f0a8efddd3abac3fe4d01f47bbf75e9f.ppt
- Количество слайдов: 69
 Treatment of Advanced Prostate Cancer: The Changing Landscape Wm. Kevin Kelly, DO Professor, Medical Oncology and Urology Director, Division of Solid Tumor Oncology Thomas Jefferson University
Treatment of Advanced Prostate Cancer: The Changing Landscape Wm. Kevin Kelly, DO Professor, Medical Oncology and Urology Director, Division of Solid Tumor Oncology Thomas Jefferson University
 Disclosures Sanofi – Aventis: Research support
Disclosures Sanofi – Aventis: Research support
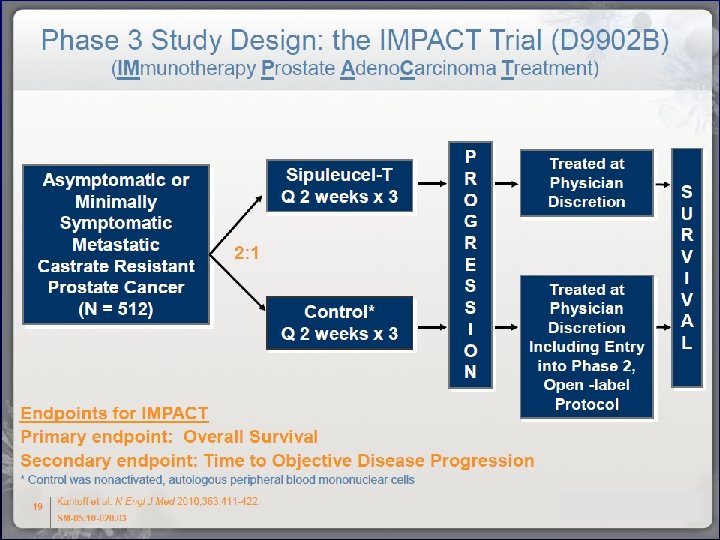
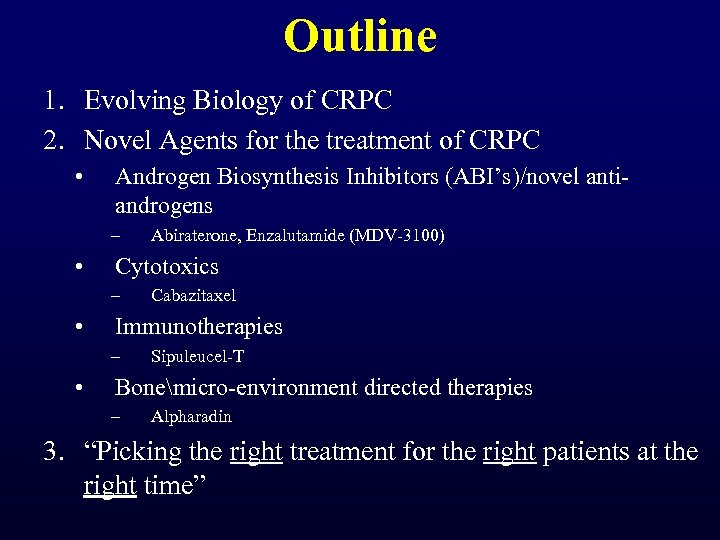 Outline 1. Evolving Biology of CRPC 2. Novel Agents for the treatment of CRPC • Androgen Biosynthesis Inhibitors (ABI’s)/novel antiandrogens – • Cytotoxics – • Cabazitaxel Immunotherapies – • Abiraterone, Enzalutamide (MDV-3100) Sipuleucel-T Bonemicro-environment directed therapies – Alpharadin 3. “Picking the right treatment for the right patients at the right time”
Outline 1. Evolving Biology of CRPC 2. Novel Agents for the treatment of CRPC • Androgen Biosynthesis Inhibitors (ABI’s)/novel antiandrogens – • Cytotoxics – • Cabazitaxel Immunotherapies – • Abiraterone, Enzalutamide (MDV-3100) Sipuleucel-T Bonemicro-environment directed therapies – Alpharadin 3. “Picking the right treatment for the right patients at the right time”
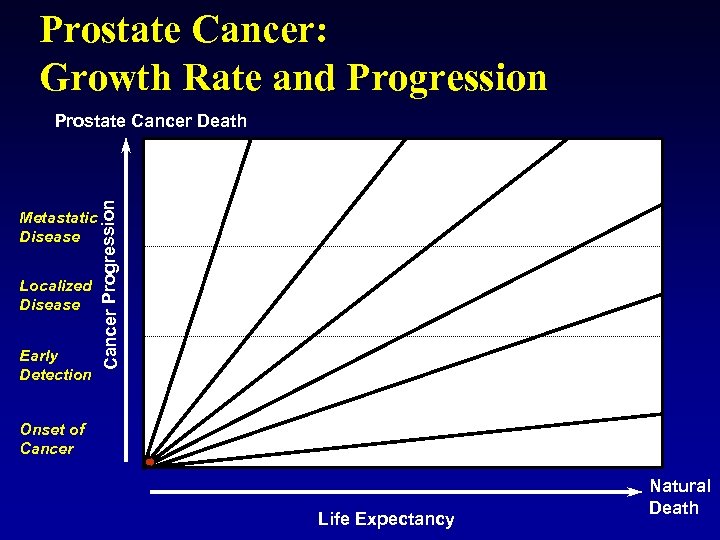 Prostate Cancer: Growth Rate and Progression Metastatic Disease Localized Disease Early Detection Cancer Progression Prostate Cancer Death Onset of Cancer Life Expectancy Natural Death
Prostate Cancer: Growth Rate and Progression Metastatic Disease Localized Disease Early Detection Cancer Progression Prostate Cancer Death Onset of Cancer Life Expectancy Natural Death
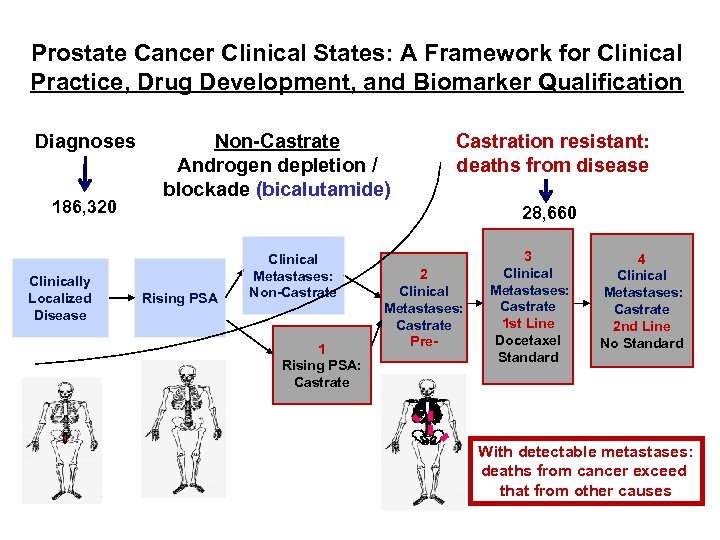 Prostate Cancer Clinical States: A Framework for Clinical Practice, Drug Development, and Biomarker Qualification Diagnoses 186, 320 Clinically Localized Disease Non-Castrate Androgen depletion / blockade (bicalutamide) Castration resistant: deaths from disease 28, 660 Rising PSA Clinical Metastases: Non-Castrate 1 Rising PSA: Castrate 2 Clinical Metastases: Castrate Pre- 3 Clinical Metastases: Castrate 1 st Line Docetaxel Standard 4 Clinical Metastases: Castrate 2 nd Line No Standard With detectable metastases: deaths from cancer exceed that from other causes
Prostate Cancer Clinical States: A Framework for Clinical Practice, Drug Development, and Biomarker Qualification Diagnoses 186, 320 Clinically Localized Disease Non-Castrate Androgen depletion / blockade (bicalutamide) Castration resistant: deaths from disease 28, 660 Rising PSA Clinical Metastases: Non-Castrate 1 Rising PSA: Castrate 2 Clinical Metastases: Castrate Pre- 3 Clinical Metastases: Castrate 1 st Line Docetaxel Standard 4 Clinical Metastases: Castrate 2 nd Line No Standard With detectable metastases: deaths from cancer exceed that from other causes
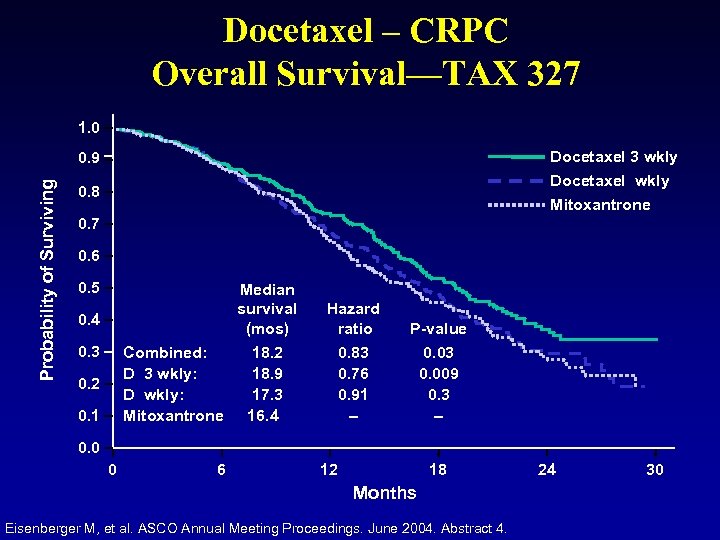 Docetaxel – CRPC Overall Survival—TAX 327 1. 0 Docetaxel 3 wkly Probability of Surviving 0. 9 Docetaxel wkly 0. 8 Mitoxantrone 0. 7 0. 6 0. 5 Median survival (mos) 0. 4 0. 3 Combined: D 3 wkly: D wkly: Mitoxantrone 0. 2 0. 1 Hazard ratio P-value 18. 2 18. 9 17. 3 16. 4 0. 83 0. 76 0. 91 – 0. 03 0. 009 0. 3 – 0. 0 0 6 12 18 Months Eisenberger M, et al. ASCO Annual Meeting Proceedings. June 2004. Abstract 4. 24 30
Docetaxel – CRPC Overall Survival—TAX 327 1. 0 Docetaxel 3 wkly Probability of Surviving 0. 9 Docetaxel wkly 0. 8 Mitoxantrone 0. 7 0. 6 0. 5 Median survival (mos) 0. 4 0. 3 Combined: D 3 wkly: D wkly: Mitoxantrone 0. 2 0. 1 Hazard ratio P-value 18. 2 18. 9 17. 3 16. 4 0. 83 0. 76 0. 91 – 0. 03 0. 009 0. 3 – 0. 0 0 6 12 18 Months Eisenberger M, et al. ASCO Annual Meeting Proceedings. June 2004. Abstract 4. 24 30
 What to do when first line Docetaxel treatment fails? Three years ago the choices were limited VS. Palliative Chemotherapy Phase I study Beach
What to do when first line Docetaxel treatment fails? Three years ago the choices were limited VS. Palliative Chemotherapy Phase I study Beach
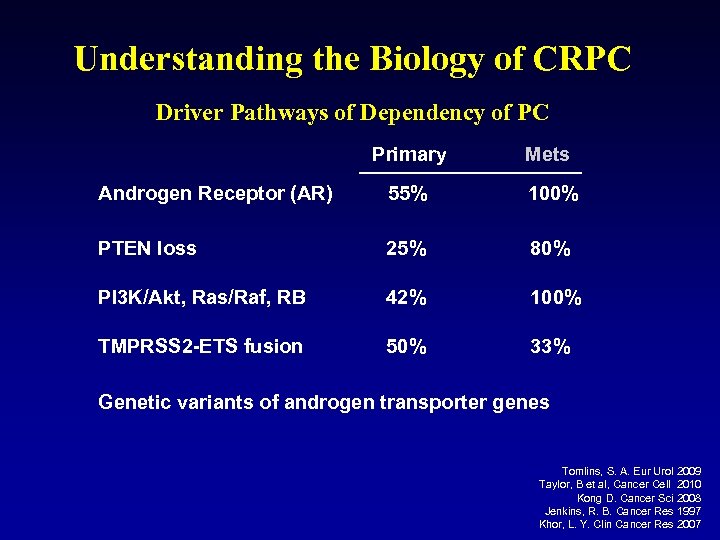 Understanding the Biology of CRPC Driver Pathways of Dependency of PC Primary Mets Androgen Receptor (AR) 55% 100% PTEN loss 25% 80% PI 3 K/Akt, Ras/Raf, RB 42% 100% TMPRSS 2 -ETS fusion 50% 33% Genetic variants of androgen transporter genes Tomlins, S. A. Eur Urol 2009 Taylor, B et al, Cancer Cell 2010 Kong D. Cancer Sci 2008 Jenkins, R. B. Cancer Res 1997 Khor, L. Y. Clin Cancer Res 2007
Understanding the Biology of CRPC Driver Pathways of Dependency of PC Primary Mets Androgen Receptor (AR) 55% 100% PTEN loss 25% 80% PI 3 K/Akt, Ras/Raf, RB 42% 100% TMPRSS 2 -ETS fusion 50% 33% Genetic variants of androgen transporter genes Tomlins, S. A. Eur Urol 2009 Taylor, B et al, Cancer Cell 2010 Kong D. Cancer Sci 2008 Jenkins, R. B. Cancer Res 1997 Khor, L. Y. Clin Cancer Res 2007
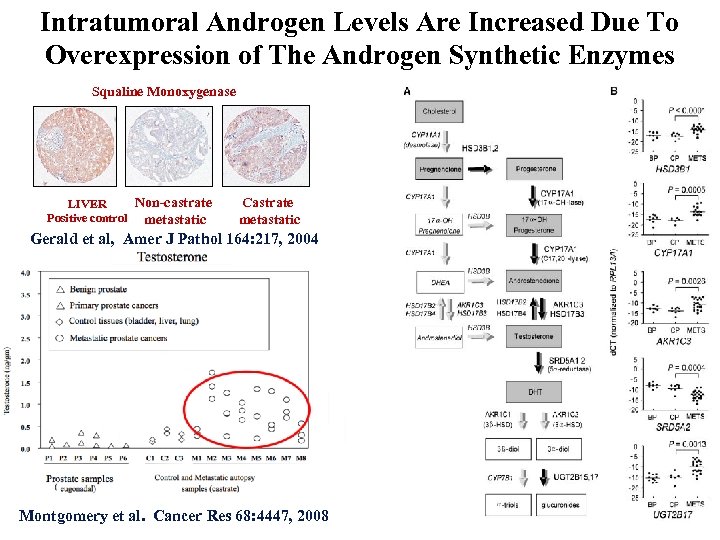 Intratumoral Androgen Levels Are Increased Due To Overexpression of The Androgen Synthetic Enzymes Squaline Monoxygenase Non-castrate LIVER Positive control metastatic Castrate metastatic Gerald et al, Amer J Pathol 164: 217, 2004 Steroid content Montgomery et al. Cancer Res 68: 4447, 2008
Intratumoral Androgen Levels Are Increased Due To Overexpression of The Androgen Synthetic Enzymes Squaline Monoxygenase Non-castrate LIVER Positive control metastatic Castrate metastatic Gerald et al, Amer J Pathol 164: 217, 2004 Steroid content Montgomery et al. Cancer Res 68: 4447, 2008
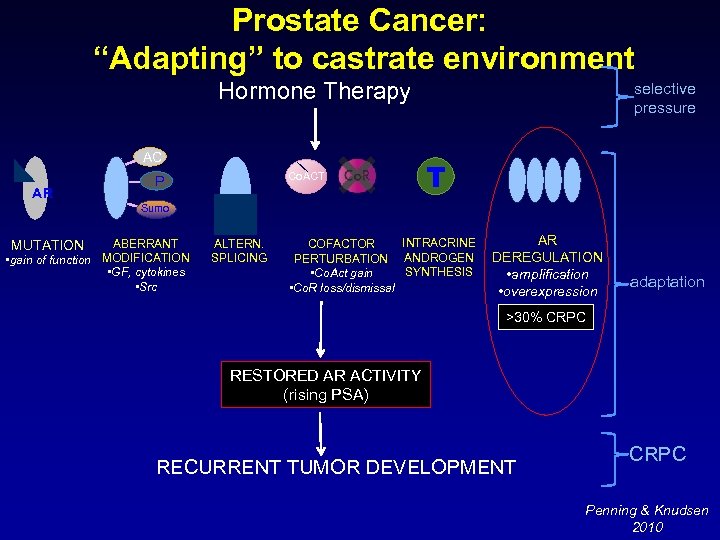 Prostate Cancer: “Adapting” to castrate environment Hormone Therapy AC AR Co. ACT P selective pressure T Sumo ABERRANT • gain of function MODIFICATION • GF, cytokines • Src MUTATION ALTERN. SPLICING INTRACRINE COFACTOR PERTURBATION ANDROGEN SYNTHESIS • Co. Act gain • Co. R loss/dismissal AR DEREGULATION • amplification • overexpression adaptation >30% CRPC RESTORED AR ACTIVITY (rising PSA) RECURRENT TUMOR DEVELOPMENT CRPC Penning & Knudsen 2010
Prostate Cancer: “Adapting” to castrate environment Hormone Therapy AC AR Co. ACT P selective pressure T Sumo ABERRANT • gain of function MODIFICATION • GF, cytokines • Src MUTATION ALTERN. SPLICING INTRACRINE COFACTOR PERTURBATION ANDROGEN SYNTHESIS • Co. Act gain • Co. R loss/dismissal AR DEREGULATION • amplification • overexpression adaptation >30% CRPC RESTORED AR ACTIVITY (rising PSA) RECURRENT TUMOR DEVELOPMENT CRPC Penning & Knudsen 2010
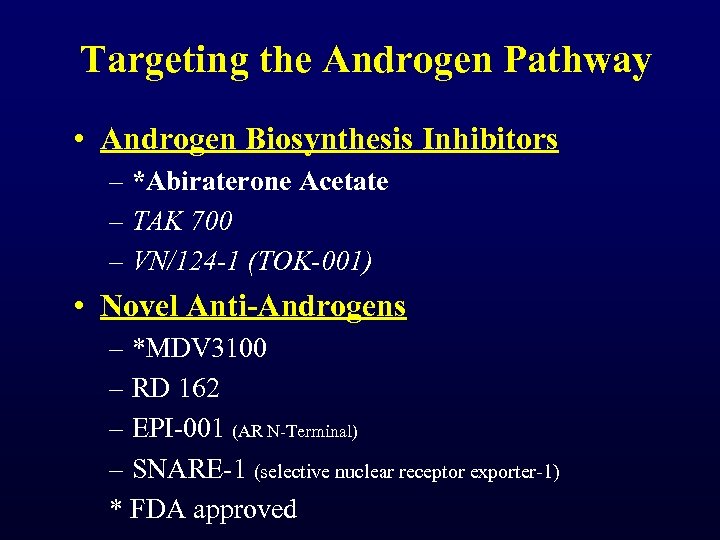 Targeting the Androgen Pathway • Androgen Biosynthesis Inhibitors – *Abiraterone Acetate – TAK 700 – VN/124 -1 (TOK-001) • Novel Anti-Androgens – *MDV 3100 – RD 162 – EPI-001 (AR N-Terminal) – SNARE-1 (selective nuclear receptor exporter-1) * FDA approved
Targeting the Androgen Pathway • Androgen Biosynthesis Inhibitors – *Abiraterone Acetate – TAK 700 – VN/124 -1 (TOK-001) • Novel Anti-Androgens – *MDV 3100 – RD 162 – EPI-001 (AR N-Terminal) – SNARE-1 (selective nuclear receptor exporter-1) * FDA approved
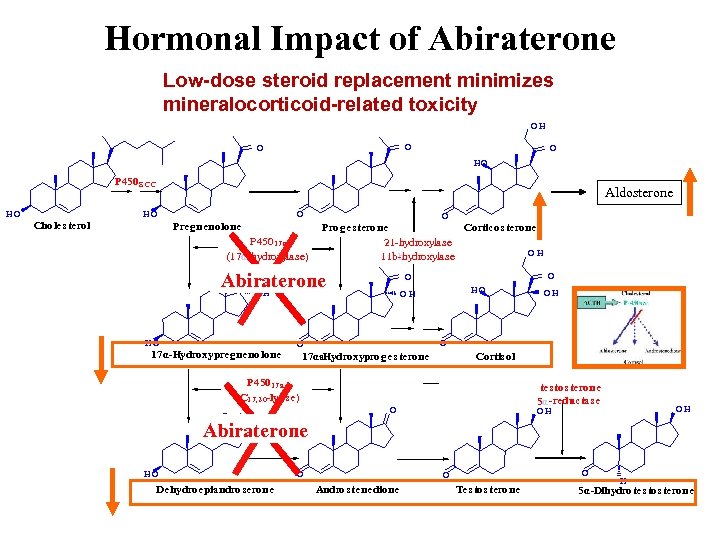 Hormonal Impact of Abiraterone Low-dose steroid replacement minimizes mineralocorticoid-related toxicity OH O O O HO P 450 S CC HO Aldosterone HO Choles terol O Pregnenolone P 45017 a (17α-hydroxylase) O Proges terone Corticos terone 21 -hydroxylase OH 11 bbhydroxylase - O Abiraterone OH HO 17α-Hydroxypregnenolone O O HO OH O O 17αa. Hydroxyproges terone - Cortis ol P 45017 a (C 17, 20 -lyas e) O OH tes tos terone 5α-reductas e O OH OH Abiraterone HO Dehydroepiandroserone O O O Andros tenedione Tes tos terone H 5α-Dihydrotes tos terone
