IVb kvira.ppt
- Количество слайдов: 82

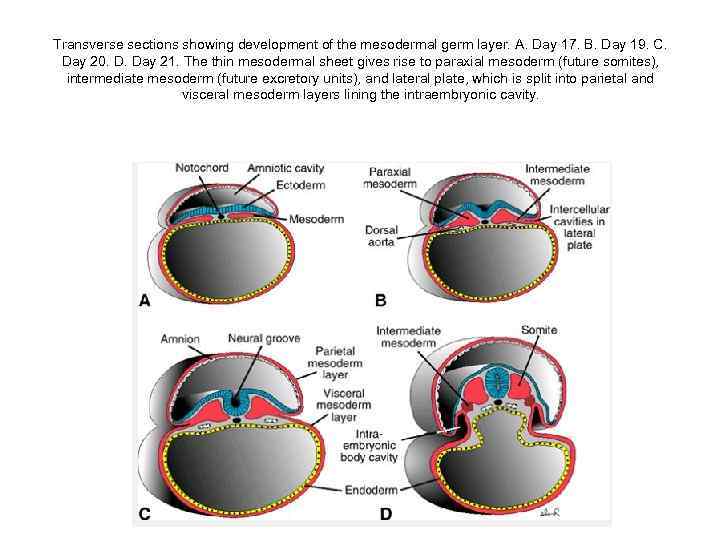 Transverse sections showing development of the mesodermal germ layer. A. Day 17. B. Day 19. C. Day 20. D. Day 21. The thin mesodermal sheet gives rise to paraxial mesoderm (future somites), intermediate mesoderm (future excretory units), and lateral plate, which is split into parietal and visceral mesoderm layers lining the intraembryonic cavity.
Transverse sections showing development of the mesodermal germ layer. A. Day 17. B. Day 19. C. Day 20. D. Day 21. The thin mesodermal sheet gives rise to paraxial mesoderm (future somites), intermediate mesoderm (future excretory units), and lateral plate, which is split into parietal and visceral mesoderm layers lining the intraembryonic cavity.
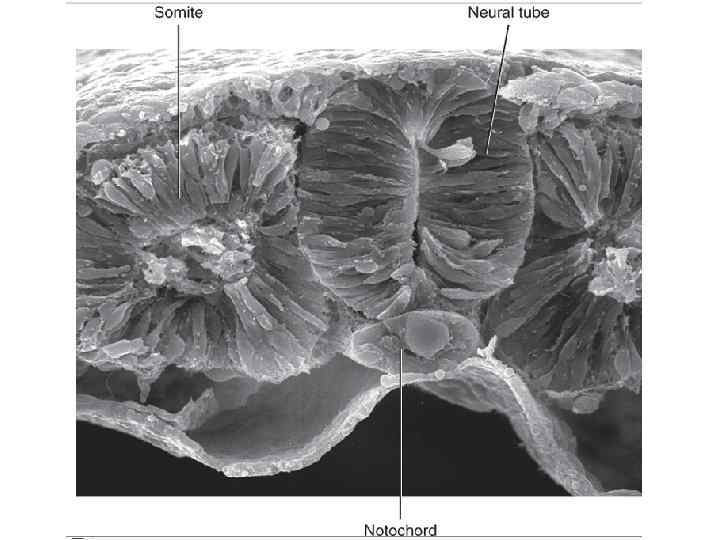
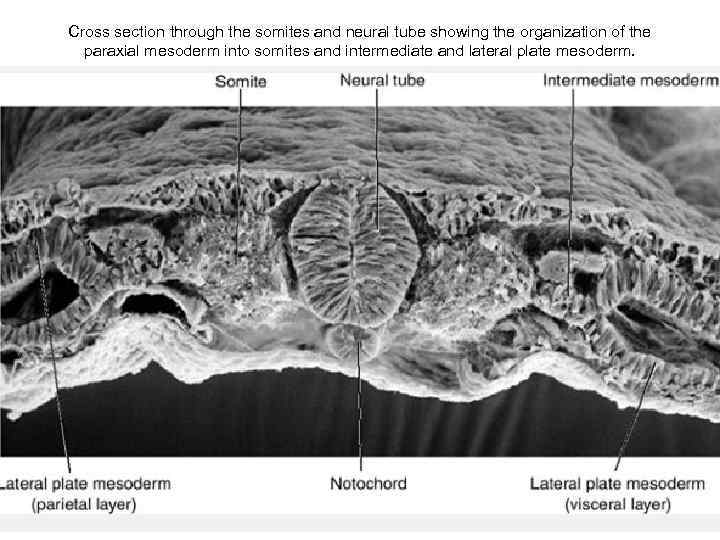 Cross section through the somites and neural tube showing the organization of the paraxial mesoderm into somites and intermediate and lateral plate mesoderm.
Cross section through the somites and neural tube showing the organization of the paraxial mesoderm into somites and intermediate and lateral plate mesoderm.
 nervuli lula, qorda da qordisaxlo mezoderma
nervuli lula, qorda da qordisaxlo mezoderma
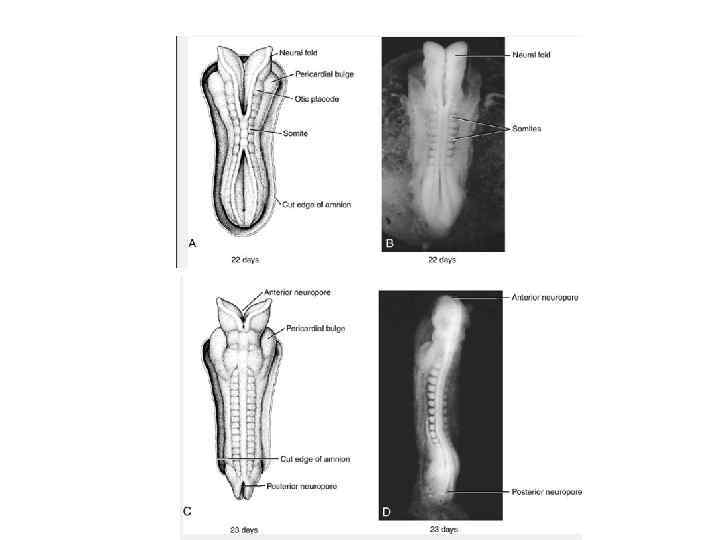
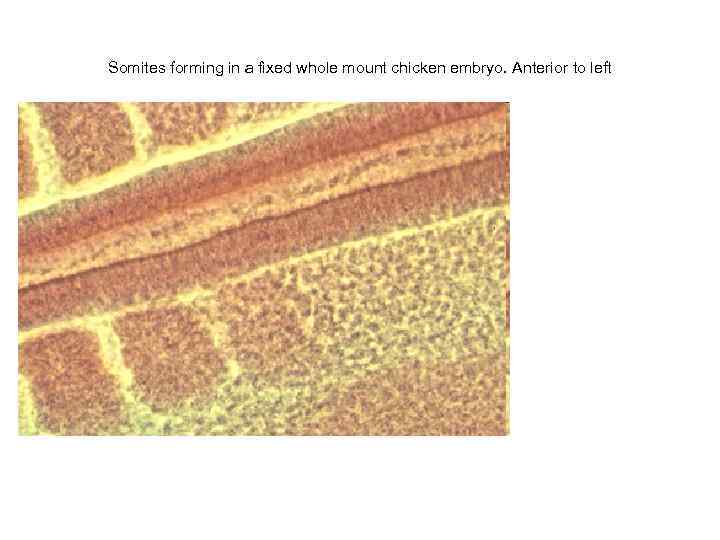 Somites forming in a fixed whole mount chicken embryo. Anterior to left
Somites forming in a fixed whole mount chicken embryo. Anterior to left
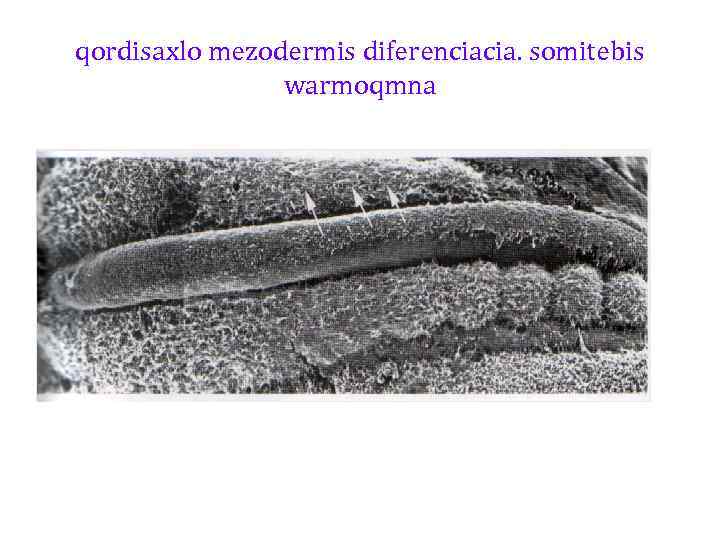 qordisaxlo mezodermis diferenciacia. somitebis warmoqmna
qordisaxlo mezodermis diferenciacia. somitebis warmoqmna
