4e688184ee96eefcf08de660abda7f17.ppt
- Количество слайдов: 43
 Translational Research for Myocardial Regeneration Timothy D. Henry, MD Director of Research Minneapolis Heart Institute Foundation
Translational Research for Myocardial Regeneration Timothy D. Henry, MD Director of Research Minneapolis Heart Institute Foundation
 Timothy D. Henry, MD Grant Support: Aastrom Biosciences, Inc. , Baxter International, Inc. , Mesoblast and Capricor. Off-Label: All cell therapy is currently research and therefore off-label
Timothy D. Henry, MD Grant Support: Aastrom Biosciences, Inc. , Baxter International, Inc. , Mesoblast and Capricor. Off-Label: All cell therapy is currently research and therefore off-label
 Cardiovascular Disease Targets ¨ Refractory angina ¨ Acute myocardial infarction ¨ Congestive heart failure ¨ Ongoing ischemia ¨ Previous MI ¨ Nonischemic ¨ Peripheral arterial disease ¨ CLI ¨ Claudication
Cardiovascular Disease Targets ¨ Refractory angina ¨ Acute myocardial infarction ¨ Congestive heart failure ¨ Ongoing ischemia ¨ Previous MI ¨ Nonischemic ¨ Peripheral arterial disease ¨ CLI ¨ Claudication
 Refractory Angina/Ischemia
Refractory Angina/Ischemia
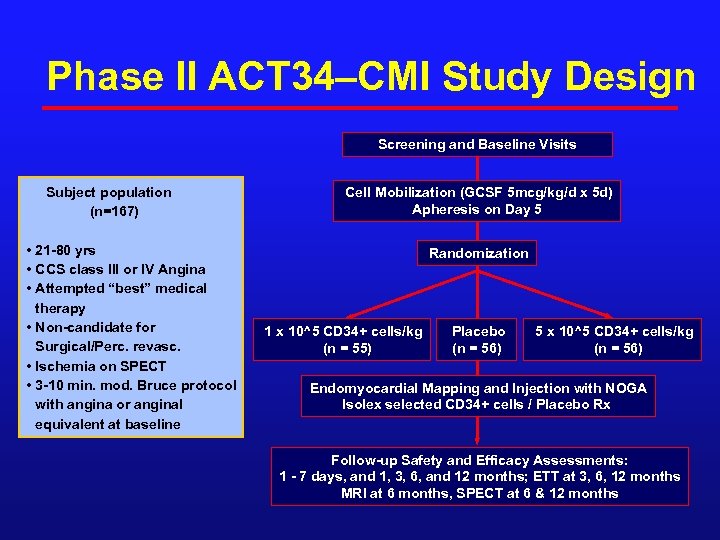 Phase II ACT 34–CMI Study Design Screening and Baseline Visits Subject population (n=167) • 21 -80 yrs • CCS class III or IV Angina • Attempted “best” medical therapy • Non-candidate for Surgical/Perc. revasc. • Ischemia on SPECT • 3 -10 min. mod. Bruce protocol with angina or anginal equivalent at baseline Cell Mobilization (GCSF 5 mcg/kg/d x 5 d) Apheresis on Day 5 Randomization 1 x 10^5 CD 34+ cells/kg (n = 55) Placebo (n = 56) 5 x 10^5 CD 34+ cells/kg (n = 56) Endomyocardial Mapping and Injection with NOGA Isolex selected CD 34+ cells / Placebo Rx Follow-up Safety and Efficacy Assessments: 1 - 7 days, and 1, 3, 6, and 12 months; ETT at 3, 6, 12 months MRI at 6 months, SPECT at 6 & 12 months
Phase II ACT 34–CMI Study Design Screening and Baseline Visits Subject population (n=167) • 21 -80 yrs • CCS class III or IV Angina • Attempted “best” medical therapy • Non-candidate for Surgical/Perc. revasc. • Ischemia on SPECT • 3 -10 min. mod. Bruce protocol with angina or anginal equivalent at baseline Cell Mobilization (GCSF 5 mcg/kg/d x 5 d) Apheresis on Day 5 Randomization 1 x 10^5 CD 34+ cells/kg (n = 55) Placebo (n = 56) 5 x 10^5 CD 34+ cells/kg (n = 56) Endomyocardial Mapping and Injection with NOGA Isolex selected CD 34+ cells / Placebo Rx Follow-up Safety and Efficacy Assessments: 1 - 7 days, and 1, 3, 6, and 12 months; ETT at 3, 6, 12 months MRI at 6 months, SPECT at 6 & 12 months
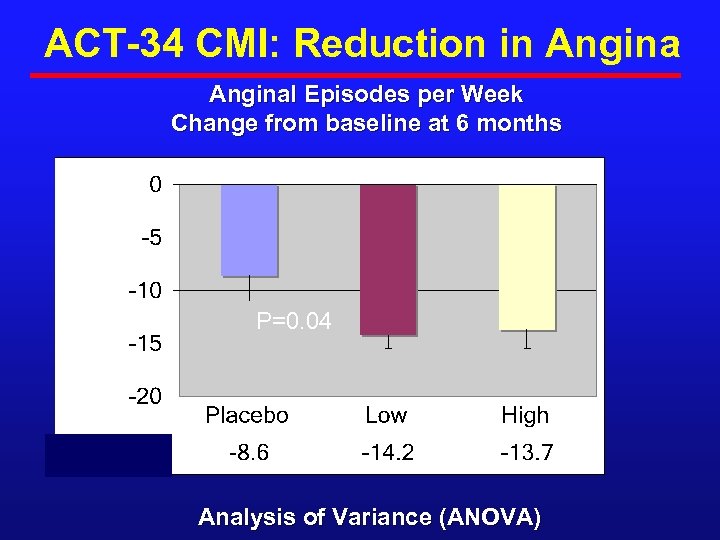 ACT-34 CMI: Reduction in Anginal Episodes per Week Change from baseline at 6 months P=0. 04 Analysis of Variance (ANOVA)
ACT-34 CMI: Reduction in Anginal Episodes per Week Change from baseline at 6 months P=0. 04 Analysis of Variance (ANOVA)
