208e06daadbeffb72eb3edeacfe00567.ppt
- Количество слайдов: 56
 Translational Research for Myocardial Regeneration 2012: Are we making progress? Timothy D. Henry, MD Director of Research Minneapolis Heart Institute Foundation
Translational Research for Myocardial Regeneration 2012: Are we making progress? Timothy D. Henry, MD Director of Research Minneapolis Heart Institute Foundation
 Timothy D. Henry, MD § Contracted Research / Grant Support: § Mesoblast My presentation will include off-label discussions: All cell therapy is currently research and therefore off-label.
Timothy D. Henry, MD § Contracted Research / Grant Support: § Mesoblast My presentation will include off-label discussions: All cell therapy is currently research and therefore off-label.
 Still not available at the florist yet…. GETTING CLOSER!
Still not available at the florist yet…. GETTING CLOSER!
 Cardiovascular Disease Targets ¨ Refractory angina ¨ Acute myocardial infarction ¨ Congestive heart failure ¨ Ongoing ischemia ¨ Previous MI ¨ Nonischemic ¨ Peripheral arterial disease ¨ CLI ¨ Claudication
Cardiovascular Disease Targets ¨ Refractory angina ¨ Acute myocardial infarction ¨ Congestive heart failure ¨ Ongoing ischemia ¨ Previous MI ¨ Nonischemic ¨ Peripheral arterial disease ¨ CLI ¨ Claudication
 Refractory Angina/Ischemia
Refractory Angina/Ischemia
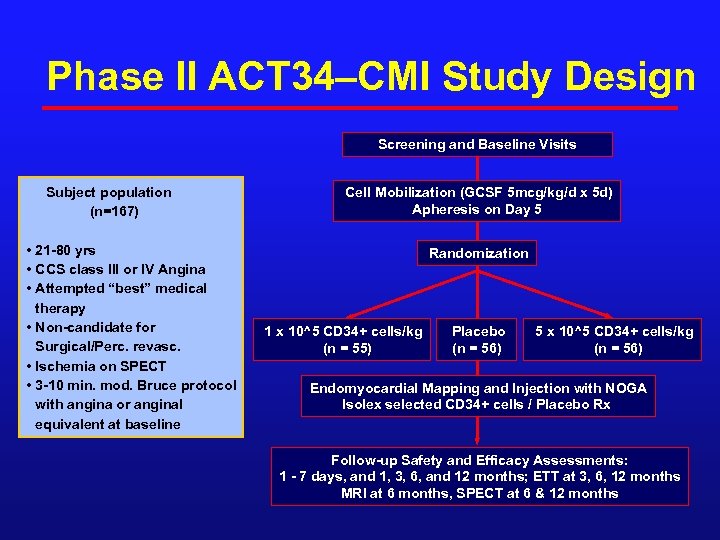 Phase II ACT 34–CMI Study Design Screening and Baseline Visits Subject population (n=167) • 21 -80 yrs • CCS class III or IV Angina • Attempted “best” medical therapy • Non-candidate for Surgical/Perc. revasc. • Ischemia on SPECT • 3 -10 min. mod. Bruce protocol with angina or anginal equivalent at baseline Cell Mobilization (GCSF 5 mcg/kg/d x 5 d) Apheresis on Day 5 Randomization 1 x 10^5 CD 34+ cells/kg (n = 55) Placebo (n = 56) 5 x 10^5 CD 34+ cells/kg (n = 56) Endomyocardial Mapping and Injection with NOGA Isolex selected CD 34+ cells / Placebo Rx Follow-up Safety and Efficacy Assessments: 1 - 7 days, and 1, 3, 6, and 12 months; ETT at 3, 6, 12 months MRI at 6 months, SPECT at 6 & 12 months
Phase II ACT 34–CMI Study Design Screening and Baseline Visits Subject population (n=167) • 21 -80 yrs • CCS class III or IV Angina • Attempted “best” medical therapy • Non-candidate for Surgical/Perc. revasc. • Ischemia on SPECT • 3 -10 min. mod. Bruce protocol with angina or anginal equivalent at baseline Cell Mobilization (GCSF 5 mcg/kg/d x 5 d) Apheresis on Day 5 Randomization 1 x 10^5 CD 34+ cells/kg (n = 55) Placebo (n = 56) 5 x 10^5 CD 34+ cells/kg (n = 56) Endomyocardial Mapping and Injection with NOGA Isolex selected CD 34+ cells / Placebo Rx Follow-up Safety and Efficacy Assessments: 1 - 7 days, and 1, 3, 6, and 12 months; ETT at 3, 6, 12 months MRI at 6 months, SPECT at 6 & 12 months
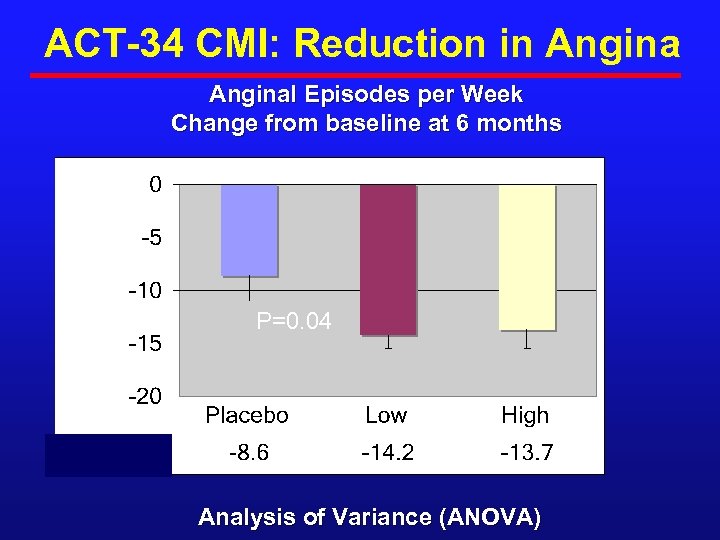 ACT-34 CMI: Reduction in Anginal Episodes per Week Change from baseline at 6 months P=0. 04 Analysis of Variance (ANOVA)
ACT-34 CMI: Reduction in Anginal Episodes per Week Change from baseline at 6 months P=0. 04 Analysis of Variance (ANOVA)
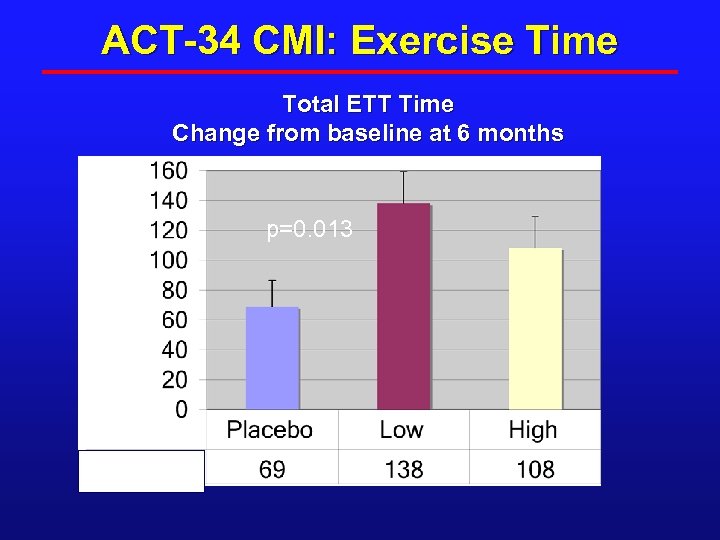 ACT-34 CMI: Exercise Time Total ETT Time Change from baseline at 6 months Seconds p=0. 013