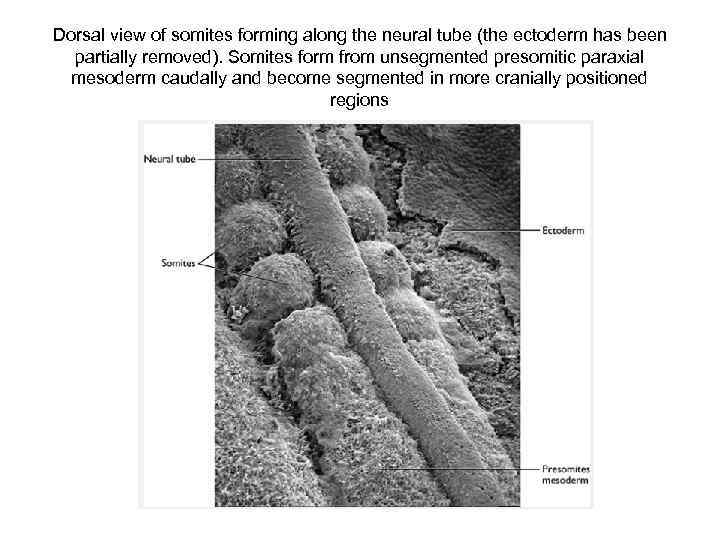 Dorsal view of somites forming along the neural tube (the ectoderm has been partially removed). Somites form from unsegmented presomitic paraxial mesoderm caudally and become segmented in more cranially positioned regions
Dorsal view of somites forming along the neural tube (the ectoderm has been partially removed). Somites form from unsegmented presomitic paraxial mesoderm caudally and become segmented in more cranially positioned regions
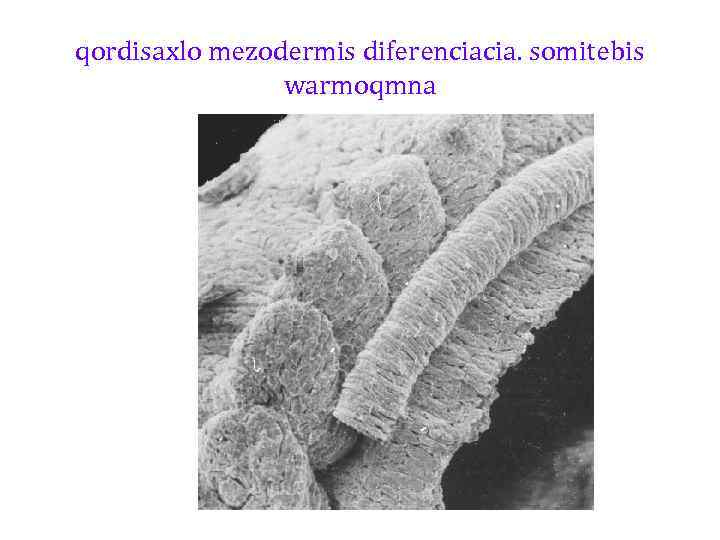 qordisaxlo mezodermis diferenciacia. somitebis warmoqmna
qordisaxlo mezodermis diferenciacia. somitebis warmoqmna
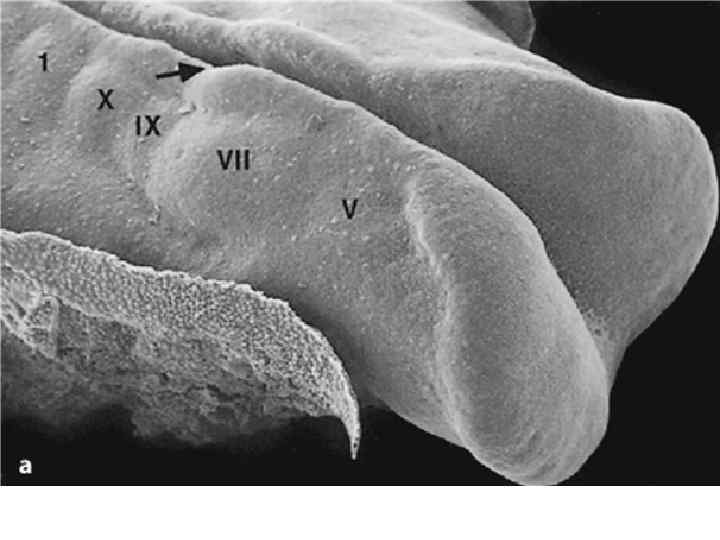
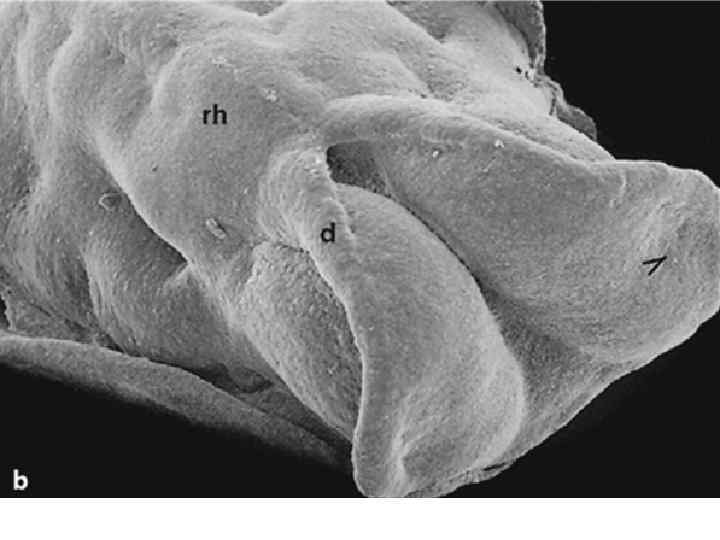
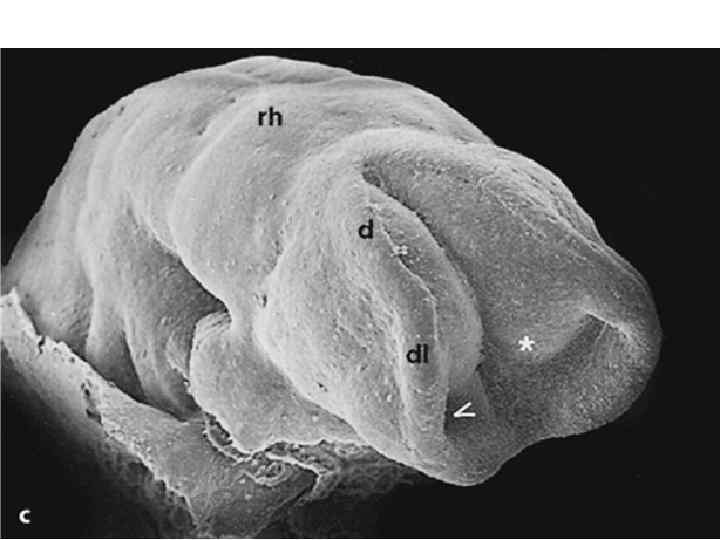
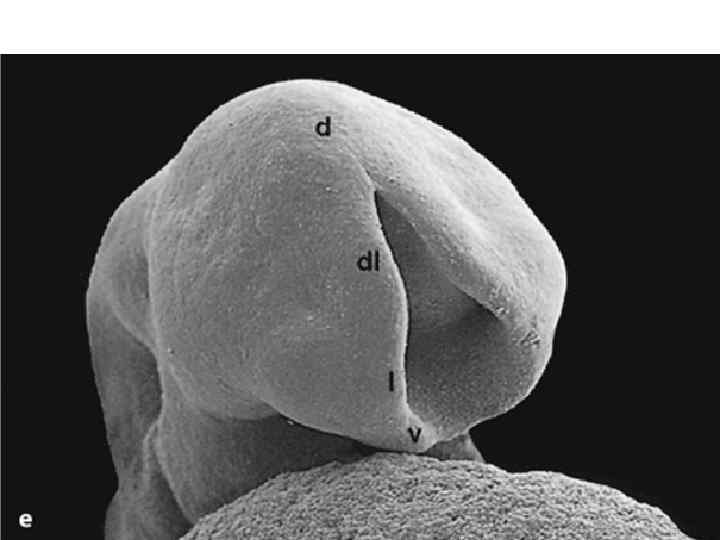
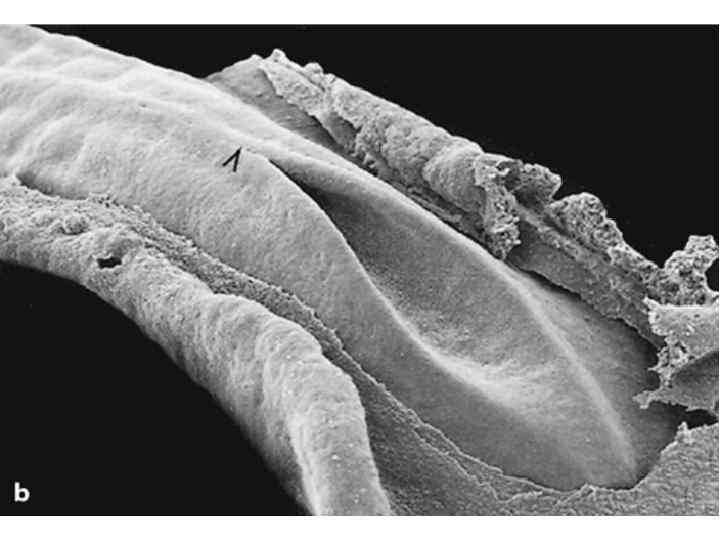
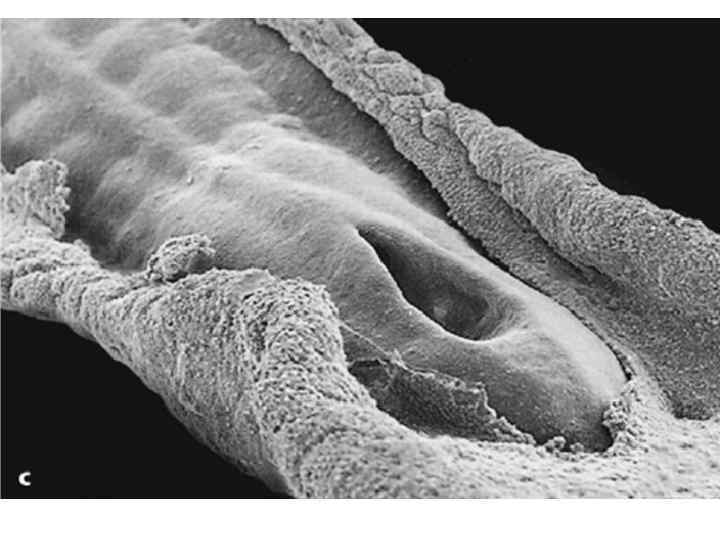
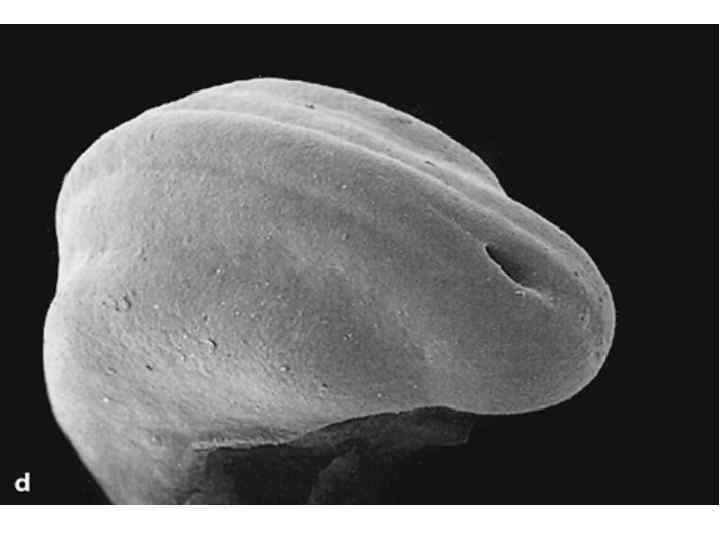
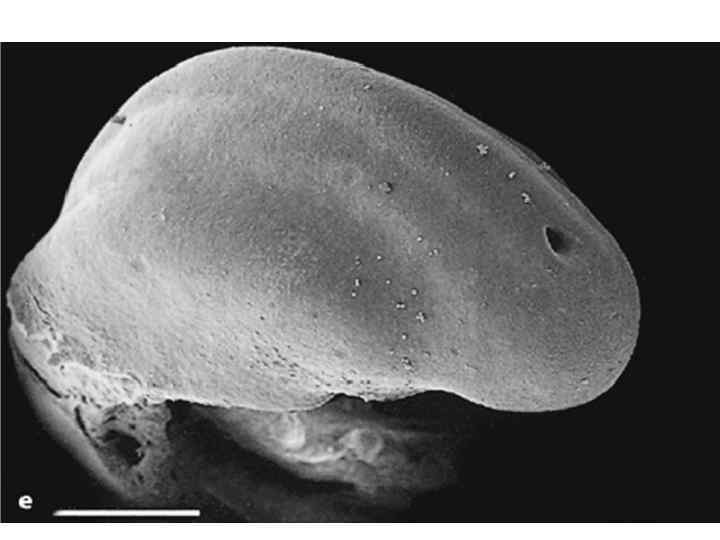
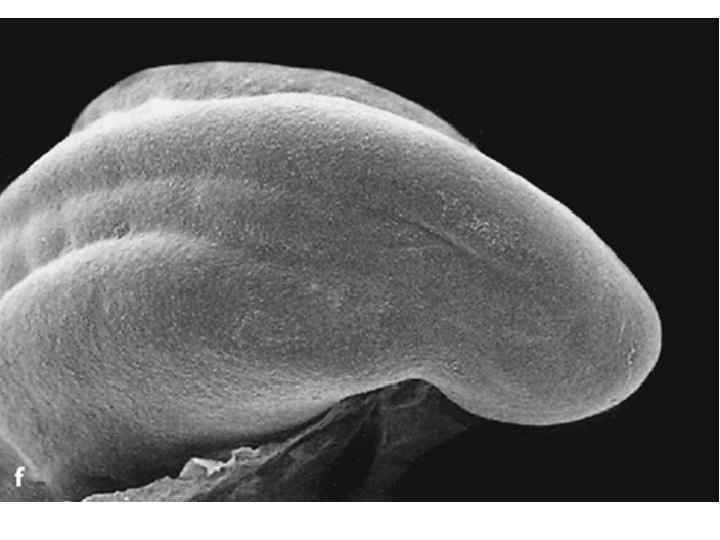
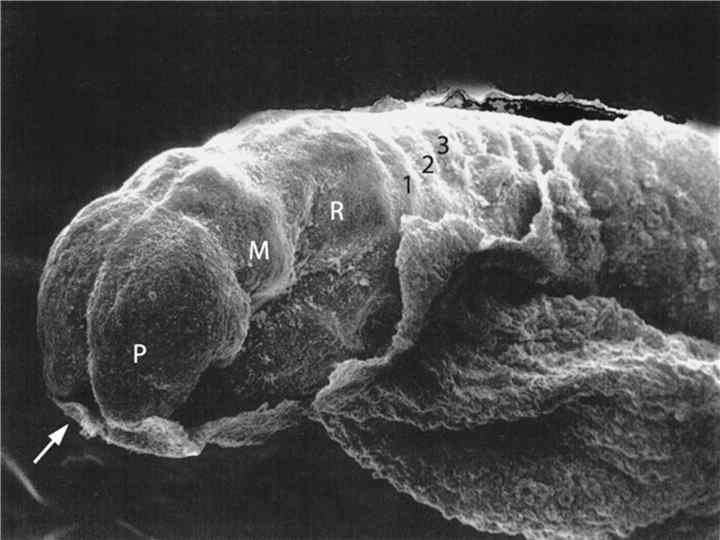
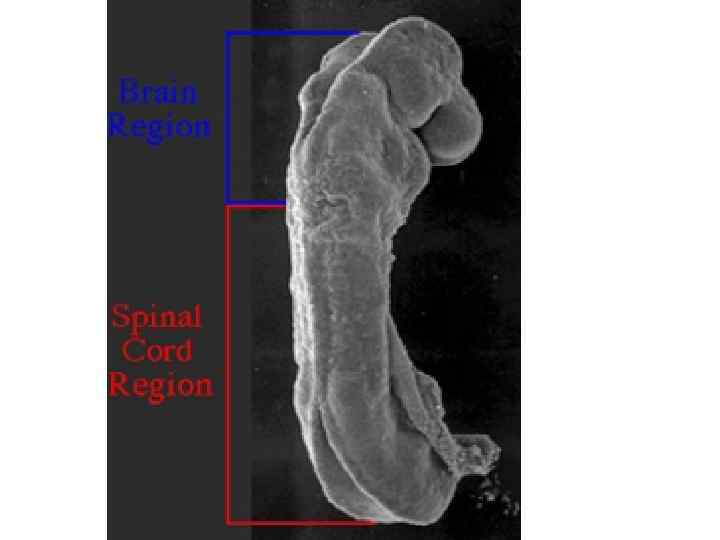
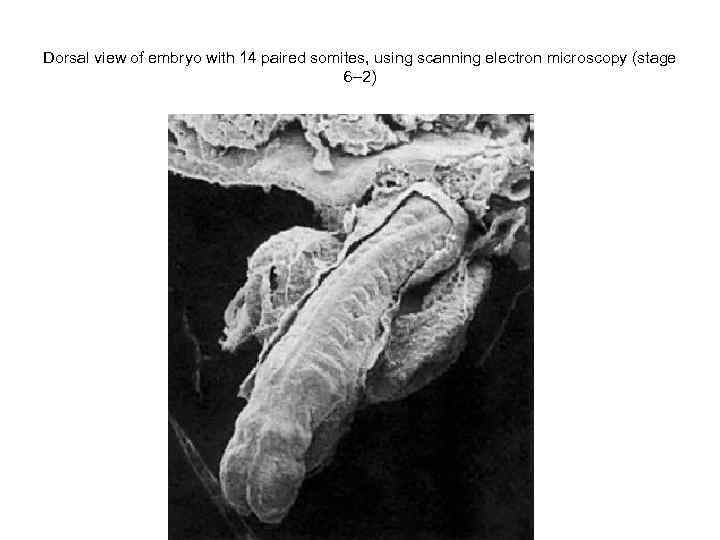 Dorsal view of embryo with 14 paired somites, using scanning electron microscopy (stage 6– 2)
Dorsal view of embryo with 14 paired somites, using scanning electron microscopy (stage 6– 2)
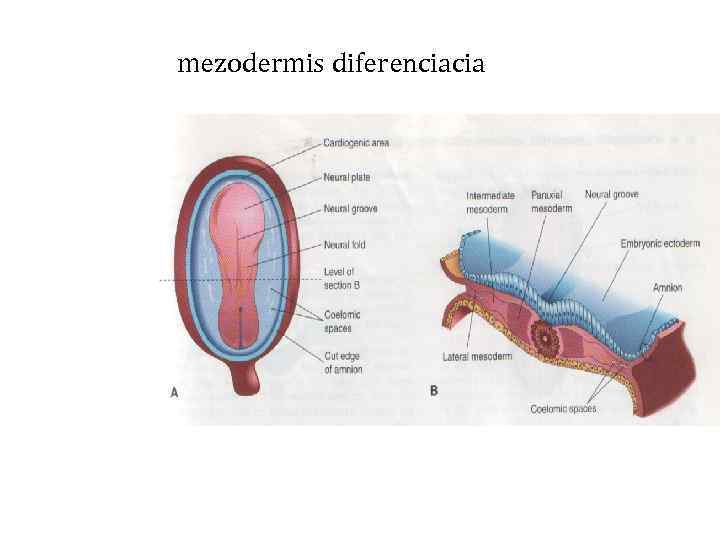 mezodermis diferenciacia
mezodermis diferenciacia
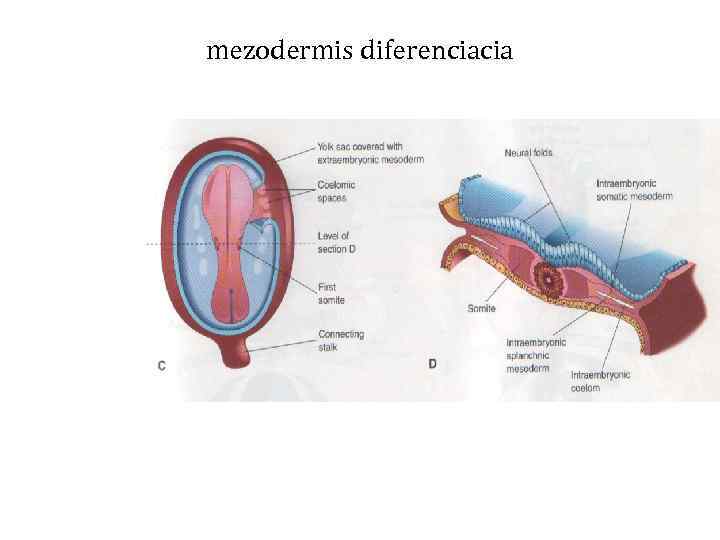 mezodermis diferenciacia
mezodermis diferenciacia
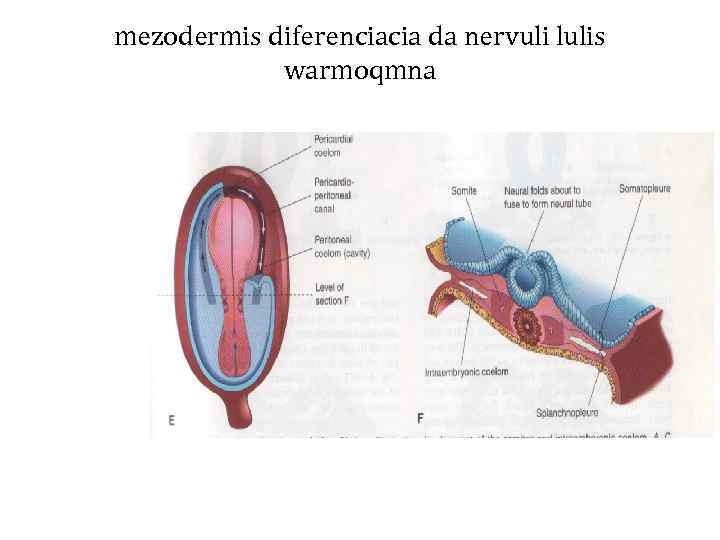 mezodermis diferenciacia da nervuli lulis warmoqmna
mezodermis diferenciacia da nervuli lulis warmoqmna
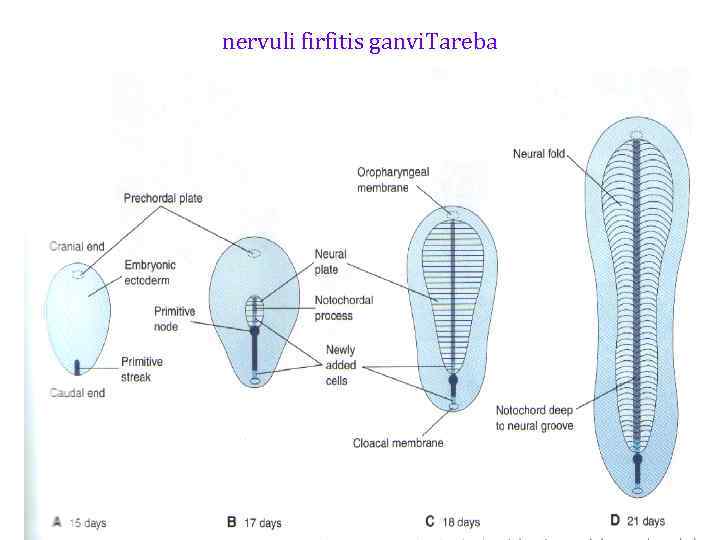 nervuli firfitis ganvi. Tareba