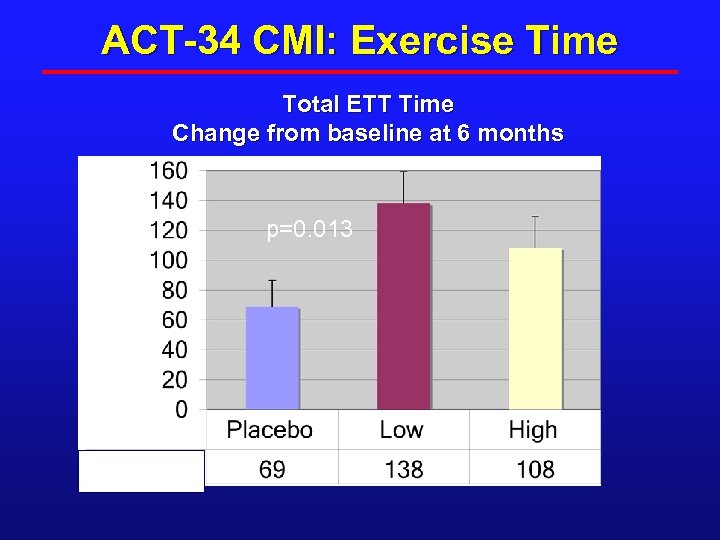 ACT-34 CMI: Exercise Time Total ETT Time Change from baseline at 6 months Seconds p=0. 013
ACT-34 CMI: Exercise Time Total ETT Time Change from baseline at 6 months Seconds p=0. 013
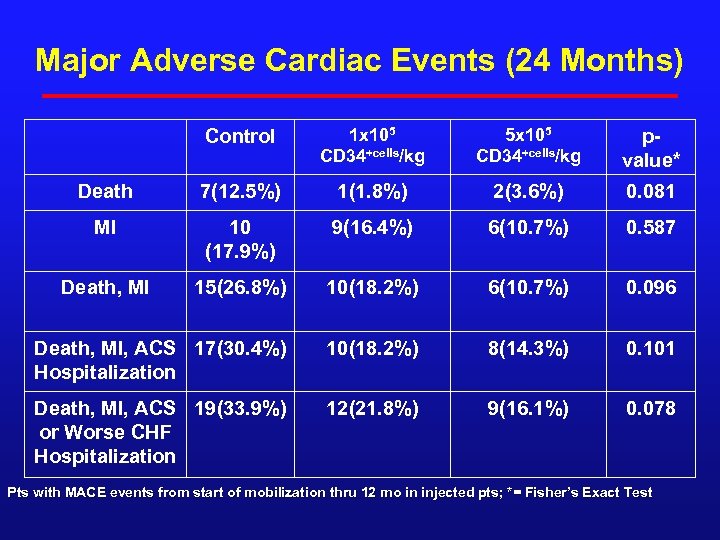 Major Adverse Cardiac Events (24 Months) Control 1 x 105 CD 34+cells/kg 5 x 105 CD 34+cells/kg pvalue* Death 7(12. 5%) 1(1. 8%) 2(3. 6%) 0. 081 MI 10 (17. 9%) 9(16. 4%) 6(10. 7%) 0. 587 Death, MI 15(26. 8%) 10(18. 2%) 6(10. 7%) 0. 096 Death, MI, ACS 17(30. 4%) Hospitalization 10(18. 2%) 8(14. 3%) 0. 101 Death, MI, ACS 19(33. 9%) or Worse CHF Hospitalization 12(21. 8%) 9(16. 1%) 0. 078 Pts with MACE events from start of mobilization thru 12 mo in injected pts; *= Fisher’s Exact Test
Major Adverse Cardiac Events (24 Months) Control 1 x 105 CD 34+cells/kg 5 x 105 CD 34+cells/kg pvalue* Death 7(12. 5%) 1(1. 8%) 2(3. 6%) 0. 081 MI 10 (17. 9%) 9(16. 4%) 6(10. 7%) 0. 587 Death, MI 15(26. 8%) 10(18. 2%) 6(10. 7%) 0. 096 Death, MI, ACS 17(30. 4%) Hospitalization 10(18. 2%) 8(14. 3%) 0. 101 Death, MI, ACS 19(33. 9%) or Worse CHF Hospitalization 12(21. 8%) 9(16. 1%) 0. 078 Pts with MACE events from start of mobilization thru 12 mo in injected pts; *= Fisher’s Exact Test
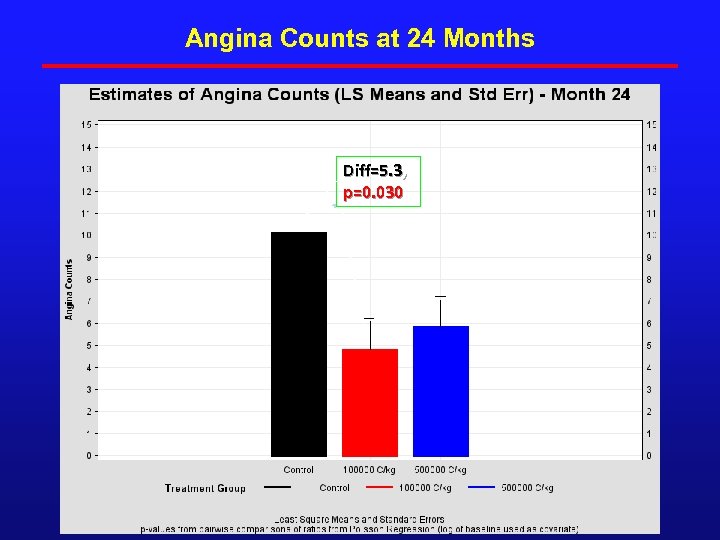 Angina Counts at 24 Months Diff=5. 3, p=0. 030
Angina Counts at 24 Months Diff=5. 3, p=0. 030
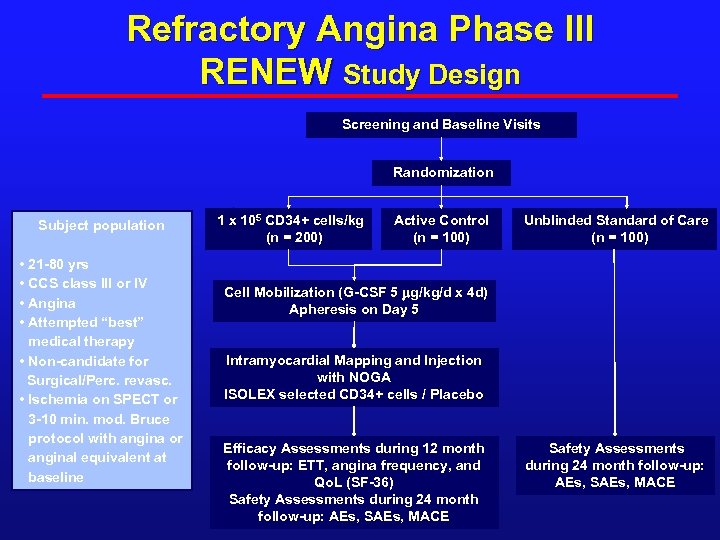 Refractory Angina Phase III RENEW Study Design Screening and Baseline Visits Randomization Subject population • 21 -80 yrs • CCS class III or IV • Angina • Attempted “best” medical therapy • Non-candidate for Surgical/Perc. revasc. • Ischemia on SPECT or 3 -10 min. mod. Bruce protocol with angina or anginal equivalent at baseline 1 x 105 CD 34+ cells/kg (n = 200) Active Control (n = 100) Unblinded Standard of Care (n = 100) Cell Mobilization (G-CSF 5 mg/kg/d x 4 d) Apheresis on Day 5 Intramyocardial Mapping and Injection with NOGA ISOLEX selected CD 34+ cells / Placebo Efficacy Assessments during 12 month follow-up: ETT, angina frequency, and Qo. L (SF-36) Safety Assessments during 24 month follow-up: AEs, SAEs, MACE
Refractory Angina Phase III RENEW Study Design Screening and Baseline Visits Randomization Subject population • 21 -80 yrs • CCS class III or IV • Angina • Attempted “best” medical therapy • Non-candidate for Surgical/Perc. revasc. • Ischemia on SPECT or 3 -10 min. mod. Bruce protocol with angina or anginal equivalent at baseline 1 x 105 CD 34+ cells/kg (n = 200) Active Control (n = 100) Unblinded Standard of Care (n = 100) Cell Mobilization (G-CSF 5 mg/kg/d x 4 d) Apheresis on Day 5 Intramyocardial Mapping and Injection with NOGA ISOLEX selected CD 34+ cells / Placebo Efficacy Assessments during 12 month follow-up: ETT, angina frequency, and Qo. L (SF-36) Safety Assessments during 24 month follow-up: AEs, SAEs, MACE
 Still not available at the florist yet…. GETTING CLOSER!
Still not available at the florist yet…. GETTING CLOSER!