ACT-34 CMI: Exercise Time Total ETT Time Change from baseline at 6 months Seconds p=0. 013
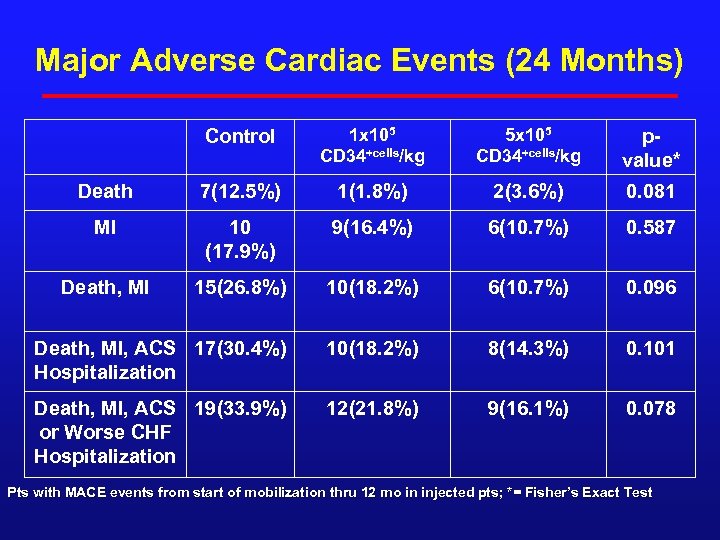 Major Adverse Cardiac Events (24 Months) Control 1 x 105 CD 34+cells/kg 5 x 105 CD 34+cells/kg pvalue* Death 7(12. 5%) 1(1. 8%) 2(3. 6%) 0. 081 MI 10 (17. 9%) 9(16. 4%) 6(10. 7%) 0. 587 Death, MI 15(26. 8%) 10(18. 2%) 6(10. 7%) 0. 096 Death, MI, ACS 17(30. 4%) Hospitalization 10(18. 2%) 8(14. 3%) 0. 101 Death, MI, ACS 19(33. 9%) or Worse CHF Hospitalization 12(21. 8%) 9(16. 1%) 0. 078 Pts with MACE events from start of mobilization thru 12 mo in injected pts; *= Fisher’s Exact Test
Major Adverse Cardiac Events (24 Months) Control 1 x 105 CD 34+cells/kg 5 x 105 CD 34+cells/kg pvalue* Death 7(12. 5%) 1(1. 8%) 2(3. 6%) 0. 081 MI 10 (17. 9%) 9(16. 4%) 6(10. 7%) 0. 587 Death, MI 15(26. 8%) 10(18. 2%) 6(10. 7%) 0. 096 Death, MI, ACS 17(30. 4%) Hospitalization 10(18. 2%) 8(14. 3%) 0. 101 Death, MI, ACS 19(33. 9%) or Worse CHF Hospitalization 12(21. 8%) 9(16. 1%) 0. 078 Pts with MACE events from start of mobilization thru 12 mo in injected pts; *= Fisher’s Exact Test
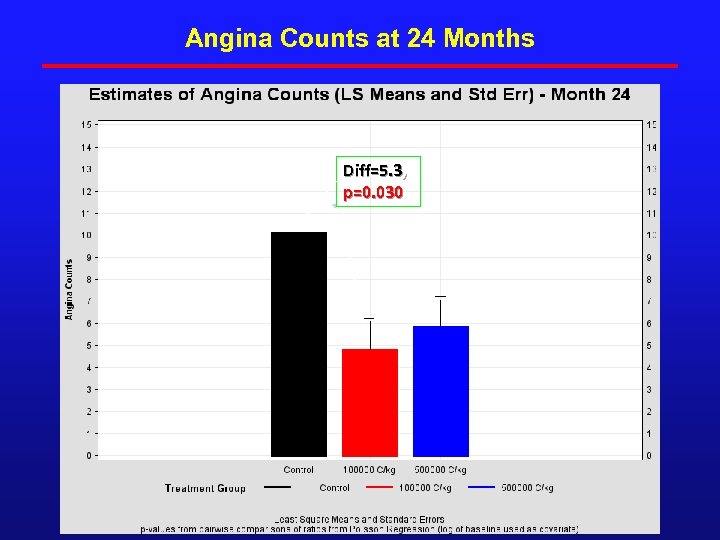 Angina Counts at 24 Months Diff=5. 3, p=0. 030
Angina Counts at 24 Months Diff=5. 3, p=0. 030
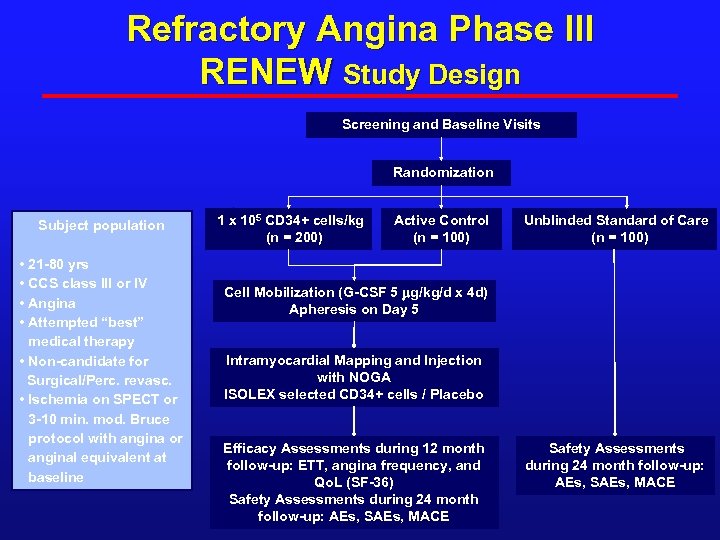 Refractory Angina Phase III RENEW Study Design Screening and Baseline Visits Randomization Subject population • 21 -80 yrs • CCS class III or IV • Angina • Attempted “best” medical therapy • Non-candidate for Surgical/Perc. revasc. • Ischemia on SPECT or 3 -10 min. mod. Bruce protocol with angina or anginal equivalent at baseline 1 x 105 CD 34+ cells/kg (n = 200) Active Control (n = 100) Unblinded Standard of Care (n = 100) Cell Mobilization (G-CSF 5 mg/kg/d x 4 d) Apheresis on Day 5 Intramyocardial Mapping and Injection with NOGA ISOLEX selected CD 34+ cells / Placebo Efficacy Assessments during 12 month follow-up: ETT, angina frequency, and Qo. L (SF-36) Safety Assessments during 24 month follow-up: AEs, SAEs, MACE
Refractory Angina Phase III RENEW Study Design Screening and Baseline Visits Randomization Subject population • 21 -80 yrs • CCS class III or IV • Angina • Attempted “best” medical therapy • Non-candidate for Surgical/Perc. revasc. • Ischemia on SPECT or 3 -10 min. mod. Bruce protocol with angina or anginal equivalent at baseline 1 x 105 CD 34+ cells/kg (n = 200) Active Control (n = 100) Unblinded Standard of Care (n = 100) Cell Mobilization (G-CSF 5 mg/kg/d x 4 d) Apheresis on Day 5 Intramyocardial Mapping and Injection with NOGA ISOLEX selected CD 34+ cells / Placebo Efficacy Assessments during 12 month follow-up: ETT, angina frequency, and Qo. L (SF-36) Safety Assessments during 24 month follow-up: AEs, SAEs, MACE
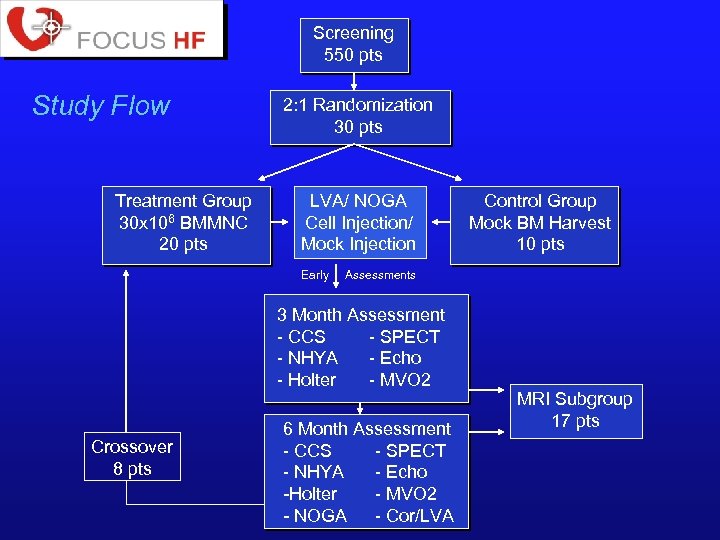 Screening 550 pts Study Flow Treatment Group 30 x 106 BMMNC 20 pts 2: 1 Randomization 30 pts LVA/ NOGA Cell Injection/ Mock Injection Early Assessments 3 Month Assessment - CCS - SPECT - NHYA - Echo - Holter - MVO 2 Crossover 8 pts Control Group Mock BM Harvest 10 pts 6 Month Assessment - CCS - SPECT - NHYA - Echo -Holter - MVO 2 - NOGA - Cor/LVA MRI Subgroup 17 pts