nervuli firfitis ganvi. Tareba
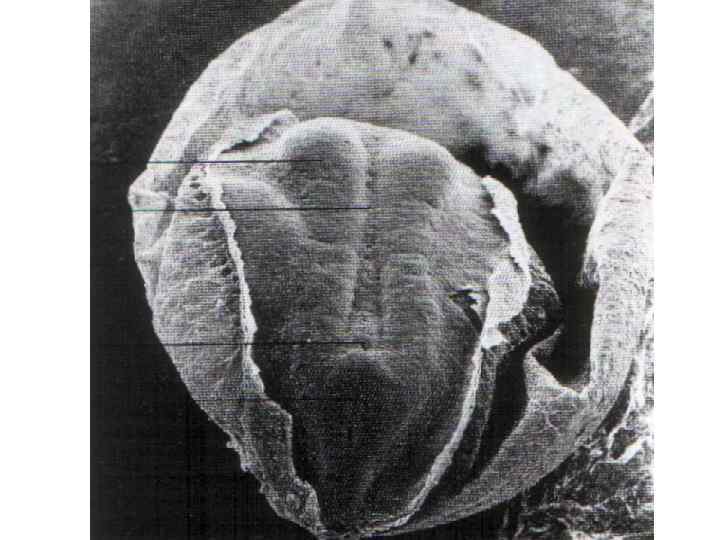
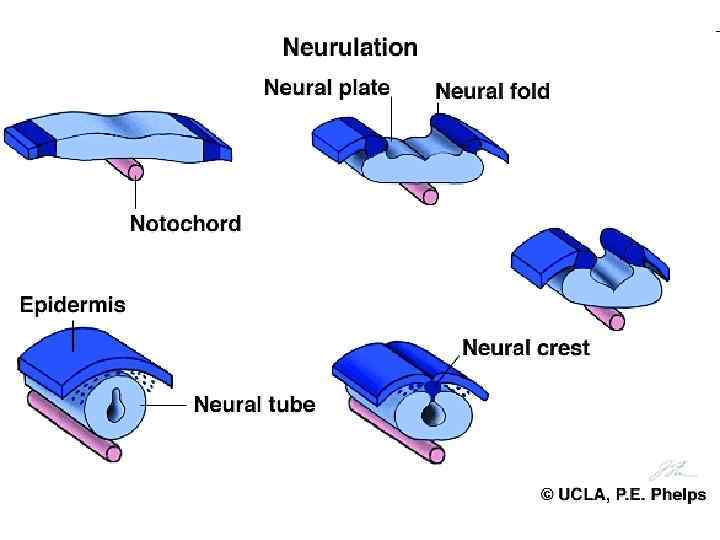
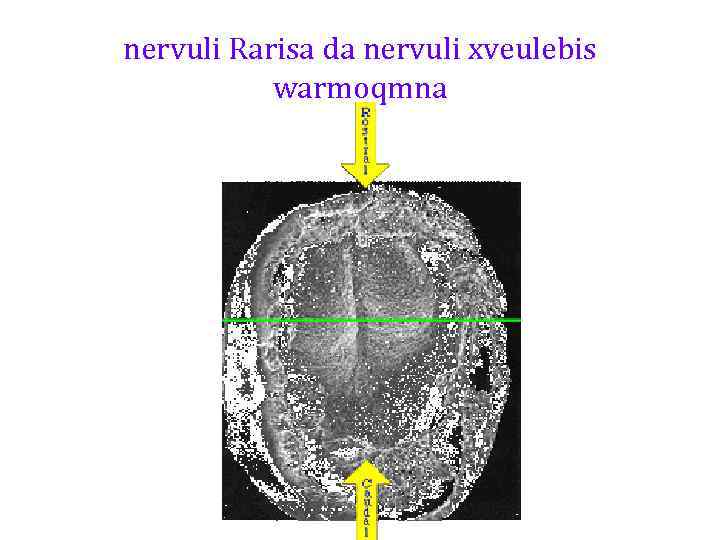 nervuli Rarisa da nervuli xveulebis warmoqmna
nervuli Rarisa da nervuli xveulebis warmoqmna
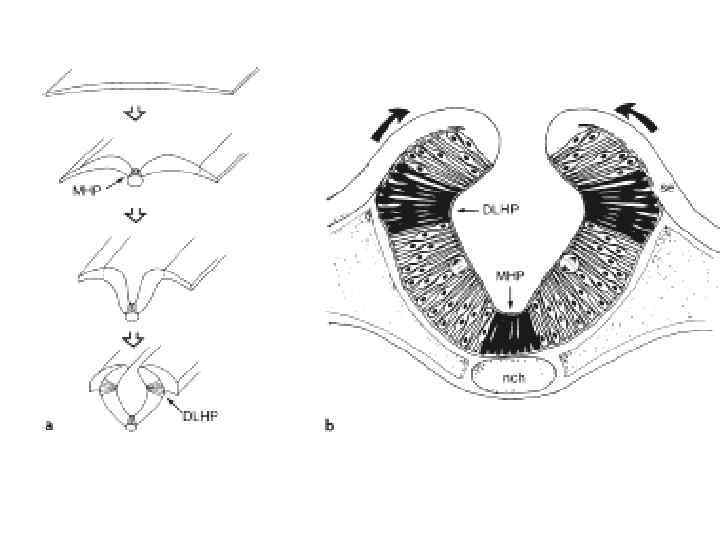
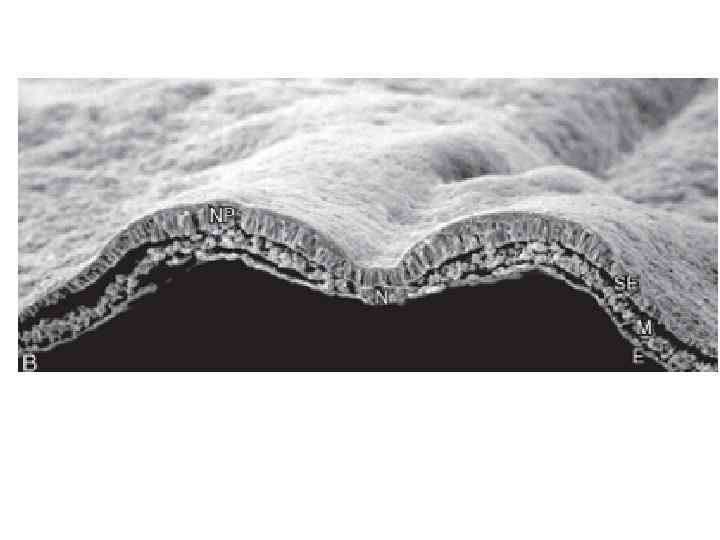
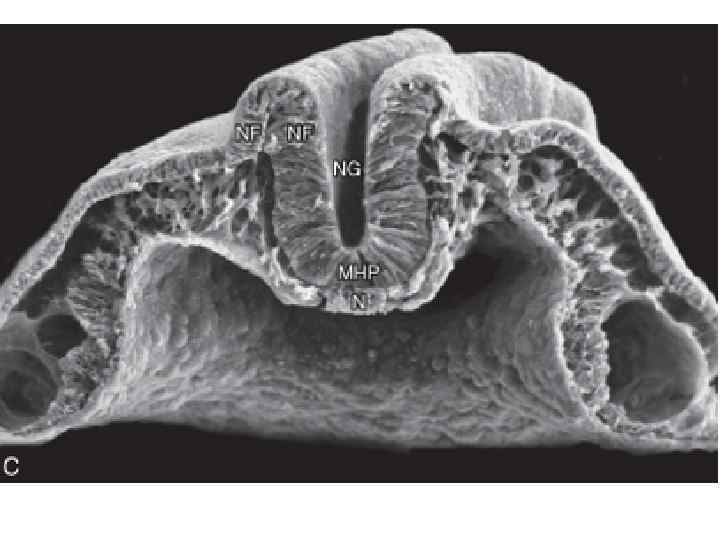
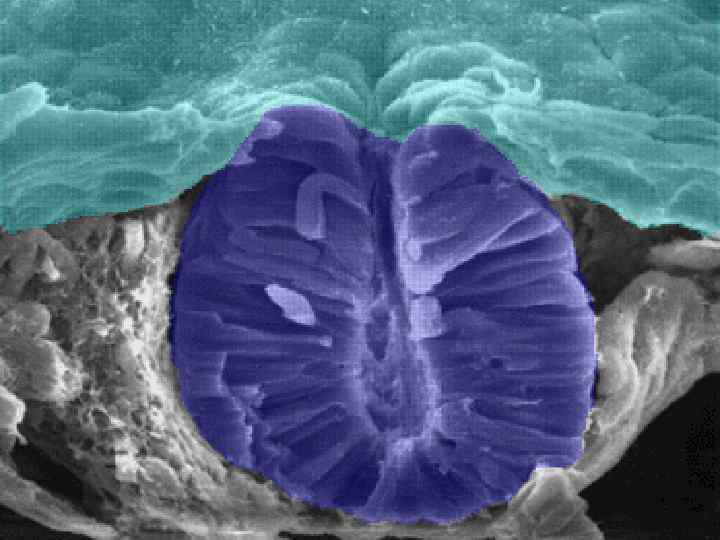
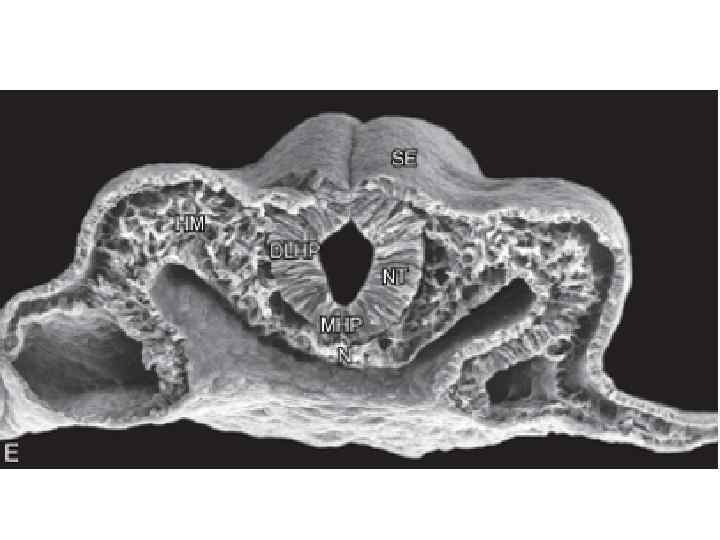
 Neurulation in the chick. A, Dorsal view showing that neurulation occurs in cranialto-caudal sequence such that at the level of the line in A, the neural plate is forming. More cranially (arrow), the neural plate is shaping, and still more cranially the neural plate is bending (asterisk) and a neural groove and paired neural folds have formed. B, Level of the forming neural plate (NP) at level of line in A. E, endoderm; SE, surface ectoderm; M, mesoderm; N, notochord. C, Transverse section through neural groove (future midbrain level) at a stage midway between A and D. MHP, median hinge point; N, notochord; NF, neural fold; NG, neural groove. D, Dorsal view during closure of the neural groove. In contrast to humans, the neural groove in chick first closes at the future midbrain level (rather than at the occipitocervical level) and then progresses cranially and caudally to close, respectively, the small cranial neuropore and elongated caudal neuropore/neural groove. Line indicates level of transverse section in E. E, Transverse section through the incipient neural tube (NT). DLHP, dorsolateral hinge point; HM, head mesoderm; N, notochord; MHP, median hinge point; SE, surface ectoderm.
