f58e8b1265769bc2dfebe2723f656905.ppt
- Количество слайдов: 53

Treatment in premature Menopause Serge Rozenberg - BMS, Brussels Serge Rozenberg CHU Saint-Pierre Department of Gynaecology and Obstetrics, Free Universities of Brussels (ULB-VUB) Belgium. Serge. rozenberg@skynet. be Together, we could fight Breast cancer!
Menopause, Premature • The premature cessation of menses (MENSTRUATION) when the last menstrual period occurs in a woman under the age of 40. • It is due to the depletion of OVARIAN FOLLICLES. Premature MENOPAUSE can be caused by diseases; OVARIECTOMY; RADIATION; chemicals; and chromosomal abnormalities. • Medline Mesh
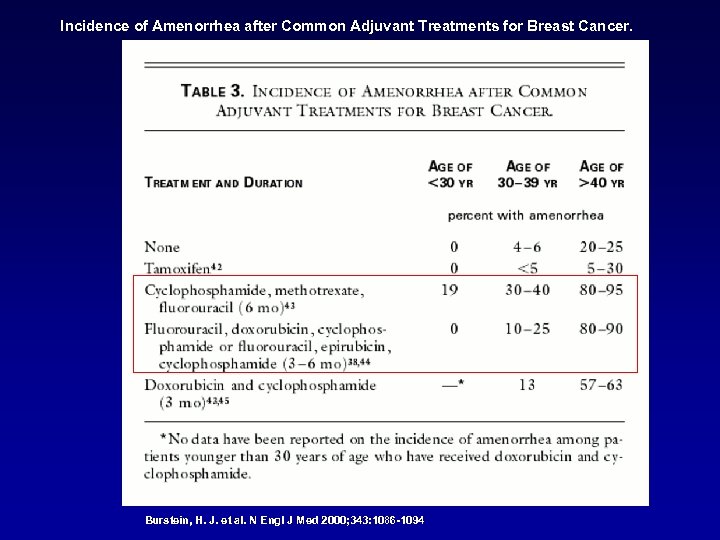
Incidence of Amenorrhea after Common Adjuvant Treatments for Breast Cancer. Burstein, H. J. et al. N Engl J Med 2000; 343: 1086 -1094

Treatment of Menopause in breast cancer survivors whith premature menopause • Medline search : number of articles • 0
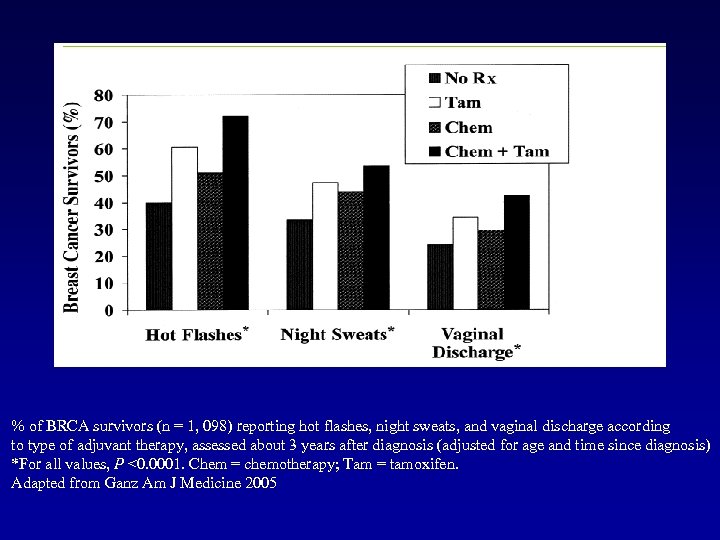
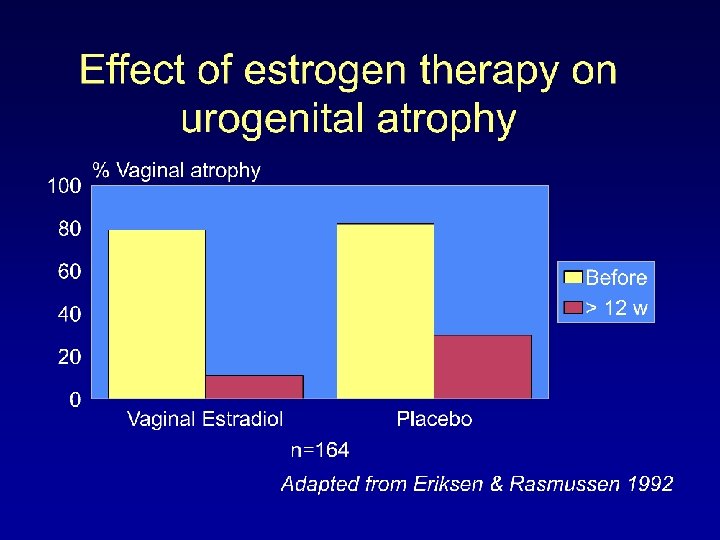
% of BRCA survivors (n = 1, 098) reporting hot flashes, night sweats, and vaginal discharge according to type of adjuvant therapy, assessed about 3 years after diagnosis (adjusted for age and time since diagnosis) *For all values, P <0. 0001. Chem = chemotherapy; Tam = tamoxifen. Adapted from Ganz Am J Medicine 2005
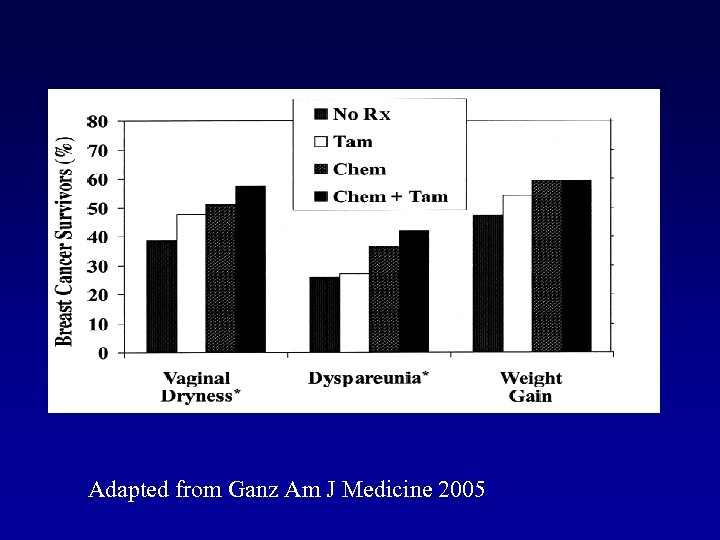
Adapted from Ganz Am J Medicine 2005


Randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. Loprinzi et al J Clin Oncol. 1997 • vaginal dryness decreased by 62% and 64% in the placebo and Replens groups • Average dyspareunia scores also improved by 41% and 60%
• Up to 20% of patients with breast cancer consider stopping or actually cease endocrine treatment because of menopausal symptoms • Fellowes et al 2001.

HRT After Breast Cancer: A Systematic Review and Quantitative Assessment of Risk • RR = 0. 64, 95% CI, 0. 36 -1. 15 Col et al Journal of Clinical Oncology, 2001

Safety of menopausal treatments after breast cancer: a qualitative systematic review Caroline Antoine, Fabienne Liebens, Birgit Carly, Ann Pastijn, Serge Rozenberg CHU Saint-Pierre Department of Gynaecology and Obstetrics, Free Universities of Brussels (ULB-VUB) Belgium EMAS, 7 th European Congress on Menopause, June 2006, Istanbul Antoine et al Human Reproduction in press
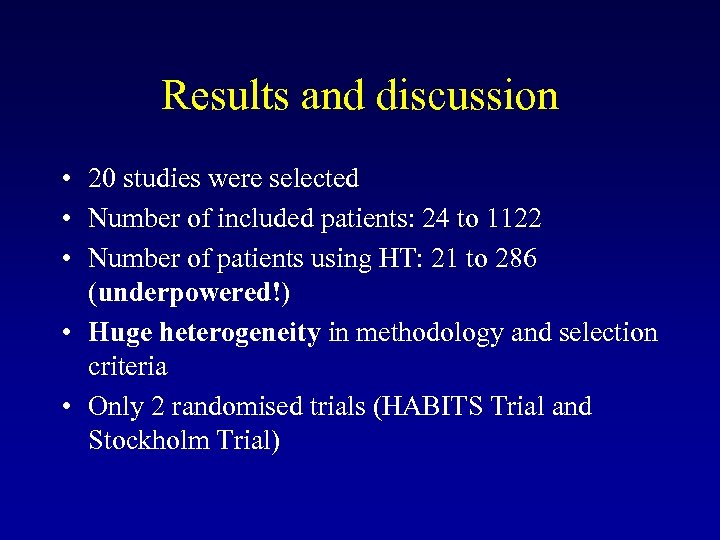
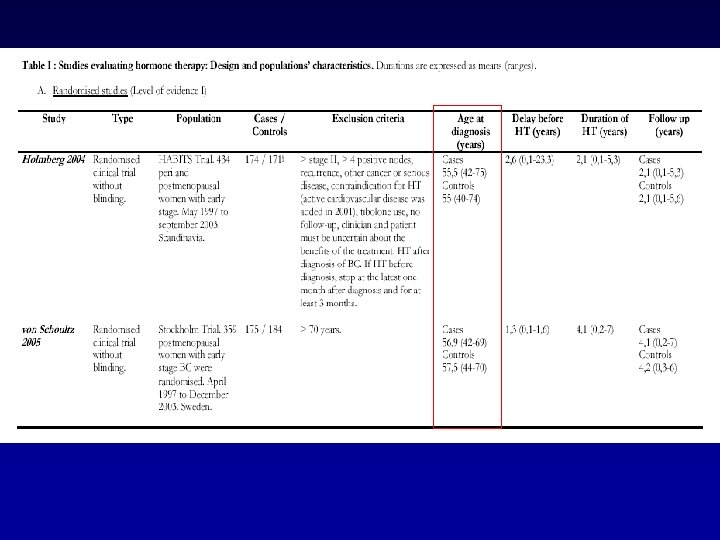
Results and discussion • 20 studies were selected • Number of included patients: 24 to 1122 • Number of patients using HT: 21 to 286 (underpowered!) • Huge heterogeneity in methodology and selection criteria • Only 2 randomised trials (HABITS Trial and Stockholm Trial)

