabrata isolates and 47. 6% of C. krusei isolates appeared resistant with MICs 2 g/ml when tested by the NCCLS method. As reported previously, the agreement between Etest and broth microdilution results was excellent, 98. 7% within ± 2 log 2 dilutions (12). INTRODUCTION The proliferation of antimicrobial resistance surveillance programs has provided useful information regarding resistance trends and pathogen distribution among various countries and types of infection (7). Although most surveillance programs have focused on bacterial pathogens, several have provided information on invasive candidiasis as well. The population based surveillance study conducted by the Centers for Disease Control (CDC) (5), the NEMIS Study (2, 9, 16, 19), the SCOPE study (4) and the SENTRY program (3, 11, 13 -15) have all contributed information regarding species distribution and antifungal susceptibilities of blood stream infection (BSI) isolates of Candida spp. over the decade 1990 -2000. Although the CDC and NEMIS studies provided some information on Candida BSI in both adult and neonatal populations, none of the Candida surveillance studies have provided information on the distribution of species and antifungal susceptibility profiles of BSI pathogens stratified according to the age of the patient. The SENTRY Antifungal Surveillance Program has been active continuously since January 1997 and has reported antifungal susceptibility and pathogen data for more than 2, 000 episodes of Candida BSI in 72 medical centers internationally (3, 11, 13 -15). Participating centers submit isolates representing consecutive episodes of BSI each month along with limited demographic data including age, sex, medical service and ICU stay. In the present report, we examine the frequency of pathogen occurrence and antifungal susceptibility profiles of the various species against both licensed and investigational antifungal agents as they occur in four different age groups: 1 yr, 2 -15 yr, 16 -64 yr, and 65 yr. The BSI isolates include all those submitted to the central monitoring laboratory from North America, Latin American and European SENTRY participants during the fouryear period from 1997 -2000. MATERIALS AND METHODS Study Design: The SENTRY Program was established in 1997 to monitor the predominant pathogens and antimicrobial resistance patterns of nosocomial and community acquired infections via a broad network of sentinel hospitals categorized by geographic location and size (3, 11, 13 -15). The present report focuses on BSI due to Candida spp. from US, Canadian, Latin American, and European sites. BSI due to Candida spp. were reported from 32 monitored medical centers in the US, 23 in Europe, nine in Latin America, and seven in Canada over the four-year period from January, 1997 through December, 2000. Each participant hospital contributed results (organism identification, date of isolation, and hospital location) for consecutive blood culture isolates (one isolate per patient) of Candida spp. judged to be clinically significant by local criteria, detected in each calendar month during the study period. All isolates were stored on agar slants and sent on a regular basis to the University of Iowa College of Medicine (Iowa City, IA) for storage and further characterization by reference identification and susceptibility testing (8, 22). Organism Identification: All fungal blood culture isolates were identified at the participating institutions by the routine method in use at each laboratory. Upon receipt at the monitoring site, the isolates were subcultured