Hormonal Impact of Abiraterone Low-dose steroid replacement minimizes mineralocorticoid-related toxicity OH O O O HO P 450 S CC HO Aldosterone HO Choles terol O Pregnenolone P 45017 a (17α-hydroxylase) O Proges terone Corticos terone 21 -hydroxylase OH 11 bbhydroxylase - O Abiraterone OH HO 17α-Hydroxypregnenolone O O HO OH O O 17αa. Hydroxyproges terone - Cortis ol P 45017 a (C 17, 20 -lyas e) O OH tes tos terone 5α-reductas e O OH OH Abiraterone HO Dehydroepiandroserone O O O Andros tenedione Tes tos terone H 5α-Dihydrotes tos terone
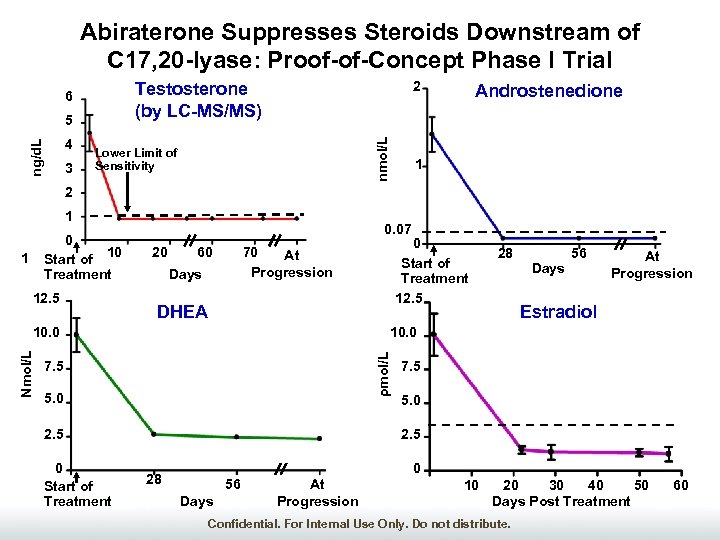 Abiraterone Suppresses Steroids Downstream of C 17, 20 -lyase: Proof-of-Concept Phase I Trial 3 nmol/L ng/d. L 5 4 2 Testosterone (by LC-MS/MS) 6 Lower Limit of Sensitivity Androstenedione 1 2 1 0 Start of 10 Treatment 1 12. 5 0. 07 20 60 Days DHEA 28 56 Days At Progression Estradiol 10. 0 ρmol/L Nmol/L 10. 0 7. 5 5. 0 2. 5 0 Start of Treatment 12. 5 70 At Progression 7. 5 5. 0 2. 5 0 28 56 Days At Progression 10 20 30 40 50 Days Post Treatment Confidential. For Internal Use Only. Do not distribute. 60
Abiraterone Suppresses Steroids Downstream of C 17, 20 -lyase: Proof-of-Concept Phase I Trial 3 nmol/L ng/d. L 5 4 2 Testosterone (by LC-MS/MS) 6 Lower Limit of Sensitivity Androstenedione 1 2 1 0 Start of 10 Treatment 1 12. 5 0. 07 20 60 Days DHEA 28 56 Days At Progression Estradiol 10. 0 ρmol/L Nmol/L 10. 0 7. 5 5. 0 2. 5 0 Start of Treatment 12. 5 70 At Progression 7. 5 5. 0 2. 5 0 28 56 Days At Progression 10 20 30 40 50 Days Post Treatment Confidential. For Internal Use Only. Do not distribute. 60
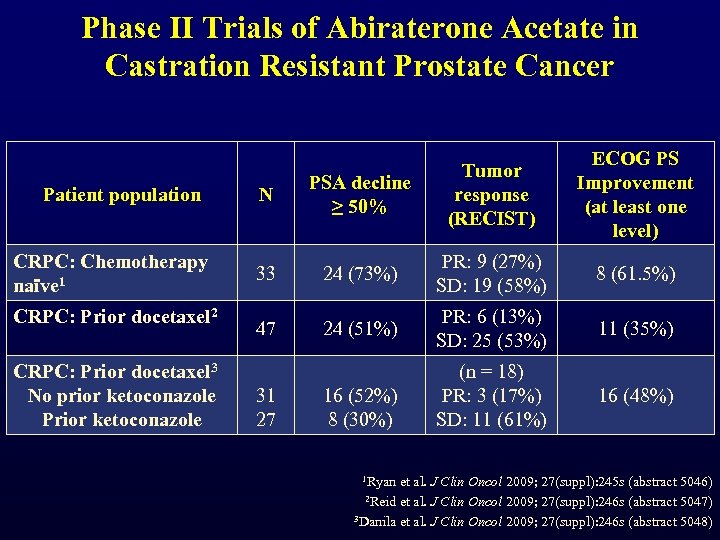 Phase II Trials of Abiraterone Acetate in Castration Resistant Prostate Cancer Patient population CRPC: Chemotherapy naïve 1 CRPC: Prior docetaxel 2 CRPC: Prior docetaxel 3 No prior ketoconazole Prior ketoconazole ECOG PS Improvement (at least one level) N PSA decline ≥ 50% Tumor response (RECIST) 33 24 (73%) PR: 9 (27%) SD: 19 (58%) 8 (61. 5%) 47 24 (51%) PR: 6 (13%) SD: 25 (53%) 11 (35%) (n = 18) PR: 3 (17%) SD: 11 (61%) 16 (48%) 31 27 16 (52%) 8 (30%) 1 Ryan et al. J Clin Oncol 2009; 27(suppl): 245 s (abstract 5046) 2 Reid et al. J Clin Oncol 2009; 27(suppl): 246 s (abstract 5047) 3 Danila et al. J Clin Oncol 2009; 27(suppl): 246 s (abstract 5048)
Phase II Trials of Abiraterone Acetate in Castration Resistant Prostate Cancer Patient population CRPC: Chemotherapy naïve 1 CRPC: Prior docetaxel 2 CRPC: Prior docetaxel 3 No prior ketoconazole Prior ketoconazole ECOG PS Improvement (at least one level) N PSA decline ≥ 50% Tumor response (RECIST) 33 24 (73%) PR: 9 (27%) SD: 19 (58%) 8 (61. 5%) 47 24 (51%) PR: 6 (13%) SD: 25 (53%) 11 (35%) (n = 18) PR: 3 (17%) SD: 11 (61%) 16 (48%) 31 27 16 (52%) 8 (30%) 1 Ryan et al. J Clin Oncol 2009; 27(suppl): 245 s (abstract 5046) 2 Reid et al. J Clin Oncol 2009; 27(suppl): 246 s (abstract 5047) 3 Danila et al. J Clin Oncol 2009; 27(suppl): 246 s (abstract 5048)
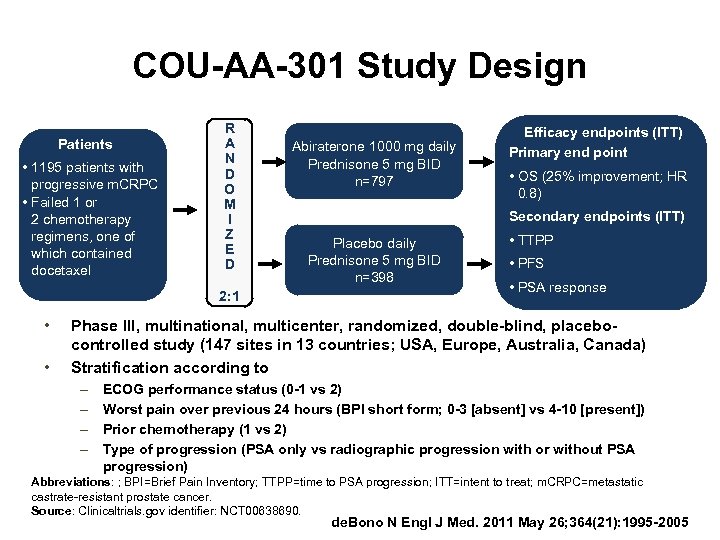 COU-AA-301 Study Design Patients • 1195 patients with progressive m. CRPC • Failed 1 or 2 chemotherapy regimens, one of which contained docetaxel R A N D O M I Z E D 2: 1 • • Abiraterone 1000 mg daily Prednisone 5 mg BID n=797 Efficacy endpoints (ITT) Primary end point • OS (25% improvement; HR 0. 8) Secondary endpoints (ITT) Placebo daily Prednisone 5 mg BID n=398 • TTPP • PFS • PSA response Phase III, multinational, multicenter, randomized, double-blind, placebocontrolled study (147 sites in 13 countries; USA, Europe, Australia, Canada) Stratification according to – – ECOG performance status (0 -1 vs 2) Worst pain over previous 24 hours (BPI short form; 0 -3 [absent] vs 4 -10 [present]) Prior chemotherapy (1 vs 2) Type of progression (PSA only vs radiographic progression with or without PSA progression) Abbreviations: ; BPI=Brief Pain Inventory; TTPP=time to PSA progression; ITT=intent to treat; m. CRPC=metastatic castrate-resistant prostate cancer. Source: Clinicaltrials. gov identifier: NCT 00638690. de. Bono N Engl J Med. 2011 May 26; 364(21): 1995 -2005
COU-AA-301 Study Design Patients • 1195 patients with progressive m. CRPC • Failed 1 or 2 chemotherapy regimens, one of which contained docetaxel R A N D O M I Z E D 2: 1 • • Abiraterone 1000 mg daily Prednisone 5 mg BID n=797 Efficacy endpoints (ITT) Primary end point • OS (25% improvement; HR 0. 8) Secondary endpoints (ITT) Placebo daily Prednisone 5 mg BID n=398 • TTPP • PFS • PSA response Phase III, multinational, multicenter, randomized, double-blind, placebocontrolled study (147 sites in 13 countries; USA, Europe, Australia, Canada) Stratification according to – – ECOG performance status (0 -1 vs 2) Worst pain over previous 24 hours (BPI short form; 0 -3 [absent] vs 4 -10 [present]) Prior chemotherapy (1 vs 2) Type of progression (PSA only vs radiographic progression with or without PSA progression) Abbreviations: ; BPI=Brief Pain Inventory; TTPP=time to PSA progression; ITT=intent to treat; m. CRPC=metastatic castrate-resistant prostate cancer. Source: Clinicaltrials. gov identifier: NCT 00638690. de. Bono N Engl J Med. 2011 May 26; 364(21): 1995 -2005
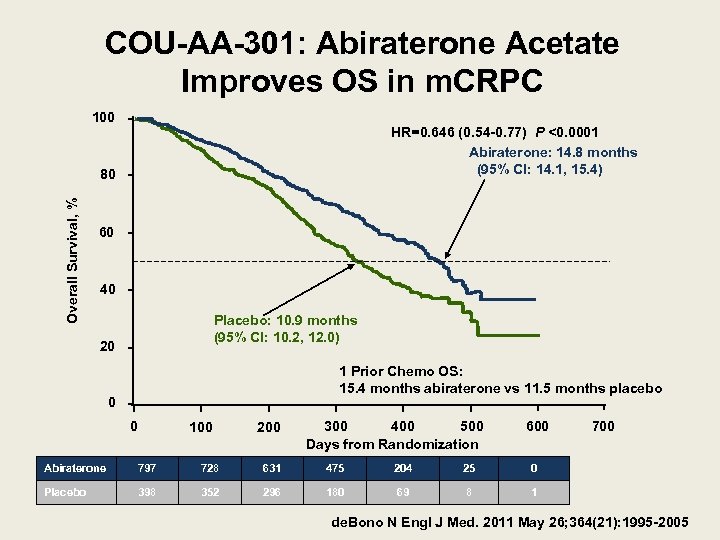 COU-AA-301: Abiraterone Acetate Improves OS in m. CRPC 100 HR=0. 646 (0. 54 -0. 77) P <0. 0001 Abiraterone: 14. 8 months (95% CI: 14. 1, 15. 4) Overall Survival, % 80 60 40 Placebo: 10. 9 months (95% CI: 10. 2, 12. 0) 20 1 Prior Chemo OS: 15. 4 months abiraterone vs 11. 5 months placebo 0 0 100 200 300 500 400 Days from Randomization 600 Abiraterone 797 728 631 475 204 25 0 Placebo 398 352 296 180 69 8 700 1 de. Bono N Engl J Med. 2011 May 26; 364(21): 1995 -2005
COU-AA-301: Abiraterone Acetate Improves OS in m. CRPC 100 HR=0. 646 (0. 54 -0. 77) P <0. 0001 Abiraterone: 14. 8 months (95% CI: 14. 1, 15. 4) Overall Survival, % 80 60 40 Placebo: 10. 9 months (95% CI: 10. 2, 12. 0) 20 1 Prior Chemo OS: 15. 4 months abiraterone vs 11. 5 months placebo 0 0 100 200 300 500 400 Days from Randomization 600 Abiraterone 797 728 631 475 204 25 0 Placebo 398 352 296 180 69 8 700 1 de. Bono N Engl J Med. 2011 May 26; 364(21): 1995 -2005
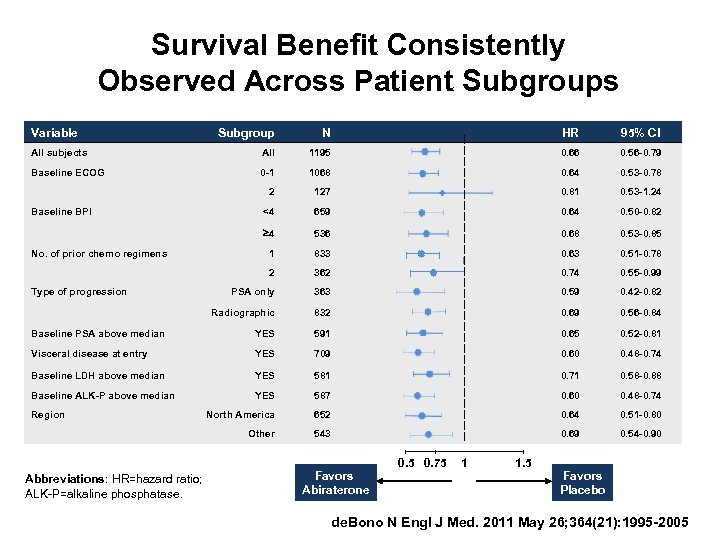 Survival Benefit Consistently Observed Across Patient Subgroups Variable Subgroup N HR 95% CI All subjects All 1195 0. 66 0. 56 -0. 79 Baseline ECOG 0 -1 1068 0. 64 0. 53 -0. 78 2 127 0. 81 0. 53 -1. 24 <4 659 0. 64 0. 50 -0. 82 4 536 0. 68 0. 53 -0. 85 1 833 0. 63 0. 51 -0. 78 2 362 0. 74 0. 55 -0. 99 PSA only 363 0. 59 0. 42 -0. 82 Radiographic 832 0. 69 0. 56 -0. 84 Baseline PSA above median YES 591 0. 65 0. 52 -0. 81 Visceral disease at entry YES 709 0. 60 0. 48 -0. 74 Baseline LDH above median YES 581 0. 71 0. 58 -0. 88 Baseline ALK-P above median YES 587 0. 60 0. 48 -0. 74 North America 652 0. 64 0. 51 -0. 80 Other 543 0. 69 0. 54 -0. 90 Baseline BPI No. of prior chemo regimens Type of progression Region Abbreviations: HR=hazard ratio; ALK-P=alkaline phosphatase. Favors Abiraterone 0. 5 0. 75 1 1. 5 Favors Placebo de. Bono N Engl J Med. 2011 May 26; 364(21): 1995 -2005