Results and discussion d. Patients’ outcome • Most observational studies concluded that HT had no negative influence on breast cancer prognosis. • Stockholm randomized trial: – RH = 0. 82, 95% CI = 0. 35 to 1. 9.
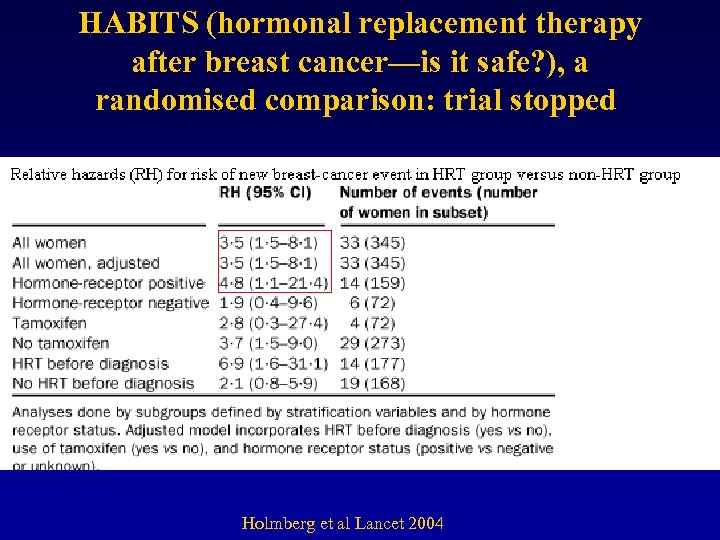
HABITS (hormonal replacement therapy after breast cancer—is it safe? ), a randomised comparison: trial stopped Holmberg et al Lancet 2004
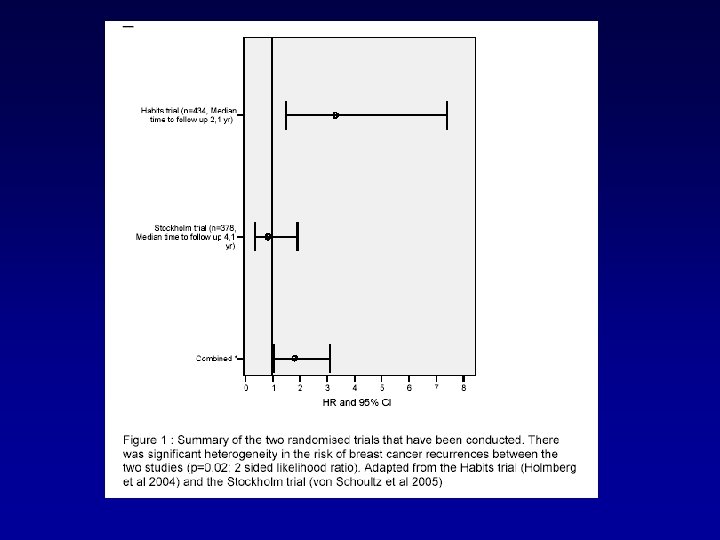
Results and discussion • The HABITS Trial and the Stockholm Trial have a higher level of evidence – Difference in results may be due to • Heterogeneity in the assessed patients (higher number of involved nodes and a lower proportion of tamoxifen treated patients) in the HABITS trial • Heterogeneity in the regimens used (more often combined regimens) in the HABITS trial