onto potato dextrose agar (Remel, Lenexa, KS) and CHROMagar Candida medium (Hardy Laboratories, Santa Maria, CA) to ensure viability and purity. Confirmation of species identification was performed with Vitek and API Susceptibility Testing: Antifungal susceptibility testing of isolates of Candida spp. was performed by the reference broth microdilution method described by the National Committee for Clinical Laboratory Standards (NCCLS) (8). Susceptibility of isolates to amphotericin B was determined using Etest (AB BIODISK, Solna, Sweden) and RPMI 1640 agar with 2% glucose (Remel, Lenexa, KS) as described previously (12). In addition, a subset of 1142 isolates was also tested against amphotericin B using the NCCLS broth microdilution method. Standard powders of fluconazole (Pfizer, Inc. , New York, NY), voriconazole (Pfizer), ravuconazole (Bristol-Myers Squibb, Wallingford, CT), posaconazole (Schering-Plough, Kenilworth, NJ), itraconazole (Janssen, Beerse, Belgium), amphotericin B and flucytosine (Sigma, St. Louis, MO) were obtained from their respective manufacturers. Following incubation at 35ºC for 48 h, the MICs of fluconazole, voriconazole, ravuconazole, posaconazole, itraconazole, and 5 FC were read as the lowest concentration at which a prominent decrease in turbidity relative to the growth control well was observed (8). Amphotericin B MICs determined by Etest were read after 48 h incubation at 35ºC and were determined to be at 100% inhibition of growth where the border of the elliptical inhibition zone intercepted the scale on the strip edge (12). Amphotericin B MICs determined by the NCCLS method were read at 100% inhibition of growth. Quality Control (QC) was ensured by testing the NCCLS (8) recommended strains, C. krusei ATCC 6258 and C. parasilosis ATCC 22019. Interpretive criteria for susceptibility to fluconazole (susceptible, 8 g/ml), itraconazole (susceptible, 0. 12 g/ml), and 5 FC (susceptible, 4 g/ml) were those published by Rex et al. (17) and the NCCLS (8). These breakpoints apply to all Candida spp. (including C. glabrata) with the exception of C. krusei which is considered inherently resistant to fluconazole regardless of the MIC value obtained (8). The investigational triazoles, posaconazole, voriconazole and ravuconazole, have not been assigned interpretive breakpoints. For purposes of comparison, and because preliminary pharmacokinetic data indicates that achievable serum levels for these agents may range from 2 to 6 g/ml depending on the dosing regimen (20), we have employed a susceptible breakpoint of 1 g/ml for all three agents. Interpretive criteria have not yet been defined for amphotericin B; however for comparison purposes we have determined the percentage of isolates inhibited by 2 g/ml to be resistant in this surveillance study. It appears that the differences in susceptibility to amphotericin B and the azoles, among the four age groups as shown in Table 2 may be largely due to differences in species distribution in the younger age groups compared to the older age groups. The 1 yr and 2 -15 yr age groups were dominated by C. albicans and C. parapsilosis both of which were considerably more susceptible to the tested antifungal agents than C. glabrata. The older age groups, and particularly the 65 yr group, had relatively fewer infections due to C. albicans and significantly more infections due to C. glabrata than the younger age groups. The decreased susceptibility of C. glabrata to both the azoles and amphotericin B is clearly evident in Tables 3 and 4 and likely accounted for the differences