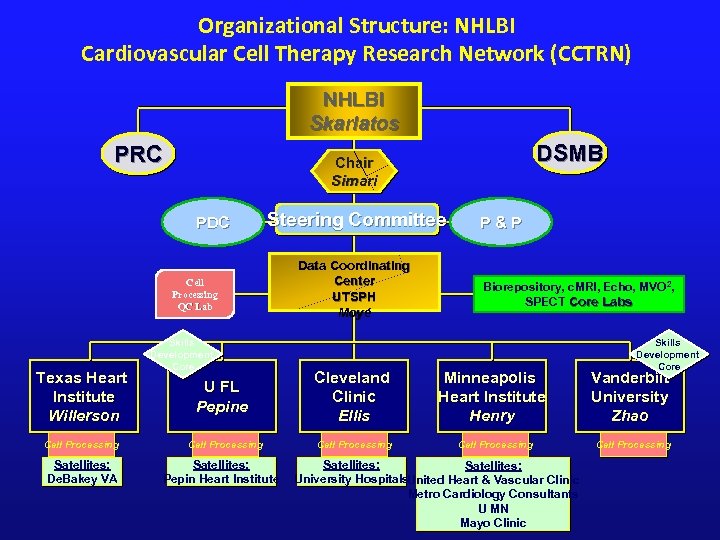 Organizational Structure: NHLBI Cardiovascular Cell Therapy Research Network (CCTRN) NHLBI Skarlatos PRC PDC Steering Committee Cell Processing QC Lab Texas Heart Institute Willerson Cell Processing Satellites: De. Bakey VA DSMB Chair Simari Skills Development Core Data Coordinating Center UTSPH Moyé P&P Biorepository, c. MRI, Echo, MVO 2, SPECT Core Labs Skills Development Core U FL Pepine Cleveland Clinic Ellis Minneapolis Heart Institute Henry Vanderbilt University Zhao Cell Processing Satellites: Pepin Heart Institute Satellites: University Hospitals. United Heart & Vascular Clinic Metro Cardiology Consultants U MN Mayo Clinic
Organizational Structure: NHLBI Cardiovascular Cell Therapy Research Network (CCTRN) NHLBI Skarlatos PRC PDC Steering Committee Cell Processing QC Lab Texas Heart Institute Willerson Cell Processing Satellites: De. Bakey VA DSMB Chair Simari Skills Development Core Data Coordinating Center UTSPH Moyé P&P Biorepository, c. MRI, Echo, MVO 2, SPECT Core Labs Skills Development Core U FL Pepine Cleveland Clinic Ellis Minneapolis Heart Institute Henry Vanderbilt University Zhao Cell Processing Satellites: Pepin Heart Institute Satellites: University Hospitals. United Heart & Vascular Clinic Metro Cardiology Consultants U MN Mayo Clinic
 Published Online First March 24, 2012 Available at www. jama. com
Published Online First March 24, 2012 Available at www. jama. com
 FOCUS-2 Rationale-Endpoints ¨ Severe ischemic LV dysfunction (<45%) n= 86 pts ¨ Increased dose (100 M cells) ¨ Multicenter-CCTRN ¨ Placebo controlled ¨ Combined Endpoints = SPECT, Echo (LVV), MVO 2 (all core assessed) ¨ Late Breaking Trials ACC 2012 Perin EC, et al. JAMA 2012 Apr 25; 307(16): 1717 -26
FOCUS-2 Rationale-Endpoints ¨ Severe ischemic LV dysfunction (<45%) n= 86 pts ¨ Increased dose (100 M cells) ¨ Multicenter-CCTRN ¨ Placebo controlled ¨ Combined Endpoints = SPECT, Echo (LVV), MVO 2 (all core assessed) ¨ Late Breaking Trials ACC 2012 Perin EC, et al. JAMA 2012 Apr 25; 307(16): 1717 -26
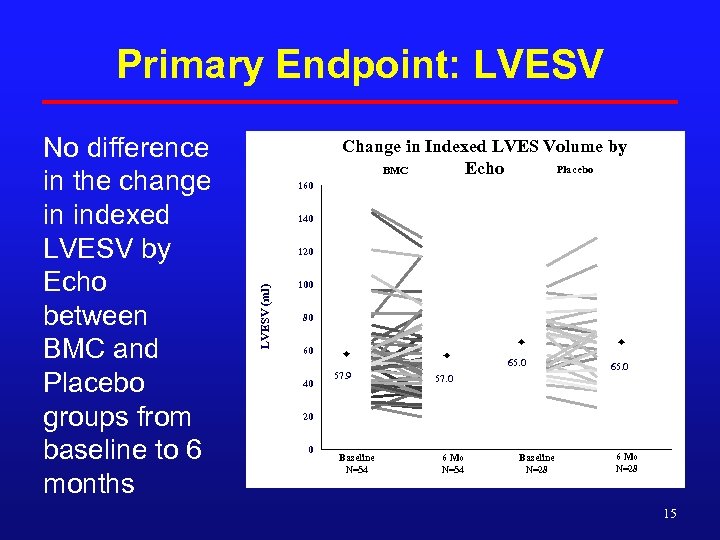 Primary Endpoint: LVESV Change in Indexed LVES Volume by Placebo BMC Echo 160 140 120 LVESV (ml) No difference in the change in indexed LVESV by Echo between BMC and Placebo groups from baseline to 6 months 100 80 60 65. 0 40 57. 9 65. 0 57. 0 20 0 Baseline N=54 6 Mo N=54 Baseline N=28 6 Mo N=28 15
Primary Endpoint: LVESV Change in Indexed LVES Volume by Placebo BMC Echo 160 140 120 LVESV (ml) No difference in the change in indexed LVESV by Echo between BMC and Placebo groups from baseline to 6 months 100 80 60 65. 0 40 57. 9 65. 0 57. 0 20 0 Baseline N=54 6 Mo N=54 Baseline N=28 6 Mo N=28 15