Survival Benefit Consistently Observed Across Patient Subgroups Variable Subgroup N HR 95% CI All subjects All 1195 0. 66 0. 56 -0. 79 Baseline ECOG 0 -1 1068 0. 64 0. 53 -0. 78 2 127 0. 81 0. 53 -1. 24 <4 659 0. 64 0. 50 -0. 82 4 536 0. 68 0. 53 -0. 85 1 833 0. 63 0. 51 -0. 78 2 362 0. 74 0. 55 -0. 99 PSA only 363 0. 59 0. 42 -0. 82 Radiographic 832 0. 69 0. 56 -0. 84 Baseline PSA above median YES 591 0. 65 0. 52 -0. 81 Visceral disease at entry YES 709 0. 60 0. 48 -0. 74 Baseline LDH above median YES 581 0. 71 0. 58 -0. 88 Baseline ALK-P above median YES 587 0. 60 0. 48 -0. 74 North America 652 0. 64 0. 51 -0. 80 Other 543 0. 69 0. 54 -0. 90 Baseline BPI No. of prior chemo regimens Type of progression Region Abbreviations: HR=hazard ratio; ALK-P=alkaline phosphatase. Favors Abiraterone 0. 5 0. 75 1 1. 5 Favors Placebo de. Bono N Engl J Med. 2011 May 26; 364(21): 1995 -2005
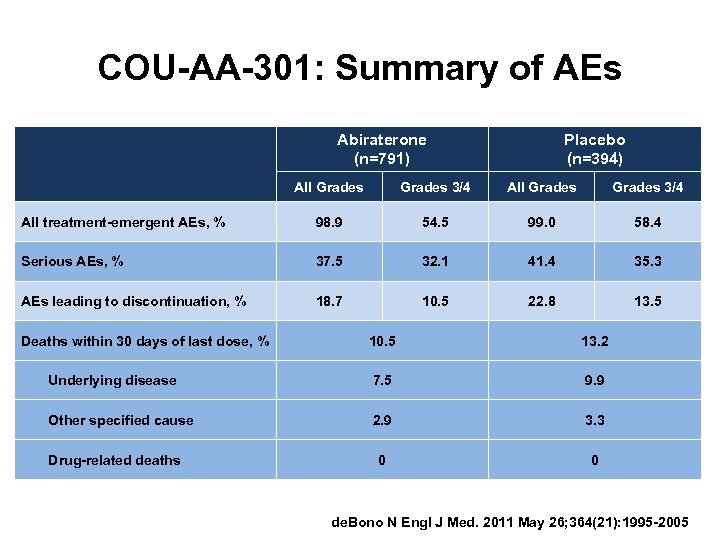 COU-AA-301: Summary of AEs Abiraterone (n=791) Placebo (n=394) All Grades 3/4 All treatment-emergent AEs, % 98. 9 54. 5 99. 0 58. 4 Serious AEs, % 37. 5 32. 1 41. 4 35. 3 AEs leading to discontinuation, % 18. 7 10. 5 22. 8 13. 5 Deaths within 30 days of last dose, % 10. 5 13. 2 Underlying disease 7. 5 9. 9 Other specified cause 2. 9 3. 3 0 0 Drug-related deaths de. Bono N Engl J Med. 2011 May 26; 364(21): 1995 -2005
COU-AA-301: Summary of AEs Abiraterone (n=791) Placebo (n=394) All Grades 3/4 All treatment-emergent AEs, % 98. 9 54. 5 99. 0 58. 4 Serious AEs, % 37. 5 32. 1 41. 4 35. 3 AEs leading to discontinuation, % 18. 7 10. 5 22. 8 13. 5 Deaths within 30 days of last dose, % 10. 5 13. 2 Underlying disease 7. 5 9. 9 Other specified cause 2. 9 3. 3 0 0 Drug-related deaths de. Bono N Engl J Med. 2011 May 26; 364(21): 1995 -2005
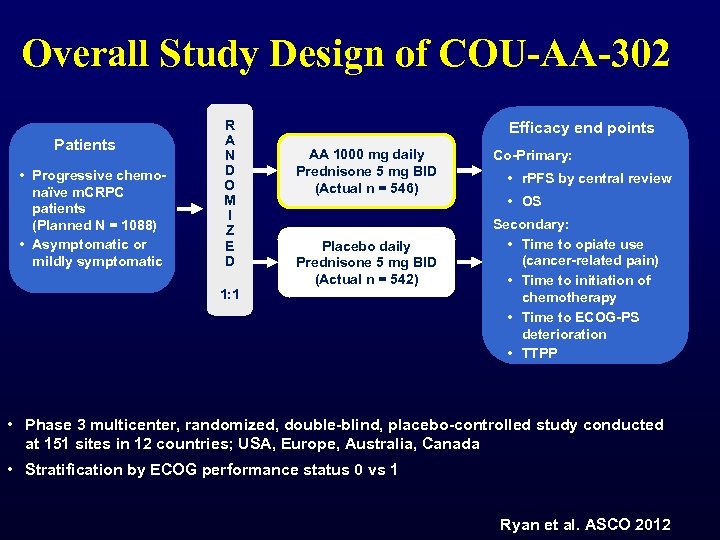 Overall Study Design of COU-AA-302 Patients • Progressive chemonaïve m. CRPC patients (Planned N = 1088) • Asymptomatic or mildly symptomatic R A N D O M I Z E D 1: 1 Efficacy end points AA 1000 mg daily Prednisone 5 mg BID (Actual n = 546) Placebo daily Prednisone 5 mg BID (Actual n = 542) Co-Primary: • r. PFS by central review • OS Secondary: • Time to opiate use (cancer-related pain) • Time to initiation of chemotherapy • Time to ECOG-PS deterioration • TTPP • Phase 3 multicenter, randomized, double-blind, placebo-controlled study conducted at 151 sites in 12 countries; USA, Europe, Australia, Canada • Stratification by ECOG performance status 0 vs 1 Ryan et al. ASCO 2012
Overall Study Design of COU-AA-302 Patients • Progressive chemonaïve m. CRPC patients (Planned N = 1088) • Asymptomatic or mildly symptomatic R A N D O M I Z E D 1: 1 Efficacy end points AA 1000 mg daily Prednisone 5 mg BID (Actual n = 546) Placebo daily Prednisone 5 mg BID (Actual n = 542) Co-Primary: • r. PFS by central review • OS Secondary: • Time to opiate use (cancer-related pain) • Time to initiation of chemotherapy • Time to ECOG-PS deterioration • TTPP • Phase 3 multicenter, randomized, double-blind, placebo-controlled study conducted at 151 sites in 12 countries; USA, Europe, Australia, Canada • Stratification by ECOG performance status 0 vs 1 Ryan et al. ASCO 2012
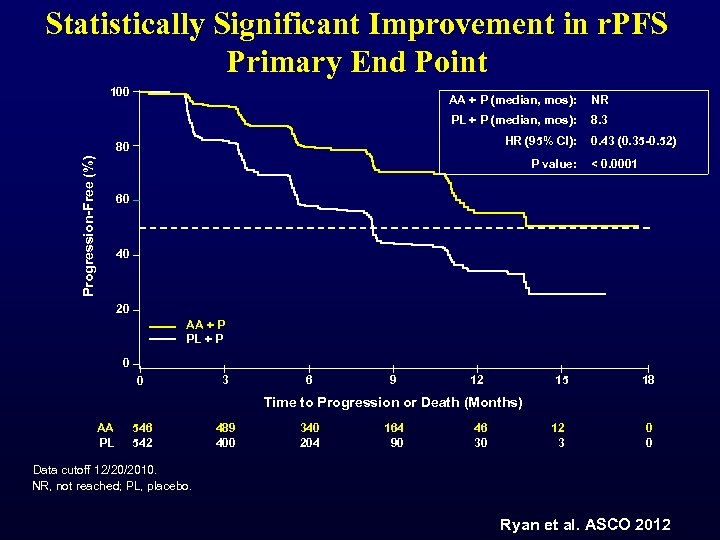 Statistically Significant Improvement in r. PFS Primary End Point 100 AA + P (median, mos): NR PL + P (median, mos): 8. 3 HR (95% CI): Progression-Free (%) 80 P value: 0. 43 (0. 35 -0. 52) < 0. 0001 60 40 20 AA + P PL + P 0 0 3 6 9 12 15 18 Time to Progression or Death (Months) AA PL 546 542 489 400 340 204 164 90 46 30 12 3 0 0 Data cutoff 12/20/2010. NR, not reached; PL, placebo. Ryan et al. ASCO 2012
Statistically Significant Improvement in r. PFS Primary End Point 100 AA + P (median, mos): NR PL + P (median, mos): 8. 3 HR (95% CI): Progression-Free (%) 80 P value: 0. 43 (0. 35 -0. 52) < 0. 0001 60 40 20 AA + P PL + P 0 0 3 6 9 12 15 18 Time to Progression or Death (Months) AA PL 546 542 489 400 340 204 164 90 46 30 12 3 0 0 Data cutoff 12/20/2010. NR, not reached; PL, placebo. Ryan et al. ASCO 2012
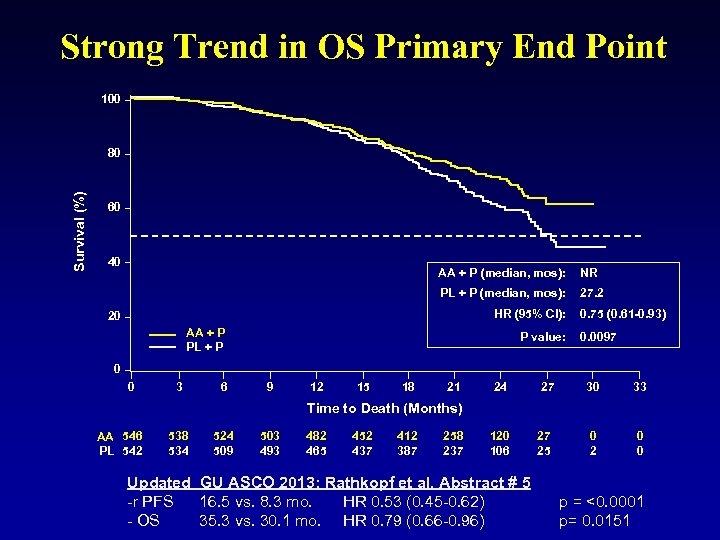 Strong Trend in OS Primary End Point 100 Survival (%) 80 60 40 AA + P (median, mos): NR PL + P (median, mos): 27. 2 HR (95% CI): 20 AA + P PL + P P value: 0. 75 (0. 61 -0. 93) 0. 0097 0 0 3 6 9 12 15 18 21 24 27 30 33 0 2 0 0 Time to Death (Months) AA 546 PL 542 538 534 524 509 503 493 482 465 452 437 412 387 258 237 120 106 Updated GU ASCO 2013: Rathkopf et al. Abstract # 5 -r PFS 16. 5 vs. 8. 3 mo. HR 0. 53 (0. 45 -0. 62) - OS 35. 3 vs. 30. 1 mo. HR 0. 79 (0. 66 -0. 96) 27 25 p = <0. 0001 p= 0. 0151
Strong Trend in OS Primary End Point 100 Survival (%) 80 60 40 AA + P (median, mos): NR PL + P (median, mos): 27. 2 HR (95% CI): 20 AA + P PL + P P value: 0. 75 (0. 61 -0. 93) 0. 0097 0 0 3 6 9 12 15 18 21 24 27 30 33 0 2 0 0 Time to Death (Months) AA 546 PL 542 538 534 524 509 503 493 482 465 452 437 412 387 258 237 120 106 Updated GU ASCO 2013: Rathkopf et al. Abstract # 5 -r PFS 16. 5 vs. 8. 3 mo. HR 0. 53 (0. 45 -0. 62) - OS 35. 3 vs. 30. 1 mo. HR 0. 79 (0. 66 -0. 96) 27 25 p = <0. 0001 p= 0. 0151
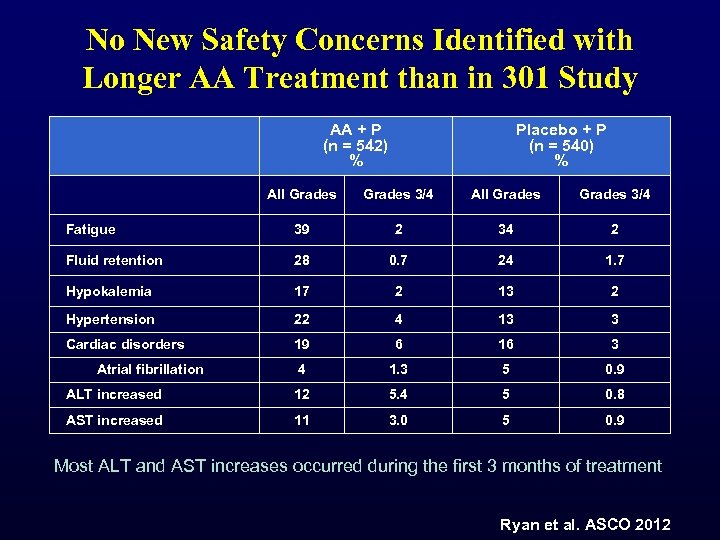 No New Safety Concerns Identified with Longer AA Treatment than in 301 Study AA + P (n = 542) % Placebo + P (n = 540) % All Grades 3/4 Fatigue 39 2 34 2 Fluid retention 28 0. 7 24 1. 7 Hypokalemia 17 2 13 2 Hypertension 22 4 13 3 Cardiac disorders 19 6 16 3 4 1. 3 5 0. 9 ALT increased 12 5. 4 5 0. 8 AST increased 11 3. 0 5 0. 9 Atrial fibrillation Most ALT and AST increases occurred during the first 3 months of treatment Ryan et al. ASCO 2012
No New Safety Concerns Identified with Longer AA Treatment than in 301 Study AA + P (n = 542) % Placebo + P (n = 540) % All Grades 3/4 Fatigue 39 2 34 2 Fluid retention 28 0. 7 24 1. 7 Hypokalemia 17 2 13 2 Hypertension 22 4 13 3 Cardiac disorders 19 6 16 3 4 1. 3 5 0. 9 ALT increased 12 5. 4 5 0. 8 AST increased 11 3. 0 5 0. 9 Atrial fibrillation Most ALT and AST increases occurred during the first 3 months of treatment Ryan et al. ASCO 2012
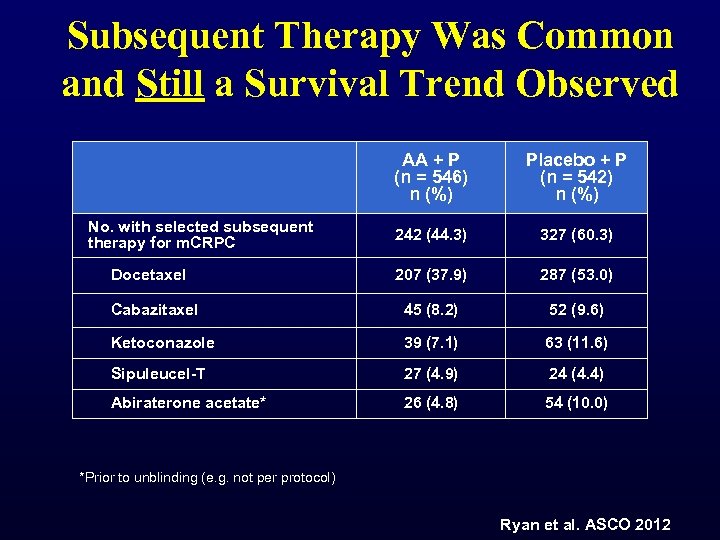 Subsequent Therapy Was Common and Still a Survival Trend Observed AA + P (n = 546) n (%) Placebo + P (n = 542) n (%) 242 (44. 3) 327 (60. 3) 207 (37. 9) 287 (53. 0) Cabazitaxel 45 (8. 2) 52 (9. 6) Ketoconazole 39 (7. 1) 63 (11. 6) Sipuleucel-T 27 (4. 9) 24 (4. 4) Abiraterone acetate* 26 (4. 8) 54 (10. 0) No. with selected subsequent therapy for m. CRPC Docetaxel *Prior to unblinding (e. g. not per protocol) Ryan et al. ASCO 2012
Subsequent Therapy Was Common and Still a Survival Trend Observed AA + P (n = 546) n (%) Placebo + P (n = 542) n (%) 242 (44. 3) 327 (60. 3) 207 (37. 9) 287 (53. 0) Cabazitaxel 45 (8. 2) 52 (9. 6) Ketoconazole 39 (7. 1) 63 (11. 6) Sipuleucel-T 27 (4. 9) 24 (4. 4) Abiraterone acetate* 26 (4. 8) 54 (10. 0) No. with selected subsequent therapy for m. CRPC Docetaxel *Prior to unblinding (e. g. not per protocol) Ryan et al. ASCO 2012
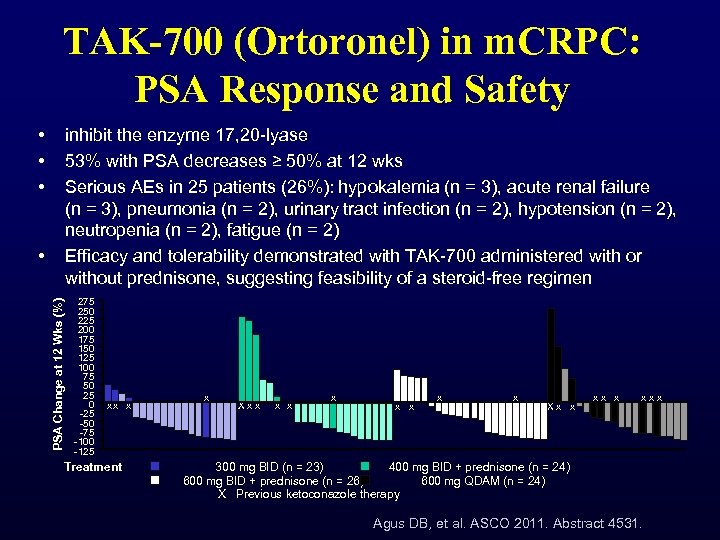 TAK-700 (Ortoronel) in m. CRPC: PSA Response and Safety • • • inhibit the enzyme 17, 20 -lyase 53% with PSA decreases ≥ 50% at 12 wks Serious AEs in 25 patients (26%): hypokalemia (n = 3), acute renal failure (n = 3), pneumonia (n = 2), urinary tract infection (n = 2), hypotension (n = 2), neutropenia (n = 2), fatigue (n = 2) Efficacy and tolerability demonstrated with TAK-700 administered with or without prednisone, suggesting feasibility of a steroid-free regimen PSA Change at 12 Wks (%) • 275 250 225 200 175 150 125 100 75 50 25 0 -25 -50 -75 -100 -125 xx x Treatment x X x x x x x X x x x x 300 mg BID (n = 23) 400 mg BID + prednisone (n = 24) 600 mg BID + prednisone (n = 26) 600 mg QDAM (n = 24) X Previous ketoconazole therapy Agus DB, et al. ASCO 2011. Abstract 4531.