Neurulation in the chick. A, Dorsal view showing that neurulation occurs in cranialto-caudal sequence such that at the level of the line in A, the neural plate is forming. More cranially (arrow), the neural plate is shaping, and still more cranially the neural plate is bending (asterisk) and a neural groove and paired neural folds have formed. B, Level of the forming neural plate (NP) at level of line in A. E, endoderm; SE, surface ectoderm; M, mesoderm; N, notochord. C, Transverse section through neural groove (future midbrain level) at a stage midway between A and D. MHP, median hinge point; N, notochord; NF, neural fold; NG, neural groove. D, Dorsal view during closure of the neural groove. In contrast to humans, the neural groove in chick first closes at the future midbrain level (rather than at the occipitocervical level) and then progresses cranially and caudally to close, respectively, the small cranial neuropore and elongated caudal neuropore/neural groove. Line indicates level of transverse section in E. E, Transverse section through the incipient neural tube (NT). DLHP, dorsolateral hinge point; HM, head mesoderm; N, notochord; MHP, median hinge point; SE, surface ectoderm.
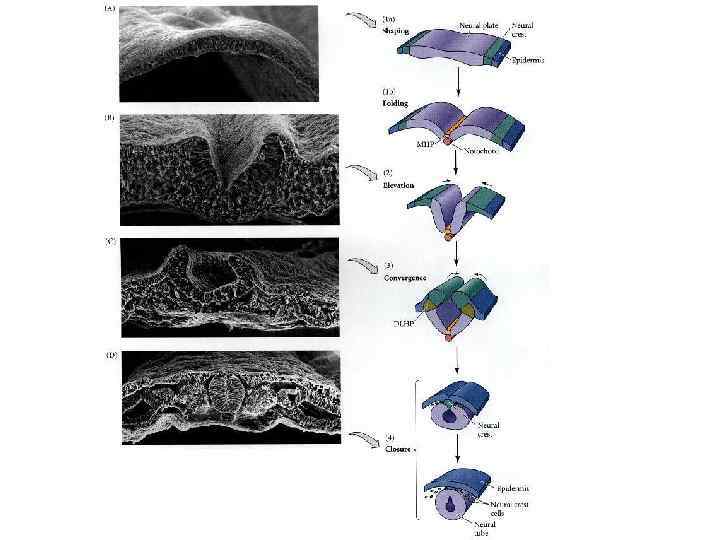
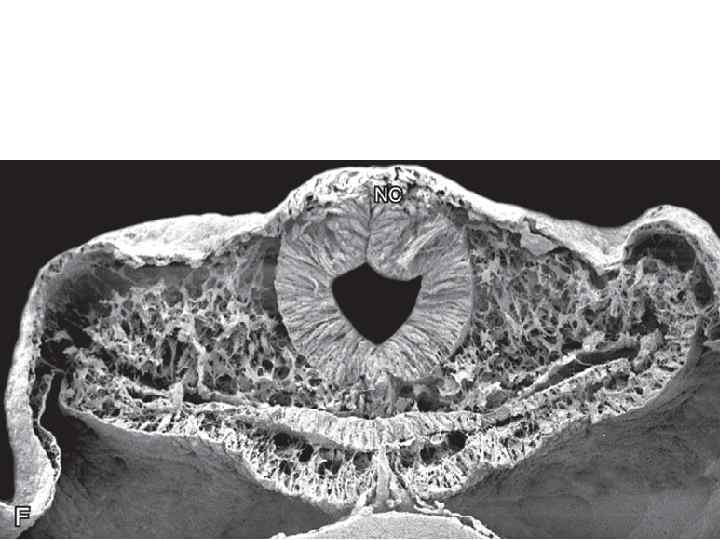
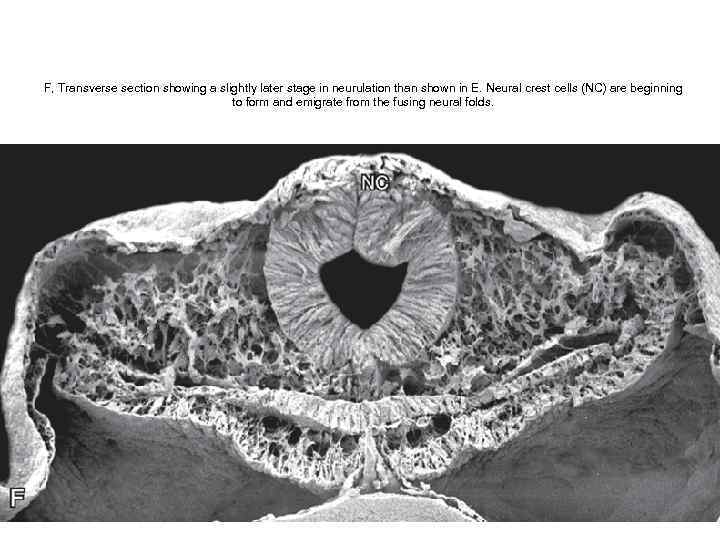 F, Transverse section showing a slightly later stage in neurulation than shown in E. Neural crest cells (NC) are beginning to form and emigrate from the fusing neural folds.
F, Transverse section showing a slightly later stage in neurulation than shown in E. Neural crest cells (NC) are beginning to form and emigrate from the fusing neural folds.
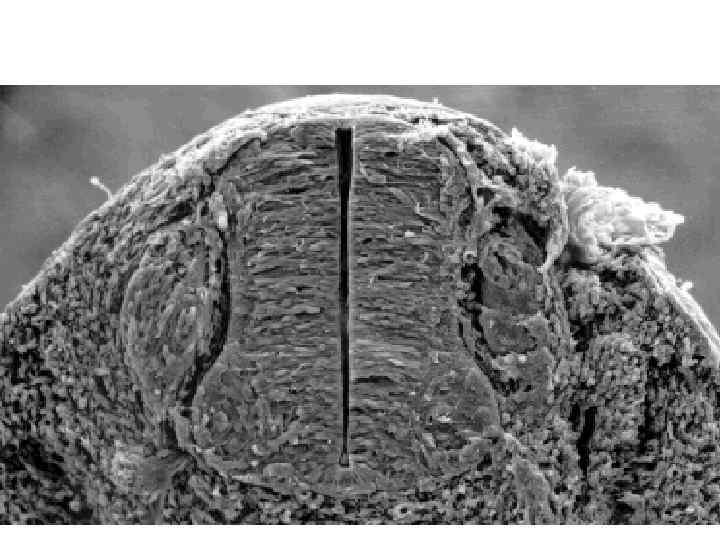
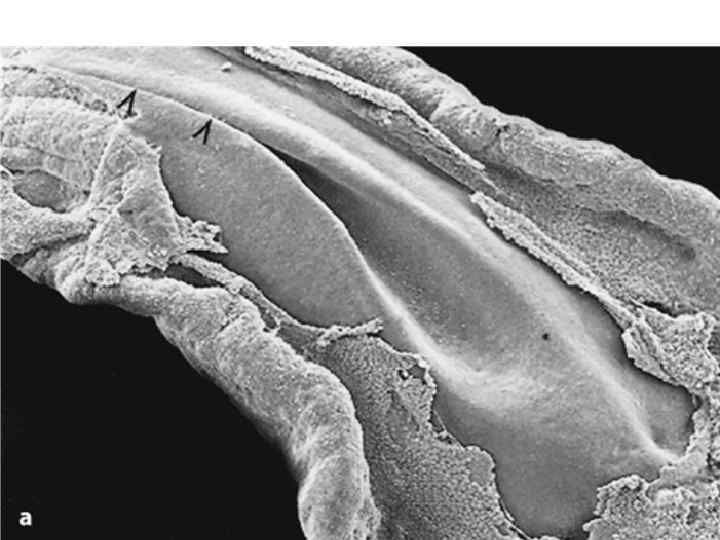
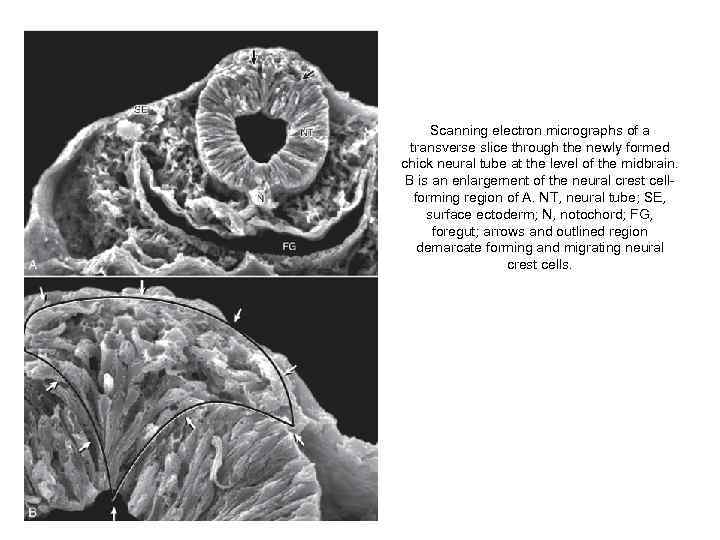 Scanning electron micrographs of a transverse slice through the newly formed chick neural tube at the level of the midbrain. B is an enlargement of the neural crest cellforming region of A. NT, neural tube; SE, surface ectoderm; N, notochord; FG, foregut; arrows and outlined region demarcate forming and migrating neural crest cells.
Scanning electron micrographs of a transverse slice through the newly formed chick neural tube at the level of the midbrain. B is an enlargement of the neural crest cellforming region of A. NT, neural tube; SE, surface ectoderm; N, notochord; FG, foregut; arrows and outlined region demarcate forming and migrating neural crest cells.