Screening 550 pts Study Flow Treatment Group 30 x 106 BMMNC 20 pts 2: 1 Randomization 30 pts LVA/ NOGA Cell Injection/ Mock Injection Early Assessments 3 Month Assessment - CCS - SPECT - NHYA - Echo - Holter - MVO 2 Crossover 8 pts Control Group Mock BM Harvest 10 pts 6 Month Assessment - CCS - SPECT - NHYA - Echo -Holter - MVO 2 - NOGA - Cor/LVA MRI Subgroup 17 pts
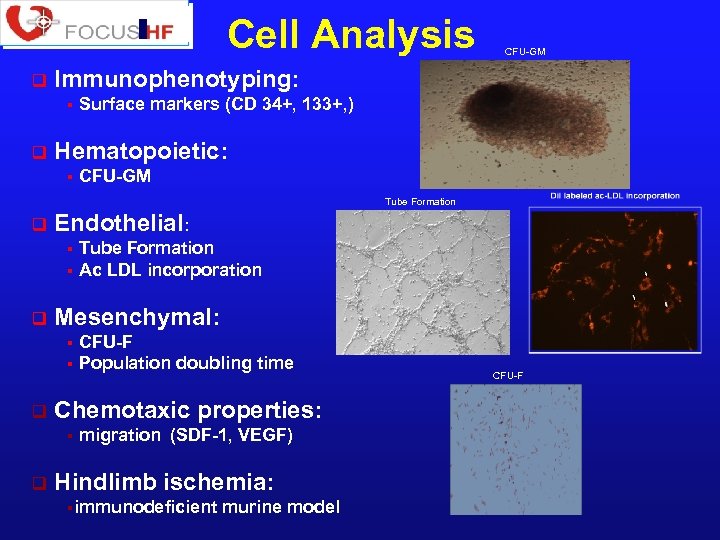 Cell Analysis q Immunophenotyping: § q CFU-GM Surface markers (CD 34+, 133+, ) Hematopoietic: § CFU-GM Tube Formation q Endothelial: Tube Formation § Ac LDL incorporation § q Mesenchymal: CFU-F § Population doubling time § q Chemotaxic properties: § q migration (SDF-1, VEGF) Hindlimb ischemia: §immunodeficient murine model CFU-F
Cell Analysis q Immunophenotyping: § q CFU-GM Surface markers (CD 34+, 133+, ) Hematopoietic: § CFU-GM Tube Formation q Endothelial: Tube Formation § Ac LDL incorporation § q Mesenchymal: CFU-F § Population doubling time § q Chemotaxic properties: § q migration (SDF-1, VEGF) Hindlimb ischemia: §immunodeficient murine model CFU-F
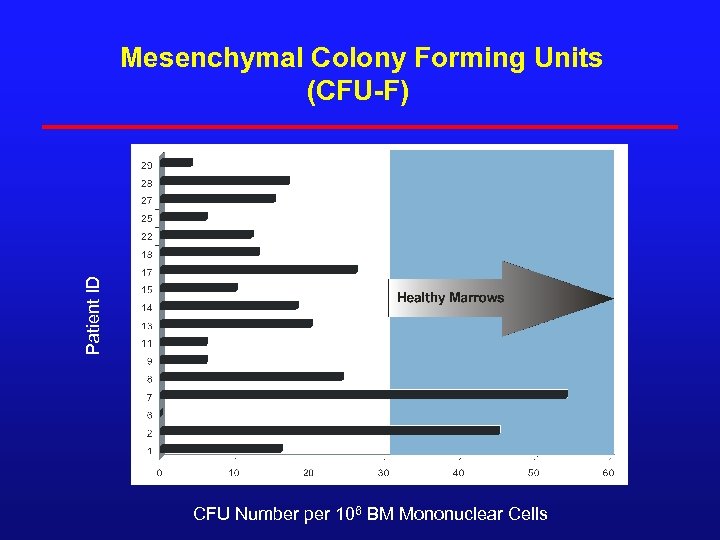 Patient ID Mesenchymal Colony Forming Units (CFU-F) 180 CFU Number per 106 BM Mononuclear Cells
Patient ID Mesenchymal Colony Forming Units (CFU-F) 180 CFU Number per 106 BM Mononuclear Cells
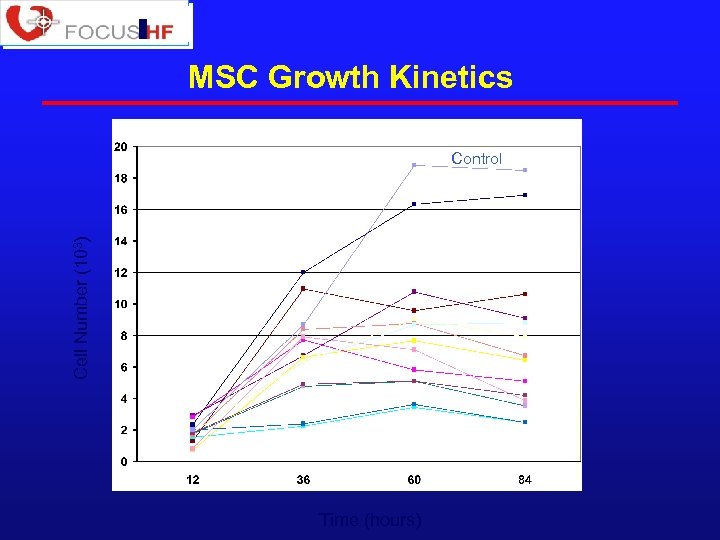 MSC Growth Kinetics Cell Number (103) Control Time (hours)
MSC Growth Kinetics Cell Number (103) Control Time (hours)
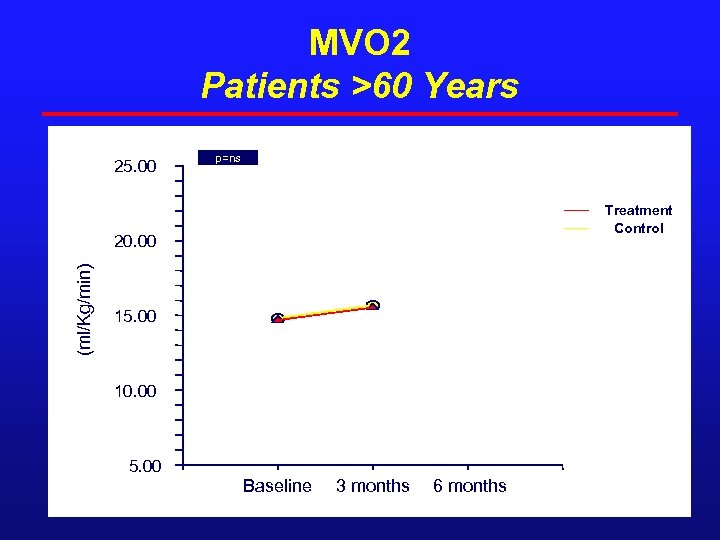 MVO 2 Patients >60 Years 25. 00 p=ns Treatment Control (ml/Kg/min) 20. 00 15. 00 10. 00 5. 00 Baseline 3 months 6 months
MVO 2 Patients >60 Years 25. 00 p=ns Treatment Control (ml/Kg/min) 20. 00 15. 00 10. 00 5. 00 Baseline 3 months 6 months
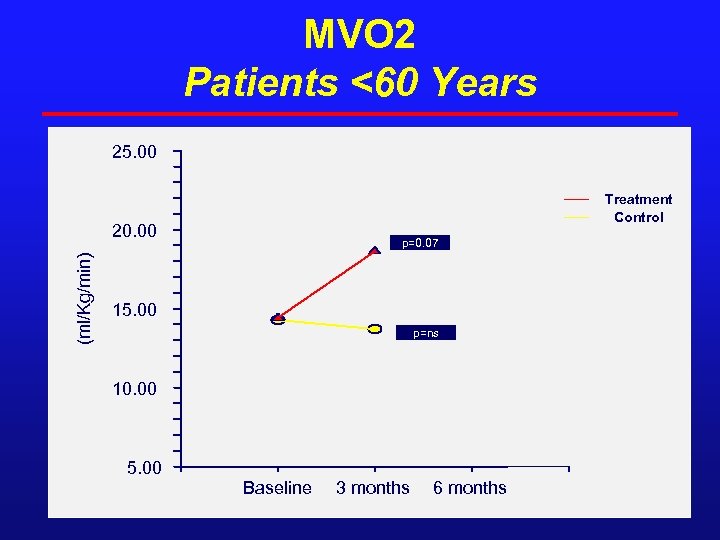 MVO 2 Patients <60 Years 25. 00 Treatment Control (ml/Kg/min) 20. 00 p=0. 07 15. 00 p=ns 10. 00 5. 00 Baseline 3 months 6 months
MVO 2 Patients <60 Years 25. 00 Treatment Control (ml/Kg/min) 20. 00 p=0. 07 15. 00 p=ns 10. 00 5. 00 Baseline 3 months 6 months
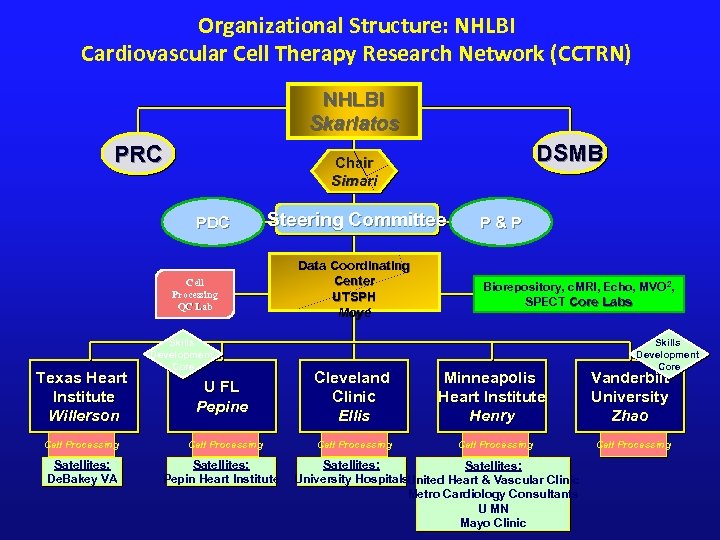 Organizational Structure: NHLBI Cardiovascular Cell Therapy Research Network (CCTRN) NHLBI Skarlatos PRC PDC Steering Committee Cell Processing QC Lab Texas Heart Institute Willerson Cell Processing Satellites: De. Bakey VA DSMB Chair Simari Skills Development Core Data Coordinating Center UTSPH Moyé P&P Biorepository, c. MRI, Echo, MVO 2, SPECT Core Labs Skills Development Core U FL Pepine Cleveland Clinic Ellis Minneapolis Heart Institute Henry Vanderbilt University Zhao Cell Processing Satellites: Pepin Heart Institute Satellites: University Hospitals. United Heart & Vascular Clinic Metro Cardiology Consultants U MN Mayo Clinic
Organizational Structure: NHLBI Cardiovascular Cell Therapy Research Network (CCTRN) NHLBI Skarlatos PRC PDC Steering Committee Cell Processing QC Lab Texas Heart Institute Willerson Cell Processing Satellites: De. Bakey VA DSMB Chair Simari Skills Development Core Data Coordinating Center UTSPH Moyé P&P Biorepository, c. MRI, Echo, MVO 2, SPECT Core Labs Skills Development Core U FL Pepine Cleveland Clinic Ellis Minneapolis Heart Institute Henry Vanderbilt University Zhao Cell Processing Satellites: Pepin Heart Institute Satellites: University Hospitals. United Heart & Vascular Clinic Metro Cardiology Consultants U MN Mayo Clinic