TAK-700 (Ortoronel) in m. CRPC: PSA Response and Safety • • • inhibit the enzyme 17, 20 -lyase 53% with PSA decreases ≥ 50% at 12 wks Serious AEs in 25 patients (26%): hypokalemia (n = 3), acute renal failure (n = 3), pneumonia (n = 2), urinary tract infection (n = 2), hypotension (n = 2), neutropenia (n = 2), fatigue (n = 2) Efficacy and tolerability demonstrated with TAK-700 administered with or without prednisone, suggesting feasibility of a steroid-free regimen PSA Change at 12 Wks (%) • 275 250 225 200 175 150 125 100 75 50 25 0 -25 -50 -75 -100 -125 xx x Treatment x X x x x x x X x x x x 300 mg BID (n = 23) 400 mg BID + prednisone (n = 24) 600 mg BID + prednisone (n = 26) 600 mg QDAM (n = 24) X Previous ketoconazole therapy Agus DB, et al. ASCO 2011. Abstract 4531.
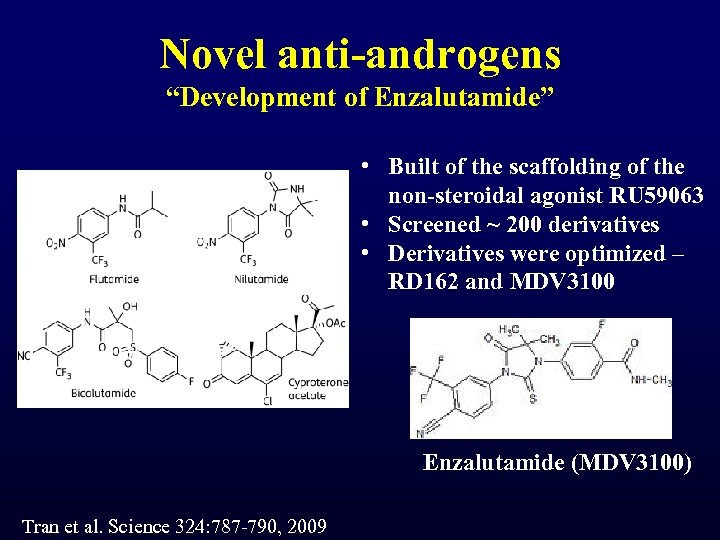 Novel anti-androgens “Development of Enzalutamide” • Built of the scaffolding of the non-steroidal agonist RU 59063 • Screened ~ 200 derivatives • Derivatives were optimized – RD 162 and MDV 3100 Enzalutamide (MDV 3100) Tran et al. Science 324: 787 -790, 2009
Novel anti-androgens “Development of Enzalutamide” • Built of the scaffolding of the non-steroidal agonist RU 59063 • Screened ~ 200 derivatives • Derivatives were optimized – RD 162 and MDV 3100 Enzalutamide (MDV 3100) Tran et al. Science 324: 787 -790, 2009
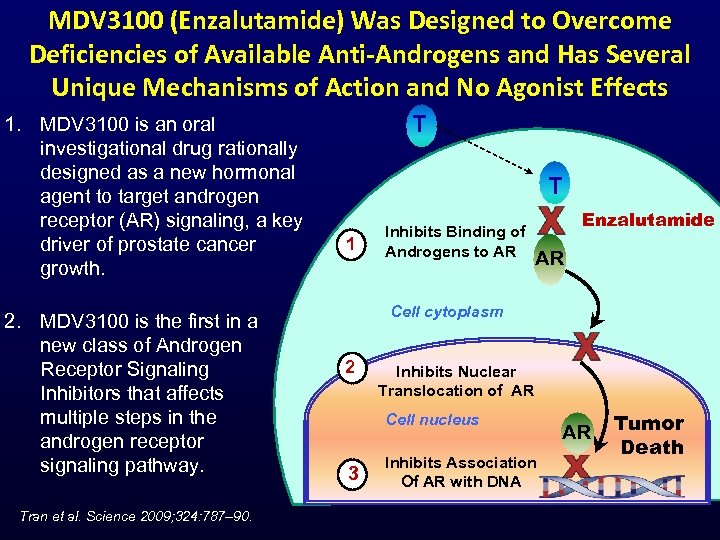 MDV 3100 (Enzalutamide) Was Designed to Overcome Deficiencies of Available Anti-Androgens and Has Several Unique Mechanisms of Action and No Agonist Effects 1. MDV 3100 is an oral investigational drug rationally designed as a new hormonal agent to target androgen receptor (AR) signaling, a key driver of prostate cancer growth. 2. MDV 3100 is the first in a new class of Androgen Receptor Signaling Inhibitors that affects multiple steps in the androgen receptor signaling pathway. Tran et al. Science 2009; 324: 787– 90. T T 1 Inhibits Binding of Androgens to AR Enzalutamide AR Cell cytoplasm 2 Inhibits Nuclear Translocation of AR Cell nucleus 3 Inhibits Association Of AR with DNA AR Tumor Death
MDV 3100 (Enzalutamide) Was Designed to Overcome Deficiencies of Available Anti-Androgens and Has Several Unique Mechanisms of Action and No Agonist Effects 1. MDV 3100 is an oral investigational drug rationally designed as a new hormonal agent to target androgen receptor (AR) signaling, a key driver of prostate cancer growth. 2. MDV 3100 is the first in a new class of Androgen Receptor Signaling Inhibitors that affects multiple steps in the androgen receptor signaling pathway. Tran et al. Science 2009; 324: 787– 90. T T 1 Inhibits Binding of Androgens to AR Enzalutamide AR Cell cytoplasm 2 Inhibits Nuclear Translocation of AR Cell nucleus 3 Inhibits Association Of AR with DNA AR Tumor Death
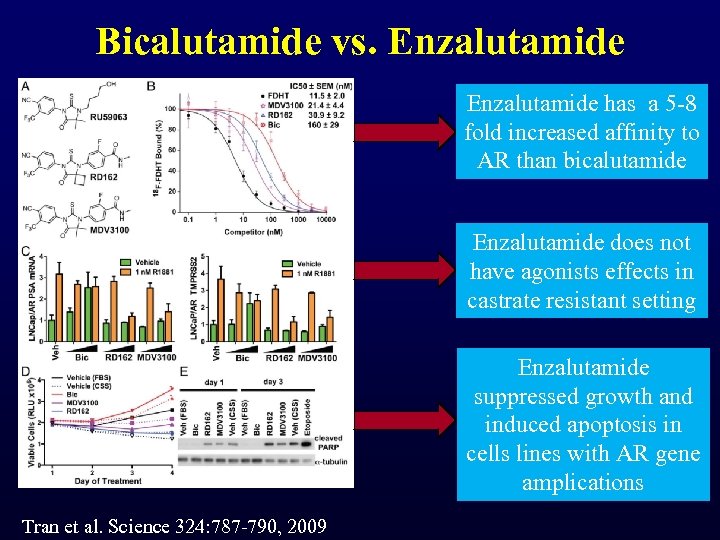 Bicalutamide vs. Enzalutamide has a 5 -8 fold increased affinity to AR than bicalutamide Enzalutamide does not have agonists effects in castrate resistant setting Enzalutamide suppressed growth and induced apoptosis in cells lines with AR gene amplications Tran et al. Science 324: 787 -790, 2009
Bicalutamide vs. Enzalutamide has a 5 -8 fold increased affinity to AR than bicalutamide Enzalutamide does not have agonists effects in castrate resistant setting Enzalutamide suppressed growth and induced apoptosis in cells lines with AR gene amplications Tran et al. Science 324: 787 -790, 2009
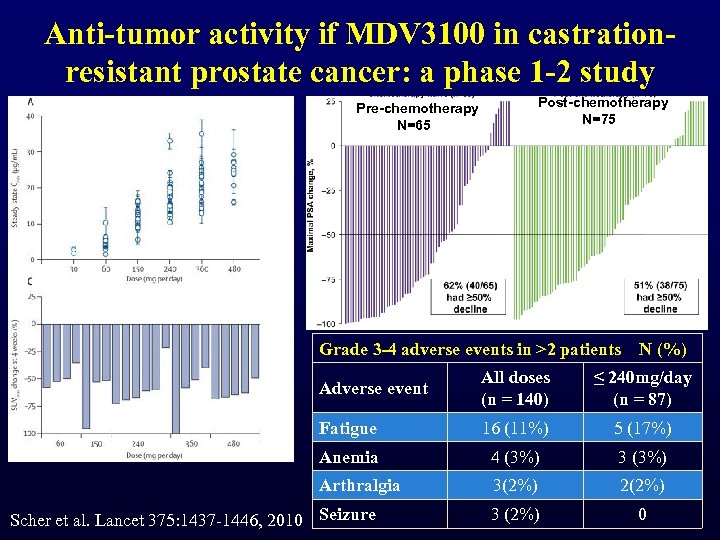 Anti-tumor activity if MDV 3100 in castrationresistant prostate cancer: a phase 1 -2 study Pre-chemotherapy N=65 Post-chemotherapy N=75 Grade 3 -4 adverse events in >2 patients N (%) Adverse event All doses (n = 140) ≤ 240 mg/day (n = 87) Fatigue 16 (11%) 5 (17%) Anemia 4 (3%) 3 (3%) Arthralgia 3(2%) 2(2%) 3 (2%) 0 Scher et al. Lancet 375: 1437 -1446, 2010 Seizure
Anti-tumor activity if MDV 3100 in castrationresistant prostate cancer: a phase 1 -2 study Pre-chemotherapy N=65 Post-chemotherapy N=75 Grade 3 -4 adverse events in >2 patients N (%) Adverse event All doses (n = 140) ≤ 240 mg/day (n = 87) Fatigue 16 (11%) 5 (17%) Anemia 4 (3%) 3 (3%) Arthralgia 3(2%) 2(2%) 3 (2%) 0 Scher et al. Lancet 375: 1437 -1446, 2010 Seizure
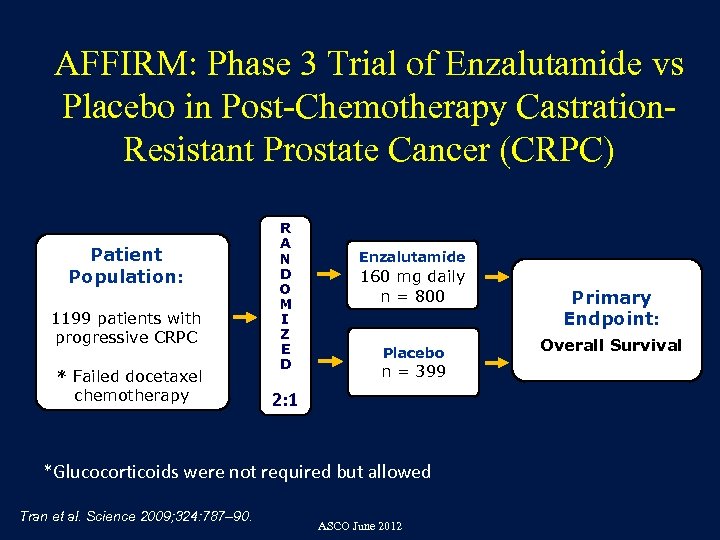 AFFIRM: Phase 3 Trial of Enzalutamide vs Placebo in Post-Chemotherapy Castration. Resistant Prostate Cancer (CRPC) Patient Population: 1199 patients with progressive CRPC * Failed docetaxel chemotherapy R A N D O M I Z E D Enzalutamide 160 mg daily n = 800 Placebo n = 399 2: 1 *Glucocorticoids were not required but allowed Tran et al. Science 2009; 324: 787– 90. ASCO June 2012 Primary Endpoint: Overall Survival
AFFIRM: Phase 3 Trial of Enzalutamide vs Placebo in Post-Chemotherapy Castration. Resistant Prostate Cancer (CRPC) Patient Population: 1199 patients with progressive CRPC * Failed docetaxel chemotherapy R A N D O M I Z E D Enzalutamide 160 mg daily n = 800 Placebo n = 399 2: 1 *Glucocorticoids were not required but allowed Tran et al. Science 2009; 324: 787– 90. ASCO June 2012 Primary Endpoint: Overall Survival
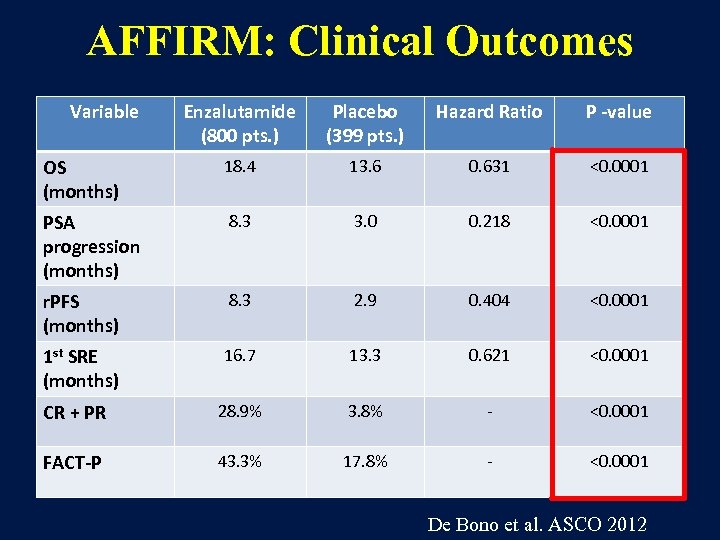 AFFIRM: Clinical Outcomes Variable Enzalutamide (800 pts. ) Placebo (399 pts. ) Hazard Ratio P -value OS (months) 18. 4 13. 6 0. 631 <0. 0001 PSA progression (months) 8. 3 3. 0 0. 218 <0. 0001 r. PFS (months) 8. 3 2. 9 0. 404 <0. 0001 1 st SRE (months) 16. 7 13. 3 0. 621 <0. 0001 CR + PR 28. 9% 3. 8% - <0. 0001 FACT-P 43. 3% 17. 8% - <0. 0001 De Bono et al. ASCO 2012
AFFIRM: Clinical Outcomes Variable Enzalutamide (800 pts. ) Placebo (399 pts. ) Hazard Ratio P -value OS (months) 18. 4 13. 6 0. 631 <0. 0001 PSA progression (months) 8. 3 3. 0 0. 218 <0. 0001 r. PFS (months) 8. 3 2. 9 0. 404 <0. 0001 1 st SRE (months) 16. 7 13. 3 0. 621 <0. 0001 CR + PR 28. 9% 3. 8% - <0. 0001 FACT-P 43. 3% 17. 8% - <0. 0001 De Bono et al. ASCO 2012
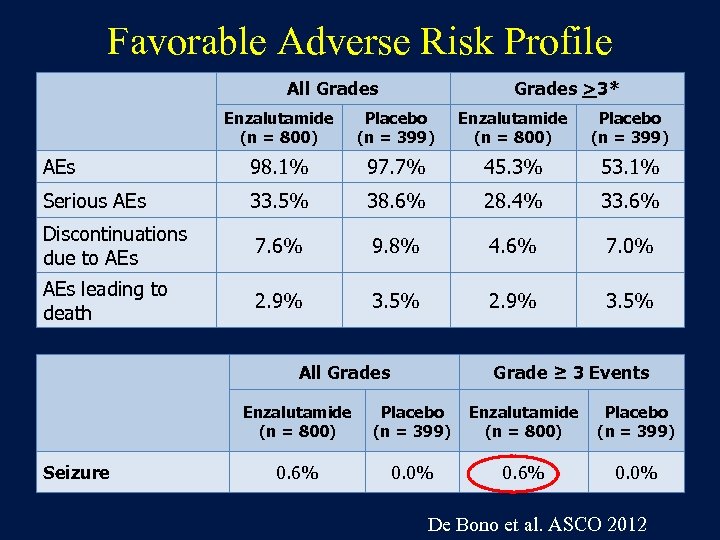 Favorable Adverse Risk Profile All Grades >3* Enzalutamide (n = 800) Placebo (n = 399) AEs 98. 1% 97. 7% 45. 3% 53. 1% Serious AEs 33. 5% 38. 6% 28. 4% 33. 6% Discontinuations due to AEs 7. 6% 9. 8% 4. 6% 7. 0% AEs leading to death 2. 9% 3. 5% All Grades Grade ≥ 3 Events Enzalutamide (n = 800) Seizure Placebo (n = 399) Enzalutamide (n = 800) Placebo (n = 399) 0. 6% 0. 0% De Bono et al. ASCO 2012
Favorable Adverse Risk Profile All Grades >3* Enzalutamide (n = 800) Placebo (n = 399) AEs 98. 1% 97. 7% 45. 3% 53. 1% Serious AEs 33. 5% 38. 6% 28. 4% 33. 6% Discontinuations due to AEs 7. 6% 9. 8% 4. 6% 7. 0% AEs leading to death 2. 9% 3. 5% All Grades Grade ≥ 3 Events Enzalutamide (n = 800) Seizure Placebo (n = 399) Enzalutamide (n = 800) Placebo (n = 399) 0. 6% 0. 0% De Bono et al. ASCO 2012
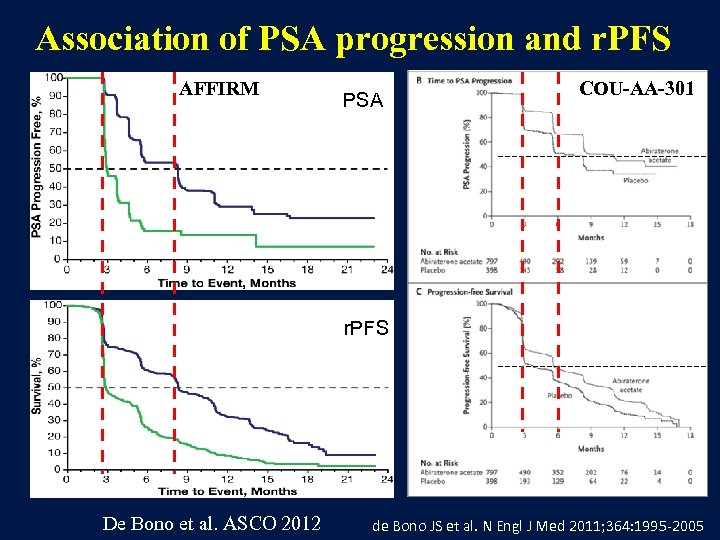 Association of PSA progression and r. PFS AFFIRM PSA COU-AA-301 r. PFS De Bono et al. ASCO 2012 de Bono JS et al. N Engl J Med 2011; 364: 1995 -2005
Association of PSA progression and r. PFS AFFIRM PSA COU-AA-301 r. PFS De Bono et al. ASCO 2012 de Bono JS et al. N Engl J Med 2011; 364: 1995 -2005
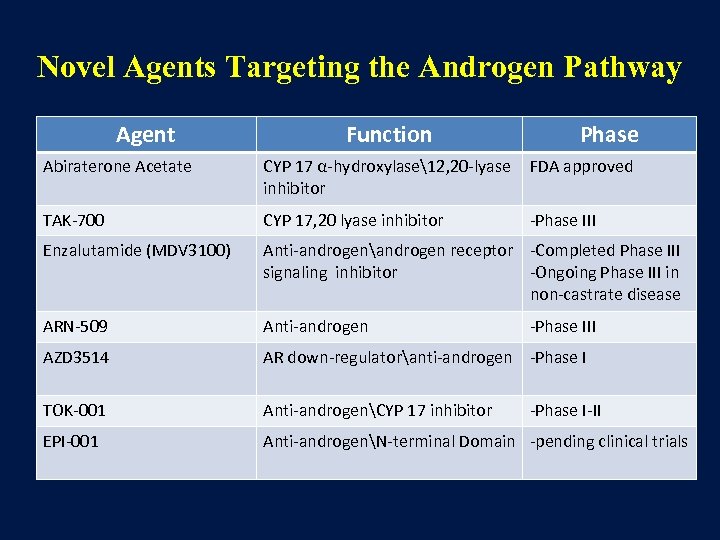 Novel Agents Targeting the Androgen Pathway Agent Function Phase Abiraterone Acetate CYP 17 α-hydroxylase12, 20 -lyase inhibitor FDA approved TAK-700 CYP 17, 20 lyase inhibitor -Phase III Enzalutamide (MDV 3100) Anti-androgenandrogen receptor -Completed Phase III signaling inhibitor -Ongoing Phase III in non-castrate disease ARN-509 Anti-androgen AZD 3514 AR down-regulatoranti-androgen -Phase I TOK-001 Anti-androgenCYP 17 inhibitor EPI-001 Anti-androgenN-terminal Domain -pending clinical trials -Phase III -Phase I-II
Novel Agents Targeting the Androgen Pathway Agent Function Phase Abiraterone Acetate CYP 17 α-hydroxylase12, 20 -lyase inhibitor FDA approved TAK-700 CYP 17, 20 lyase inhibitor -Phase III Enzalutamide (MDV 3100) Anti-androgenandrogen receptor -Completed Phase III signaling inhibitor -Ongoing Phase III in non-castrate disease ARN-509 Anti-androgen AZD 3514 AR down-regulatoranti-androgen -Phase I TOK-001 Anti-androgenCYP 17 inhibitor EPI-001 Anti-androgenN-terminal Domain -pending clinical trials -Phase III -Phase I-II
 Cabazitaxel • Microtubule stabilizer • Developed in docetaxel-resistant prostate cancer cell lines • a favorable pharmacokinetic and safety profile • decreased propensity for Pglycoprotein (Pgp)-mediated drug resistance. • inhibited cell growth in a wide range of human cancer cell lines, including tumor models expressing Pgp.