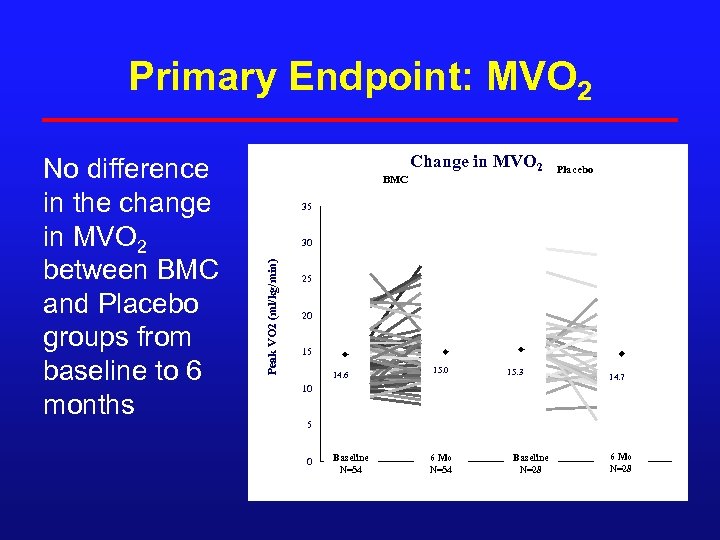 Primary Endpoint: MVO 2 BMC Change in MVO 2 Placebo 35 30 Peak VO 2 (ml/kg/min) No difference in the change in MVO 2 between BMC and Placebo groups from baseline to 6 months 25 20 15 14. 6 15. 0 15. 3 14. 7 10 5 0 Baseline N=54 6 Mo N=54 Baseline N=28 6 Mo N=28
Primary Endpoint: MVO 2 BMC Change in MVO 2 Placebo 35 30 Peak VO 2 (ml/kg/min) No difference in the change in MVO 2 between BMC and Placebo groups from baseline to 6 months 25 20 15 14. 6 15. 0 15. 3 14. 7 10 5 0 Baseline N=54 6 Mo N=54 Baseline N=28 6 Mo N=28
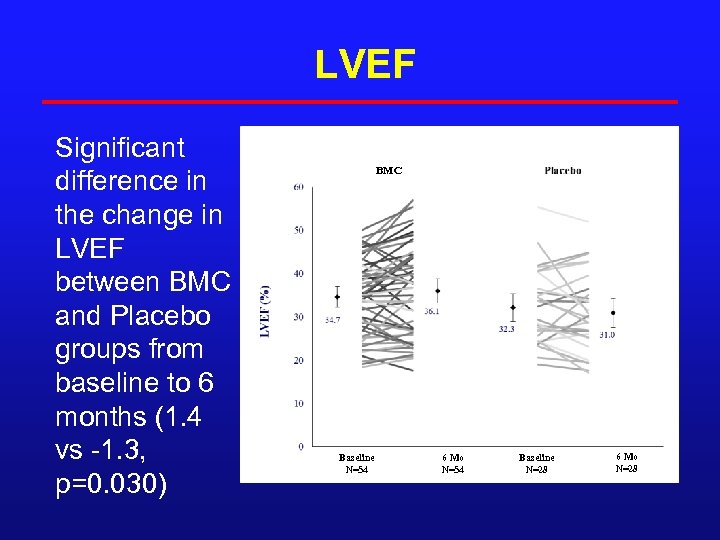 LVEF Significant difference in the change in LVEF between BMC and Placebo groups from baseline to 6 months (1. 4 vs -1. 3, p=0. 030) BMC Baseline N=54 6 Mo N=54 Baseline N=28 6 Mo N=28
LVEF Significant difference in the change in LVEF between BMC and Placebo groups from baseline to 6 months (1. 4 vs -1. 3, p=0. 030) BMC Baseline N=54 6 Mo N=54 Baseline N=28 6 Mo N=28
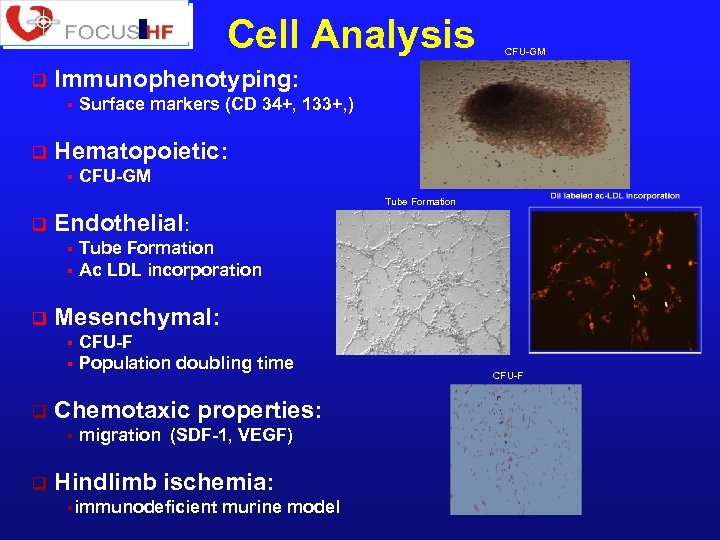 Cell Analysis q Immunophenotyping: § q CFU-GM Surface markers (CD 34+, 133+, ) Hematopoietic: § CFU-GM Tube Formation q Endothelial: Tube Formation § Ac LDL incorporation § q Mesenchymal: CFU-F § Population doubling time § q Chemotaxic properties: § q migration (SDF-1, VEGF) Hindlimb ischemia: §immunodeficient murine model CFU-F
Cell Analysis q Immunophenotyping: § q CFU-GM Surface markers (CD 34+, 133+, ) Hematopoietic: § CFU-GM Tube Formation q Endothelial: Tube Formation § Ac LDL incorporation § q Mesenchymal: CFU-F § Population doubling time § q Chemotaxic properties: § q migration (SDF-1, VEGF) Hindlimb ischemia: §immunodeficient murine model CFU-F
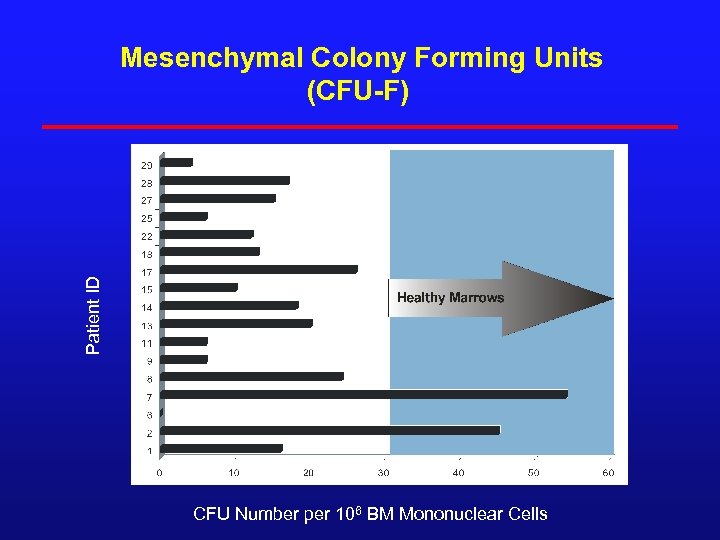 Patient ID Mesenchymal Colony Forming Units (CFU-F) 180 CFU Number per 106 BM Mononuclear Cells
Patient ID Mesenchymal Colony Forming Units (CFU-F) 180 CFU Number per 106 BM Mononuclear Cells
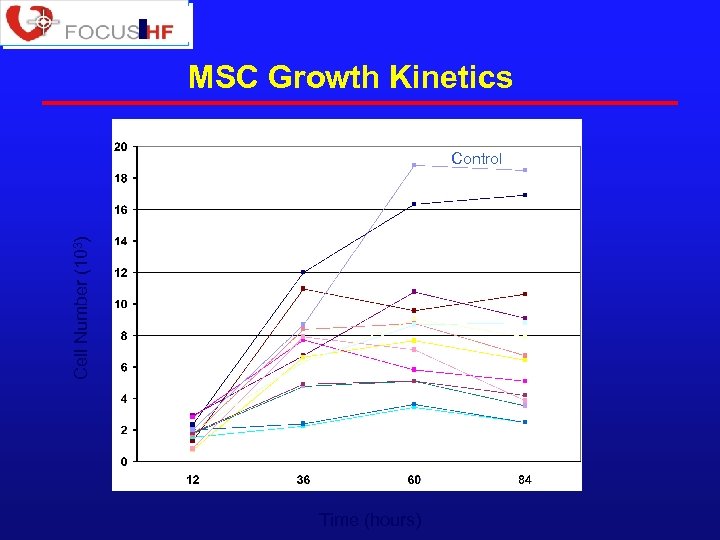 MSC Growth Kinetics Cell Number (103) Control Time (hours)
MSC Growth Kinetics Cell Number (103) Control Time (hours)
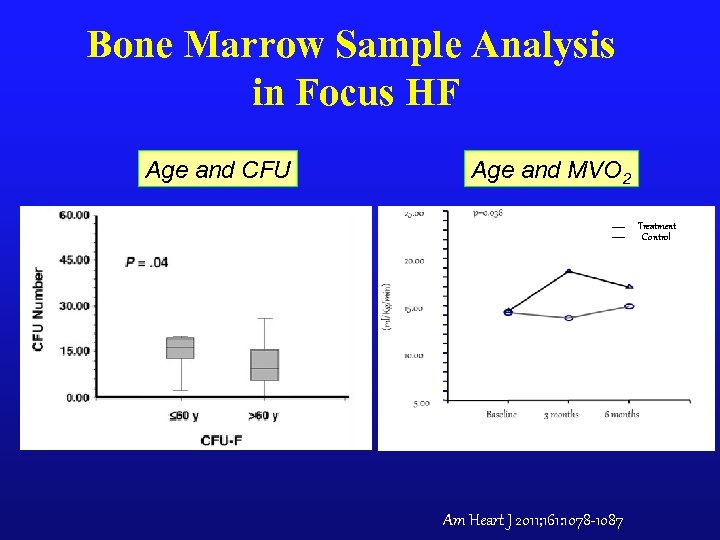 Bone Marrow Sample Analysis in Focus HF Age and CFU Age and MVO 2 Treatment Control Am Heart J 2011; 161: 1078 -1087
Bone Marrow Sample Analysis in Focus HF Age and CFU Age and MVO 2 Treatment Control Am Heart J 2011; 161: 1078 -1087
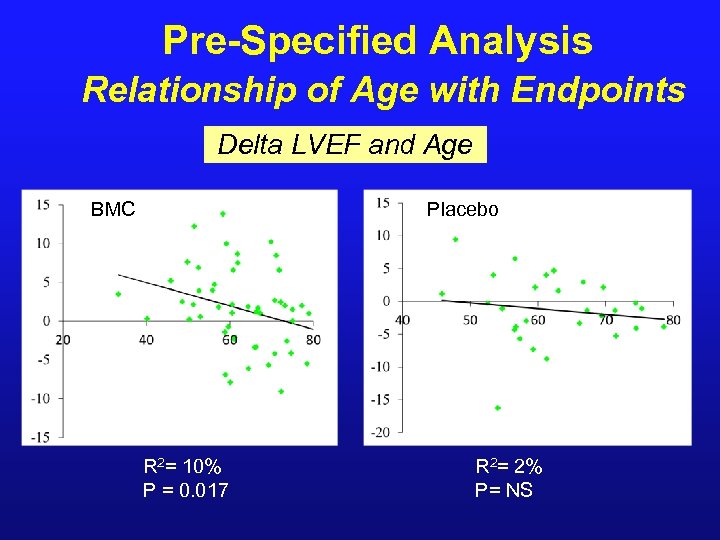 Pre-Specified Analysis Relationship of Age with Endpoints Delta LVEF and Age BMC Placebo R 2= 10% P = 0. 017 R 2= 2% P= NS
Pre-Specified Analysis Relationship of Age with Endpoints Delta LVEF and Age BMC Placebo R 2= 10% P = 0. 017 R 2= 2% P= NS
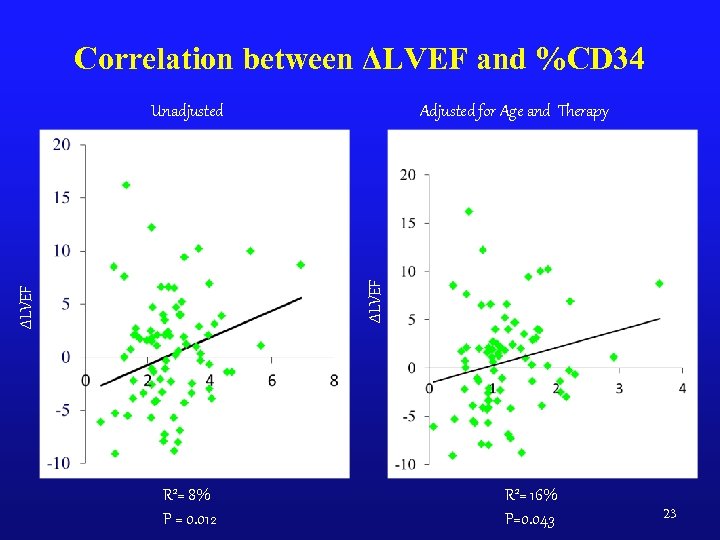 Correlation between ΔLVEF and %CD 34 Adjusted for Age and Therapy ΔLVEF Unadjusted %CD 34 R 2= 8% P = 0. 012 R 2= 16% P=0. 043 23