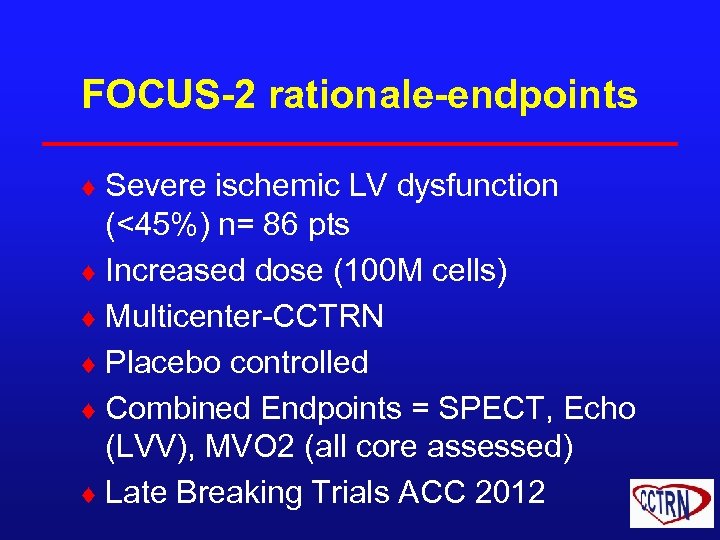 FOCUS-2 rationale-endpoints ¨ Severe ischemic LV dysfunction (<45%) n= 86 pts ¨ Increased dose (100 M cells) ¨ Multicenter-CCTRN ¨ Placebo controlled ¨ Combined Endpoints = SPECT, Echo (LVV), MVO 2 (all core assessed) ¨ Late Breaking Trials ACC 2012
FOCUS-2 rationale-endpoints ¨ Severe ischemic LV dysfunction (<45%) n= 86 pts ¨ Increased dose (100 M cells) ¨ Multicenter-CCTRN ¨ Placebo controlled ¨ Combined Endpoints = SPECT, Echo (LVV), MVO 2 (all core assessed) ¨ Late Breaking Trials ACC 2012
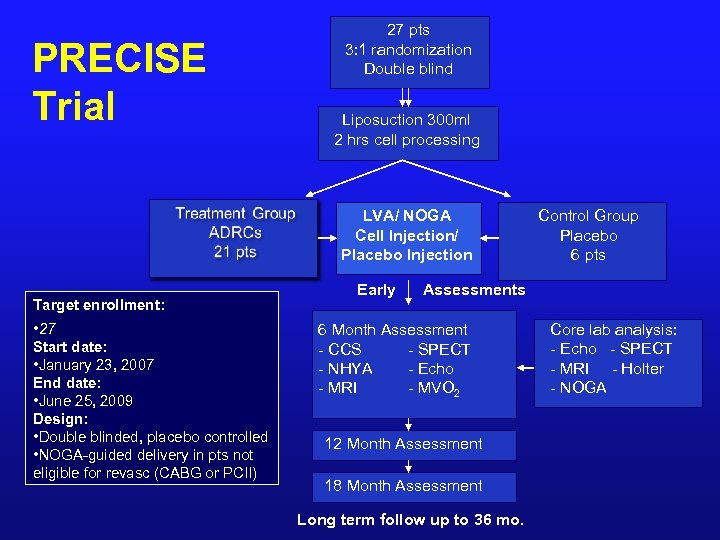 PRECISE Trial 27 pts 3: 1 randomization Double blind Liposuction 300 ml 2 hrs cell processing LVA/ NOGA Cell Injection/ Placebo Injection Target enrollment: • 27 Start date: • January 23, 2007 End date: • June 25, 2009 Design: • Double blinded, placebo controlled • NOGA-guided delivery in pts not eligible for revasc (CABG or PCII) Early Control Group Placebo 6 pts Assessments 6 Month Assessment - CCS - SPECT - NHYA - Echo - MRI - MVO 2 12 Month Assessment 18 Month Assessment Long term follow up to 36 mo. Core lab analysis: - Echo - SPECT - MRI - Holter - NOGA
PRECISE Trial 27 pts 3: 1 randomization Double blind Liposuction 300 ml 2 hrs cell processing LVA/ NOGA Cell Injection/ Placebo Injection Target enrollment: • 27 Start date: • January 23, 2007 End date: • June 25, 2009 Design: • Double blinded, placebo controlled • NOGA-guided delivery in pts not eligible for revasc (CABG or PCII) Early Control Group Placebo 6 pts Assessments 6 Month Assessment - CCS - SPECT - NHYA - Echo - MRI - MVO 2 12 Month Assessment 18 Month Assessment Long term follow up to 36 mo. Core lab analysis: - Echo - SPECT - MRI - Holter - NOGA
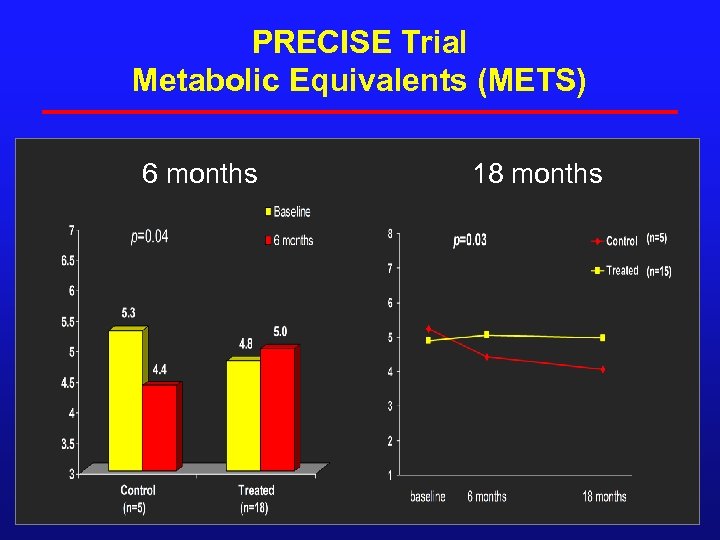 PRECISE Trial Metabolic Equivalents (METS) 6 months 18 months
PRECISE Trial Metabolic Equivalents (METS) 6 months 18 months
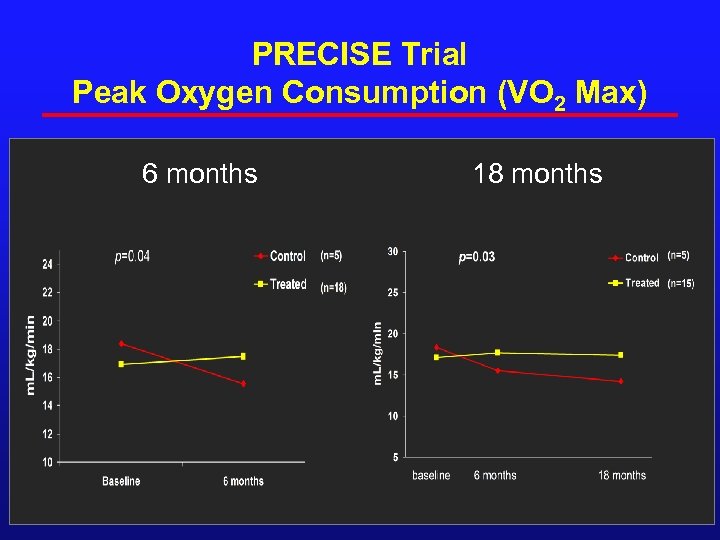 PRECISE Trial Peak Oxygen Consumption (VO 2 Max) 6 months 18 months
PRECISE Trial Peak Oxygen Consumption (VO 2 Max) 6 months 18 months
 Adipose Derived MSC’s ¨ Cytori: ¨ CE mark based on PRECISE ¨ U. S. trial to begin soon
Adipose Derived MSC’s ¨ Cytori: ¨ CE mark based on PRECISE ¨ U. S. trial to begin soon
 AMI The Problem The Solution
AMI The Problem The Solution
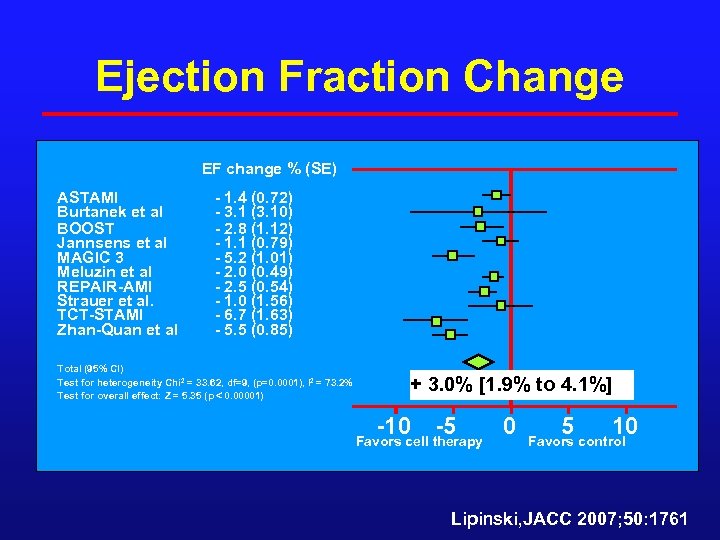 Ejection Fraction Change EF change % (SE) ASTAMI Burtanek et al BOOST Jannsens et al MAGIC 3 Meluzin et al REPAIR-AMI Strauer et al. TCT-STAMI Zhan-Quan et al - 1. 4 (0. 72) - 3. 1 (3. 10) - 2. 8 (1. 12) - 1. 1 (0. 79) - 5. 2 (1. 01) - 2. 0 (0. 49) - 2. 5 (0. 54) - 1. 0 (1. 56) - 6. 7 (1. 63) - 5. 5 (0. 85) Total (95% CI) Test for heterogeneity Chi 2 = 33. 62, df=9, (p=0. 0001), I 2 = 73. 2% Test for overall effect: Z = 5. 35 (p 0. 00001) + 3. 0% [1. 9% to 4. 1%] -10 -5 Favors cell therapy 0 5 10 Favors control Lipinski, JACC 2007; 50: 1761
Ejection Fraction Change EF change % (SE) ASTAMI Burtanek et al BOOST Jannsens et al MAGIC 3 Meluzin et al REPAIR-AMI Strauer et al. TCT-STAMI Zhan-Quan et al - 1. 4 (0. 72) - 3. 1 (3. 10) - 2. 8 (1. 12) - 1. 1 (0. 79) - 5. 2 (1. 01) - 2. 0 (0. 49) - 2. 5 (0. 54) - 1. 0 (1. 56) - 6. 7 (1. 63) - 5. 5 (0. 85) Total (95% CI) Test for heterogeneity Chi 2 = 33. 62, df=9, (p=0. 0001), I 2 = 73. 2% Test for overall effect: Z = 5. 35 (p 0. 00001) + 3. 0% [1. 9% to 4. 1%] -10 -5 Favors cell therapy 0 5 10 Favors control Lipinski, JACC 2007; 50: 1761