Cabazitaxel • Microtubule stabilizer • Developed in docetaxel-resistant prostate cancer cell lines • a favorable pharmacokinetic and safety profile • decreased propensity for Pglycoprotein (Pgp)-mediated drug resistance. • inhibited cell growth in a wide range of human cancer cell lines, including tumor models expressing Pgp.
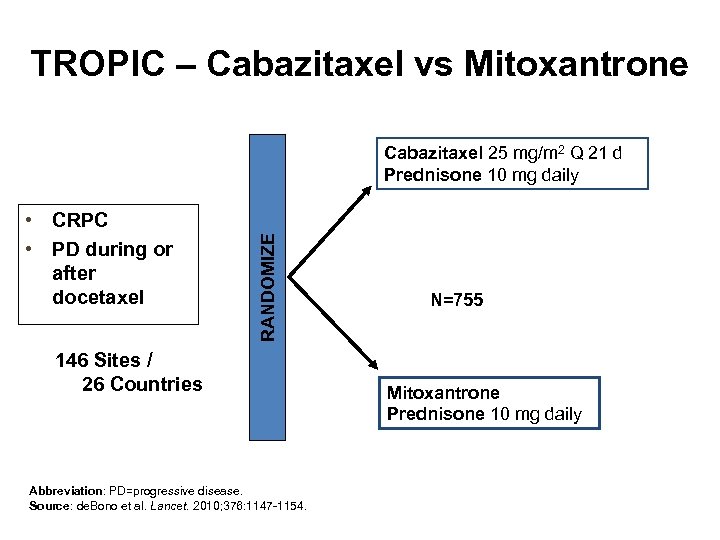 TROPIC – Cabazitaxel vs Mitoxantrone • CRPC • PD during or after docetaxel RANDOMIZE Cabazitaxel 25 mg/m 2 Q 21 d Prednisone 10 mg daily 146 Sites / 26 Countries Abbreviation: PD=progressive disease. Source: de. Bono et al. Lancet. 2010; 376: 1147 -1154. N=755 Mitoxantrone Prednisone 10 mg daily
TROPIC – Cabazitaxel vs Mitoxantrone • CRPC • PD during or after docetaxel RANDOMIZE Cabazitaxel 25 mg/m 2 Q 21 d Prednisone 10 mg daily 146 Sites / 26 Countries Abbreviation: PD=progressive disease. Source: de. Bono et al. Lancet. 2010; 376: 1147 -1154. N=755 Mitoxantrone Prednisone 10 mg daily
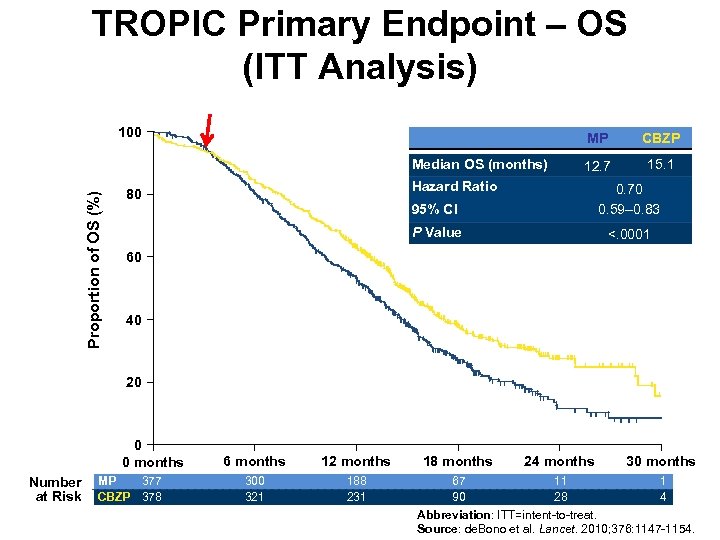 TROPIC Primary Endpoint – OS (ITT Analysis) 100 MP Proportion of OS (%) Median OS (months) CBZP 12. 7 15. 1 Hazard Ratio 95% CI 0. 70 0. 59– 0. 83 P Value 80 <. 0001 60 40 20 0 0 months Number at Risk MP 377 CBZP 378 6 months 12 months 300 321 188 231 18 months 24 months 30 months 67 11 1 90 28 4 Abbreviation: ITT=intent-to-treat. Source: de. Bono et al. Lancet. 2010; 376: 1147 -1154.
TROPIC Primary Endpoint – OS (ITT Analysis) 100 MP Proportion of OS (%) Median OS (months) CBZP 12. 7 15. 1 Hazard Ratio 95% CI 0. 70 0. 59– 0. 83 P Value 80 <. 0001 60 40 20 0 0 months Number at Risk MP 377 CBZP 378 6 months 12 months 300 321 188 231 18 months 24 months 30 months 67 11 1 90 28 4 Abbreviation: ITT=intent-to-treat. Source: de. Bono et al. Lancet. 2010; 376: 1147 -1154.
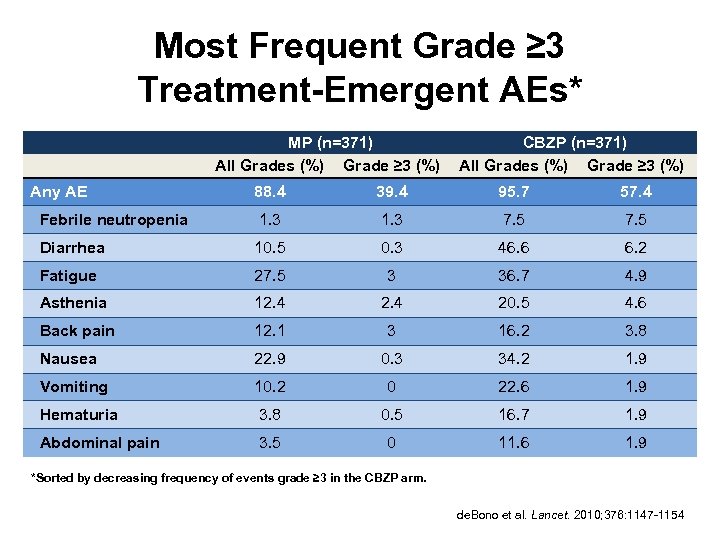 Most Frequent Grade ≥ 3 Treatment-Emergent AEs* MP (n=371) All Grades (%) Grade ≥ 3 (%) CBZP (n=371) All Grades (%) Grade ≥ 3 (%) 88. 4 39. 4 95. 7 57. 4 Febrile neutropenia 1. 3 7. 5 Diarrhea 10. 5 0. 3 46. 6 6. 2 Fatigue 27. 5 3 36. 7 4. 9 Asthenia 12. 4 20. 5 4. 6 Back pain 12. 1 3 16. 2 3. 8 Nausea 22. 9 0. 3 34. 2 1. 9 Vomiting 10. 2 0 22. 6 1. 9 Hematuria 3. 8 0. 5 16. 7 1. 9 Abdominal pain 3. 5 0 11. 6 1. 9 Any AE *Sorted by decreasing frequency of events grade ≥ 3 in the CBZP arm. de. Bono et al. Lancet. 2010; 376: 1147 -1154
Most Frequent Grade ≥ 3 Treatment-Emergent AEs* MP (n=371) All Grades (%) Grade ≥ 3 (%) CBZP (n=371) All Grades (%) Grade ≥ 3 (%) 88. 4 39. 4 95. 7 57. 4 Febrile neutropenia 1. 3 7. 5 Diarrhea 10. 5 0. 3 46. 6 6. 2 Fatigue 27. 5 3 36. 7 4. 9 Asthenia 12. 4 20. 5 4. 6 Back pain 12. 1 3 16. 2 3. 8 Nausea 22. 9 0. 3 34. 2 1. 9 Vomiting 10. 2 0 22. 6 1. 9 Hematuria 3. 8 0. 5 16. 7 1. 9 Abdominal pain 3. 5 0 11. 6 1. 9 Any AE *Sorted by decreasing frequency of events grade ≥ 3 in the CBZP arm. de. Bono et al. Lancet. 2010; 376: 1147 -1154
 Immunotherapy Approaches in PC § Active immunotherapy v tumor associated antigen is directly targeted by loading in that antigen in APC or into vaccine vector at protein or DNA level v Antigen specific immunotherapy § Sipuleucel-T § Poxvirus-based vectors § DNA based vaccines § Passive immunotherapy v Antibodies to specific receptors/antigens § Prostate Specific Membrane Antigen (PSMA) § Immune Checkpoint Inhibitors v Strategies to maintain activated tumor specific T-cells by neutralizing coinhibitory receptors v Anti-cytotoxic T lymphocyte protein 4 (CTLA 4) § Ipilumimab, tremelimumab v Anti- program death 1 (PD-1) § MDX-1106
Immunotherapy Approaches in PC § Active immunotherapy v tumor associated antigen is directly targeted by loading in that antigen in APC or into vaccine vector at protein or DNA level v Antigen specific immunotherapy § Sipuleucel-T § Poxvirus-based vectors § DNA based vaccines § Passive immunotherapy v Antibodies to specific receptors/antigens § Prostate Specific Membrane Antigen (PSMA) § Immune Checkpoint Inhibitors v Strategies to maintain activated tumor specific T-cells by neutralizing coinhibitory receptors v Anti-cytotoxic T lymphocyte protein 4 (CTLA 4) § Ipilumimab, tremelimumab v Anti- program death 1 (PD-1) § MDX-1106
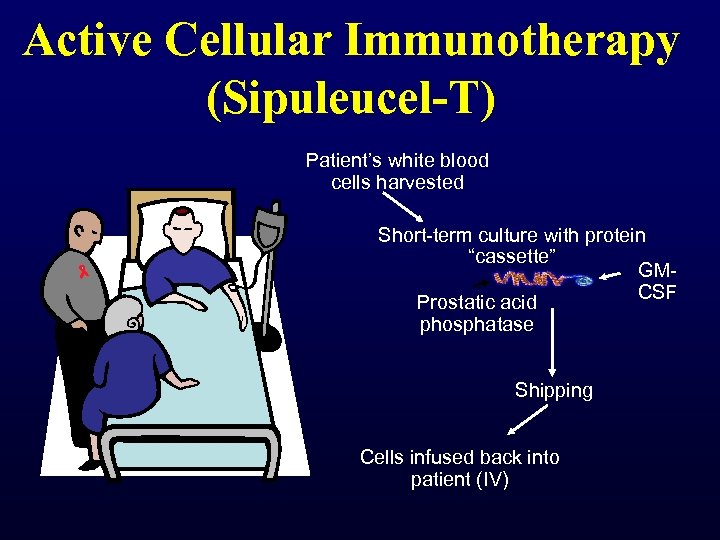 Active Cellular Immunotherapy (Sipuleucel-T) Patient’s white blood cells harvested Short-term culture with protein “cassette” GMCSF Prostatic acid phosphatase Shipping Cells infused back into patient (IV)
Active Cellular Immunotherapy (Sipuleucel-T) Patient’s white blood cells harvested Short-term culture with protein “cassette” GMCSF Prostatic acid phosphatase Shipping Cells infused back into patient (IV)
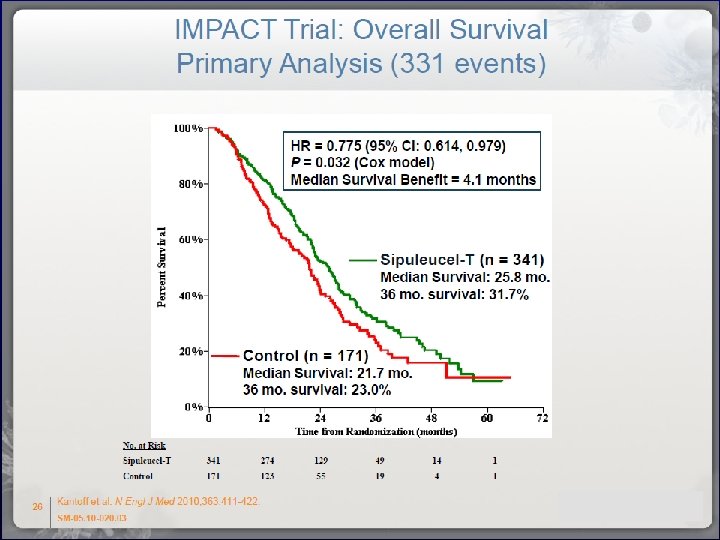
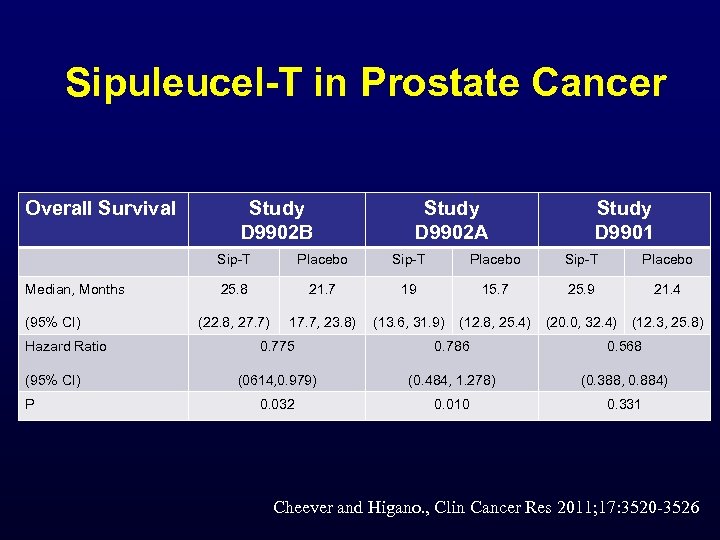 Sipuleucel-T in Prostate Cancer Overall Survival Study D 9902 B Study D 9902 A Study D 9901 Sip-T Median, Months (95% CI) Hazard Ratio (95% CI) P Placebo Sip-T Placebo 25. 8 21. 7 19 15. 7 25. 9 21. 4 (22. 8, 27. 7) 17. 7, 23. 8) (13. 6, 31. 9) (12. 8, 25. 4) (20. 0, 32. 4) (12. 3, 25. 8) 0. 775 0. 786 0. 568 (0614, 0. 979) (0. 484, 1. 278) (0. 388, 0. 884) 0. 032 0. 010 0. 331 Cheever and Higano. , Clin Cancer Res 2011; 17: 3520 -3526
Sipuleucel-T in Prostate Cancer Overall Survival Study D 9902 B Study D 9902 A Study D 9901 Sip-T Median, Months (95% CI) Hazard Ratio (95% CI) P Placebo Sip-T Placebo 25. 8 21. 7 19 15. 7 25. 9 21. 4 (22. 8, 27. 7) 17. 7, 23. 8) (13. 6, 31. 9) (12. 8, 25. 4) (20. 0, 32. 4) (12. 3, 25. 8) 0. 775 0. 786 0. 568 (0614, 0. 979) (0. 484, 1. 278) (0. 388, 0. 884) 0. 032 0. 010 0. 331 Cheever and Higano. , Clin Cancer Res 2011; 17: 3520 -3526
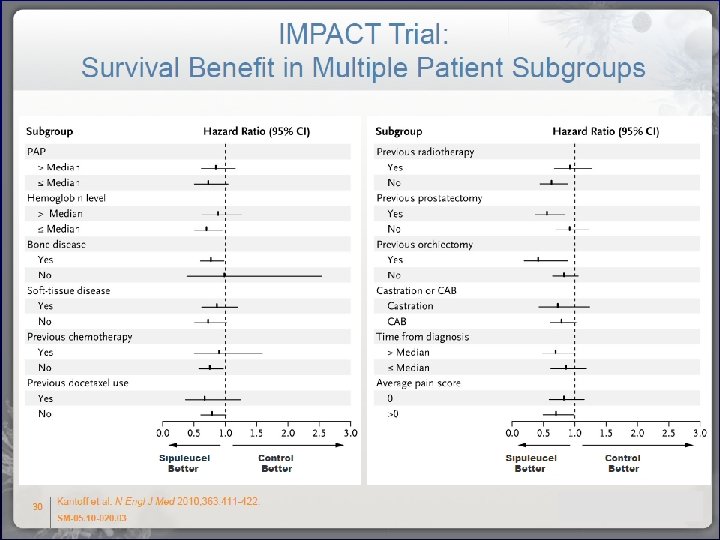
 CD 54 is a surrogate marker of antigen presenting cell activation Serum cytokines or humoral responses by ELISA have limited utility
CD 54 is a surrogate marker of antigen presenting cell activation Serum cytokines or humoral responses by ELISA have limited utility
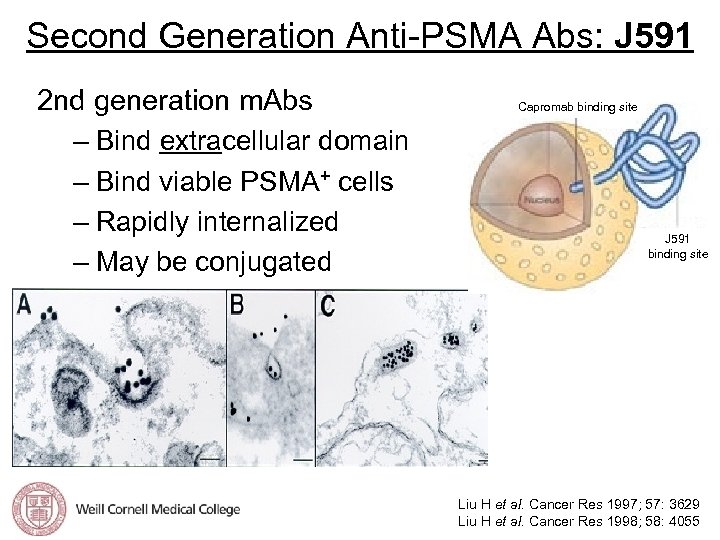 Second Generation Anti-PSMA Abs: J 591 2 nd generation m. Abs – Bind extracellular domain – Bind viable PSMA+ cells – Rapidly internalized – May be conjugated Capromab binding site J 591 binding site Liu H et al. Cancer Res 1997; 57: 3629 Liu H et al. Cancer Res 1998; 58: 4055
Second Generation Anti-PSMA Abs: J 591 2 nd generation m. Abs – Bind extracellular domain – Bind viable PSMA+ cells – Rapidly internalized – May be conjugated Capromab binding site J 591 binding site Liu H et al. Cancer Res 1997; 57: 3629 Liu H et al. Cancer Res 1998; 58: 4055