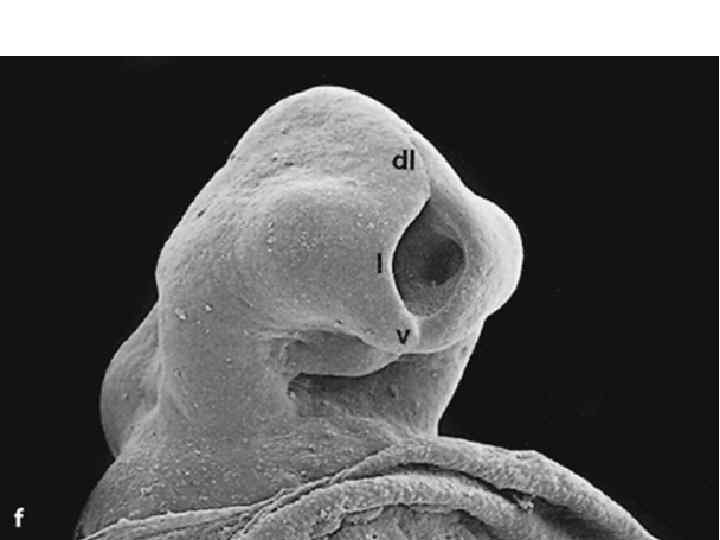
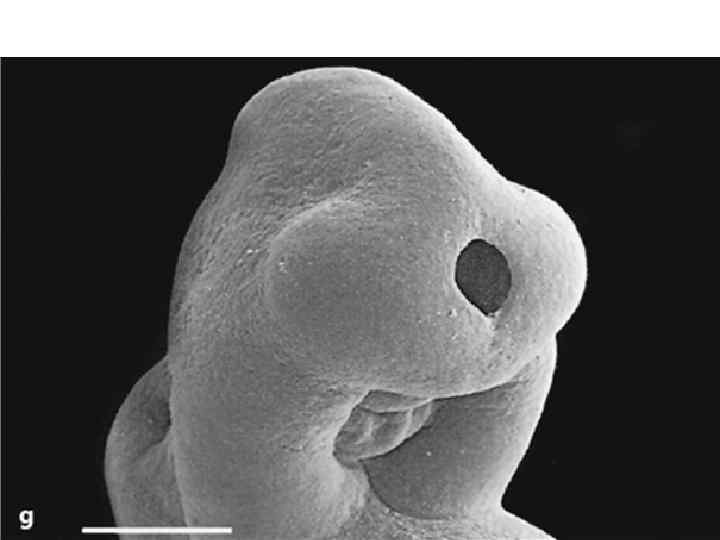
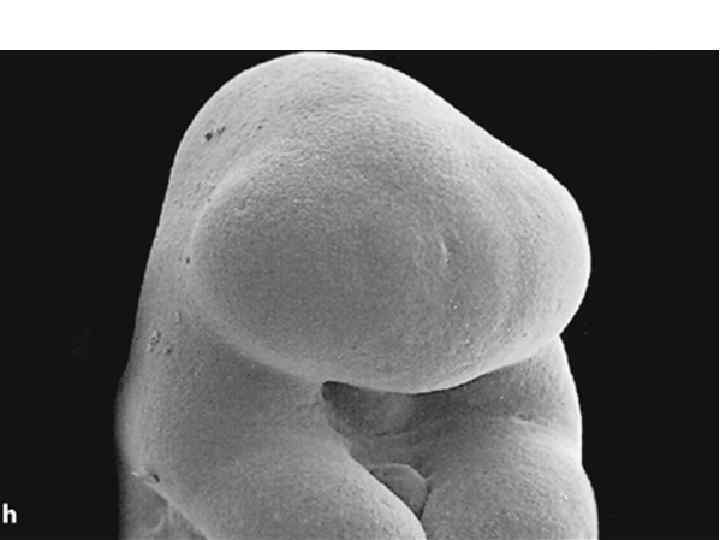
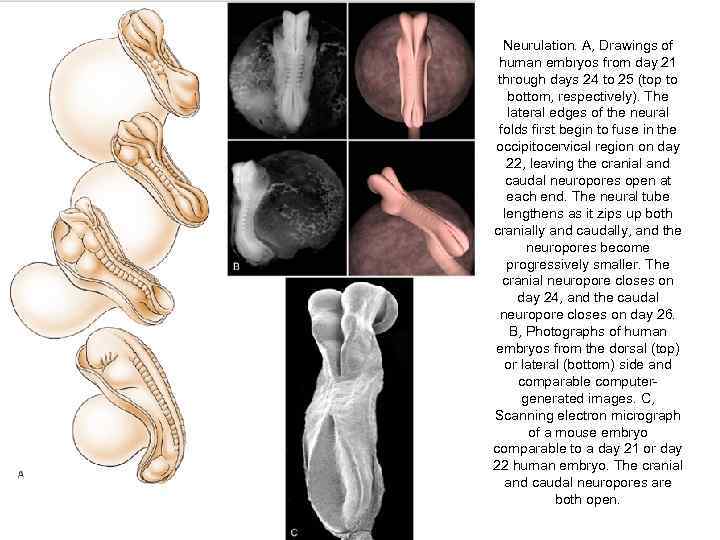 Neurulation. A, Drawings of human embryos from day 21 through days 24 to 25 (top to bottom, respectively). The lateral edges of the neural folds first begin to fuse in the occipitocervical region on day 22, leaving the cranial and caudal neuropores open at each end. The neural tube lengthens as it zips up both cranially and caudally, and the neuropores become progressively smaller. The cranial neuropore closes on day 24, and the caudal neuropore closes on day 26. B, Photographs of human embryos from the dorsal (top) or lateral (bottom) side and comparable computergenerated images. C, Scanning electron micrograph of a mouse embryo comparable to a day 21 or day 22 human embryo. The cranial and caudal neuropores are both open.
Neurulation. A, Drawings of human embryos from day 21 through days 24 to 25 (top to bottom, respectively). The lateral edges of the neural folds first begin to fuse in the occipitocervical region on day 22, leaving the cranial and caudal neuropores open at each end. The neural tube lengthens as it zips up both cranially and caudally, and the neuropores become progressively smaller. The cranial neuropore closes on day 24, and the caudal neuropore closes on day 26. B, Photographs of human embryos from the dorsal (top) or lateral (bottom) side and comparable computergenerated images. C, Scanning electron micrograph of a mouse embryo comparable to a day 21 or day 22 human embryo. The cranial and caudal neuropores are both open.
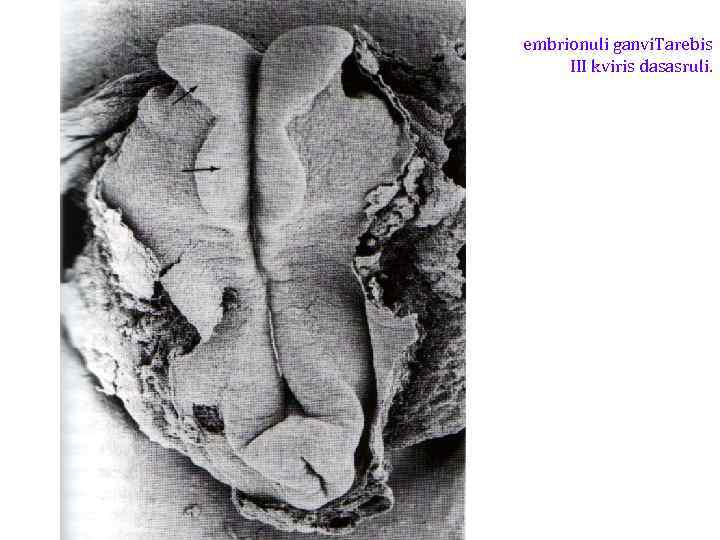 embrionuli ganvi. Tarebis III kviris dasasruli.
embrionuli ganvi. Tarebis III kviris dasasruli.
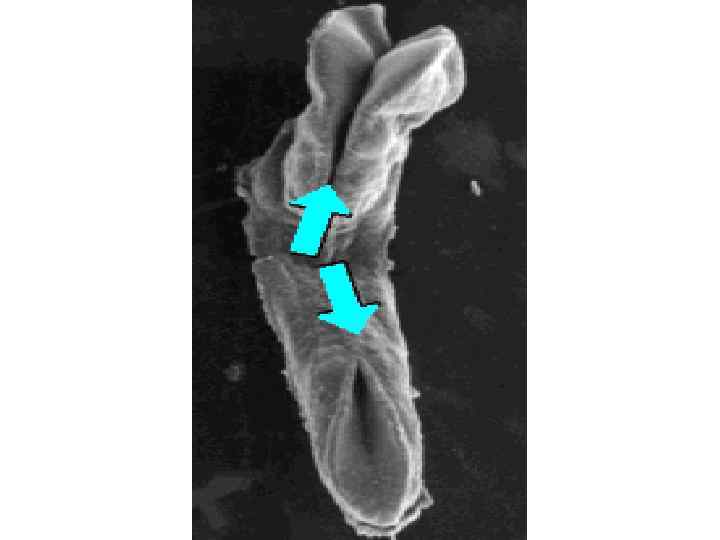
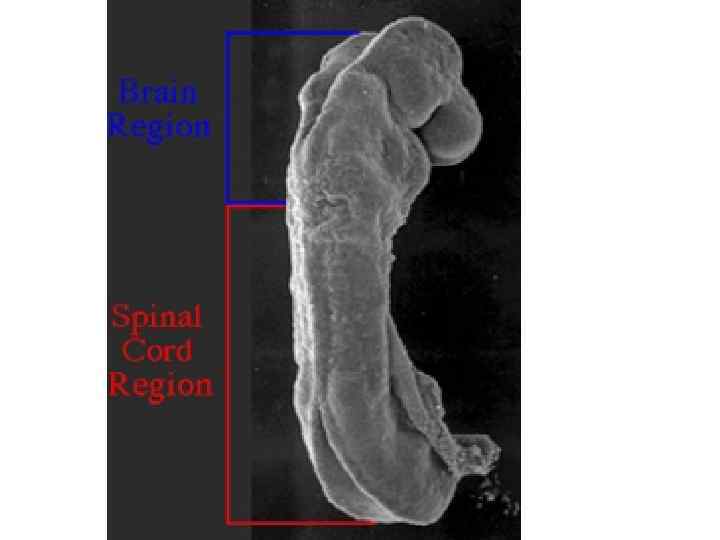

 2, 5 mm
2, 5 mm
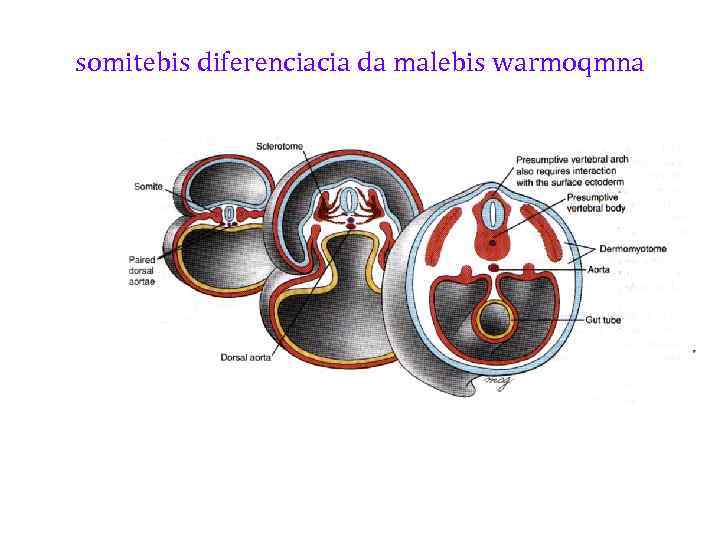 somitebis diferenciacia da malebis warmoqmna
somitebis diferenciacia da malebis warmoqmna
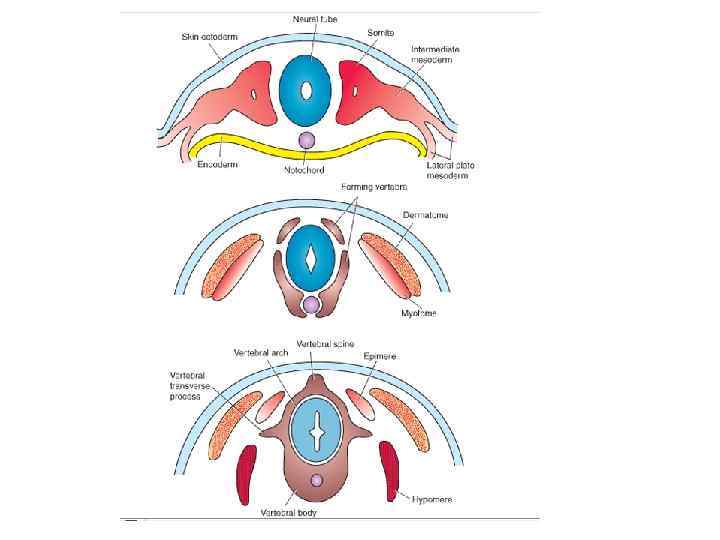
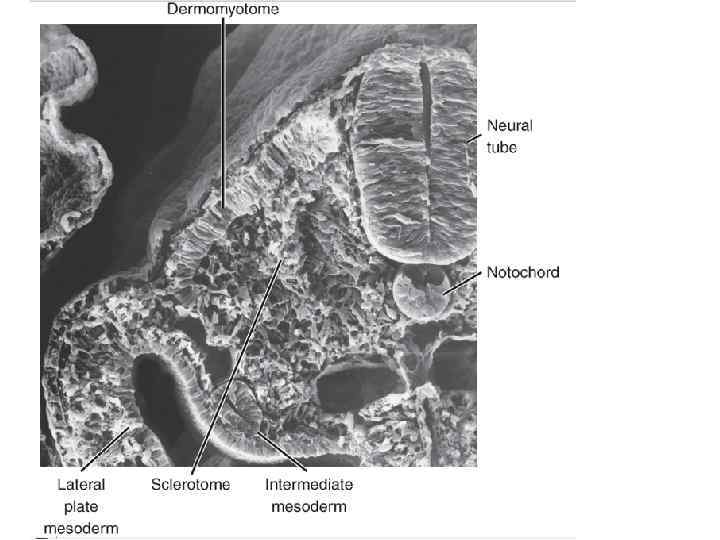
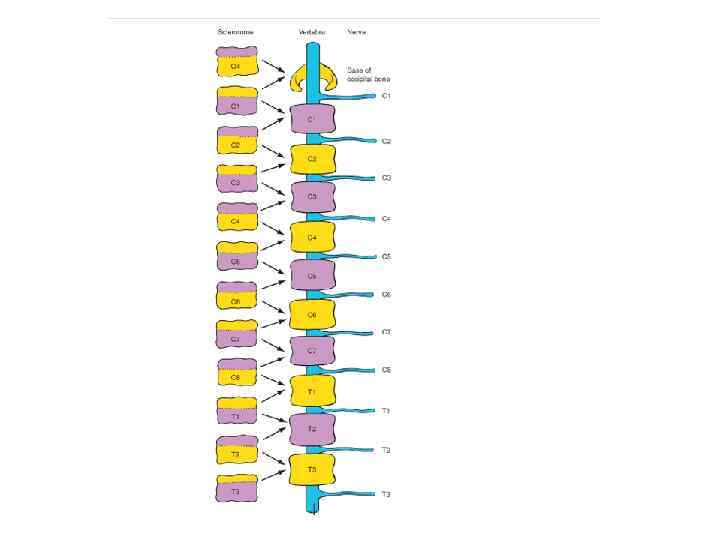
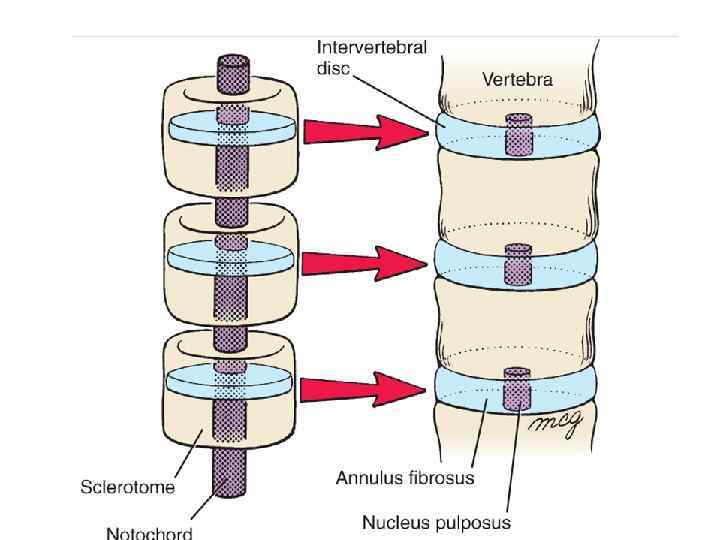
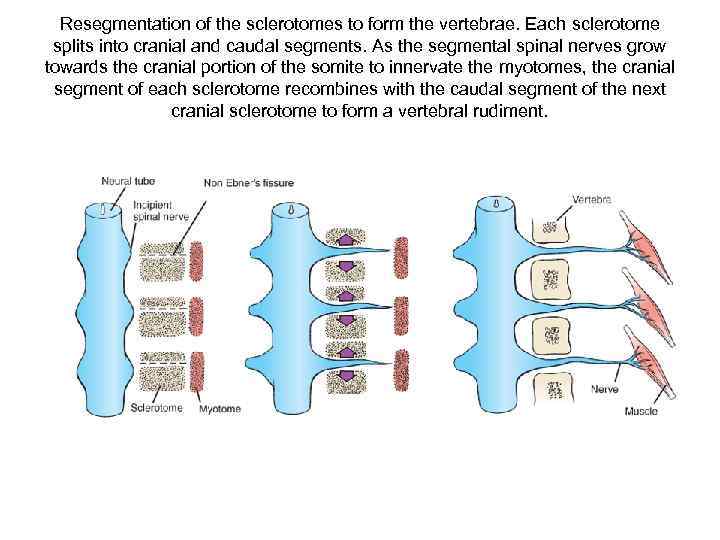 Resegmentation of the sclerotomes to form the vertebrae. Each sclerotome splits into cranial and caudal segments. As the segmental spinal nerves grow towards the cranial portion of the somite to innervate the myotomes, the cranial segment of each sclerotome recombines with the caudal segment of the next cranial sclerotome to form a vertebral rudiment.
Resegmentation of the sclerotomes to form the vertebrae. Each sclerotome splits into cranial and caudal segments. As the segmental spinal nerves grow towards the cranial portion of the somite to innervate the myotomes, the cranial segment of each sclerotome recombines with the caudal segment of the next cranial sclerotome to form a vertebral rudiment.