in susceptibility among the age groups noted in Table 2. The predominance of C. albicans and C. parapsilosis as the etiologic agents of fungemia in neonates has been noted previously by Kao et al (5) and Samian et al (19) among others; however, the lack of infections with C. glabrata and C. krusei in this age group is less well appreciated. The increasing importance of C. glabrata in the adult population is well known and the proportion of BSI due to C. glabrata has been noted to be considerably higher in individuals > 60 yrs of age in some institutions (6, 15). The reasons for these differences are speculative but may relate to the tendency for neonatologists and pediatricians to use amphotericin B preferentially over fluconazole in the treatment of documented or suspected candidemia (18) and the pervasive use of fluconazole in the adult hospital population (1). Furthermore, vertical transmission of C. albicans from mother to infant and horizontal transmission of C. parapsilosis from patient-topatient in the neonatal intensive care unit environment is well documented and may also account for the predominance of these two species in infants and children (9, 21). TABLE 1: Species Distribution of Candida Blood Stream Isolates by Age Group (yr): SENTRY, 1997 - 2000 RESULTS AND DISCUSSION 1 Species Frequency of Occurrence of Blood Stream Pathogens: During the four-year study period (January 1997 to December 2000), a total of 2, 047 BSI due to Candida spp. were reported by SENTRY participants. Table 1 compares the frequencies of occurrence of the seven most commonly isolated pathogens during this time period. These seven species accounted for 99% of all Candida BSI reported from SENTRY study sites. Overall, the rank order of the top five species was unchanged from year-to-year. These five species (C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei) accounted for 97% of all Candida BSI. C. albicans C. glabrata C. parapsilosis C. tropicalis C. krusei C. guilliermondii C. lusitaniae Candida spp. Comparing the frequency of isolation of different species by age group, we found an identical rank order in the 16 -64 yr and 65 yr age groups with C. albicans > C. glabrata > C. parapsilosis > C. tropicalis > C. krusei. The rank order of species in the 1 yr and 2 -15 yr age groups was considerably different from that of the two older age groups. The infant and pediatric age groups were dominated by C. albicans and C. parapsilosis and very few infections were due to C. glabrata and C. krusei. Although C. albicans was the most common species in all age groups, the percentage of BSI due to this species decreased from 60% in the 1 yr group to 50% in the 65 yr group. C. glabrata was the second most common species overall causing 17 -23% of BSI in the 16 -64 yr and 65 yr age groups. In contrast, C. glabrata accounted for only 3% of BSI in 1 yr and 2 -15 yr age groups and was surpassed by both C. parapsilosis (21 -24%) and C. tropicalis (7 -10%). % of Isolates by Age Group (Yr, No. Tested) 2 - 15 16 - 64 65 Total (124) (1028) (622) (2047) 60 3 24 7 3 2 1 55 3 21 10 4 2 2 3 55 17 12 11 2 1 1 1 50 23 12 10 2 1 1 1 Antifungal Agents Fluconazole Age No Group(Yr) Tested 0. 12 1 273 18 2 -15 124 14 16 -64 1028 14 65 622 16 Itraconazole 1 273 84 b 2 -15 124 78 b 16 -64 1028 70 b 65 622 67 b Amphotericin B 1 273 1 2 -15 124 1 16 -64 1028 0. 