Recommendation • Combined HRT cannot be considered as first-line management for menopausal symptoms after breast cancer. • Uncertainty about whether type or regimen or receptor status have implications for the safety ? • HRT could be justified for quality of life when other interventions have failed and the woman is clearly informed of the risk • Hickey, et al Lancet 2005
Other molecules Progestagens • Prospective randomised trial breast cancer patients (most taking tamoxifen) – 85% reduction in hot flashes (20 mg megestrol acetate 2 X/d) vs 21% with placebo. – Loprinzi et al. N Eng J Med 1994

Safety of progestagens ? • Has not been established after breast cancer. – could be mitogenic in the breast ? – Addition of progestagen to oestrogen was associated with an increased risk of breast cancer in several trals (WHI) – Interactions between progestagens and antioestrogens ?

Other molecules Tibolone • Only 4 studies • No increase of a new breast cancer event • Very small number of patients (underpowered!) • Ongoing trials: LIBERATE Trial in 2007 & Dana-Farber Cancer Institute Brigham and Women's Hospital Beth Israel Deaconess Medical Center Massachusetts
Non-hormonal treatments • Strong placebo effects – 40% in vasomotor symptoms, can persist for several weeks • Few non-hormonal agents have been tested for hot flashes in breast cancer patients. • Generally poor quality of studies • Minimum recommended period by FDA/ EMEA is 12 weeks, but most studies of non-hormonal agents 4– 6 weeks. • Most studies: mixture of patients with/without breast cancer (some of whom were taking anti-oestrogens).