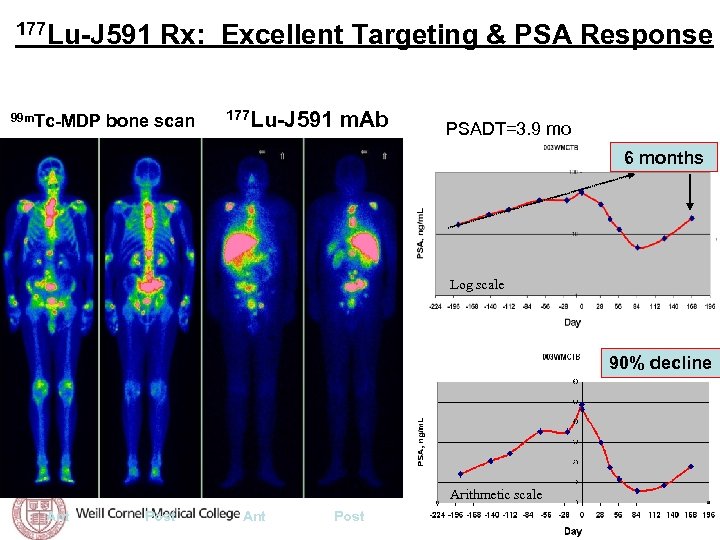 Tagawa et al, ASCO 2008 177 Lu-J 591 Rx: Excellent Targeting & PSA Response 30/32 (94%) with accurate targeting of known sites of disease 99 m. Tc-MDP bone scan 177 Lu-J 591 m. Ab PSADT=3. 9 mo 6 months Log scale 90% decline Arithmetic scale Ant Post
Tagawa et al, ASCO 2008 177 Lu-J 591 Rx: Excellent Targeting & PSA Response 30/32 (94%) with accurate targeting of known sites of disease 99 m. Tc-MDP bone scan 177 Lu-J 591 m. Ab PSADT=3. 9 mo 6 months Log scale 90% decline Arithmetic scale Ant Post
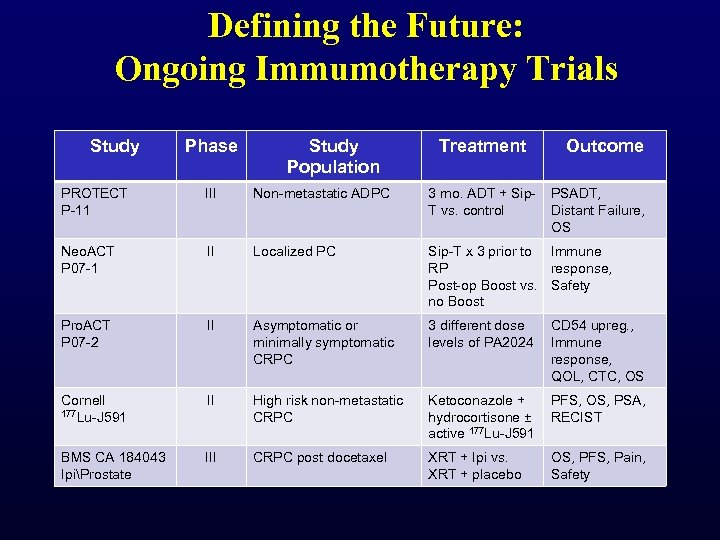 Defining the Future: Ongoing Immumotherapy Trials Study Phase Study Population Treatment Outcome PROTECT P-11 III Non-metastatic ADPC 3 mo. ADT + Sip. T vs. control PSADT, Distant Failure, OS Neo. ACT P 07 -1 II Localized PC Sip-T x 3 prior to Immune RP response, Post-op Boost vs. Safety no Boost Pro. ACT P 07 -2 II Asymptomatic or minimally symptomatic CRPC 3 different dose levels of PA 2024 CD 54 upreg. , Immune response, QOL, CTC, OS Cornell 177 Lu-J 591 II High risk non-metastatic CRPC Ketoconazole + hydrocortisone ± active 177 Lu-J 591 PFS, OS, PSA, RECIST BMS CA 184043 IpiProstate III CRPC post docetaxel XRT + Ipi vs. XRT + placebo OS, PFS, Pain, Safety
Defining the Future: Ongoing Immumotherapy Trials Study Phase Study Population Treatment Outcome PROTECT P-11 III Non-metastatic ADPC 3 mo. ADT + Sip. T vs. control PSADT, Distant Failure, OS Neo. ACT P 07 -1 II Localized PC Sip-T x 3 prior to Immune RP response, Post-op Boost vs. Safety no Boost Pro. ACT P 07 -2 II Asymptomatic or minimally symptomatic CRPC 3 different dose levels of PA 2024 CD 54 upreg. , Immune response, QOL, CTC, OS Cornell 177 Lu-J 591 II High risk non-metastatic CRPC Ketoconazole + hydrocortisone ± active 177 Lu-J 591 PFS, OS, PSA, RECIST BMS CA 184043 IpiProstate III CRPC post docetaxel XRT + Ipi vs. XRT + placebo OS, PFS, Pain, Safety
 Radioisotopes Targets Bone Metastases • Naturally targets new bone growth in and around bone metastases • Most acts as a calcium mimic • Strontium-89 • Samrarium-153 • Radium-223 Ca Ra
Radioisotopes Targets Bone Metastases • Naturally targets new bone growth in and around bone metastases • Most acts as a calcium mimic • Strontium-89 • Samrarium-153 • Radium-223 Ca Ra
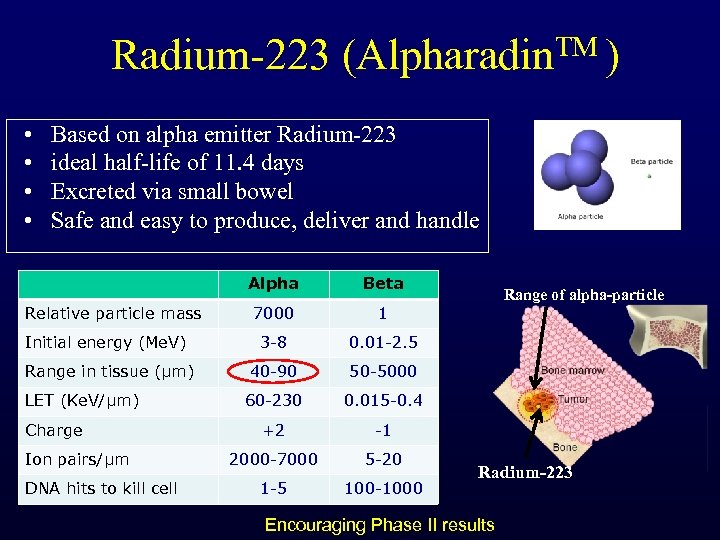 Radium-223 (Alpharadin. TM ) • • Based on alpha emitter Radium-223 ideal half-life of 11. 4 days Excreted via small bowel Safe and easy to produce, deliver and handle Alpha Beta 7000 1 Initial energy (Me. V) 3 -8 0. 01 -2. 5 Range in tissue (µm) 40 -90 50 -5000 60 -230 0. 015 -0. 4 +2 -1 2000 -7000 5 -20 1 -5 100 -1000 Relative particle mass LET (Ke. V/µm) Charge Ion pairs/µm DNA hits to kill cell Range of alpha-particle Radium-223 Encouraging Phase II results
Radium-223 (Alpharadin. TM ) • • Based on alpha emitter Radium-223 ideal half-life of 11. 4 days Excreted via small bowel Safe and easy to produce, deliver and handle Alpha Beta 7000 1 Initial energy (Me. V) 3 -8 0. 01 -2. 5 Range in tissue (µm) 40 -90 50 -5000 60 -230 0. 015 -0. 4 +2 -1 2000 -7000 5 -20 1 -5 100 -1000 Relative particle mass LET (Ke. V/µm) Charge Ion pairs/µm DNA hits to kill cell Range of alpha-particle Radium-223 Encouraging Phase II results
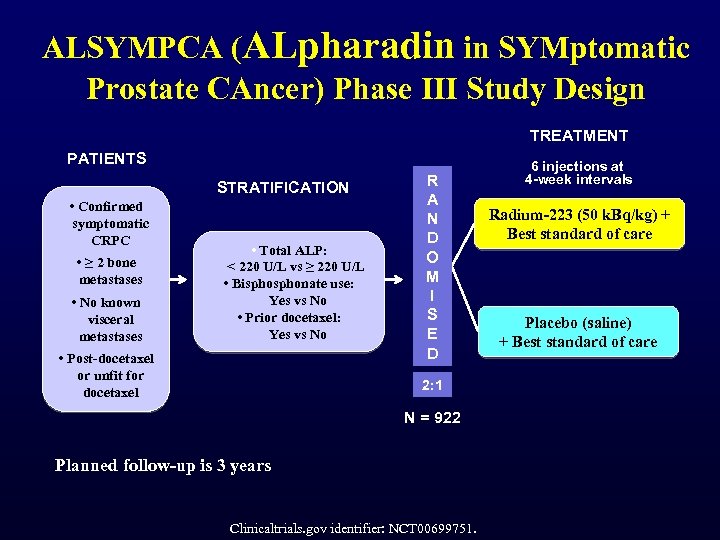 ALSYMPCA (ALpharadin in SYMptomatic Prostate CAncer) Phase III Study Design TREATMENT PATIENTS STRATIFICATION • Confirmed symptomatic CRPC • ≥ 2 bone metastases • No known visceral metastases • Total ALP: < 220 U/L vs ≥ 220 U/L • Bisphonate use: Yes vs No • Prior docetaxel: Yes vs No • Post-docetaxel or unfit for docetaxel R A N D O M I S E D 2: 1 N = 922 Planned follow-up is 3 years Clinicaltrials. gov identifier: NCT 00699751. 6 injections at 4 -week intervals Radium-223 (50 k. Bq/kg) + Best standard of care Placebo (saline) + Best standard of care
ALSYMPCA (ALpharadin in SYMptomatic Prostate CAncer) Phase III Study Design TREATMENT PATIENTS STRATIFICATION • Confirmed symptomatic CRPC • ≥ 2 bone metastases • No known visceral metastases • Total ALP: < 220 U/L vs ≥ 220 U/L • Bisphonate use: Yes vs No • Prior docetaxel: Yes vs No • Post-docetaxel or unfit for docetaxel R A N D O M I S E D 2: 1 N = 922 Planned follow-up is 3 years Clinicaltrials. gov identifier: NCT 00699751. 6 injections at 4 -week intervals Radium-223 (50 k. Bq/kg) + Best standard of care Placebo (saline) + Best standard of care
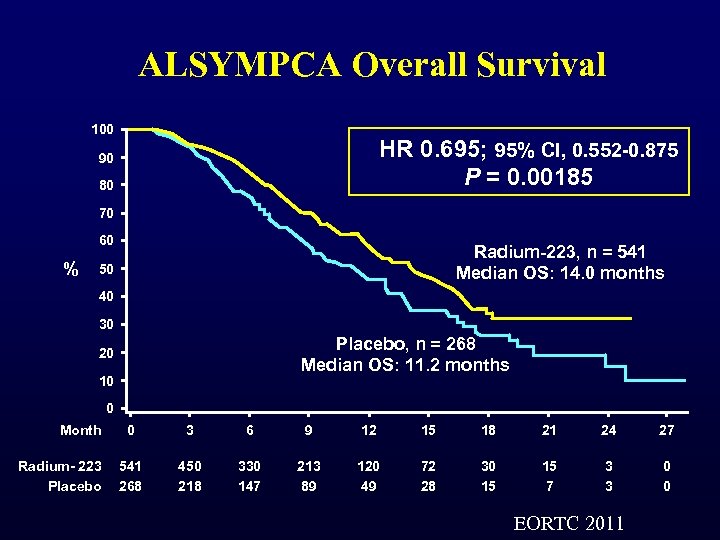 ALSYMPCA Overall Survival 100 HR 0. 695; 95% CI, 0. 552 -0. 875 P = 0. 00185 90 80 70 60 % Radium-223, n = 541 Median OS: 14. 0 months 50 40 30 Placebo, n = 268 Median OS: 11. 2 months 20 10 0 Month Radium- 223 Placebo 0 3 6 9 12 15 18 21 24 27 541 268 450 218 330 147 213 89 120 49 72 28 30 15 15 7 3 3 0 0 EORTC 2011
ALSYMPCA Overall Survival 100 HR 0. 695; 95% CI, 0. 552 -0. 875 P = 0. 00185 90 80 70 60 % Radium-223, n = 541 Median OS: 14. 0 months 50 40 30 Placebo, n = 268 Median OS: 11. 2 months 20 10 0 Month Radium- 223 Placebo 0 3 6 9 12 15 18 21 24 27 541 268 450 218 330 147 213 89 120 49 72 28 30 15 15 7 3 3 0 0 EORTC 2011
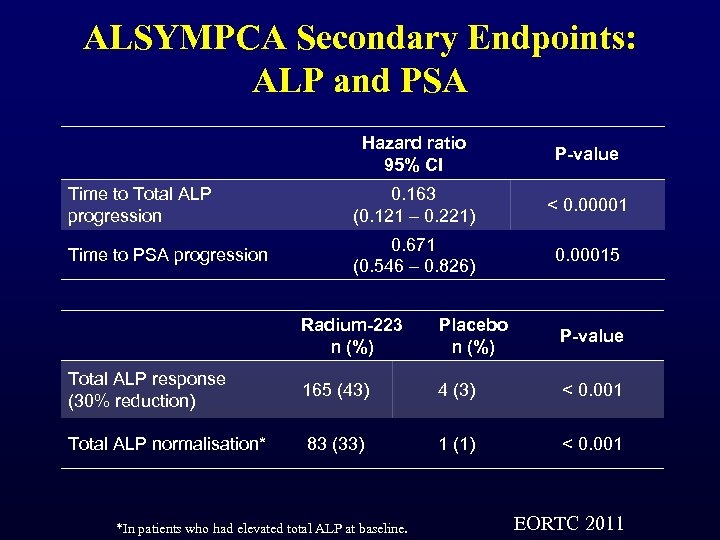 ALSYMPCA Secondary Endpoints: ALP and PSA Hazard ratio 95% CI P-value Time to Total ALP progression 0. 163 (0. 121 – 0. 221) < 0. 00001 Time to PSA progression 0. 671 (0. 546 – 0. 826) 0. 00015 Radium-223 n (%) Placebo n (%) P-value Total ALP response (30% reduction) 165 (43) 4 (3) < 0. 001 Total ALP normalisation* 83 (33) 1 (1) < 0. 001 *In patients who had elevated total ALP at baseline. EORTC 2011
ALSYMPCA Secondary Endpoints: ALP and PSA Hazard ratio 95% CI P-value Time to Total ALP progression 0. 163 (0. 121 – 0. 221) < 0. 00001 Time to PSA progression 0. 671 (0. 546 – 0. 826) 0. 00015 Radium-223 n (%) Placebo n (%) P-value Total ALP response (30% reduction) 165 (43) 4 (3) < 0. 001 Total ALP normalisation* 83 (33) 1 (1) < 0. 001 *In patients who had elevated total ALP at baseline. EORTC 2011
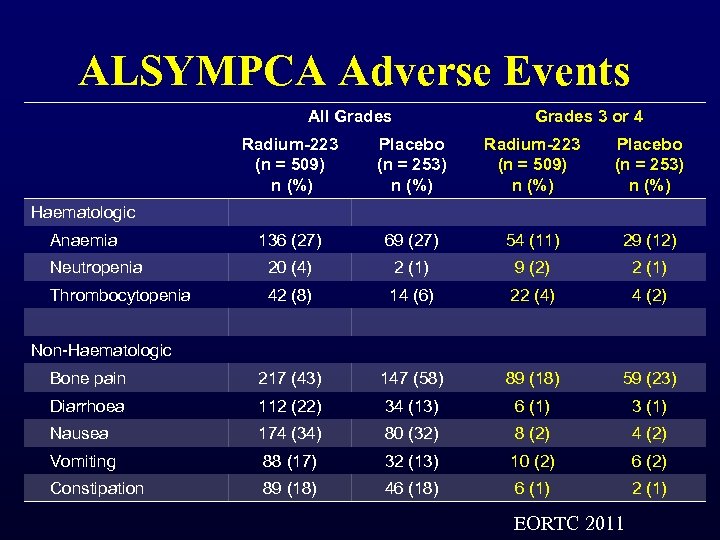 ALSYMPCA Adverse Events All Grades 3 or 4 Radium-223 (n = 509) n (%) Placebo (n = 253) n (%) 136 (27) 69 (27) 54 (11) 29 (12) Neutropenia 20 (4) 2 (1) 9 (2) 2 (1) Thrombocytopenia 42 (8) 14 (6) 22 (4) 4 (2) Bone pain 217 (43) 147 (58) 89 (18) 59 (23) Diarrhoea 112 (22) 34 (13) 6 (1) 3 (1) Nausea 174 (34) 80 (32) 8 (2) 4 (2) Vomiting 88 (17) 32 (13) 10 (2) 6 (2) Constipation 89 (18) 46 (18) 6 (1) 2 (1) Haematologic Anaemia Non-Haematologic EORTC 2011