Correlation between ΔLVEF and %CD 34 Adjusted for Age and Therapy ΔLVEF Unadjusted %CD 34 R 2= 8% P = 0. 012 R 2= 16% P=0. 043 23
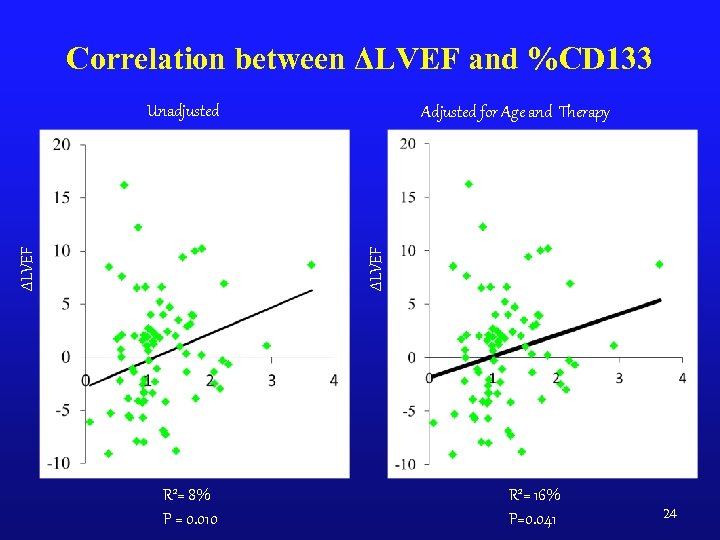 Correlation between ΔLVEF and %CD 133 Adjusted for Age and Therapy ΔLVEF Unadjusted R 2= 8% P = 0. 010 R 2= 16% P=0. 041 24
Correlation between ΔLVEF and %CD 133 Adjusted for Age and Therapy ΔLVEF Unadjusted R 2= 8% P = 0. 010 R 2= 16% P=0. 041 24
 AMI The Problem The Solution
AMI The Problem The Solution
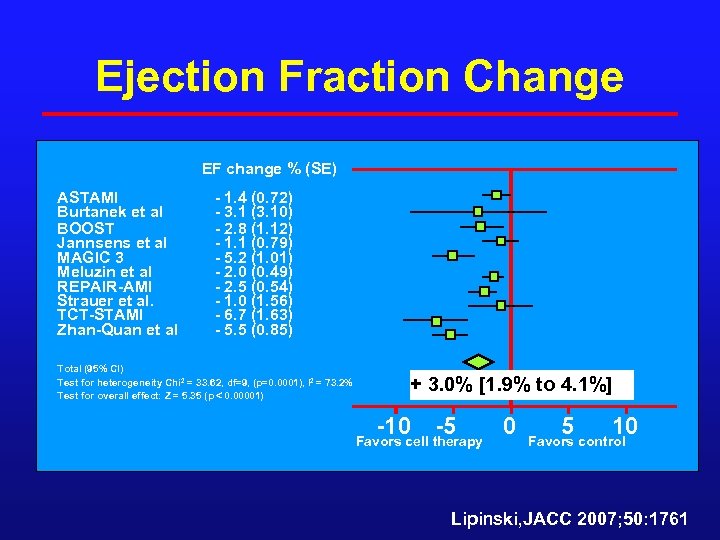 Ejection Fraction Change EF change % (SE) ASTAMI Burtanek et al BOOST Jannsens et al MAGIC 3 Meluzin et al REPAIR-AMI Strauer et al. TCT-STAMI Zhan-Quan et al - 1. 4 (0. 72) - 3. 1 (3. 10) - 2. 8 (1. 12) - 1. 1 (0. 79) - 5. 2 (1. 01) - 2. 0 (0. 49) - 2. 5 (0. 54) - 1. 0 (1. 56) - 6. 7 (1. 63) - 5. 5 (0. 85) Total (95% CI) Test for heterogeneity Chi 2 = 33. 62, df=9, (p=0. 0001), I 2 = 73. 2% Test for overall effect: Z = 5. 35 (p 0. 00001) + 3. 0% [1. 9% to 4. 1%] -10 -5 Favors cell therapy 0 5 10 Favors control Lipinski, JACC 2007; 50: 1761
Ejection Fraction Change EF change % (SE) ASTAMI Burtanek et al BOOST Jannsens et al MAGIC 3 Meluzin et al REPAIR-AMI Strauer et al. TCT-STAMI Zhan-Quan et al - 1. 4 (0. 72) - 3. 1 (3. 10) - 2. 8 (1. 12) - 1. 1 (0. 79) - 5. 2 (1. 01) - 2. 0 (0. 49) - 2. 5 (0. 54) - 1. 0 (1. 56) - 6. 7 (1. 63) - 5. 5 (0. 85) Total (95% CI) Test for heterogeneity Chi 2 = 33. 62, df=9, (p=0. 0001), I 2 = 73. 2% Test for overall effect: Z = 5. 35 (p 0. 00001) + 3. 0% [1. 9% to 4. 1%] -10 -5 Favors cell therapy 0 5 10 Favors control Lipinski, JACC 2007; 50: 1761
![Controls BMMNC P< 0. 001 LVESV -7. 4 ml [-12. 2 to -2. 7] Controls BMMNC P< 0. 001 LVESV -7. 4 ml [-12. 2 to -2. 7]](https://present5.com/presentation/4e688184ee96eefcf08de660abda7f17/image-27.jpg) Controls BMMNC P< 0. 001 LVESV -7. 4 ml [-12. 2 to -2. 7] MI size -5. 6% [-8. 7% to -2. 5%] P= 0. 002 Lipinski, JACC 2007; 50: 1761
Controls BMMNC P< 0. 001 LVESV -7. 4 ml [-12. 2 to -2. 7] MI size -5. 6% [-8. 7% to -2. 5%] P= 0. 002 Lipinski, JACC 2007; 50: 1761
 REPAIR-AMI Team Hamburg Mathey Bad Oeynhausen Horstkotte / Farr Lippe-Detmold Tebbe / Cueno Bochum Mügge / Germing Echo Core Lab Giessen Hölschermann Bad Nauheim Hamm / Elsässer Dill MRT Core Lab Mainz Münzel / Horstick Homburg / Saar Böhm / Nickenig Ludwigshafen Senges / Mark Mannheim Haase / Süsselbeck Zürich Lüscher / Corti Berlin Rutsch Suhl Haberbosch Bad Berka Lauer Leipzig Hambrecht / Schuler / Erbs Doppler Core Lab Frankfurt / Rotes Kreuz Haase Frankfurt / J. W. Goethe Uni Zeiher / Schächinger Dimmeler / Martin Coordination / Angio Core Lab BSD Hessen Tonn / Krzossok / Seifried Cell Processing Center