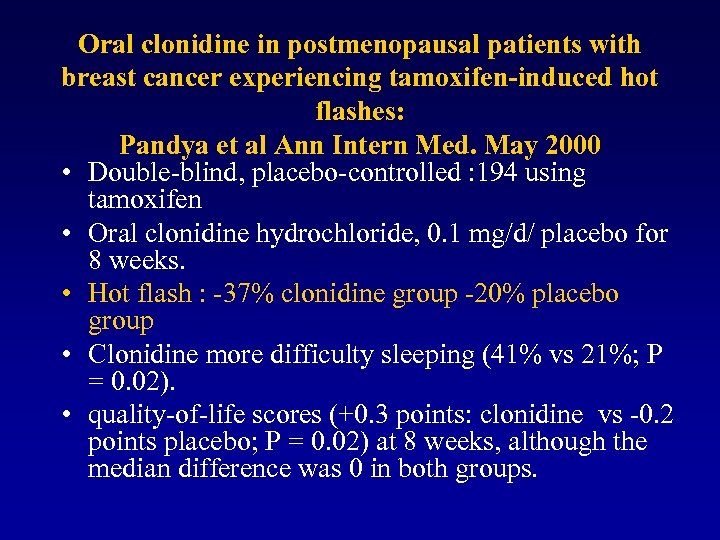
Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: Pandya et al Ann Intern Med. May 2000 • Double-blind, placebo-controlled : 194 using tamoxifen • Oral clonidine hydrochloride, 0. 1 mg/d/ placebo for 8 weeks. • Hot flash : -37% clonidine group -20% placebo group • Clonidine more difficulty sleeping (41% vs 21%; P = 0. 02). • quality-of-life scores (+0. 3 points: clonidine vs -0. 2 points placebo; P = 0. 02) at 8 weeks, although the median difference was 0 in both groups.
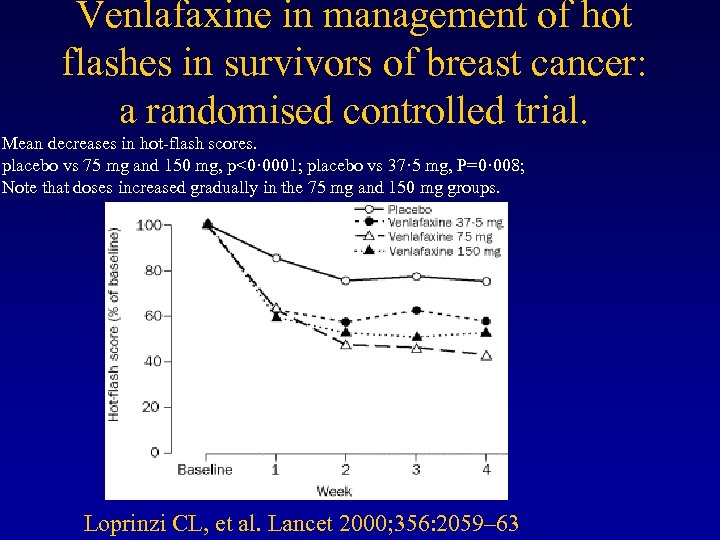
Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Mean decreases in hot-flash scores. placebo vs 75 mg and 150 mg, p<0· 0001; placebo vs 37· 5 mg, P=0· 008; Note that doses increased gradually in the 75 mg and 150 mg groups. Loprinzi CL, et al. Lancet 2000; 356: 2059– 63
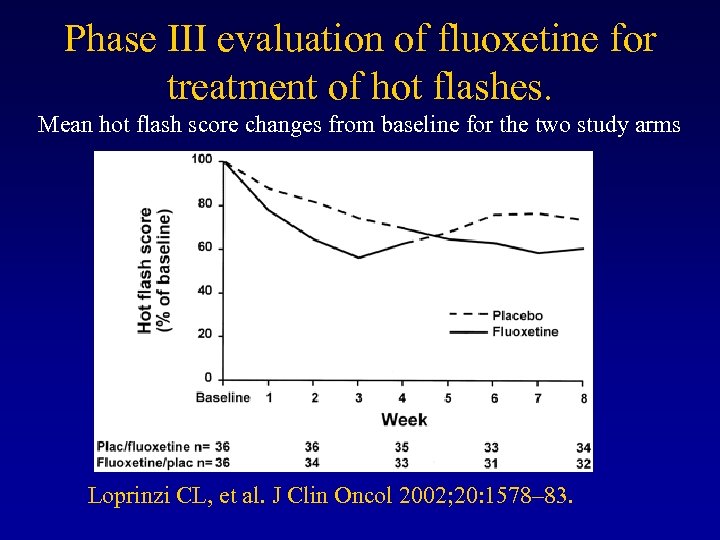
Phase III evaluation of fluoxetine for treatment of hot flashes. Mean hot flash score changes from baseline for the two study arms Loprinzi CL, et al. J Clin Oncol 2002; 20: 1578– 83.
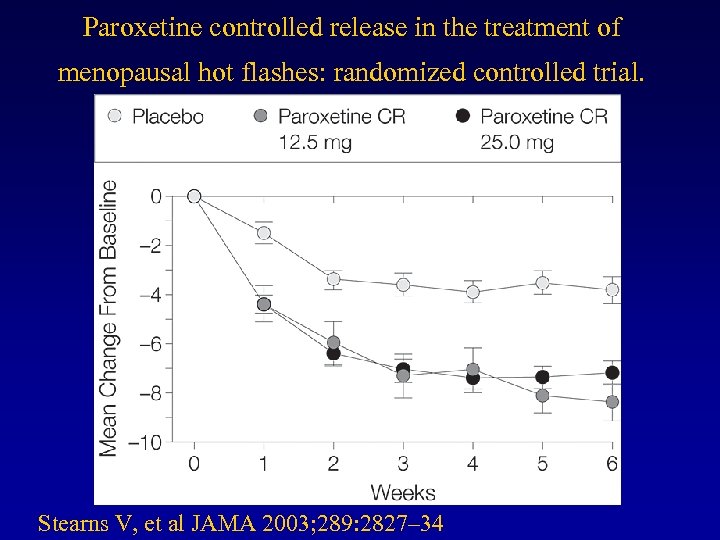
Paroxetine controlled release in the treatment of menopausal hot flashes: randomized controlled trial. Stearns V, et al JAMA 2003; 289: 2827– 34