![Controls BMMNC P< 0. 001 LVESV -7. 4 ml [-12. 2 to -2. 7] Controls BMMNC P< 0. 001 LVESV -7. 4 ml [-12. 2 to -2. 7]](https://present5.com/presentation/208e06daadbeffb72eb3edeacfe00567/image-26.jpg) Controls BMMNC P< 0. 001 LVESV -7. 4 ml [-12. 2 to -2. 7] MI size -5. 6% [-8. 7% to -2. 5%] P= 0. 002 Lipinski, JACC 2007; 50: 1761
Controls BMMNC P< 0. 001 LVESV -7. 4 ml [-12. 2 to -2. 7] MI size -5. 6% [-8. 7% to -2. 5%] P= 0. 002 Lipinski, JACC 2007; 50: 1761
 REPAIR-AMI Team Hamburg Mathey Bad Oeynhausen Horstkotte / Farr Lippe-Detmold Tebbe / Cueno Bochum Mügge / Germing Echo Core Lab Giessen Hölschermann Bad Nauheim Hamm / Elsässer Dill MRT Core Lab Mainz Münzel / Horstick Homburg / Saar Böhm / Nickenig Ludwigshafen Senges / Mark Mannheim Haase / Süsselbeck Zürich Lüscher / Corti Berlin Rutsch Suhl Haberbosch Bad Berka Lauer Leipzig Hambrecht / Schuler / Erbs Doppler Core Lab Frankfurt / Rotes Kreuz Haase Frankfurt / J. W. Goethe Uni Zeiher / Schächinger Dimmeler / Martin Coordination / Angio Core Lab BSD Hessen Tonn / Krzossok / Seifried Cell Processing Center
REPAIR-AMI Team Hamburg Mathey Bad Oeynhausen Horstkotte / Farr Lippe-Detmold Tebbe / Cueno Bochum Mügge / Germing Echo Core Lab Giessen Hölschermann Bad Nauheim Hamm / Elsässer Dill MRT Core Lab Mainz Münzel / Horstick Homburg / Saar Böhm / Nickenig Ludwigshafen Senges / Mark Mannheim Haase / Süsselbeck Zürich Lüscher / Corti Berlin Rutsch Suhl Haberbosch Bad Berka Lauer Leipzig Hambrecht / Schuler / Erbs Doppler Core Lab Frankfurt / Rotes Kreuz Haase Frankfurt / J. W. Goethe Uni Zeiher / Schächinger Dimmeler / Martin Coordination / Angio Core Lab BSD Hessen Tonn / Krzossok / Seifried Cell Processing Center
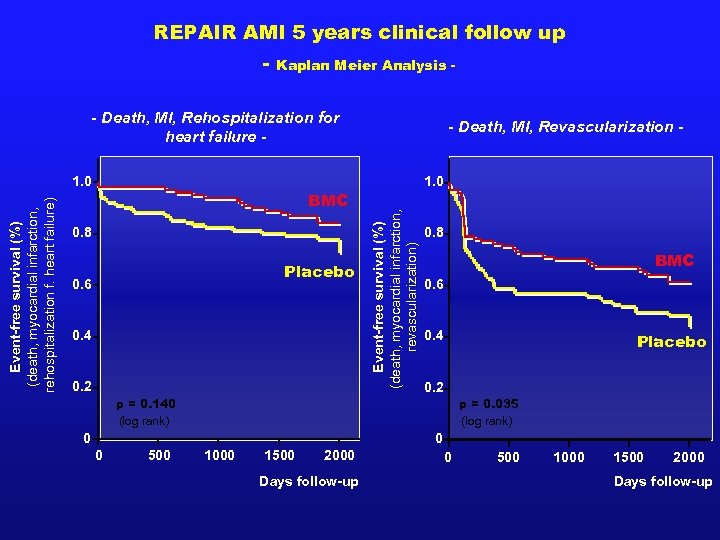 REPAIR AMI 5 years clinical follow up - Kaplan Meier Analysis - - Death, MI, Rehospitalization for heart failure - BMC 0. 8 Placebo 0. 6 0. 4 0. 2 1. 0 Event-free survival (%) (death, myocardial infarction, revascularization) Event-free survival (%) (death, myocardial infarction, rehospitalization f. heart failure) 1. 0 - Death, MI, Revascularization - 0. 8 BMC 0. 6 0. 4 Placebo 0. 2 p = 0. 140 (log rank) p = 0. 035 (log rank) 0 0 0 500 1000 1500 2000 Days follow-up
REPAIR AMI 5 years clinical follow up - Kaplan Meier Analysis - - Death, MI, Rehospitalization for heart failure - BMC 0. 8 Placebo 0. 6 0. 4 0. 2 1. 0 Event-free survival (%) (death, myocardial infarction, revascularization) Event-free survival (%) (death, myocardial infarction, rehospitalization f. heart failure) 1. 0 - Death, MI, Revascularization - 0. 8 BMC 0. 6 0. 4 Placebo 0. 2 p = 0. 140 (log rank) p = 0. 035 (log rank) 0 0 0 500 1000 1500 2000 Days follow-up

 Rationale for Late. TIME • Optimal timing for cell delivery post-AMI is unknown • Almost all BMC Trials delivered cells ≤ 7 days post-AMI • Cell delivery 2 -3 wks post-MI may be an important time period for patients: -initially too sick, or -presenting to hospitals without these capabilities JAMA 2011; 306: 2110 -19