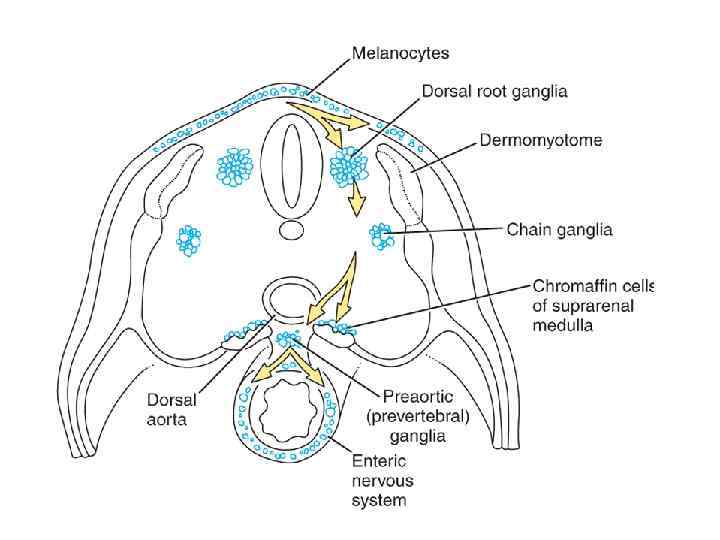
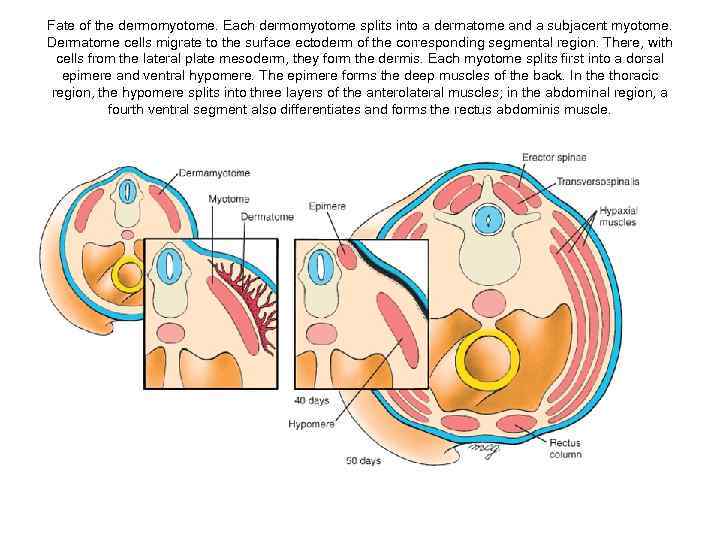 Fate of the dermomyotome. Each dermomyotome splits into a dermatome and a subjacent myotome. Dermatome cells migrate to the surface ectoderm of the corresponding segmental region. There, with cells from the lateral plate mesoderm, they form the dermis. Each myotome splits first into a dorsal epimere and ventral hypomere. The epimere forms the deep muscles of the back. In the thoracic region, the hypomere splits into three layers of the anterolateral muscles; in the abdominal region, a fourth ventral segment also differentiates and forms the rectus abdominis muscle.
Fate of the dermomyotome. Each dermomyotome splits into a dermatome and a subjacent myotome. Dermatome cells migrate to the surface ectoderm of the corresponding segmental region. There, with cells from the lateral plate mesoderm, they form the dermis. Each myotome splits first into a dorsal epimere and ventral hypomere. The epimere forms the deep muscles of the back. In the thoracic region, the hypomere splits into three layers of the anterolateral muscles; in the abdominal region, a fourth ventral segment also differentiates and forms the rectus abdominis muscle.
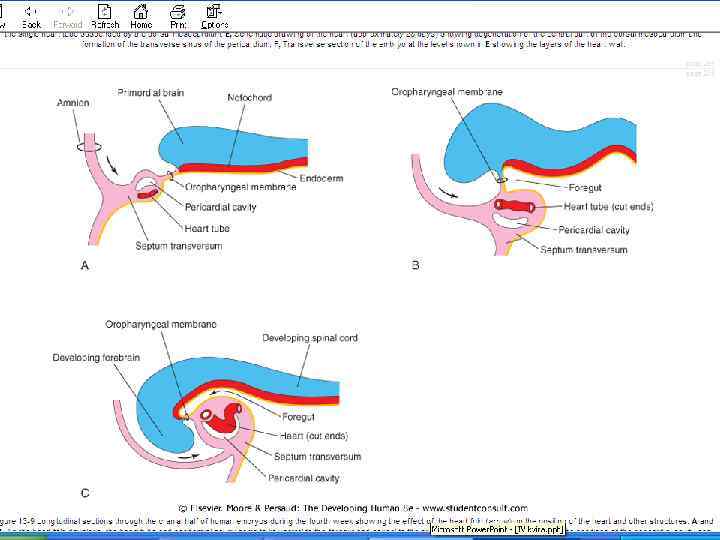
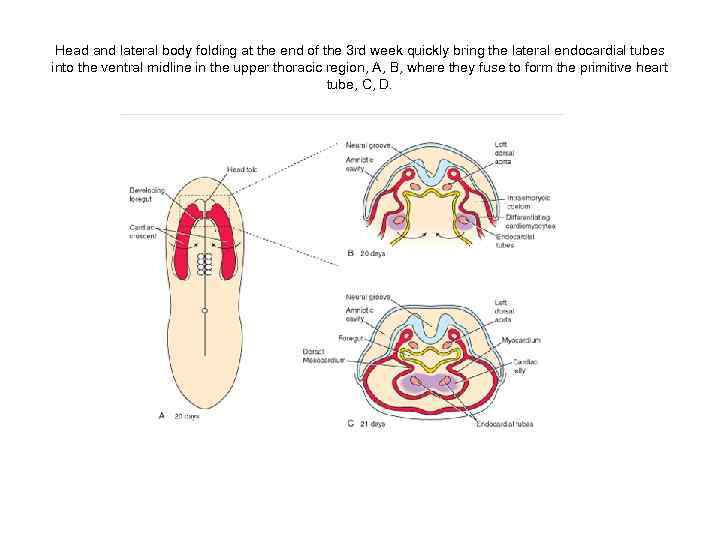 Head and lateral body folding at the end of the 3 rd week quickly bring the lateral endocardial tubes into the ventral midline in the upper thoracic region, A, B, where they fuse to form the primitive heart tube, C, D.
Head and lateral body folding at the end of the 3 rd week quickly bring the lateral endocardial tubes into the ventral midline in the upper thoracic region, A, B, where they fuse to form the primitive heart tube, C, D.
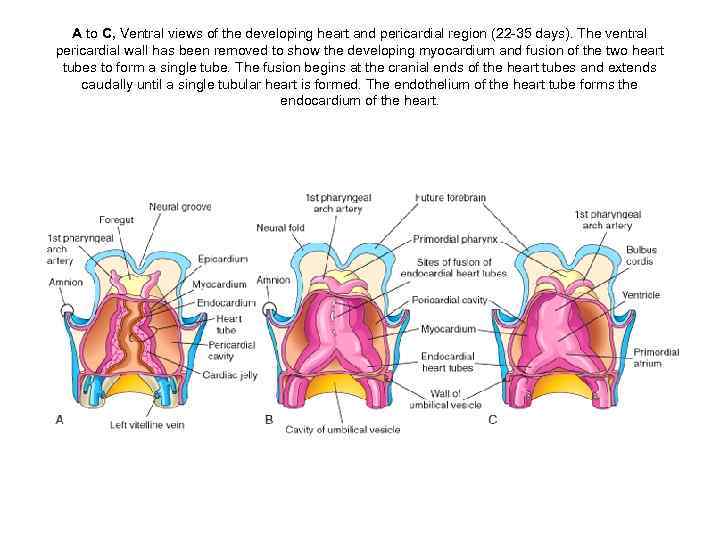 A to C, Ventral views of the developing heart and pericardial region (22 -35 days). The ventral pericardial wall has been removed to show the developing myocardium and fusion of the two heart tubes to form a single tube. The fusion begins at the cranial ends of the heart tubes and extends caudally until a single tubular heart is formed. The endothelium of the heart tube forms the endocardium of the heart.
A to C, Ventral views of the developing heart and pericardial region (22 -35 days). The ventral pericardial wall has been removed to show the developing myocardium and fusion of the two heart tubes to form a single tube. The fusion begins at the cranial ends of the heart tubes and extends caudally until a single tubular heart is formed. The endothelium of the heart tube forms the endocardium of the heart.
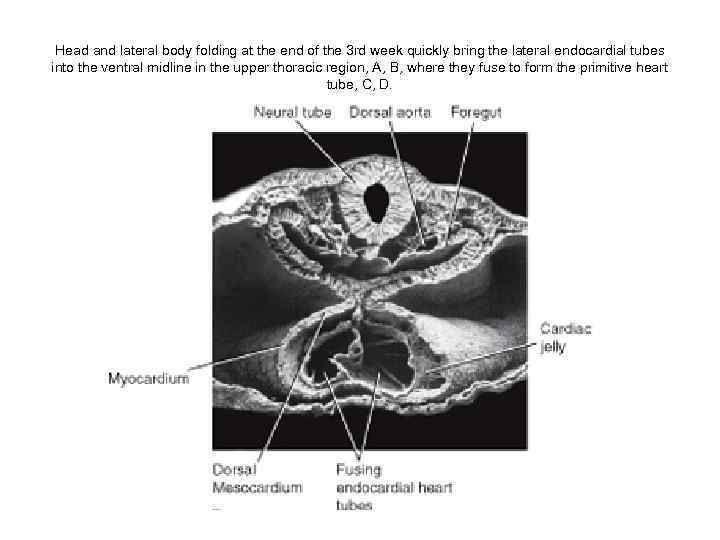 Head and lateral body folding at the end of the 3 rd week quickly bring the lateral endocardial tubes into the ventral midline in the upper thoracic region, A, B, where they fuse to form the primitive heart tube, C, D.