3 65 622 1 Flucytosine 1 273 60 2 -15 124 68 16 -64 1028 60 65 622 69 87 Among the licensed and investigational azoles, fluconazole, posaconazole, ravuconazole, and voriconazole were all highly active (96 -100% susceptible) against C. albicans, C. tropicalis, C. guilliermondii, and C. lusitaniae regardless of the age group (Table 3). Although fluconazole appeared less active against isolates of C. glabrata in the 1 yr age group (63% susceptible) compared with those from the 16 -64 yr (70% susceptible) and 65 yr (73% susceptible) age groups, there were no highly resistant (MIC, 64 g/ml) isolates in the 1 yr group compared to 5 -9% of isolates from the two older groups. Itraconazole was less active than all of the other azoles against all species with the exception of C. albicans. Consistent with previous reports (3, 10, 13, 15), all three investigational triazoles were highly active against all species from all age groups with the exception of C. glabrata isolates from the 16 -64 yr group where 88 -90% of isolates were susceptible at 1 g/ml to the three agents compared to 95 -100% of isolates from all other age groups. Cumulative % Inhibited at MIC (mg/ml) 0. 25 0. 5 1 2 4 8 16 32 59 80 91 95 97 99 a 100 48 73 85 90 94 95 a 97 98 a 55 67 74 81 87 91 95 97 50 64 71 79 86 91 a 96 98 97 98 99 100 100 94 98 99 99 84 92 97 98 99 99 83 91 96 99 99 99 3 28 80 97 99 100 3 27 72 91 98 99 2 26 70 91 98 99 2 20 67 92 99 99 80 86 95 95 96 c 96 97 97 c 80 89 94 98 98 98 99 100 c 76 84 93 95 95 96 98 98 92 96 96 97 c 97 98 99 99 Fluconazole susceptible breakpoint, < 8 g/ml (NCCLS, M 27 -A) b Itraconazole susceptible breakpoint, < 12 g/ml (NCCLS, M 27 -A) c Flucytosine susceptible breakpoint, < 4 g/ml (NCCLS M 27 -A) a Antifungal Agent N C. albicans (1114) Fluconazole Itraconazole Posaconazole Ravuconazole Voriconazole C. glabrata (334) Species (N) C. parapsilosis (301) C. tropicalis (209) <1 50/90 2 - 15 50/90 %S %S N 163 0. 25/0. 5 0. 3/0. 12 0. 03/0. 06 0. 01/0. 03 100 95 100 100 68 0. 25/0. 5 0. 03/0. 12 0. 03/0. 06 0. 01/0. 03 Fluconazole Itraconazole Posaconazole Ravuconazole Voriconazole 8 8/1/0. 25/0. 12/0. 5/- 63 13 100 100 4 2/0. 25/0. 12/0. 06/- 75 100 100 Fluconazole Itraconazole Posaconazole Ravuconazole Voriconazole 66 0. 5/2 0. 12/0. 25 0. 03/0. 12 0. 03/0. 06 100 80 100 100 27 0. 5/4 0. 12/025 0. 06/0. 12 0. 03/0. 06 100 63 100 100 Fluconazole Itraconazole Posaconazole Ravuconazole Voriconazole 20 0. 5/1 0. 12/0. 25 0. 03/0. 12 0. 03/0. 06/0. 12 100 70 100 100 13 0. 5/2 0. 12/0. 5 0. 03/0. 12 0. 03/0. 06 100 62 100 100 5 16/0. 25/0. 12/- 16 - 64 N 50/90 %S 569 0. 25/0. 5 98 0. 03/0. 12 95 0. 03/0. 06 99 0. 01/0. 03 99 180 8/32 70 0. 5/2 88 0. 25/2 89 0. 12/1 90 20 40 100 100 Fluconazole Itraconazole Posaconazole Ravuconazole Voriconazole 99 97 98 98 99 C. guilliermondii (14) Fluconazole Itraconazole Posaconazole Ravuconazole Voriconazole 6 2/0. 25/0. 12/0. 25/0. 06/- 100 0 100 75 100 2 2/0. 12/0. 060. 03/- Fluconazole Itraconazole Posaconazole Ravuconazole Voriconazole 7 0. 25/0. 12/0. 03/0. 03/- 100 71 100 100 2 0. 25/0. 06/0. 03/0. 01/- 125 0. 5/2 0. 12/0. 25 0. 06/0. 12 0. 03/0. 06 0. 5/2 0. 12/0. 5 0. 03/0. 12 99 0. 03/0. 12 97 23 32/64 0. 5/2 0. 25/0. 5 0. 25/1 100 5 2/0. 25/0. 06/0. 066 0. 25/0. 12/0. 03/0. 01/- 100 50 100 100 a% 100 64 100 100 113 100 50 100 100 C. lusitaniae (18) 54 16 15 10 2 1 1 1 SUMMARY AND CONCLUSIONS n Antifungal resistance surveillance programs provide important information, both for the development of empiric antifungal therapy recommendations and for design of programs to control antifungal resistance. n The present study demonstrates differences in the spectrum of pathogens and in MIC (mg/ml) and % susceptible (%(S)a by Age Group (Yr) C. krusei (39) 96 68 9 0 100 95 N antifungal susceptibility among the pediatric and adult age groups in the SENTRY Program. > 65 50/90 %S 314 0. 