Rationale for Late. TIME • Optimal timing for cell delivery post-AMI is unknown • Almost all BMC Trials delivered cells ≤ 7 days post-AMI • Cell delivery 2 -3 wks post-MI may be an important time period for patients: -initially too sick, or -presenting to hospitals without these capabilities JAMA 2011; 306: 2110 -19
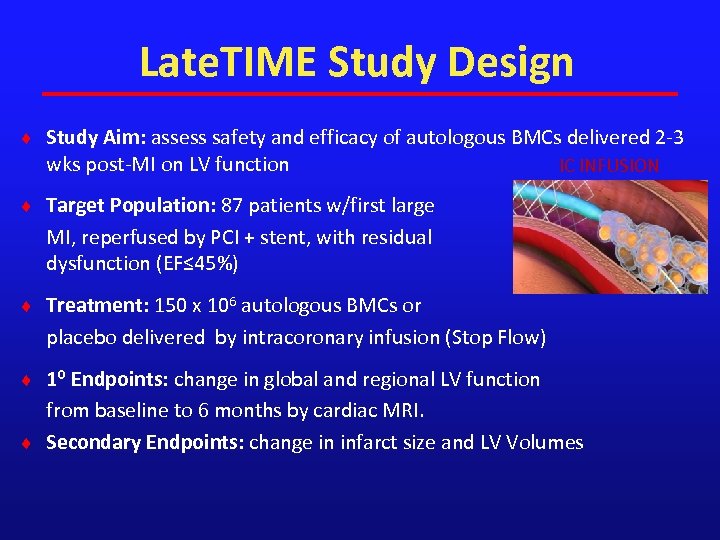 Late. TIME Study Design ¨ Study Aim: assess safety and efficacy of autologous BMCs delivered 2 -3 wks post-MI on LV function IC INFUSION ¨ Target Population: 87 patients w/first large MI, reperfused by PCI + stent, with residual dysfunction (EF≤ 45%) ¨ Treatment: 150 x 106 autologous BMCs or placebo delivered by intracoronary infusion (Stop Flow) ¨ 1⁰ Endpoints: change in global and regional LV function from baseline to 6 months by cardiac MRI. ¨ Secondary Endpoints: change in infarct size and LV Volumes LV
Late. TIME Study Design ¨ Study Aim: assess safety and efficacy of autologous BMCs delivered 2 -3 wks post-MI on LV function IC INFUSION ¨ Target Population: 87 patients w/first large MI, reperfused by PCI + stent, with residual dysfunction (EF≤ 45%) ¨ Treatment: 150 x 106 autologous BMCs or placebo delivered by intracoronary infusion (Stop Flow) ¨ 1⁰ Endpoints: change in global and regional LV function from baseline to 6 months by cardiac MRI. ¨ Secondary Endpoints: change in infarct size and LV Volumes LV
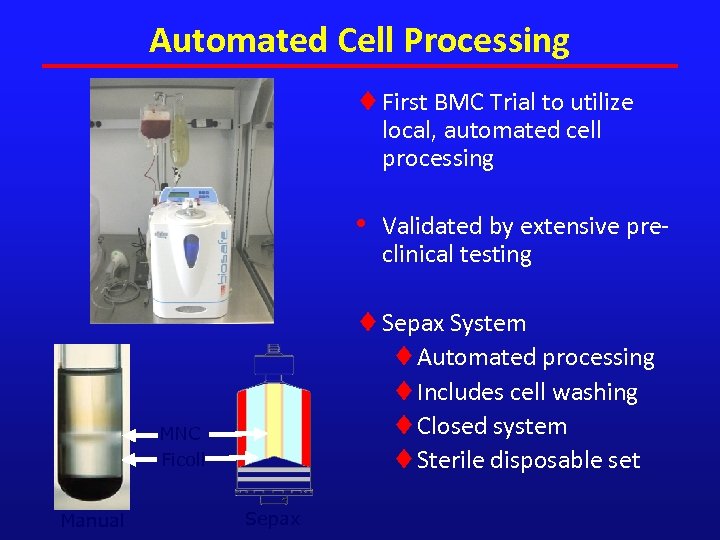 Automated Cell Processing ¨ First BMC Trial to utilize local, automated cell processing • ¨ Sepax System ¨Automated processing ¨Includes cell washing ¨Closed system ¨Sterile disposable set MNC Ficoll Manual Validated by extensive preclinical testing Sepax
Automated Cell Processing ¨ First BMC Trial to utilize local, automated cell processing • ¨ Sepax System ¨Automated processing ¨Includes cell washing ¨Closed system ¨Sterile disposable set MNC Ficoll Manual Validated by extensive preclinical testing Sepax
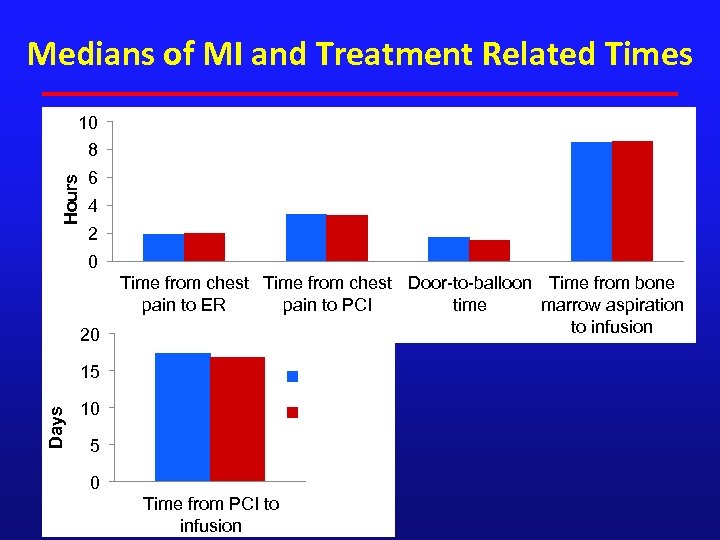 Medians of MI and Treatment Related Times 10 Hours 8 6 4 2 0 20 Time from chest Door-to-balloon Time from bone pain to ER pain to PCI time marrow aspiration to infusion 15 BMC Days (N=58) 10 Placebo (N=29) 5 0 Time from PCI to infusion
Medians of MI and Treatment Related Times 10 Hours 8 6 4 2 0 20 Time from chest Door-to-balloon Time from bone pain to ER pain to PCI time marrow aspiration to infusion 15 BMC Days (N=58) 10 Placebo (N=29) 5 0 Time from PCI to infusion
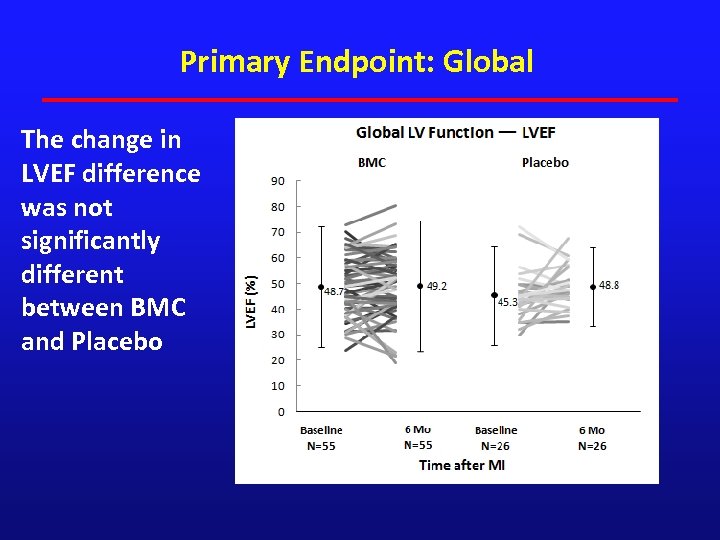 Primary Endpoint: Global The change in LVEF difference was not significantly different between BMC and Placebo
Primary Endpoint: Global The change in LVEF difference was not significantly different between BMC and Placebo
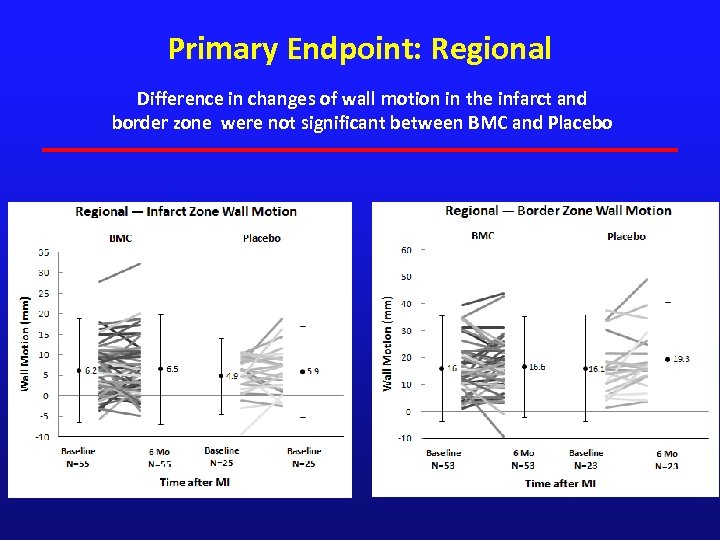 Primary Endpoint: Regional Difference in changes of wall motion in the infarct and border zone were not significant between BMC and Placebo
Primary Endpoint: Regional Difference in changes of wall motion in the infarct and border zone were not significant between BMC and Placebo
 Implications of Late. TIME ¨ Is 2 -3 weeks too late? ¨ Differences in cell number and product (Biorepository) ¨ Differences in myocardium ¨ Is the Clinical Event Reduction real? ¨ Would a different cell be better at this time point? ¨ The history of Late. TIME will be written in the context of TIME (AHA 2012).