REPAIR-AMI Team Hamburg Mathey Bad Oeynhausen Horstkotte / Farr Lippe-Detmold Tebbe / Cueno Bochum Mügge / Germing Echo Core Lab Giessen Hölschermann Bad Nauheim Hamm / Elsässer Dill MRT Core Lab Mainz Münzel / Horstick Homburg / Saar Böhm / Nickenig Ludwigshafen Senges / Mark Mannheim Haase / Süsselbeck Zürich Lüscher / Corti Berlin Rutsch Suhl Haberbosch Bad Berka Lauer Leipzig Hambrecht / Schuler / Erbs Doppler Core Lab Frankfurt / Rotes Kreuz Haase Frankfurt / J. W. Goethe Uni Zeiher / Schächinger Dimmeler / Martin Coordination / Angio Core Lab BSD Hessen Tonn / Krzossok / Seifried Cell Processing Center
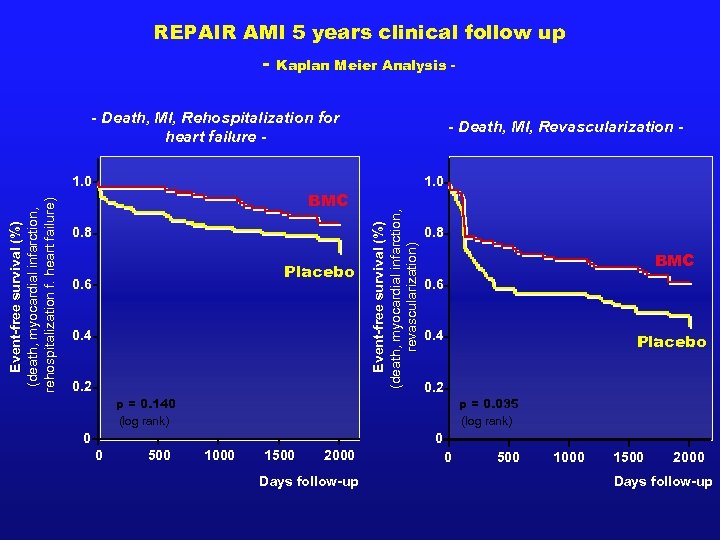 REPAIR AMI 5 years clinical follow up - Kaplan Meier Analysis - - Death, MI, Rehospitalization for heart failure - BMC 0. 8 Placebo 0. 6 0. 4 0. 2 1. 0 Event-free survival (%) (death, myocardial infarction, revascularization) Event-free survival (%) (death, myocardial infarction, rehospitalization f. heart failure) 1. 0 - Death, MI, Revascularization - 0. 8 BMC 0. 6 0. 4 Placebo 0. 2 p = 0. 140 (log rank) p = 0. 035 (log rank) 0 0 0 500 1000 1500 2000 Days follow-up
REPAIR AMI 5 years clinical follow up - Kaplan Meier Analysis - - Death, MI, Rehospitalization for heart failure - BMC 0. 8 Placebo 0. 6 0. 4 0. 2 1. 0 Event-free survival (%) (death, myocardial infarction, revascularization) Event-free survival (%) (death, myocardial infarction, rehospitalization f. heart failure) 1. 0 - Death, MI, Revascularization - 0. 8 BMC 0. 6 0. 4 Placebo 0. 2 p = 0. 140 (log rank) p = 0. 035 (log rank) 0 0 0 500 1000 1500 2000 Days follow-up
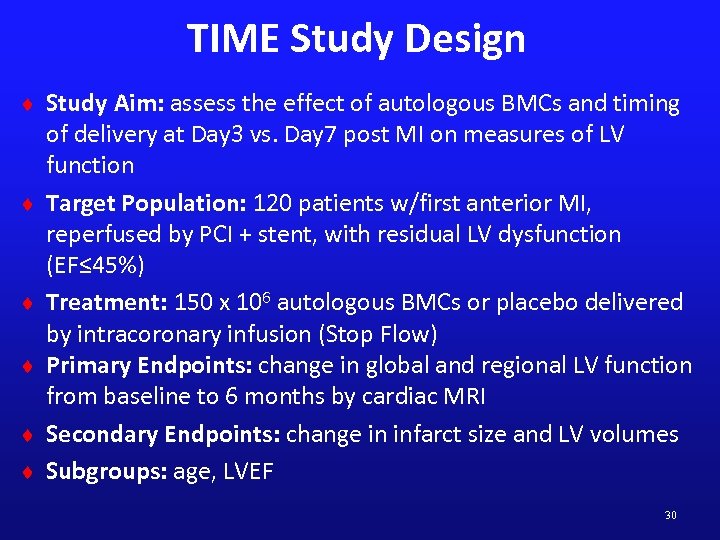 TIME Study Design ¨ Study Aim: assess the effect of autologous BMCs and timing ¨ ¨ ¨ of delivery at Day 3 vs. Day 7 post MI on measures of LV function Target Population: 120 patients w/first anterior MI, reperfused by PCI + stent, with residual LV dysfunction (EF≤ 45%) Treatment: 150 x 106 autologous BMCs or placebo delivered by intracoronary infusion (Stop Flow) Primary Endpoints: change in global and regional LV function from baseline to 6 months by cardiac MRI Secondary Endpoints: change in infarct size and LV volumes Subgroups: age, LVEF 30
TIME Study Design ¨ Study Aim: assess the effect of autologous BMCs and timing ¨ ¨ ¨ of delivery at Day 3 vs. Day 7 post MI on measures of LV function Target Population: 120 patients w/first anterior MI, reperfused by PCI + stent, with residual LV dysfunction (EF≤ 45%) Treatment: 150 x 106 autologous BMCs or placebo delivered by intracoronary infusion (Stop Flow) Primary Endpoints: change in global and regional LV function from baseline to 6 months by cardiac MRI Secondary Endpoints: change in infarct size and LV volumes Subgroups: age, LVEF 30
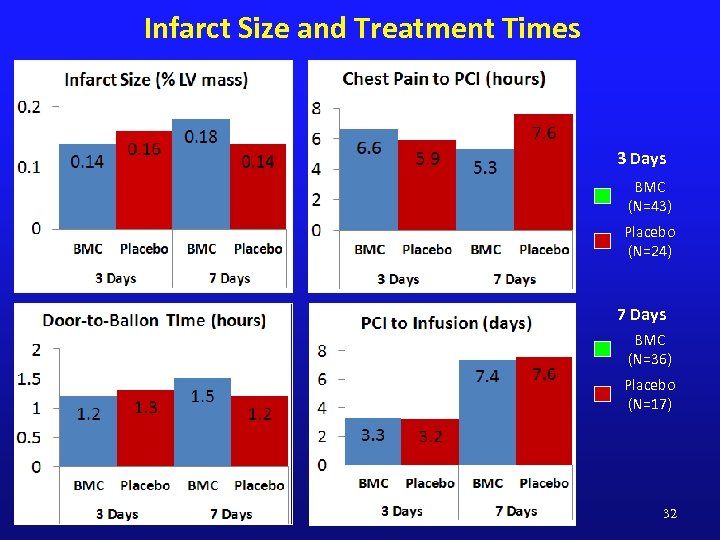 Infarct Size and Treatment Times 3 Days BMC (N=43) Placebo (N=24) 7 Days BMC (N=36) Placebo (N=17) 32