Head and lateral body folding at the end of the 3 rd week quickly bring the lateral endocardial tubes into the ventral midline in the upper thoracic region, A, B, where they fuse to form the primitive heart tube, C, D.
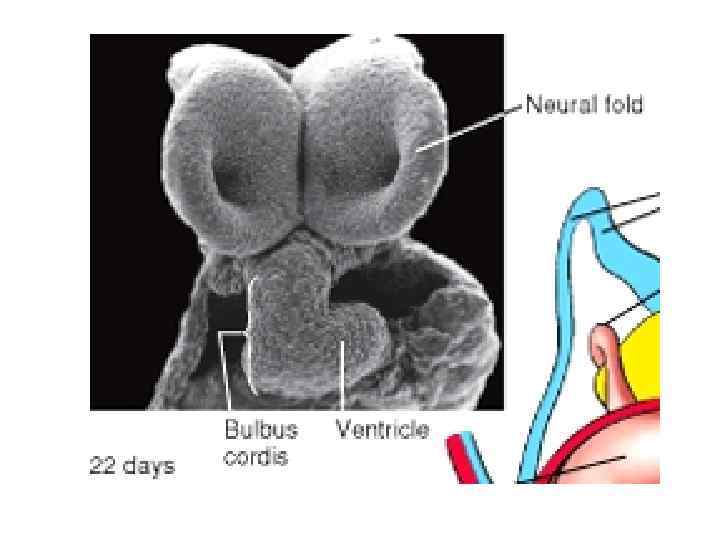
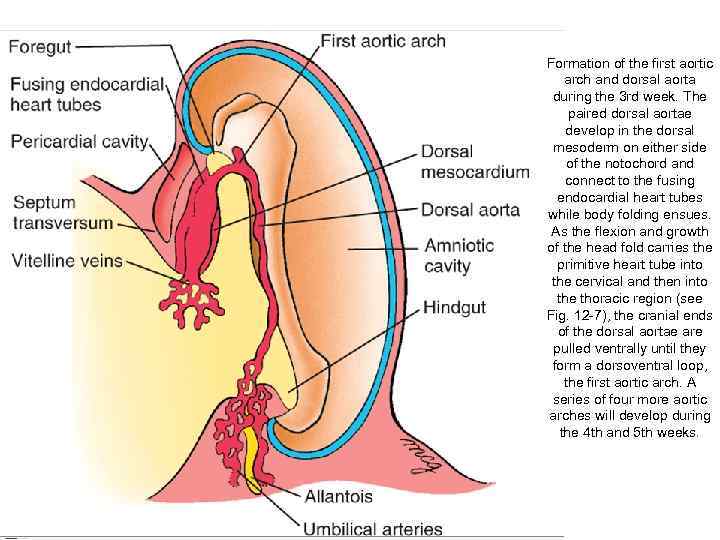 Formation of the first aortic arch and dorsal aorta during the 3 rd week. The paired dorsal aortae develop in the dorsal mesoderm on either side of the notochord and connect to the fusing endocardial heart tubes while body folding ensues. As the flexion and growth of the head fold carries the primitive heart tube into the cervical and then into the thoracic region (see Fig. 12 -7), the cranial ends of the dorsal aortae are pulled ventrally until they form a dorsoventral loop, the first aortic arch. A series of four more aortic arches will develop during the 4 th and 5 th weeks.
Formation of the first aortic arch and dorsal aorta during the 3 rd week. The paired dorsal aortae develop in the dorsal mesoderm on either side of the notochord and connect to the fusing endocardial heart tubes while body folding ensues. As the flexion and growth of the head fold carries the primitive heart tube into the cervical and then into the thoracic region (see Fig. 12 -7), the cranial ends of the dorsal aortae are pulled ventrally until they form a dorsoventral loop, the first aortic arch. A series of four more aortic arches will develop during the 4 th and 5 th weeks.
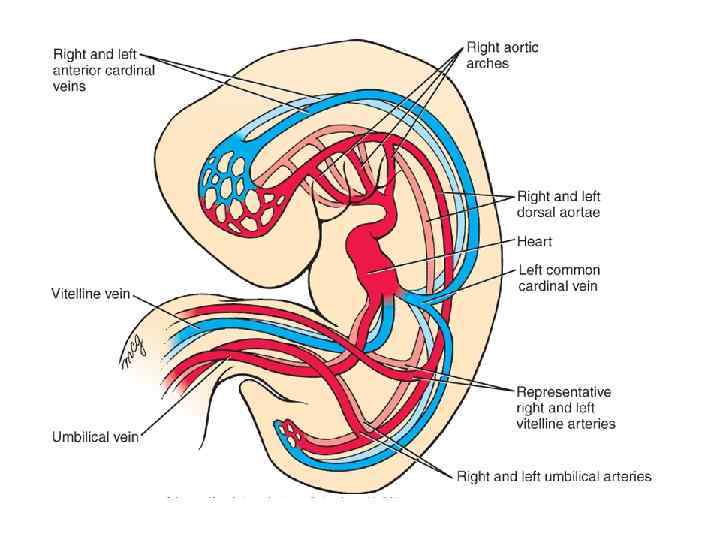
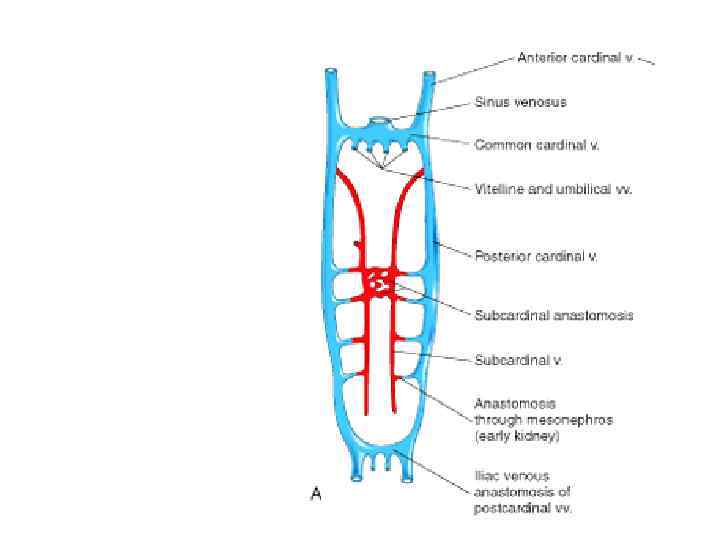
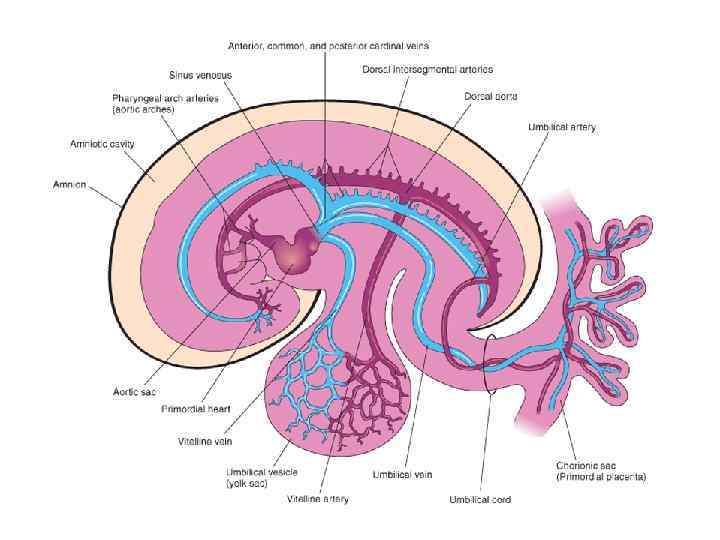
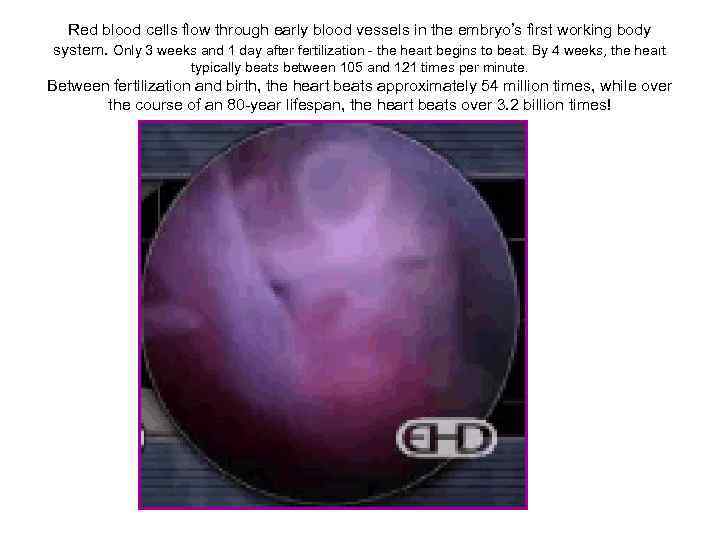 Red blood cells flow through early blood vessels in the embryo’s first working body system. Only 3 weeks and 1 day after fertilization - the heart begins to beat. By 4 weeks, the heart typically beats between 105 and 121 times per minute. Between fertilization and birth, the heart beats approximately 54 million times, while over the course of an 80 -year lifespan, the heart beats over 3. 2 billion times!
Red blood cells flow through early blood vessels in the embryo’s first working body system. Only 3 weeks and 1 day after fertilization - the heart begins to beat. By 4 weeks, the heart typically beats between 105 and 121 times per minute. Between fertilization and birth, the heart beats approximately 54 million times, while over the course of an 80 -year lifespan, the heart beats over 3. 2 billion times!
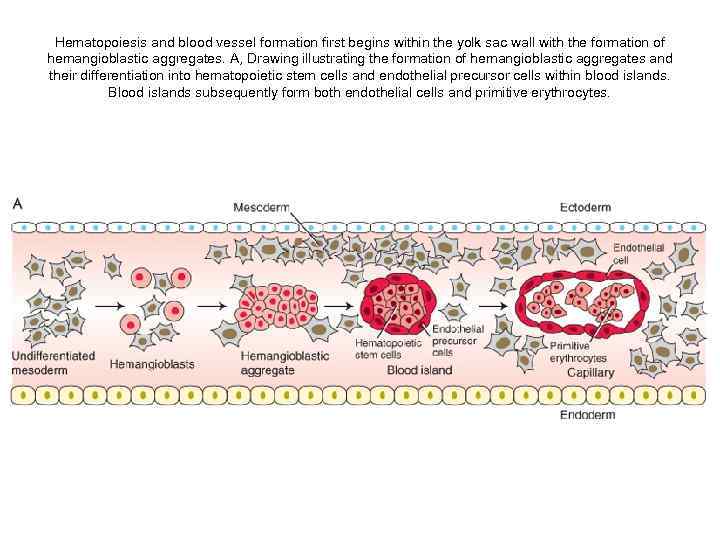 Hematopoiesis and blood vessel formation first begins within the yolk sac wall with the formation of hemangioblastic aggregates. A, Drawing illustrating the formation of hemangioblastic aggregates and their differentiation into hematopoietic stem cells and endothelial precursor cells within blood islands. Blood islands subsequently form both endothelial cells and primitive erythrocytes.
Hematopoiesis and blood vessel formation first begins within the yolk sac wall with the formation of hemangioblastic aggregates. A, Drawing illustrating the formation of hemangioblastic aggregates and their differentiation into hematopoietic stem cells and endothelial precursor cells within blood islands. Blood islands subsequently form both endothelial cells and primitive erythrocytes.
 • The yolk sac is the first supplier of blood cells to the embryonic circulation. The cells supplied are predominantly nucleated erythrocytes containing embryonic hemoglobin (primitive erythrocytes). By day 60, the yolk sac no longer serves as a hematopoietic organ. Rather, this task is relayed to intraembryonic organs, including the liver, spleen, thymus, and bone marrow. With the onset of a functional circulatory system, primitive HSCs (i. e. , those with limited pluripotential) from the extraembryonic mesoderm colonize these organs. The first organ to be colonized is the liver. This organ remains the main hematopoietic organ of the embryo and fetus until initiation of bone marrow hematopoiesis near parturition (birth). Colonization of the liver primordia by HSCs likely occurs in two waves, the first beginning at about day 23 and the other at about day 30, but these HSCs arise from two different sources (Fig. 13 -2; discussed in the following "In the Research Lab"). The shift from generating primitive nucleated erythroblasts to enucleated erythrocytes synthesizing fetal hemoglobin (definitive erythrocytes) occurs by 5 weeks of gestation. The liver is where long-term definitive HSCs arise that have the potential to generate all the hematopoietic cell lineages of the adult, with both primitive and definitive HSC production overlapping for a time. Evidence suggests that definite HSCs come to colonize the bone marrow and contribute blood cells as early as 10. 5 weeks, but the bulk of the hematopoietic burden is still carried by the liver until birth. Thus, the extraembryonic, primitive HSCs serve mainly to provide an early, but necessary, blood supply for the developing embryo until the definitive intraembryonic hematopoietic organs can assume this task.