ALSYMPCA Adverse Events All Grades 3 or 4 Radium-223 (n = 509) n (%) Placebo (n = 253) n (%) 136 (27) 69 (27) 54 (11) 29 (12) Neutropenia 20 (4) 2 (1) 9 (2) 2 (1) Thrombocytopenia 42 (8) 14 (6) 22 (4) 4 (2) Bone pain 217 (43) 147 (58) 89 (18) 59 (23) Diarrhoea 112 (22) 34 (13) 6 (1) 3 (1) Nausea 174 (34) 80 (32) 8 (2) 4 (2) Vomiting 88 (17) 32 (13) 10 (2) 6 (2) Constipation 89 (18) 46 (18) 6 (1) 2 (1) Haematologic Anaemia Non-Haematologic EORTC 2011
 Targeting the micro-environment • Bevacizumab (monoclonal VEGF) • Sunitinib (TKI VEGF 1, 2, 3) • Aflibercept (VEGF 1 and 2 domains fused to Fc portion Ig. G 1 – VENICE Trial-completed • Lenalidomide (Immunomodulatory derivative of thalidomide) – MAINSAIL Trial • Tasquinimod (quinolone-3 -carboxamide) • Cabozantinib (VEGFR 2/MET inhibitor) • Dasatanib (SRC inhibitor)
Targeting the micro-environment • Bevacizumab (monoclonal VEGF) • Sunitinib (TKI VEGF 1, 2, 3) • Aflibercept (VEGF 1 and 2 domains fused to Fc portion Ig. G 1 – VENICE Trial-completed • Lenalidomide (Immunomodulatory derivative of thalidomide) – MAINSAIL Trial • Tasquinimod (quinolone-3 -carboxamide) • Cabozantinib (VEGFR 2/MET inhibitor) • Dasatanib (SRC inhibitor)
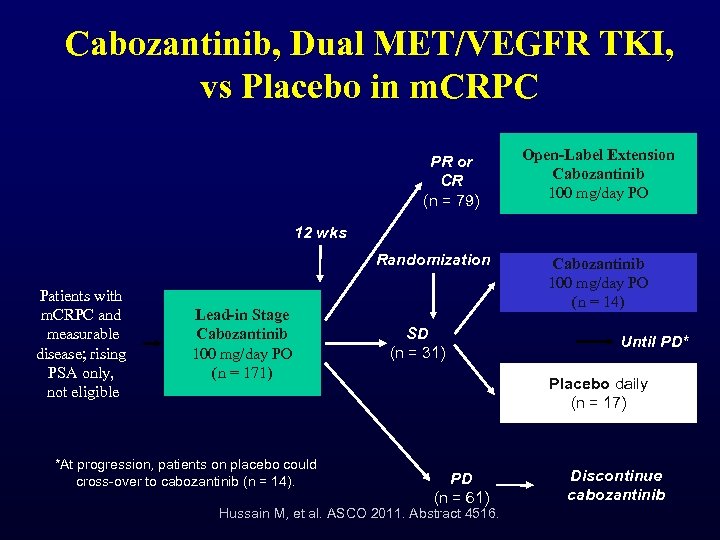 Cabozantinib, Dual MET/VEGFR TKI, vs Placebo in m. CRPC PR or CR (n = 79) Open-Label Extension Cabozantinib 100 mg/day PO 12 wks Randomization Patients with m. CRPC and measurable disease; rising PSA only, not eligible Lead-in Stage Cabozantinib 100 mg/day PO (n = 171) *At progression, patients on placebo could cross-over to cabozantinib (n = 14). SD (n = 31) Cabozantinib 100 mg/day PO (n = 14) Until PD* Placebo daily (n = 17) PD (n = 61) Hussain M, et al. ASCO 2011. Abstract 4516. Discontinue cabozantinib
Cabozantinib, Dual MET/VEGFR TKI, vs Placebo in m. CRPC PR or CR (n = 79) Open-Label Extension Cabozantinib 100 mg/day PO 12 wks Randomization Patients with m. CRPC and measurable disease; rising PSA only, not eligible Lead-in Stage Cabozantinib 100 mg/day PO (n = 171) *At progression, patients on placebo could cross-over to cabozantinib (n = 14). SD (n = 31) Cabozantinib 100 mg/day PO (n = 14) Until PD* Placebo daily (n = 17) PD (n = 61) Hussain M, et al. ASCO 2011. Abstract 4516. Discontinue cabozantinib
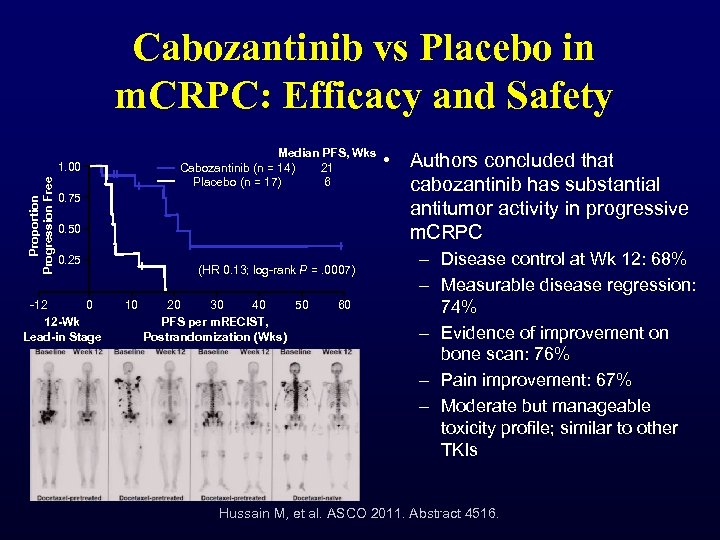 Cabozantinib vs Placebo in m. CRPC: Efficacy and Safety Median PFS, Wks Cabozantinib (n = 14) 21 Placebo (n = 17) 6 Proportion Progression Free 1. 00 0. 75 0. 50 0. 25 -12 0 12 -Wk Lead-in Stage (HR 0. 13; log-rank P =. 0007) 10 20 30 40 50 PFS per m. RECIST, Postrandomization (Wks) 60 • Authors concluded that cabozantinib has substantial antitumor activity in progressive m. CRPC – Disease control at Wk 12: 68% – Measurable disease regression: 74% – Evidence of improvement on bone scan: 76% – Pain improvement: 67% – Moderate but manageable toxicity profile; similar to other TKIs Hussain M, et al. ASCO 2011. Abstract 4516.
Cabozantinib vs Placebo in m. CRPC: Efficacy and Safety Median PFS, Wks Cabozantinib (n = 14) 21 Placebo (n = 17) 6 Proportion Progression Free 1. 00 0. 75 0. 50 0. 25 -12 0 12 -Wk Lead-in Stage (HR 0. 13; log-rank P =. 0007) 10 20 30 40 50 PFS per m. RECIST, Postrandomization (Wks) 60 • Authors concluded that cabozantinib has substantial antitumor activity in progressive m. CRPC – Disease control at Wk 12: 68% – Measurable disease regression: 74% – Evidence of improvement on bone scan: 76% – Pain improvement: 67% – Moderate but manageable toxicity profile; similar to other TKIs Hussain M, et al. ASCO 2011. Abstract 4516.
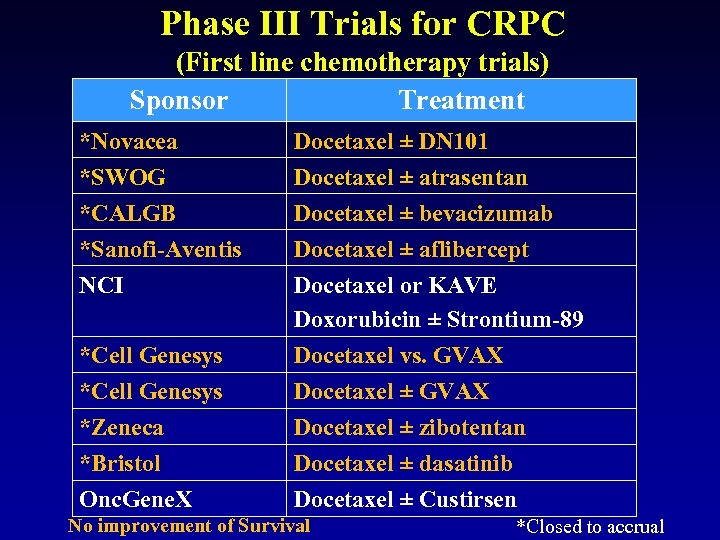 Phase III Trials for CRPC (First line chemotherapy trials) Sponsor Treatment *Novacea Docetaxel ± DN 101 *SWOG *CALGB *Sanofi-Aventis NCI Docetaxel ± atrasentan Docetaxel ± bevacizumab Docetaxel ± aflibercept Docetaxel or KAVE Doxorubicin ± Strontium-89 Docetaxel vs. GVAX Docetaxel ± zibotentan Docetaxel ± dasatinib Docetaxel ± Custirsen *Cell Genesys *Zeneca *Bristol Onc. Gene. X No improvement of Survival *Closed to accrual
Phase III Trials for CRPC (First line chemotherapy trials) Sponsor Treatment *Novacea Docetaxel ± DN 101 *SWOG *CALGB *Sanofi-Aventis NCI Docetaxel ± atrasentan Docetaxel ± bevacizumab Docetaxel ± aflibercept Docetaxel or KAVE Doxorubicin ± Strontium-89 Docetaxel vs. GVAX Docetaxel ± zibotentan Docetaxel ± dasatinib Docetaxel ± Custirsen *Cell Genesys *Zeneca *Bristol Onc. Gene. X No improvement of Survival *Closed to accrual
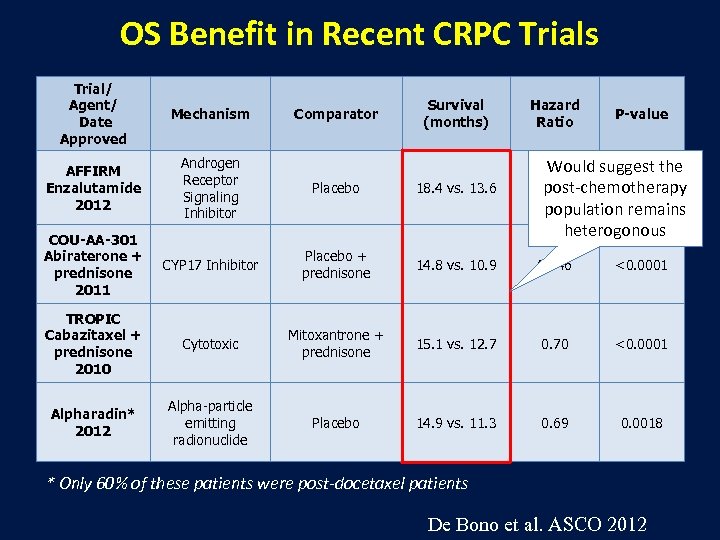 OS Benefit in Recent CRPC Trials Trial/ Agent/ Date Approved Mechanism AFFIRM Enzalutamide 2012 Androgen Receptor Signaling Inhibitor Placebo 18. 4 vs. 13. 6 COU-AA-301 Abiraterone + prednisone 2011 CYP 17 Inhibitor Placebo + prednisone 14. 8 vs. 10. 9 0. 646 <0. 0001 TROPIC Cabazitaxel + prednisone 2010 Cytotoxic Mitoxantrone + prednisone 15. 1 vs. 12. 7 0. 70 <0. 0001 Alpharadin* 2012 Alpha-particle emitting radionuclide Placebo 14. 9 vs. 11. 3 0. 69 0. 0018 Comparator Survival (months) Hazard Ratio P-value Would suggest the 0. 631 <0. 0001 post-chemotherapy population remains heterogonous * Only 60% of these patients were post-docetaxel patients De Bono et al. ASCO 2012
OS Benefit in Recent CRPC Trials Trial/ Agent/ Date Approved Mechanism AFFIRM Enzalutamide 2012 Androgen Receptor Signaling Inhibitor Placebo 18. 4 vs. 13. 6 COU-AA-301 Abiraterone + prednisone 2011 CYP 17 Inhibitor Placebo + prednisone 14. 8 vs. 10. 9 0. 646 <0. 0001 TROPIC Cabazitaxel + prednisone 2010 Cytotoxic Mitoxantrone + prednisone 15. 1 vs. 12. 7 0. 70 <0. 0001 Alpharadin* 2012 Alpha-particle emitting radionuclide Placebo 14. 9 vs. 11. 3 0. 69 0. 0018 Comparator Survival (months) Hazard Ratio P-value Would suggest the 0. 631 <0. 0001 post-chemotherapy population remains heterogonous * Only 60% of these patients were post-docetaxel patients De Bono et al. ASCO 2012
 1. Who is the right patient for which novel therapy? 2. What is the optimal sequencing of these agents? Does it matter? 3. How long do I give these agents? 4. Why are patients still relapsing?
1. Who is the right patient for which novel therapy? 2. What is the optimal sequencing of these agents? Does it matter? 3. How long do I give these agents? 4. Why are patients still relapsing?