New trials • Venlafaxine With or Without Zolpidem (NCI) Massachusetts General Hospital • Paroxetine (GSK) • DVS-233 SR (Whyeth) • Citalopram (not yet open) (NCI)
Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine statistically significantly reduced the concentrations of 4 -hydroxy-Ndesmethyl-tamoxifen (endoxifen), a metabolite resulting from CYP 2 D 6 mediated hydroxylation of N-desmethyltamoxifen. Stearns V, et al. J Natl Cancer Inst 2003; 95

Recommendations for practice • 75 mg venlafaxine effective initially in the treatment of hot flashes after breast cancer, but seems not to continue for a clinically worthwhile duration. • Other SSRIs such as fluoxetine (10– 30 mg a day), paroxetine (25 mg), and citalopram (10– 20 mg) might be effective, but there are no data to show that this effect persists beyond 6 weeks. • advise that use of SSRI and SNRI are probably associated with anticholinergic side-effects and that it could potentially interfere with the metabolism of antioestrogens.

Gabapentin • GABA analogue used in the treatment of epilepsy, neurogenic pain, restless-leg syndrome, essential tremor, bipolar disorder, and migraine prophylaxis

Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebocontrolled trial • 420 women with breast cancer • > 2 hot flashes/ day • placebo, gabapentin 300 mg/d or 900 mg/d P. O. in 3 doses for 8 weeks. • 1 -week, self-report diary before (frequency, severity, and duration) and during weeks 4 and 8 of treatment. • Analyses were by intention to treat. • Pandya Lancet 2005
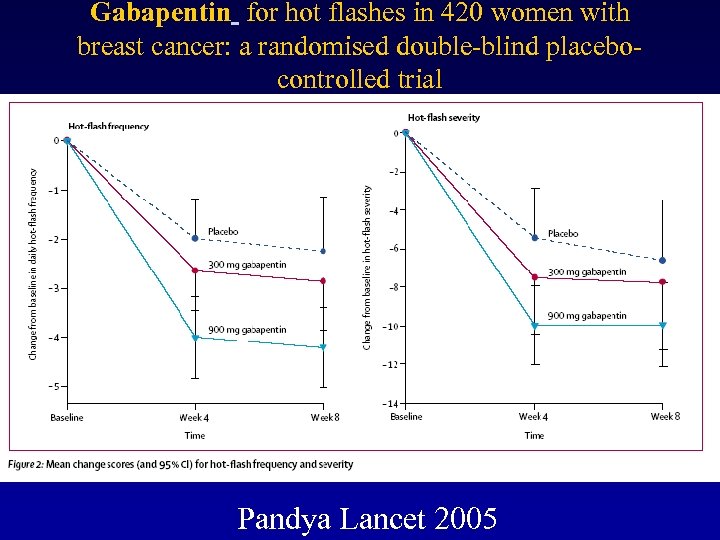
Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebocontrolled trial Pandya Lancet 2005
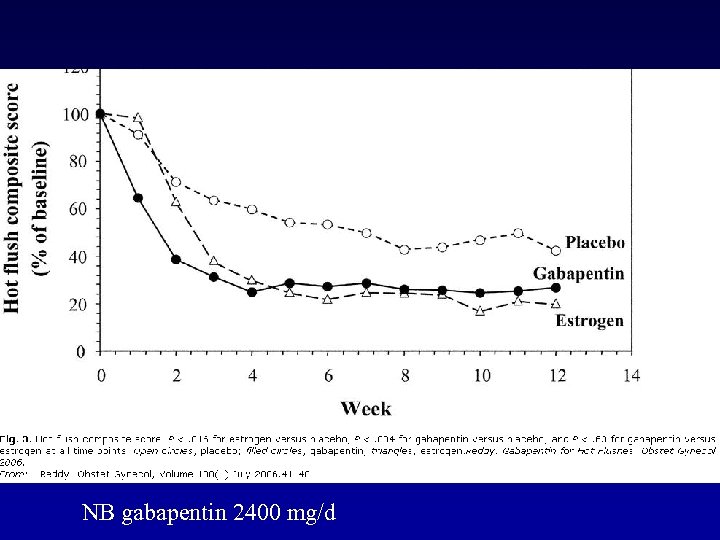
NB gabapentin 2400 mg/d

General recommendations • Achieve a normal BMI, • Identify triggers to their hot flashes (eg, alcohol, hot drinks, or spicy food) ? • Training in relaxation techniques could also be beneficial. • Stop smoking • Maintain a regular exercise


Prevalence of vertebral fracture in women with non-metastatic breast cancer. • At the time of first diagnosis= general population (age-matched sample). • Nearly 5 times greater from the time of first diagnosis OR, 4. 7; 95% CI: 2. 3 -9. 9 • Kanis et al Br J Cancer. 1999 Mar; 79(7 -8): 117981.