25/0. 5 0. 03/0. 12 0. 03/0. 06 0. 01/0. 03 142 8/16 0. 5/2 0. 5/1 0. 25/0. 5 0. 12/1 n Most common Candida BSI pathogens by age group – 1 yr: C. albicans (60%), C. parapsilosis (24%) – 2 -15 yr C. albicans (55%), C. parapsilosis (21%) – 16 -64 yr: C. albicans (55%), C. glabrata (17%) – 65 yr: C. albicans (50%), C. glabrata (23%) 99 97 99 99 98 73 6 97 97 95 83 63 0. 5/2 0. 12/0. 25 0. 06/0. 12 0. 03/0. 06 0. 03/0. 12 100 67 100 100 11 32/64 1/2 0. 25/0. 5/1 100 40 100 1 100 50 100 100 3 prominent among isolates of C. glabrata and C. krusei from all age groups. n The investigational triazoles, posaconazole, ravuconazole, and voriconazole were all highly active against all species of Candida from all age groups. 100 63 100 100 0. 5/1 0. 12/0. 2 0. 03/0. 25 0. 03/0. 12 n Decreased susceptibility to fluconazole, itraconazole, and amphotericin B was most 9 9 100 100 >64 4 >8 >8 >8 0 0 0. 5/0. 25/0. 03/0. 01/- 67 33 100 67 100 n Ongoing surveillance is essential and will enhance efforts to limit the extent of resistance among the various age groups. ACKNOWLEDGEMENTS Linda Elliott provided excellent support in the preparation of this manuscript. This study was supported in part by research grants from Bristol-Myers Squibb (SENTRY), Pfizer Pharmaceuticals, and Schering-Plough Research Institute. We express our appreciation to all SENTRY site participants. Participants contributing data/isolates to the study included: The Medical Center of Delaware, Wilmington, DE, (L. Steele-Moore); Clarion Health Methodist Hospital, Indianapolis, IN, (G. Denys); Henry Ford Hospital, Detroit, MI (C. Staley); Summa Health System, Akron, OH (J. R. Dipersio); Good Samaritan Regional Medical Center, Phoenix, AZ (M. Saubolle); Denver General Hospital, Denver, CO, (M. L. Wilson); University of New Mexico Hospital, Albuquerque, NM, (G. D. Overturf); University of Illinois at Chicago, IL, (P. C. Schreckenberger); University of Iowa Hospitals and Clinics, Iowa City, IA, (R. N. Jones); Creighton University, Omaha, NE, (S. Cavalieri); Froedtert Memorial Lutheran Hospital-East, Milwaukee, WI, (S. Kehl); Boston VAMC, Boston, MA (S. Brecher); Columbia Presbyterian Medical Center, New York, NY, (P. Della- Latta); Long Island Jewish Medical Center, New Hyde Park, NY, (H. Isenberg); Strong Memorial Hospital, Rochester, NY, (D. Hardy); Kaiser Regional Laboratory, Berkeley, CA, (J. Fusco); Sacred Heart Medical Center, Spokane, WA, (M. Hoffmann); University of Washington Medical Center, Seattle, WA, (S. Swanzy); Barnes-Jewish Hospital, St. Louis, MO, (P. R. Murray); Parkland Health & Hospital System, Dallas, TX, (P. Southern); The University of Texas Medical School, Houston, TX, (A. Wanger); University of Texas Medical Branch at Galveston, TX (B. Reisner); University of Louisville Hospital, Louisville, KY, (J. Snyder); University of Mississippi Medical Center, Jackson, MS, (J. Humphries); Carolinas Medical Center, Charlotte, NC, (S. Jenkins); University of Virginia Medical Center, Charlottesville, VA, (K. Hazen); University of Alberta Hospital, Edmonton, Alberta, CAN, ( R. Rennie); Health Sciences Centre, Winnipeg, Manitoba, CAN, ( D. Hoban); Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, CAN, (K. Forward); Ottawa General Hospital, Ottawa, Ontario, CAN, (B. Toye); Royal Victoria Hospital, Montreal, Quebec, CAN, (H. Robson); Microbiology Laboratory C. E. M. I. C. , Buenos Aires, Argentina, (J. Smayvsky); Hospital San Lucas and Olivos Community Hospital, Buenos