Infarct Size and Treatment Times 3 Days BMC (N=43) Placebo (N=24) 7 Days BMC (N=36) Placebo (N=17) 32
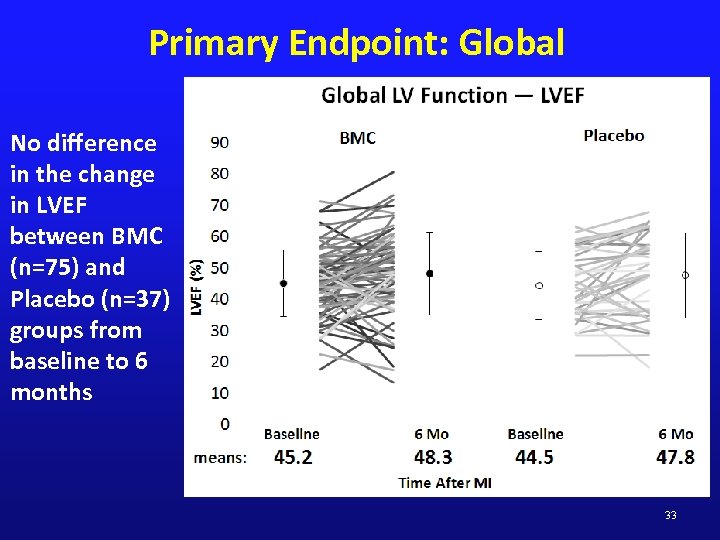 Primary Endpoint: Global No difference in the change in LVEF between BMC (n=75) and Placebo (n=37) groups from baseline to 6 months 33
Primary Endpoint: Global No difference in the change in LVEF between BMC (n=75) and Placebo (n=37) groups from baseline to 6 months 33
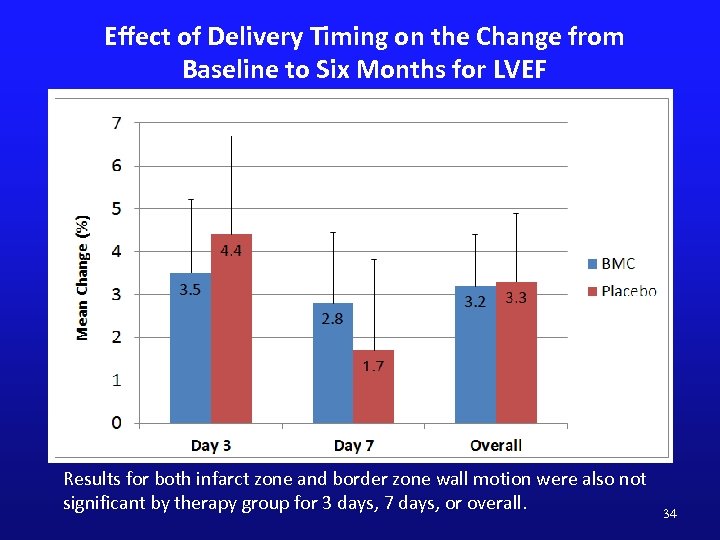 Effect of Delivery Timing on the Change from Baseline to Six Months for LVEF Results for both infarct zone and border zone wall motion were also not significant by therapy group for 3 days, 7 days, or overall. 34
Effect of Delivery Timing on the Change from Baseline to Six Months for LVEF Results for both infarct zone and border zone wall motion were also not significant by therapy group for 3 days, 7 days, or overall. 34
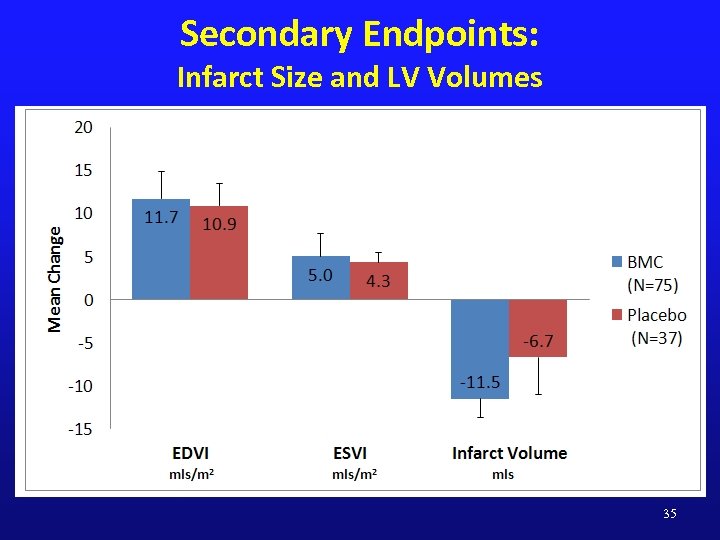 Secondary Endpoints: Infarct Size and LV Volumes 35
Secondary Endpoints: Infarct Size and LV Volumes 35
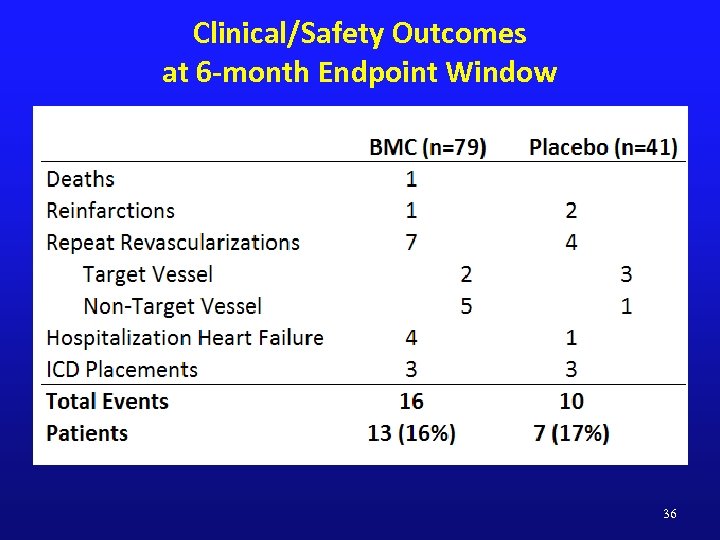 Clinical/Safety Outcomes at 6 -month Endpoint Window 36
Clinical/Safety Outcomes at 6 -month Endpoint Window 36
 Conclusions ¨ Intracoronary delivery of autologous BMCs 3 or 7 days following primary PCI + stenting after moderate to large acute MIs is safe. ¨ No improvement in global and regional LV function is observed at 6 months by c. MRI in response to intracoronary BMC delivery. ¨ Young patients at Day 7 randomized to BMCs had significant improvement with LVEF compared with placebo. 37
Conclusions ¨ Intracoronary delivery of autologous BMCs 3 or 7 days following primary PCI + stenting after moderate to large acute MIs is safe. ¨ No improvement in global and regional LV function is observed at 6 months by c. MRI in response to intracoronary BMC delivery. ¨ Young patients at Day 7 randomized to BMCs had significant improvement with LVEF compared with placebo. 37
 Strategies to Enhance Cell Therapy 1. Increase the number of cells (autologous) ¨ Whole bone marrow (Harvest) 2. Selected cells (autologous) ¨ Adipose derived cells (Cytori) ¨ CD 34+ cells (Baxter) ¨ ALD-bright (Aldagen) 3. Expand and/or enhanced cells (autologous) ¨ Aastrom Biosciences ¨ C-Cure ¨ Allogeneic ¨ MPC (Mesoblast-Teva) ¨ MSC (Osiris) ¨ MAPC (Athersys) ¨ Cardiac derived ¨ Caduceus (Capricor) ¨ SCIPIO
Strategies to Enhance Cell Therapy 1. Increase the number of cells (autologous) ¨ Whole bone marrow (Harvest) 2. Selected cells (autologous) ¨ Adipose derived cells (Cytori) ¨ CD 34+ cells (Baxter) ¨ ALD-bright (Aldagen) 3. Expand and/or enhanced cells (autologous) ¨ Aastrom Biosciences ¨ C-Cure ¨ Allogeneic ¨ MPC (Mesoblast-Teva) ¨ MSC (Osiris) ¨ MAPC (Athersys) ¨ Cardiac derived ¨ Caduceus (Capricor) ¨ SCIPIO