• The yolk sac is the first supplier of blood cells to the embryonic circulation. The cells supplied are predominantly nucleated erythrocytes containing embryonic hemoglobin (primitive erythrocytes). By day 60, the yolk sac no longer serves as a hematopoietic organ. Rather, this task is relayed to intraembryonic organs, including the liver, spleen, thymus, and bone marrow. With the onset of a functional circulatory system, primitive HSCs (i. e. , those with limited pluripotential) from the extraembryonic mesoderm colonize these organs. The first organ to be colonized is the liver. This organ remains the main hematopoietic organ of the embryo and fetus until initiation of bone marrow hematopoiesis near parturition (birth). Colonization of the liver primordia by HSCs likely occurs in two waves, the first beginning at about day 23 and the other at about day 30, but these HSCs arise from two different sources (Fig. 13 -2; discussed in the following "In the Research Lab"). The shift from generating primitive nucleated erythroblasts to enucleated erythrocytes synthesizing fetal hemoglobin (definitive erythrocytes) occurs by 5 weeks of gestation. The liver is where long-term definitive HSCs arise that have the potential to generate all the hematopoietic cell lineages of the adult, with both primitive and definitive HSC production overlapping for a time. Evidence suggests that definite HSCs come to colonize the bone marrow and contribute blood cells as early as 10. 5 weeks, but the bulk of the hematopoietic burden is still carried by the liver until birth. Thus, the extraembryonic, primitive HSCs serve mainly to provide an early, but necessary, blood supply for the developing embryo until the definitive intraembryonic hematopoietic organs can assume this task.

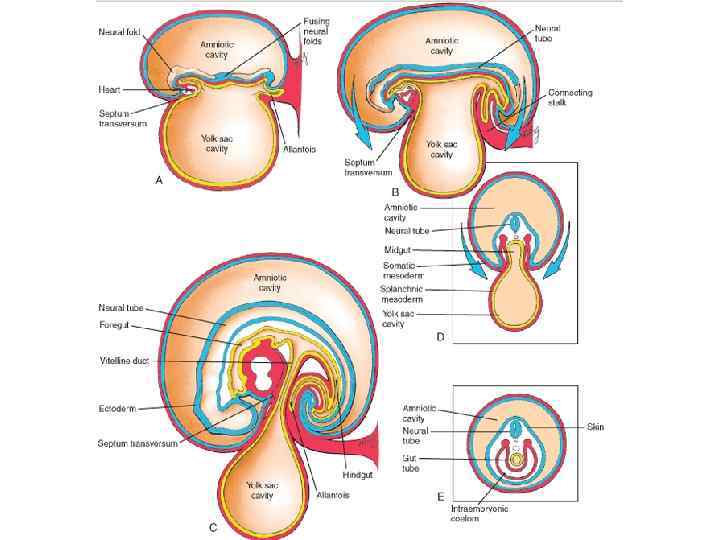
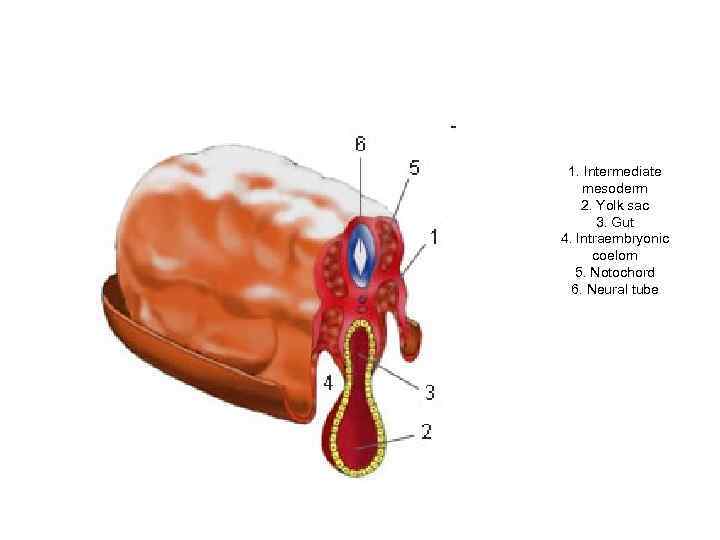 1. Intermediate mesoderm 2. Yolk sac 3. Gut 4. Intraembryonic coelom 5. Notochord 6. Neural tube
1. Intermediate mesoderm 2. Yolk sac 3. Gut 4. Intraembryonic coelom 5. Notochord 6. Neural tube
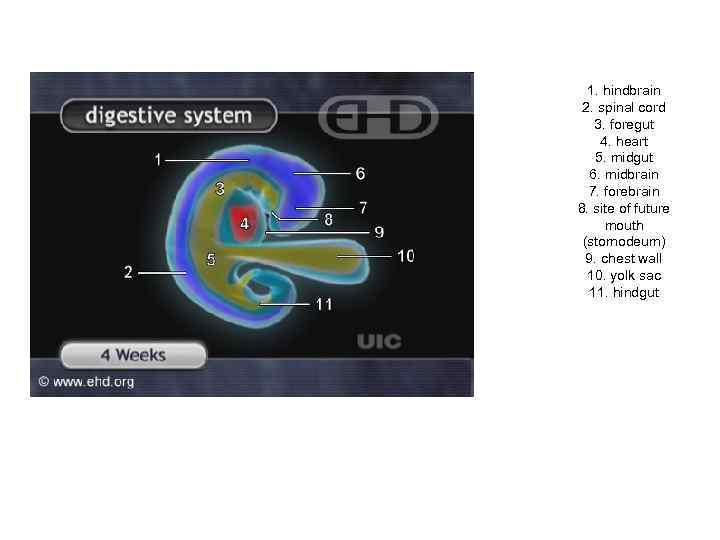 1. hindbrain 2. spinal cord 3. foregut 4. heart 5. midgut 6. midbrain 7. forebrain 8. site of future mouth (stomodeum) 9. chest wall 10. yolk sac 11. hindgut
1. hindbrain 2. spinal cord 3. foregut 4. heart 5. midgut 6. midbrain 7. forebrain 8. site of future mouth (stomodeum) 9. chest wall 10. yolk sac 11. hindgut
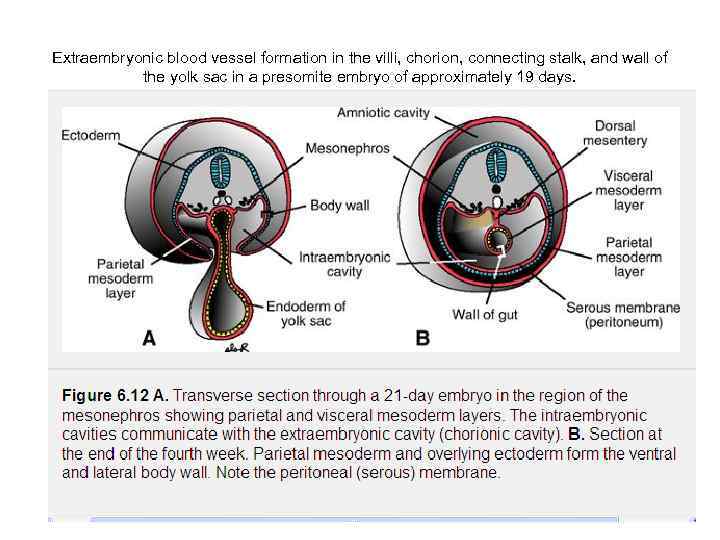 Extraembryonic blood vessel formation in the villi, chorion, connecting stalk, and wall of the yolk sac in a presomite embryo of approximately 19 days.
Extraembryonic blood vessel formation in the villi, chorion, connecting stalk, and wall of the yolk sac in a presomite embryo of approximately 19 days.

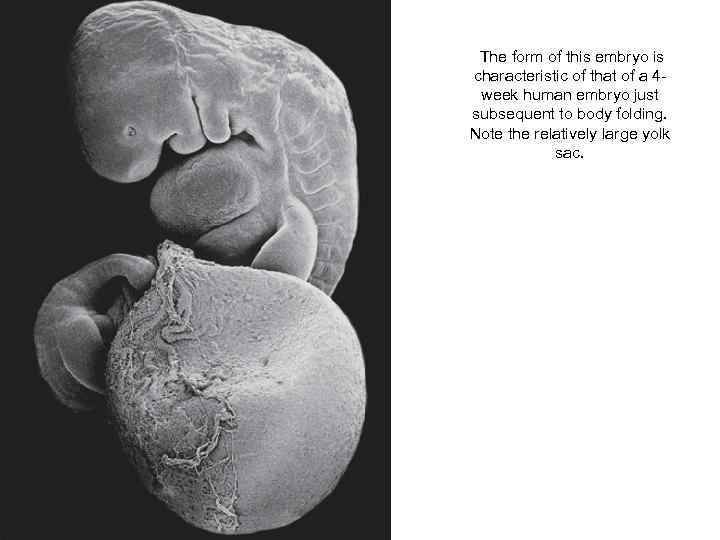 The form of this embryo is characteristic of that of a 4 week human embryo just subsequent to body folding. Note the relatively large yolk sac.
The form of this embryo is characteristic of that of a 4 week human embryo just subsequent to body folding. Note the relatively large yolk sac.
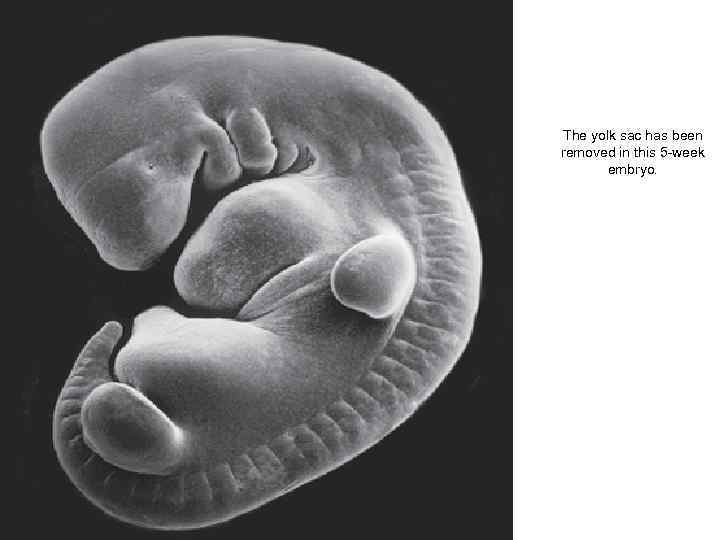 The yolk sac has been removed in this 5 -week embryo.
The yolk sac has been removed in this 5 -week embryo.
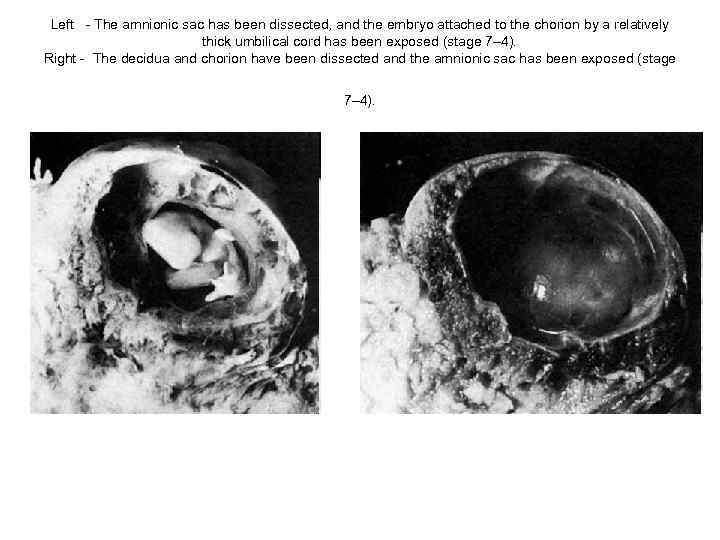 Left - The amnionic sac has been dissected, and the embryo attached to the chorion by a relatively thick umbilical cord has been exposed (stage 7– 4). Right - The decidua and chorion have been dissected and the amnionic sac has been exposed (stage 7– 4).
Left - The amnionic sac has been dissected, and the embryo attached to the chorion by a relatively thick umbilical cord has been exposed (stage 7– 4). Right - The decidua and chorion have been dissected and the amnionic sac has been exposed (stage 7– 4).

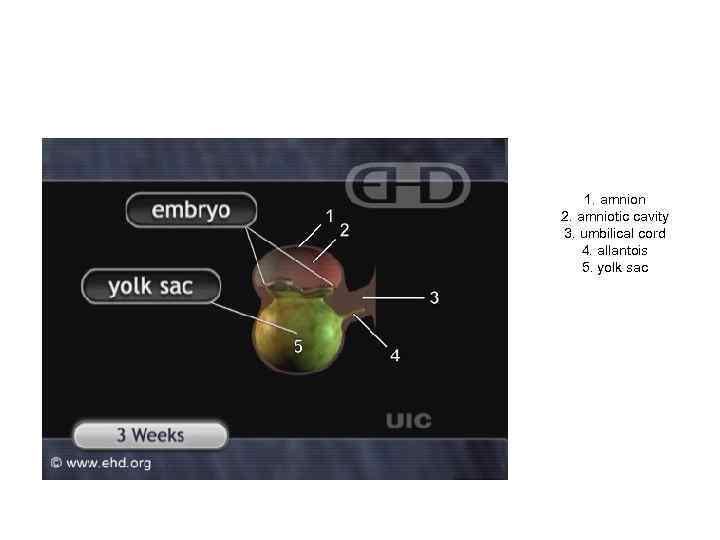 1. amnion 2. amniotic cavity 3. umbilical cord 4. allantois 5. yolk sac
1. amnion 2. amniotic cavity 3. umbilical cord 4. allantois 5. yolk sac