Aires, Argentina, (J. M. Casellas/G. Tome); Lamina LTDA, Rio De Janeiro, Brazil, (J. L. M. Sampaio); Unidad De Microbiologia Oriente, Santiago, Chile, (V. Prado); Hospital Clinico Universidad Catolica, Santiago, Chile, (E. Palavecino); Corp. Para Investig Biologicas, Medellin, Columbia, (J. A. Robledo); Instituto Nacional de la Nutricion, Mexico City, Mexico, (J. S. Osornio); Laboratorio Medico Santa Luzia, Florianopolis, Brazil; Instituto DE Doencas Infecciosas-IDIPA, Sao Paulo, Brazil, (H. S. Sader); Centro Medico De Caracas, San Bernadino, Caracas, (M. Guzman); Chru De Lille Hopital Calmette, Lille, Cedex, France (M. Roussel-Delvallez); National University of Athens Medical School, Athens, Greece (N. Legakis); Sheba Medical Center, Tel-Hashomer, Israel (N. Keller); University Hospital V. de Macarena, Sevilla, Spain (E. J. Perea); Hospital de Bellvitge, Barcelona, Spain (J. Linares); Hospital Ramon y Cajal, Madrid, Spain (R. Canton); Unite de Bacteriologie, Luasanne, Switzerland (F. Praplan); Hacettepe Universitaesi Tip Fakultesi, Ankara, Turkey (D. Gur); Universita degli Studi di Genova, Italy (E. Debbia); Azienda Policlinico Univ Catania, Italy (G. Nicoletti); Policlinico Agostino Germelli, Roma, Italy (G. Fadda); Universitat Bonn, Germany (K. P. Schaalb); J. -W. -Goethe Universitat, Frankfurt, Germany (P. Shah); University Hospital, Linkoping, Sweden (H. Hanberger); Sera & Vaccines Central Research Lab, Warsaw, Poland (W. Hryniewicz); St. Thomas Hospital, London, United Kingdom (G. French); Univ Libre de bruxelles-Hopital Erasme, Burssels, Belgium (M. J. Struelens); Marmara Universitesi Tip Fakultesi, Istanbul, Turkey (V. Korten). REFERENCES susceptible at MIC of < 8 g/ml (fluconazole), < 0. 12 g/ml (itraconazole) or < 1 g/ml (all other agents) 1. TABLE 2: Antifungal Activities of Four Licensed Systematic Antifungal Agents Against 2, 047 Invasive Isolates of Candida spp. According to Age Group: SENTRY, 1997 - 2000 Difference in Susceptibility Among Age Groups to Both Licensed and Investigational Antifungal Agents: Among the licensed antifungal agents, fluconazole, itraconazole, amphotericin B, and flucytosine, isolates of Candida spp. from patients 1 yr were more susceptible to fluconazole, itraconazole, and amphotericin B than isolates from the 16 -64 yr and 65 yr age groups (Table 2). Although flucytosine was uniformly active among all age groups overall (95 -98% susceptible), isolates of C. tropicalis from patients 1 yr were more susceptible to this agent (MIC 90, 0. 5 g/ml; 0% resistant) than those isolates from patients 65 yr (MIC 90, 32 g/ml; 11% resistant) (data not shown). The activity of amphotericin B as determined by Etest against the top five species is shown in Table 4. As expected, amphotericin B was most active against C. albicans (MIC 90, 1 g/ml). The activity of amphotericin B against the other species was significantly less than that observed with C. albicans: C. glabrata (MIC 90, 4 g/ml), C. parapsiolosis (MIC 90, 4 g/ml) and C. krusei (MIC 90, 8 g/ml). Although the Etest in our hands tended to give higher amphotericin B MICs for the non-ablicans species than for C. albicans, the same tendency was seen with (273) TABLE 3: Antifungal activities of fluconazole, itraconazole, and investigational azole antifungal agents against 2, 029 invasive isolates of Candida spp. according to patient age group: SENTRY 1997 -2000 64 100 99 98 99 TABLE 4: Antifungal activities of amphotericin B against 1, 997 invasive isolates of Candida spp. : SENTRY 1997 -2000 Species C. albicans C. glabrata C. parapsilosis C. tropicalis C. krusei a. Amphotericin No. Tested 1, 114 334 301 209 39 0. 