Implications of Late. TIME ¨ Is 2 -3 weeks too late? ¨ Differences in cell number and product (Biorepository) ¨ Differences in myocardium ¨ Is the Clinical Event Reduction real? ¨ Would a different cell be better at this time point? ¨ The history of Late. TIME will be written in the context of TIME (AHA 2012).
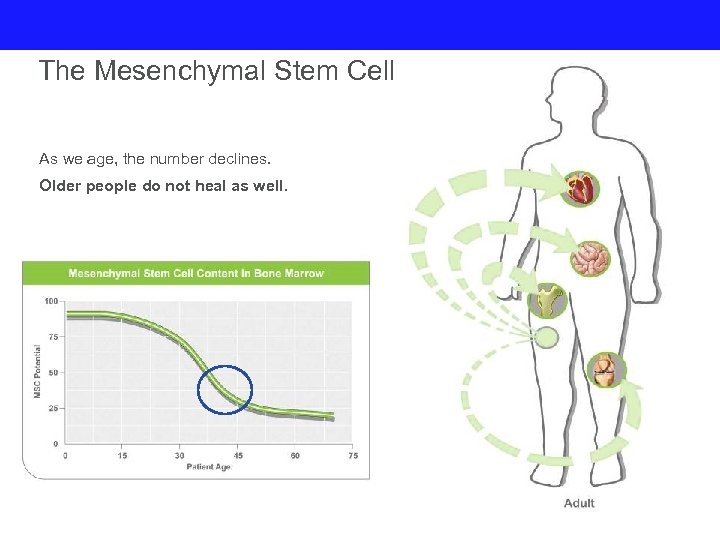 The Mesenchymal Stem Cell As we age, the number declines. Older people do not heal as well.
The Mesenchymal Stem Cell As we age, the number declines. Older people do not heal as well.

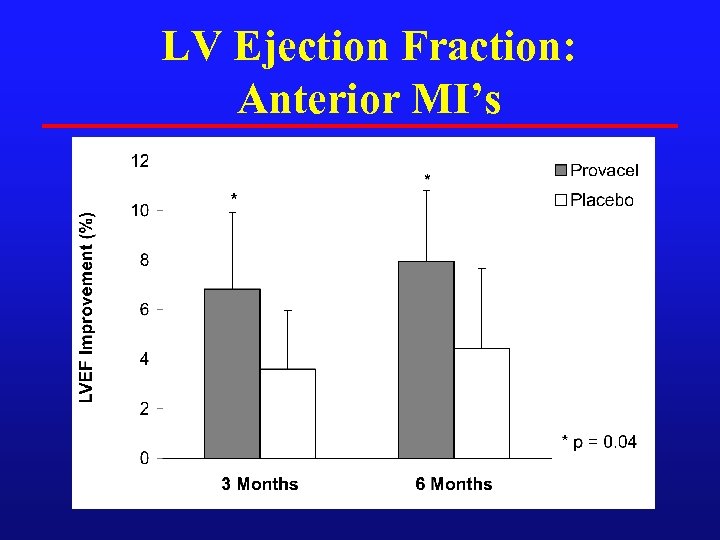 LV Ejection Fraction: Anterior MI’s
LV Ejection Fraction: Anterior MI’s
 CHF
CHF
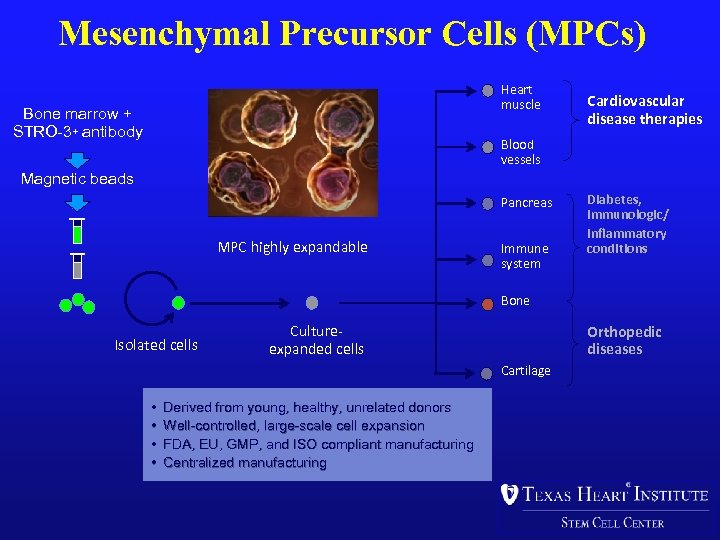 Mesenchymal Precursor Cells (MPCs) Heart muscle Bone marrow + STRO-3+ antibody Cardiovascular disease therapies Blood vessels Magnetic beads Pancreas MPC highly expandable Immune system Diabetes, immunologic/ inflammatory conditions Bone Isolated cells Cultureexpanded cells Orthopedic diseases Cartilage • • Derived from young, healthy, unrelated donors Well-controlled, large-scale cell expansion FDA, EU, GMP, and ISO compliant manufacturing Centralized manufacturing
Mesenchymal Precursor Cells (MPCs) Heart muscle Bone marrow + STRO-3+ antibody Cardiovascular disease therapies Blood vessels Magnetic beads Pancreas MPC highly expandable Immune system Diabetes, immunologic/ inflammatory conditions Bone Isolated cells Cultureexpanded cells Orthopedic diseases Cartilage • • Derived from young, healthy, unrelated donors Well-controlled, large-scale cell expansion FDA, EU, GMP, and ISO compliant manufacturing Centralized manufacturing
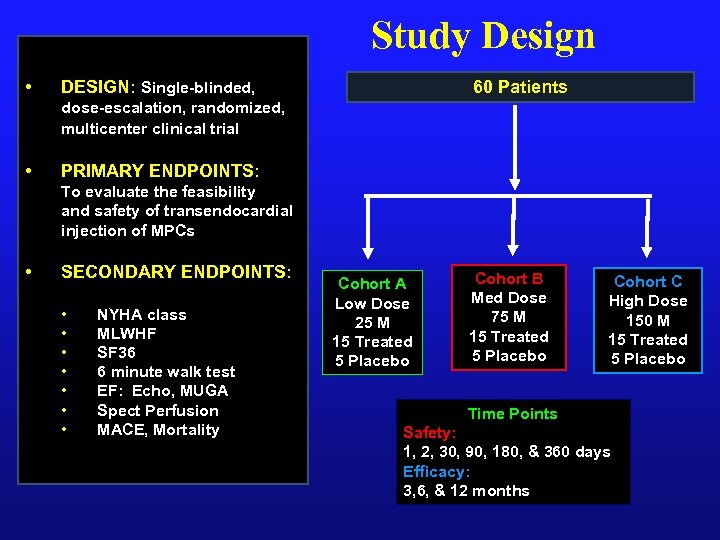 Study Design • 60 Patients DESIGN: Single-blinded, dose-escalation, randomized, multicenter clinical trial • PRIMARY ENDPOINTS: To evaluate the feasibility and safety of transendocardial injection of MPCs • SECONDARY ENDPOINTS: • • NYHA class MLWHF SF 36 6 minute walk test EF: Echo, MUGA Spect Perfusion MACE, Mortality Cohort A Low Dose 25 M 15 Treated 5 Placebo Cohort B Med Dose 75 M 15 Treated 5 Placebo Cohort C High Dose 150 M 15 Treated 5 Placebo Time Points Safety: 1, 2, 30, 90, 180, & 360 days Efficacy: 3, 6, & 12 months