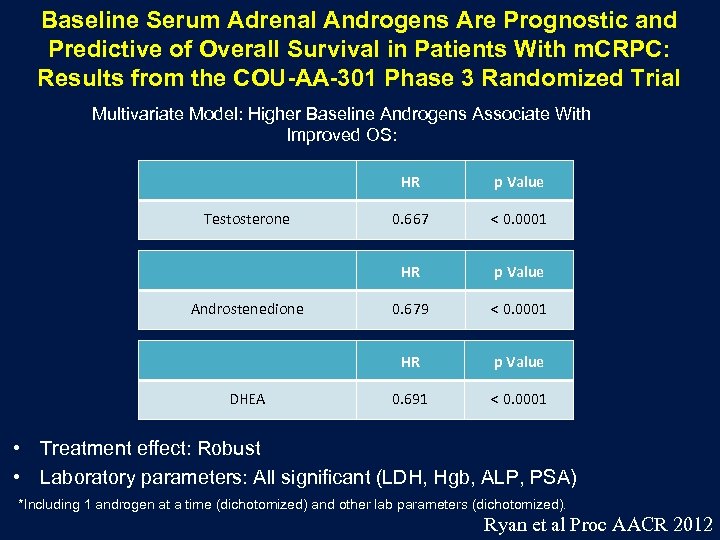 Baseline Serum Adrenal Androgens Are Prognostic and Predictive of Overall Survival in Patients With m. CRPC: Results from the COU-AA-301 Phase 3 Randomized Trial Multivariate Model: Higher Baseline Androgens Associate With Improved OS: HR DHEA < 0. 0001 p Value 0. 679 < 0. 0001 HR Androstenedione 0. 667 HR Testosterone p Value 0. 691 < 0. 0001 • Treatment effect: Robust • Laboratory parameters: All significant (LDH, Hgb, ALP, PSA) *Including 1 androgen at a time (dichotomized) and other lab parameters (dichotomized). Ryan et al Proc AACR 2012
Baseline Serum Adrenal Androgens Are Prognostic and Predictive of Overall Survival in Patients With m. CRPC: Results from the COU-AA-301 Phase 3 Randomized Trial Multivariate Model: Higher Baseline Androgens Associate With Improved OS: HR DHEA < 0. 0001 p Value 0. 679 < 0. 0001 HR Androstenedione 0. 667 HR Testosterone p Value 0. 691 < 0. 0001 • Treatment effect: Robust • Laboratory parameters: All significant (LDH, Hgb, ALP, PSA) *Including 1 androgen at a time (dichotomized) and other lab parameters (dichotomized). Ryan et al Proc AACR 2012
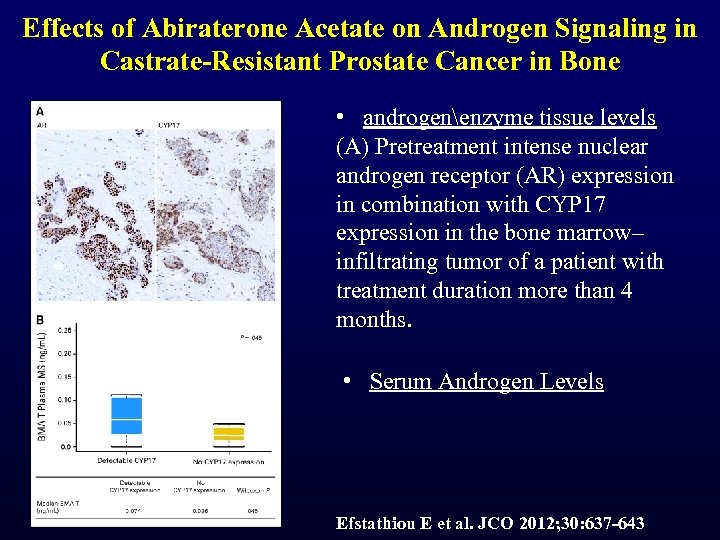 Effects of Abiraterone Acetate on Androgen Signaling in Castrate-Resistant Prostate Cancer in Bone • androgenenzyme tissue levels (A) Pretreatment intense nuclear androgen receptor (AR) expression in combination with CYP 17 expression in the bone marrow– infiltrating tumor of a patient with treatment duration more than 4 months. • Serum Androgen Levels Efstathiou E et al. JCO 2012; 30: 637 -643
Effects of Abiraterone Acetate on Androgen Signaling in Castrate-Resistant Prostate Cancer in Bone • androgenenzyme tissue levels (A) Pretreatment intense nuclear androgen receptor (AR) expression in combination with CYP 17 expression in the bone marrow– infiltrating tumor of a patient with treatment duration more than 4 months. • Serum Androgen Levels Efstathiou E et al. JCO 2012; 30: 637 -643
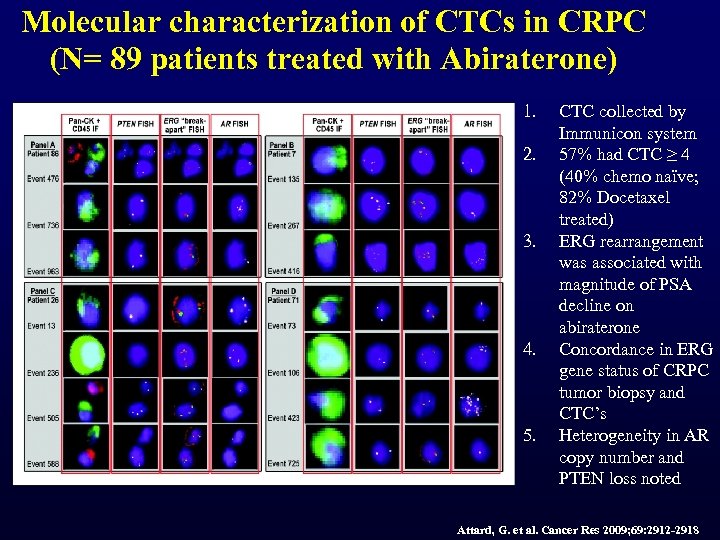 Molecular characterization of CTCs in CRPC (N= 89 patients treated with Abiraterone) 1. 2. 3. 4. 5. CTC collected by Immunicon system 57% had CTC ≥ 4 (40% chemo naïve; 82% Docetaxel treated) ERG rearrangement was associated with magnitude of PSA decline on abiraterone Concordance in ERG gene status of CRPC tumor biopsy and CTC’s Heterogeneity in AR copy number and PTEN loss noted Attard, G. et al. Cancer Res 2009; 69: 2912 -2918
Molecular characterization of CTCs in CRPC (N= 89 patients treated with Abiraterone) 1. 2. 3. 4. 5. CTC collected by Immunicon system 57% had CTC ≥ 4 (40% chemo naïve; 82% Docetaxel treated) ERG rearrangement was associated with magnitude of PSA decline on abiraterone Concordance in ERG gene status of CRPC tumor biopsy and CTC’s Heterogeneity in AR copy number and PTEN loss noted Attard, G. et al. Cancer Res 2009; 69: 2912 -2918
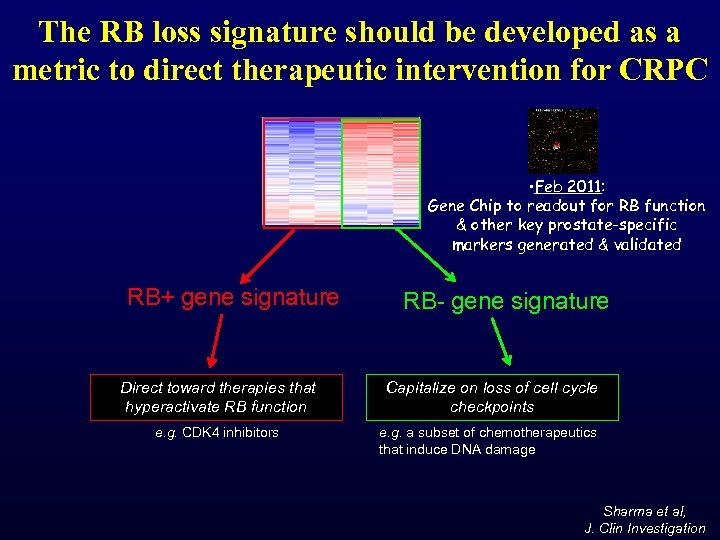 The RB loss signature should be developed as a metric to direct therapeutic intervention for CRPC • Feb 2011: Gene Chip to readout for RB function & other key prostate-specific markers generated & validated RB+ gene signature RB- gene signature Direct toward therapies that hyperactivate RB function Capitalize on loss of cell cycle checkpoints e. g. CDK 4 inhibitors e. g. a subset of chemotherapeutics that induce DNA damage Sharma et al, J. Clin Investigation
The RB loss signature should be developed as a metric to direct therapeutic intervention for CRPC • Feb 2011: Gene Chip to readout for RB function & other key prostate-specific markers generated & validated RB+ gene signature RB- gene signature Direct toward therapies that hyperactivate RB function Capitalize on loss of cell cycle checkpoints e. g. CDK 4 inhibitors e. g. a subset of chemotherapeutics that induce DNA damage Sharma et al, J. Clin Investigation
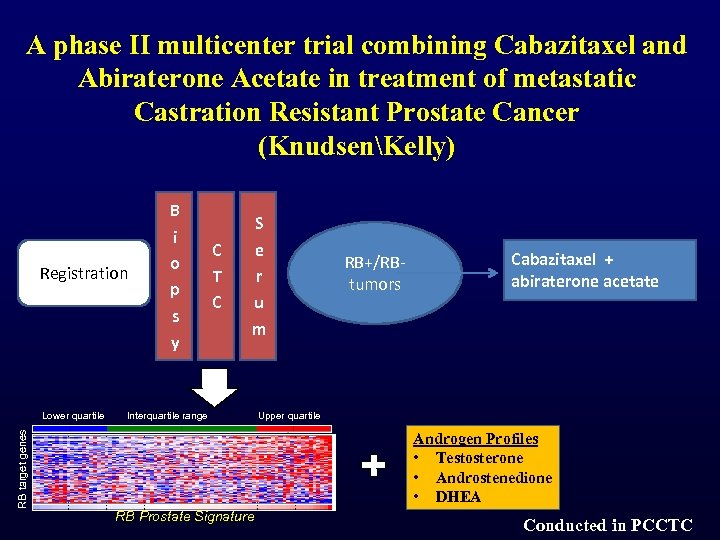 A phase II multicenter trial combining Cabazitaxel and Abiraterone Acetate in treatment of metastatic Castration Resistant Prostate Cancer (KnudsenKelly) Registration B i o p s y C T C S e r u m RB+/RBtumors Cabazitaxel + abiraterone acetate RB target genes Lower quartile Interquartile range Upper quartile Androgen Profiles • Testosterone • Androstenedione • DHEA RB Prostate Signature Conducted in PCCTC
A phase II multicenter trial combining Cabazitaxel and Abiraterone Acetate in treatment of metastatic Castration Resistant Prostate Cancer (KnudsenKelly) Registration B i o p s y C T C S e r u m RB+/RBtumors Cabazitaxel + abiraterone acetate RB target genes Lower quartile Interquartile range Upper quartile Androgen Profiles • Testosterone • Androstenedione • DHEA RB Prostate Signature Conducted in PCCTC
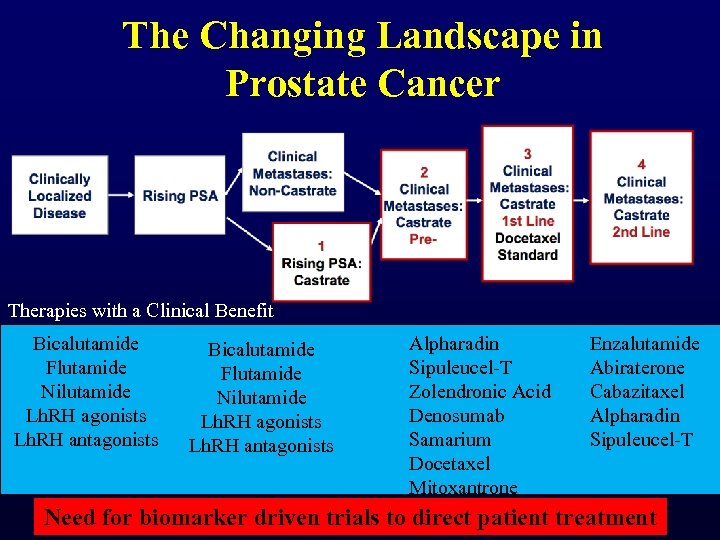 The Changing Landscape in Prostate Cancer Therapies with a Clinical Benefit Bicalutamide Flutamide Nilutamide Lh. RH agonists Lh. RH antagonists Alpharadin Sipuleucel-T Zolendronic Acid Denosumab Samarium Docetaxel Mitoxantrone Enzalutamide Abiraterone Cabazitaxel Alpharadin Sipuleucel-T Need for biomarker driven trials to direct patient treatment
The Changing Landscape in Prostate Cancer Therapies with a Clinical Benefit Bicalutamide Flutamide Nilutamide Lh. RH agonists Lh. RH antagonists Alpharadin Sipuleucel-T Zolendronic Acid Denosumab Samarium Docetaxel Mitoxantrone Enzalutamide Abiraterone Cabazitaxel Alpharadin Sipuleucel-T Need for biomarker driven trials to direct patient treatment
 Novel Agents in CRPC 17α hydrolase/17, 20 lyase inhibitor Anti-angiogenic/immunomodulatory Anti-CTLA 4PD-1 antibody Anti-CCL 2 Anti-FGFR 3 Anti-Il-6 antibody Anti-insulin-like GFR antibody Anti-integrin anti-body Anti-PSMA immunoconjugate Anti-prostate stem cell antibody Anti-VEGF Cytotoxic agent Clusterin inhibitor EGFR antibodiesTKI Faranesyl protein transferase inhibitor GMP phospodiesterase inhibitor HSP-90 inhibitor HDACi HGF inhibitor Hypoxia activated pro-drug IGF inhibotors Integrin receptor antagonist Il-11 inhibitor JAK inhibitor M-TOR inhibitor Multi-targeted TKI MMP-9 inhibitor Pro-apoptotic agent Proteosome Inhibitor RANKL inhibitor SRC kinase inhibitor Signal Transduction inhibitor Survivin suppressant Abiraterone, TAK 700 CC-4047, Lenalidomide, ABR-215050, cediranib, AS 1404 Ipilimumab, MLN 2704 CNTO 888 TKI 258 CNTO 95 IMC-A 12 CNTO 95 MLN 2704, 177 Lu-J 591 AGS-PSCA Bevacizumab, Alfibercept ABT-751, abraxane, E 7389, SB-715992, carbazitaxel Irofulven, Paclitaxel poliglumex, TPI 287, E 7389 OGX-11 Pertuzumab, Cetuximab, Erlotinib, Gefitnib, R 115777 Exisulind 17 -AAG, IPI-504 LBH 589, Vorinostat, Belinostat, SB 939 AMG 102 TH 302 Citutumumab, figitumumab, octreotide, pasireotide Cilengitide BMTP-11 INCB-18424 Temsirolimus Sunitinib, Sorafenib, CEP 701, vatalinib, DMXAA PCK 3145 AT-101 Bortezomib Denosumab KX 2 -391 PCK-3145 YM 155
Novel Agents in CRPC 17α hydrolase/17, 20 lyase inhibitor Anti-angiogenic/immunomodulatory Anti-CTLA 4PD-1 antibody Anti-CCL 2 Anti-FGFR 3 Anti-Il-6 antibody Anti-insulin-like GFR antibody Anti-integrin anti-body Anti-PSMA immunoconjugate Anti-prostate stem cell antibody Anti-VEGF Cytotoxic agent Clusterin inhibitor EGFR antibodiesTKI Faranesyl protein transferase inhibitor GMP phospodiesterase inhibitor HSP-90 inhibitor HDACi HGF inhibitor Hypoxia activated pro-drug IGF inhibotors Integrin receptor antagonist Il-11 inhibitor JAK inhibitor M-TOR inhibitor Multi-targeted TKI MMP-9 inhibitor Pro-apoptotic agent Proteosome Inhibitor RANKL inhibitor SRC kinase inhibitor Signal Transduction inhibitor Survivin suppressant Abiraterone, TAK 700 CC-4047, Lenalidomide, ABR-215050, cediranib, AS 1404 Ipilimumab, MLN 2704 CNTO 888 TKI 258 CNTO 95 IMC-A 12 CNTO 95 MLN 2704, 177 Lu-J 591 AGS-PSCA Bevacizumab, Alfibercept ABT-751, abraxane, E 7389, SB-715992, carbazitaxel Irofulven, Paclitaxel poliglumex, TPI 287, E 7389 OGX-11 Pertuzumab, Cetuximab, Erlotinib, Gefitnib, R 115777 Exisulind 17 -AAG, IPI-504 LBH 589, Vorinostat, Belinostat, SB 939 AMG 102 TH 302 Citutumumab, figitumumab, octreotide, pasireotide Cilengitide BMTP-11 INCB-18424 Temsirolimus Sunitinib, Sorafenib, CEP 701, vatalinib, DMXAA PCK 3145 AT-101 Bortezomib Denosumab KX 2 -391 PCK-3145 YM 155
 GU-Investigator Initiated Trails at KCC Phase I Trial of Weekly Cabazitaxel with Concurrent Intensity Modulated Radiation Therapy and Androgen Deprivation Therapy for the Treatment of Locally Advanced High Risk Adenocarcinoma of the Prostate. - Lin A Phase II Study of Cabazitaxel in Patients with Urothelial Carcinoma Who Have Disease Progression Following Platinum-Based Chemotherapy. -Hoffman Phase I Trial of High Dose Rate Brachytherapy Combined with Stereotactic Body Radiation Therapy for Intermediate Risk Prostate Cancer Patients. -Den A Pilot Phase II Study of Digoxin in Patients with Recurrent Prostate Cancer as Evident by a Rising PSA. -Lin A Multi-Institutional Translational Clinical Trial of Disulfiram in Men with Recurrent Prostate Cancer as Evident by a Rising PSA. -Lin A Phase I/II multicenter trial combining Cabazitaxel and Abiraterone Acetate in Treatment of Metastatic Castration Resistant Prostate Cancer. -Kelly A Phase I/II Study of MLN 8237 in combination with Abiraterone for patients with castration- resistant prostate cancer after progression on Abiraterone. -Lin
GU-Investigator Initiated Trails at KCC Phase I Trial of Weekly Cabazitaxel with Concurrent Intensity Modulated Radiation Therapy and Androgen Deprivation Therapy for the Treatment of Locally Advanced High Risk Adenocarcinoma of the Prostate. - Lin A Phase II Study of Cabazitaxel in Patients with Urothelial Carcinoma Who Have Disease Progression Following Platinum-Based Chemotherapy. -Hoffman Phase I Trial of High Dose Rate Brachytherapy Combined with Stereotactic Body Radiation Therapy for Intermediate Risk Prostate Cancer Patients. -Den A Pilot Phase II Study of Digoxin in Patients with Recurrent Prostate Cancer as Evident by a Rising PSA. -Lin A Multi-Institutional Translational Clinical Trial of Disulfiram in Men with Recurrent Prostate Cancer as Evident by a Rising PSA. -Lin A Phase I/II multicenter trial combining Cabazitaxel and Abiraterone Acetate in Treatment of Metastatic Castration Resistant Prostate Cancer. -Kelly A Phase I/II Study of MLN 8237 in combination with Abiraterone for patients with castration- resistant prostate cancer after progression on Abiraterone. -Lin
 Genitourinary Oncology Team Medical Oncology • Jean Hoffman-Censits, MD • Jianqing Lin, MD • Gwen Slakind, RN • Diane Woodford, APRN Radiation Oncology • Adam Dicker, Ph. D, MD • Robert Den, MD • Mark Hurwitz, MD Urology • Lenny Gomella, MD • Edouard Trabulsi, MD • Costas Lallas, MD Pathology • Peter Mc. Cue, MD • Ruth Birbie Clinical Trials OfficeData Collection • Monica Byrnes • Christine Huberts • Brooke Kennedy • Deborah Kilpatrick, RN • Zachary Foerst Basic Science • Allessandro Fatalis, MD • Karen Knudsen, Ph. D • Lucia Languino, Ph. D • Marja Nevalainen, Ph. D • Eric Wickstrom, Ph. D Administrative Support • Teresa Bryant • Beth Schade
Genitourinary Oncology Team Medical Oncology • Jean Hoffman-Censits, MD • Jianqing Lin, MD • Gwen Slakind, RN • Diane Woodford, APRN Radiation Oncology • Adam Dicker, Ph. D, MD • Robert Den, MD • Mark Hurwitz, MD Urology • Lenny Gomella, MD • Edouard Trabulsi, MD • Costas Lallas, MD Pathology • Peter Mc. Cue, MD • Ruth Birbie Clinical Trials OfficeData Collection • Monica Byrnes • Christine Huberts • Brooke Kennedy • Deborah Kilpatrick, RN • Zachary Foerst Basic Science • Allessandro Fatalis, MD • Karen Knudsen, Ph. D • Lucia Languino, Ph. D • Marja Nevalainen, Ph. D • Eric Wickstrom, Ph. D Administrative Support • Teresa Bryant • Beth Schade
 Thank You
Thank You