Tamoxifen and osteoporosis • Tamoxifen led to a 32% reduction in osteoporotic fractures (RR = 0. 68, 95% CI = 0. 51 to 0. 92). • Fisher J Natl Cancer Inst. 2005 Nov 16; 97(22): 1652 -62
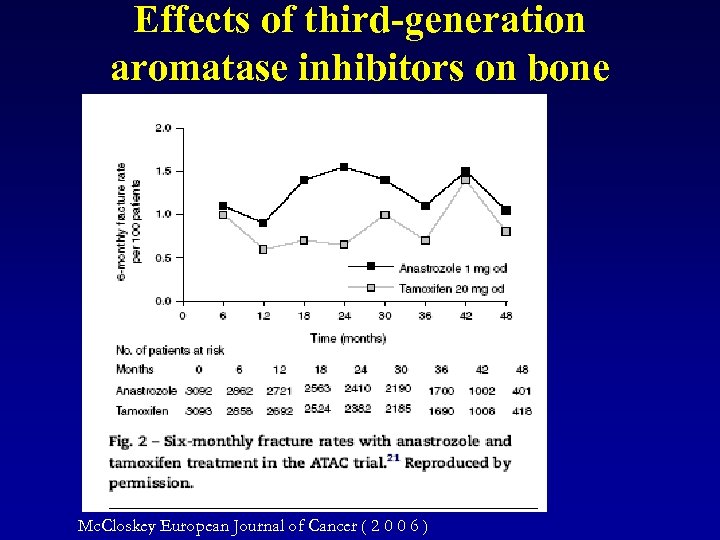
Effects of third-generation aromatase inhibitors on bone Mc. Closkey European Journal of Cancer ( 2 0 0 6 )

Prevention and treatment • Regular physical exercise • calcium 1500 mg and vitamin D 800 U daily. • T-score < 2. 5 & between -1. 5 and -2. 5 in the presence of a fragility fracture or vertebral compression fracture – Consider bisphonate therapy. • Paterson et al Clin Breast Cancer. 2005 Feb; 5 Suppl(2): S 41 -5.

Celebrate yor birthday twice a year You will live twice as long

Communicate with your physician. Together, we could fight Breast cancer!

alternative medicine • There is insufficient evidence about efficacy and safety to support the use of alternative medicine in the treatment of menopausal symptoms after breast cancer.

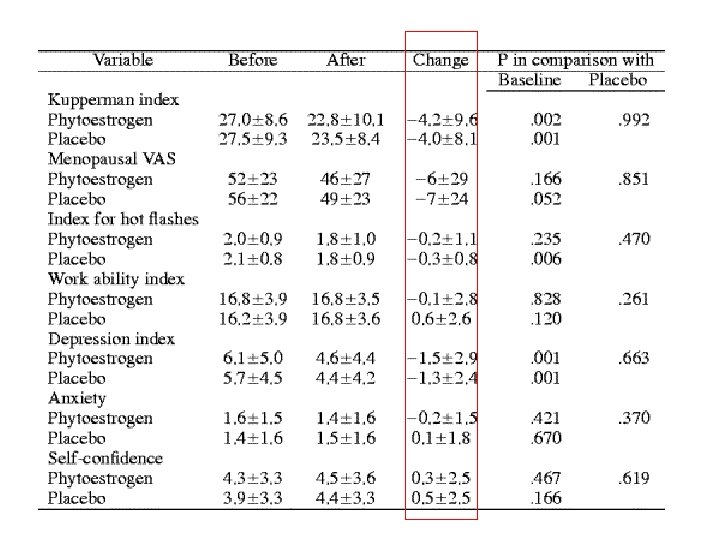
Phytoestrogens

A randomized placebo-controlled crossover trial with phytoestrogens in treatment of menopause in breast cancer patients Nikander et al Obstet & Gynecol June 2003,

New trials • St. John's Wort NCI August 2006 Wake Forest University • A Taiwan Isoflavone Multicenter Study (TIMS)