12 0. 4 0. 9 0. 0 0. 25 2. 7 1. 8 0. 3 0. 5 0. 0 0. 5 38. 2 5. 7 2. 7 8. 1 0. 0 1 92. 1 41. 4 44. 1 48. 8 10. 3 2 99. 5 83. 3 80. 9 90. 9 43. 6 2. 3. 4. 4 100 97. 9 97. 0 99. 5 82. 1 8 100 99. 7 99. 3 100 97. 4 16 100 100 100 32 100 100 100 B MICs by Etest 5. 6. 7. 8. 9. 10. 11. 12. TABLE 5: In vitro susceptibility of blood stream isolates of Candida spp. to amphotericin B as determined by Broth Microdilution (BMD) and Etest methodsa Species (N) Candida albicans (658) Candida glabrata (180) 97 100 98 Cumulative % Inhibited at (mg/ml)a 0. 06 0. 2 0. 6 0. 0 Candia parapsilosis (179) Candida tropicalis (104) Candida krusei (21) a. Overall Test Methods BMD Range 0. 015 -2 Etest BMD Etest 0. 06 -3 0. 25 -2 0. 06 -8 0. 015 -2 0. 38 -6 0. 5 -2 0. 25 -31 1 -2 1 -8 agreement between BMD and Etest, 98. 7% (± 2 dilutions) MIC (at mg/ml) 50% 90% 1 1 0. 5 1 1. 5 1 2 1 3 *Corresponding Author: Michael A. Pfaller, M. D. Medical Microbiology Division, C 606 GH, Department of Pathology University of Iowa College of Medicine, Iowa City, Iowa 52242 Phone: (319) 384 -9566 Fax: (319) 356 -4916 Email: michael-pfaller@uiowa. edu 1 2 2 1 3 1 2 2 6 % with MIC 2 1. 8 1. 2 24. 4 28. 2 9. 5 46. 6 2. 9 6. 9 47. 6 86. 7 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. Berrouane, Y. F. , L. A. Herwaldt, and M. A. Pfaller. 1999. Trends in antifungal use and epidemiology of noscocomial yeast infections in a University hospital. J. Clin. Microbiol. 37: 531 -537 Blumberg, H. M. , W. R. Jarvis, J. M. Soucie, J. E. Edwards, J. E. Patterson, M. A. Pfaller, M. S. Rangel-Frausto, M. G. Rinaldi, L. Saiman, R. T. Wiblin, R. P. Wenzel, and the NEMIS Study Group. 2001. Risk factors for candidal blood stream infections in surgical intensive care unit patients: The NEMIS Prospective Multicenter Study. Clin. Infect. Dis. 33: 177 -186. Diekema, D. J. , M. A. Pfaller, S. A. Messer, A. Houston, R. J. Hollis, G. V. Doern, R. N. Jones and The SENTRY Participants Group. 1999. In vitro activities of BMS-207 -147 against over 600 contemporary clinical blood stream isolates of Candida species from the SENTRY Antibmicrobial Surveillance Program in North America and Latin America. Antimicrob. Agents Chemother. 43: 2236 -2239. Edmond, M. B. , S. E. Wallace, D. K. Mc. Clish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nocsocomial blood stream infections in United States hospitals: A three-year analysis. Clin. Infect. Dis. 29: 239 -244. Kao, A. S. , M. E. Brandt, W. R. Pruitt, L. A. Conn, B. A. Perkins, D. S. Stevens, W. S. Baughman, A. L. Reingold, G. A. Rothrock, M. A. Pfaller, R. W. Pinner, and R. A. Hajjeh. 1999. The epidemiology of candidemia in two United States cities: Results of a population-based active surveillance. Clin. Infect. Dis. 29: 1164 -1170. Kauffman, C. A. 2001. Fungal infections in older adults. Clin. Infect. Dis. 33: In Press. Masterton, R. G. 2000. Surveillance studies: How can they help the management of infection? J. Antimicrob. Chemother. 46: 53 -58. National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M 27 -A. National Committee for Clinical Laboratory Standards, Wayne, PA. Pfaller, M. A. , S. A. Messer, A. Houston, M. S. Rangel-Frausto, T. Wiblin, H. M. Blumberg, J. E. Edwards, W. Jarvis, M. A. Martin, H. C. Neu, L. Saiman, J. E. Patterson, J. C. Dibb, C. M. Roldan, M. G. Rinaldi, and R. P. Wenzel. 