 CADUCEUS Design ¨ Post-MI (<30 days at screening) & LV dysfunction (EF 25 -45%) ¨ Randomized (2: 1), controlled, dose-escalation safety and preliminary efficacy study (MRI for scar mass, viable mass, volumes, & function) ¨ Two centers (Cedars-Sinai Heart Institute; Johns Hopkins) ¨ Endomyocardial biopsies; CDCs manufactured at Cedars-Sinai Heart Institute ¨ Intracoronary infusions of autologous CDCs
CADUCEUS Design ¨ Post-MI (<30 days at screening) & LV dysfunction (EF 25 -45%) ¨ Randomized (2: 1), controlled, dose-escalation safety and preliminary efficacy study (MRI for scar mass, viable mass, volumes, & function) ¨ Two centers (Cedars-Sinai Heart Institute; Johns Hopkins) ¨ Endomyocardial biopsies; CDCs manufactured at Cedars-Sinai Heart Institute ¨ Intracoronary infusions of autologous CDCs
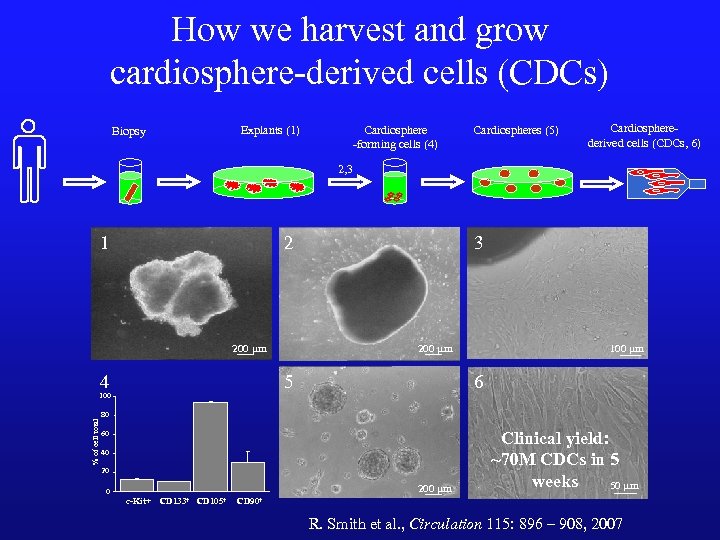 How we harvest and grow cardiosphere-derived cells (CDCs) Explants (1) Biopsy Cardiosphere -forming cells (4) Cardiospheres (5) Cardiospherederived cells (CDCs, 6) 2, 3 1 2 200 mm 4 5 100 % of cell total 3 100 mm 6 80 60 40 20 200 mm 0 c-Kit+ CD 133+ CD 105+ Clinical yield: ~70 M CDCs in 5 weeks 50 mm CD 90+ R. Smith et al. , Circulation 115: 896 – 908, 2007
How we harvest and grow cardiosphere-derived cells (CDCs) Explants (1) Biopsy Cardiosphere -forming cells (4) Cardiospheres (5) Cardiospherederived cells (CDCs, 6) 2, 3 1 2 200 mm 4 5 100 % of cell total 3 100 mm 6 80 60 40 20 200 mm 0 c-Kit+ CD 133+ CD 105+ Clinical yield: ~70 M CDCs in 5 weeks 50 mm CD 90+ R. Smith et al. , Circulation 115: 896 – 908, 2007
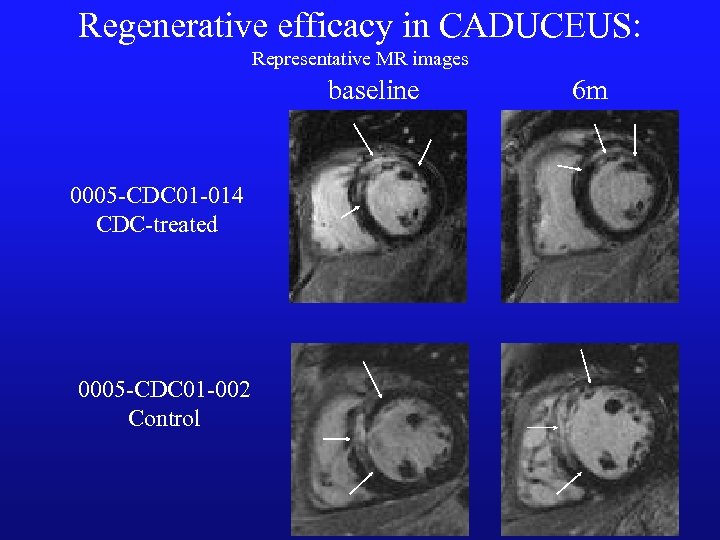 Regenerative efficacy in CADUCEUS: Representative MR images baseline 0005 -CDC 01 -014 CDC-treated 0005 -CDC 01 -002 Control 6 m
Regenerative efficacy in CADUCEUS: Representative MR images baseline 0005 -CDC 01 -014 CDC-treated 0005 -CDC 01 -002 Control 6 m
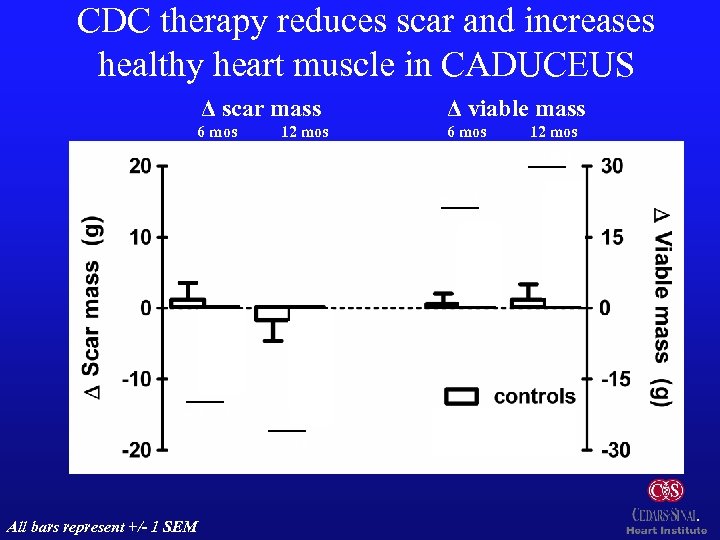 CDC therapy reduces scar and increases healthy heart muscle in CADUCEUS Δ scar mass Δ viable mass 6 mos 12 mos p=0. 001 p: 0. 01 p=0. 001 p: 0. 02 n=8 All bars represent +/- 1 SEM n=15 n=7 n=8
CDC therapy reduces scar and increases healthy heart muscle in CADUCEUS Δ scar mass Δ viable mass 6 mos 12 mos p=0. 001 p: 0. 01 p=0. 001 p: 0. 02 n=8 All bars represent +/- 1 SEM n=15 n=7 n=8
 Cardiovascular Disease Targets ¨ Refractory angina ¨ Acute myocardial infarction ¨ Congestive heart failure ¨ Ongoing ischemia ¨ Previous MI ¨ Nonischemic ¨ Peripheral arterial disease ¨ CLI ¨ Claudication
Cardiovascular Disease Targets ¨ Refractory angina ¨ Acute myocardial infarction ¨ Congestive heart failure ¨ Ongoing ischemia ¨ Previous MI ¨ Nonischemic ¨ Peripheral arterial disease ¨ CLI ¨ Claudication
 We still need better Options!! Will it be Cell Therapy? ?
We still need better Options!! Will it be Cell Therapy? ?