New trials • Menopause and Meditation University of Pittsburgh (NIH) • Hypnosis National Cancer Institute (NCI) Scott and White Cancer Institute
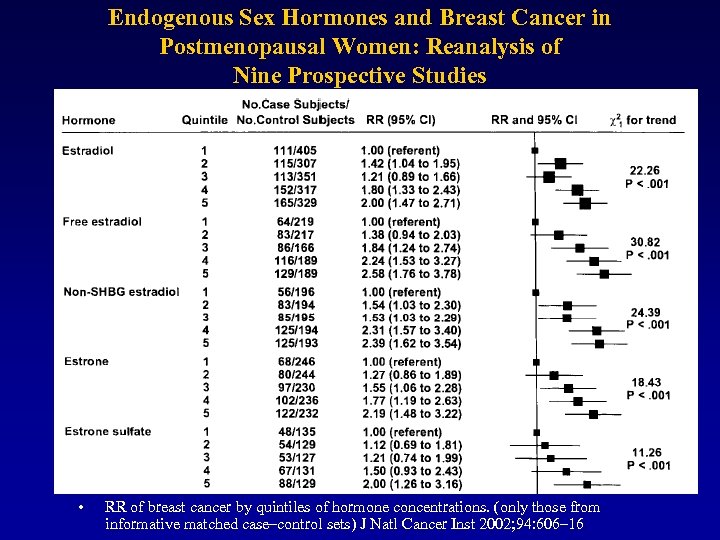
Endogenous Sex Hormones and Breast Cancer in Postmenopausal Women: Reanalysis of Nine Prospective Studies • RR of breast cancer by quintiles of hormone concentrations. (only those from informative matched case–control sets) J Natl Cancer Inst 2002; 94: 606– 16
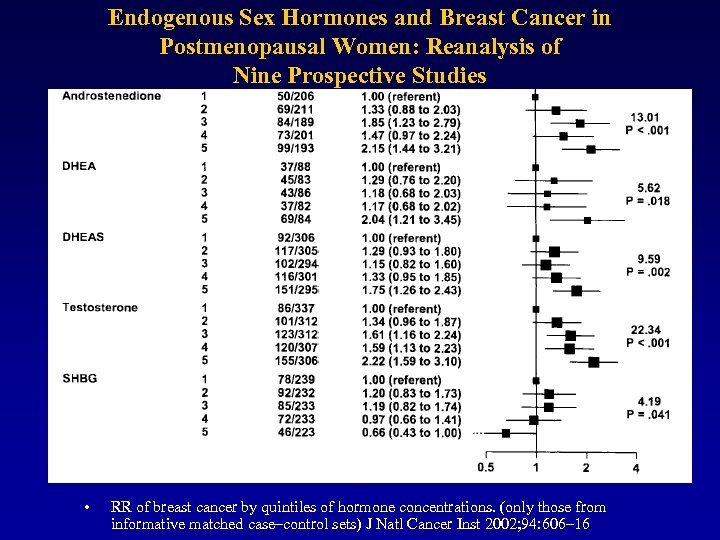
Endogenous Sex Hormones and Breast Cancer in Postmenopausal Women: Reanalysis of Nine Prospective Studies • RR of breast cancer by quintiles of hormone concentrations. (only those from informative matched case–control sets) J Natl Cancer Inst 2002; 94: 606– 16

Future perspectives of selective estrogen receptor modulators used alone and in combination with DHEA • Disclosure: Labrie is president of Endorecherche, the company responsible for the development of acolbifene and has patents on medical uses of DHEA and its combination with SERMs. • Labrie Endocr Relat Cancer. 2006 June

Future perspectives of selective estrogen receptor modulators used alone and in combination with DHEA • DHEA inhibits breast cancer : Low circulating levels of DHEA and DHEA-S in patients with breast cancer (Zumoff et al. 1981) and DHEA has been found to exert anti-oncogenic activity in a series of animal models (Schwartz et al. 1986, Gordon et al. 1987, Li et al. 1993). • Labrie Endocr Relat Cancer. 2006 June
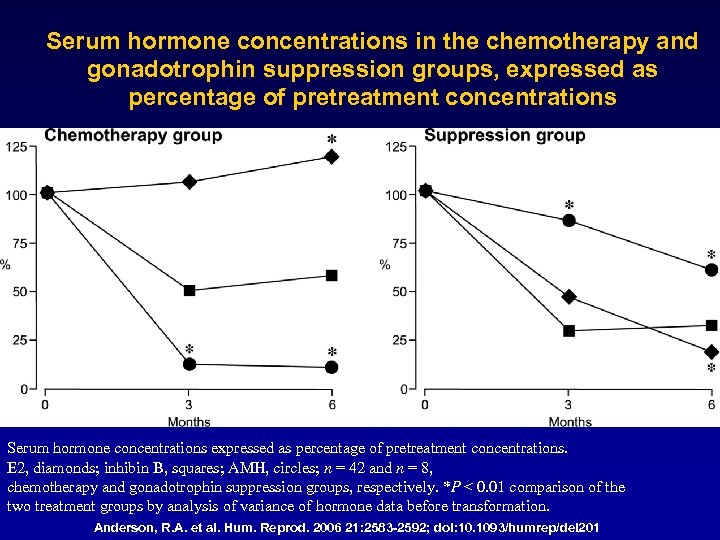
Serum hormone concentrations in the chemotherapy and gonadotrophin suppression groups, expressed as percentage of pretreatment concentrations Serum hormone concentrations expressed as percentage of pretreatment concentrations. E 2, diamonds; inhibin B, squares; AMH, circles; n = 42 and n = 8, chemotherapy and gonadotrophin suppression groups, respectively. *P < 0. 01 comparison of the two treatment groups by analysis of variance of hormone data before transformation. Anderson, R. A. et al. Hum. Reprod. 2006 21: 2583 -2592; doi: 10. 1093/humrep/del 201
f58e8b1265769bc2dfebe2723f656905.ppt