1998. National Epidemiology of Mycoses Survey: A multicenter study of strain variation and antifungal susceptibility among isolates of Candida species. Diagn. Microbiol. Infect. Dis. 31: 289 -296. Pfaller, M. A. , S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1998. In vitro susceptibilities of Candida blood stream isolates to the new triazole antifungal agents BMS-207147, Sch 56592, and voriconazole. Antimicrob. Agents Chemother. 42: 3242 -3244. Pfaller, M. A. , R. N. Jones, G. V. Doern, H. S. Sader, R. J. Hollis, and S. A. Messer for the SENTRY Participant Group. 1998. International surveillance of blood stream infections due to Candida species: Frequency of occurrence and antifungal susceptibilities of isolates collected in 1997 in the United States, Canada, and South America for the SENTRY Program. J. Clin. Microbiol. 36: 1886 -1889. Pfaller, M. A. , S. A. Messer, and A. Bolmstrom. 1998. Evaluation of Etest for determining in vitro susceptibility of yeast isolates to amphotericin B. Diagn. Microbiol Infect. Dis. 32: 223 -227. Pfaller, M. D. , R. N. Jones, G. V. Doern, A. C. Fluit, J. Verhoef, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, and R. J. Hollis for the SENTRY Participant Group (Europe). 1999. International surveillance of blood stream infections due to Candida species in the European SENTRY Program: Species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. Diagn. Microbiol. Infect. Dis. 35: 19 -25. Pfaller, M. A. , R. N. Jones, G. V. Doern, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, R. J. Hollis, and The SENTRY Participant Group. 2000. Blood stream infections due to Candida species: SENTRY Antimicrobial Surveillance Program in North America and Latin America. 1997 -1998. Antimicrob. Agents Chemother. 44: 747 -751. Pfaller, M. A. , D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, and S. A. Messer. 2001. International surveillance of blood stream infections due to Candida species: Frequency of occurrence and in vitro susceptibility to fluconazole, ravuconazole, and voriconazole among isolates collected from 1997 through 1999 in the SENTRY Antimicrobial Surveillance Program. J. Clin. Microbiol. In Press. Rangel-Frasuto, M. S. , T. Wiblin, H. M. Blumberg, L. Saiman, J. Patterson, M. Rinaldi, M. Pfaller, J. E. Edwards, Jr. , W. Jarvis, J. Dawson, and R. P. Wenzel. 1999. National epidemiology of mycoses survey (NEMIS): Variations in rates of blood stream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin. Infect. Dis. 29: 253 -258. Rex, J. H. , M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry for the Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: Conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24: 235 -247. Rowen, J. L. and J. M. Tate. 1998. Management of neonatal candidiasis. Neonatal Candidiasis Study Group. Pediatr. Infect. Dis. J. 17: 1007 -1001. Saiman, L. , E. Ludington, M. Pfaller, S. Rangel-Frausto, R. T. Wiblin, J. Dawson, H. M. Blumberg, J. E. Patterson, M. Rinaldi, J. E. Edwards, R. P. Wenzel, and W. Jarvis. 2000. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr. Infect. Dis. J. 19: 319 -324. Sheehan, D. J. , C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12: 40 -79. Waggoner-Fountain, L. A. , M. W. Walker, R. J. Hollis, M. A. Pfaller, J. E. Ferguson, 2 nd. , R. P. Wenzel, and L. G. Donowitz. 1996. Vertical and horizontal transmission of unique Candida species to premature newborns. Clin. Infec. Dis. 22: 803 -808. Warren, N. G. , and K. C. Hazen. 1999. Candida, Cryptococcus, and other yeasts of medical importance, p. 1184 -1199. In. P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed), Manual of Clinical Microbiology, 7 th ed. ASM Press, Washington, D. C. A 156 -03
384db50f7fabb76d108d6b28bd6e1cac.ppt