Study Design • 60 Patients DESIGN: Single-blinded, dose-escalation, randomized, multicenter clinical trial • PRIMARY ENDPOINTS: To evaluate the feasibility and safety of transendocardial injection of MPCs • SECONDARY ENDPOINTS: • • NYHA class MLWHF SF 36 6 minute walk test EF: Echo, MUGA Spect Perfusion MACE, Mortality Cohort A Low Dose 25 M 15 Treated 5 Placebo Cohort B Med Dose 75 M 15 Treated 5 Placebo Cohort C High Dose 150 M 15 Treated 5 Placebo Time Points Safety: 1, 2, 30, 90, 180, & 360 days Efficacy: 3, 6, & 12 months
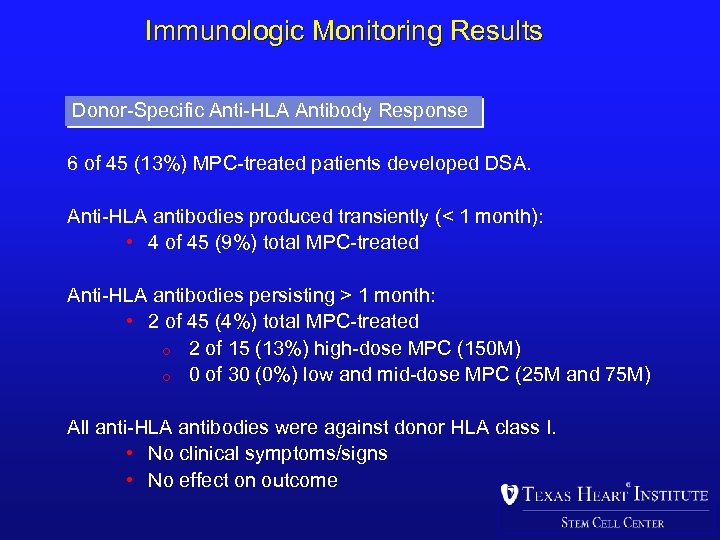 Immunologic Monitoring Results Donor-Specific Anti-HLA Antibody Response 6 of 45 (13%) MPC-treated patients developed DSA. Anti-HLA antibodies produced transiently (< 1 month): • 4 of 45 (9%) total MPC-treated Anti-HLA antibodies persisting > 1 month: • 2 of 45 (4%) total MPC-treated o 2 of 15 (13%) high-dose MPC (150 M) o 0 of 30 (0%) low and mid-dose MPC (25 M and 75 M) All anti-HLA antibodies were against donor HLA class I. • No clinical symptoms/signs • No effect on outcome
Immunologic Monitoring Results Donor-Specific Anti-HLA Antibody Response 6 of 45 (13%) MPC-treated patients developed DSA. Anti-HLA antibodies produced transiently (< 1 month): • 4 of 45 (9%) total MPC-treated Anti-HLA antibodies persisting > 1 month: • 2 of 45 (4%) total MPC-treated o 2 of 15 (13%) high-dose MPC (150 M) o 0 of 30 (0%) low and mid-dose MPC (25 M and 75 M) All anti-HLA antibodies were against donor HLA class I. • No clinical symptoms/signs • No effect on outcome
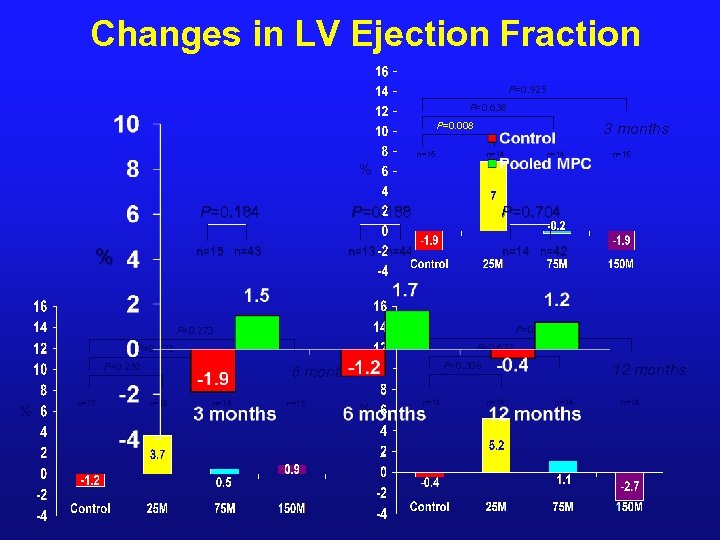 Changes in LV Ejection Fraction P=0. 925 P=0. 638 P=0. 008 3 months n=15 n=14 n=15 % P=0. 184 P=0. 704 n=15 n=43 % P=0. 188 n=13 n=44 n=14 n=42 P=0. 469 P=0. 273 P=0. 672 P=0. 472 P=0. 232 % n=13 P=0. 308 6 months n=15 n=14 n=15 % n=14 12 months n=14
Changes in LV Ejection Fraction P=0. 925 P=0. 638 P=0. 008 3 months n=15 n=14 n=15 % P=0. 184 P=0. 704 n=15 n=43 % P=0. 188 n=13 n=44 n=14 n=42 P=0. 469 P=0. 273 P=0. 672 P=0. 472 P=0. 232 % n=13 P=0. 308 6 months n=15 n=14 n=15 % n=14 12 months n=14
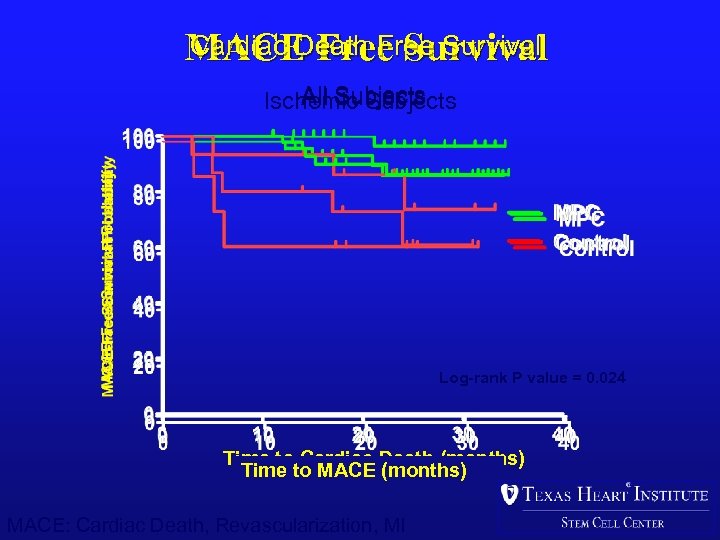 Cardiac Death Free Survival MACE Free Survival All Subjects Ischemic Subjects Log-rank P value = 0. 020 Log-rank P value = 0. 024 Log-rank P value=0. 036 Time to Cardiac Death (months) Time to MACE (months) MACE: Cardiac Death, Revascularization, MI
Cardiac Death Free Survival MACE Free Survival All Subjects Ischemic Subjects Log-rank P value = 0. 020 Log-rank P value = 0. 024 Log-rank P value=0. 036 Time to Cardiac Death (months) Time to MACE (months) MACE: Cardiac Death, Revascularization, MI
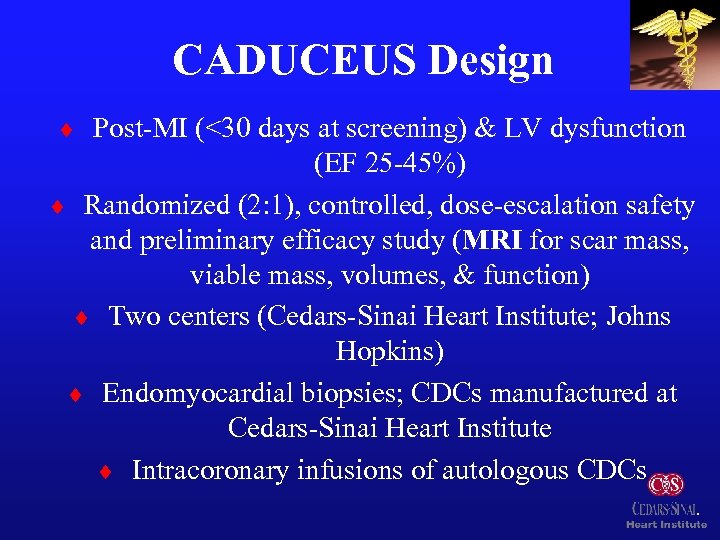 CADUCEUS Design ¨ Post-MI (<30 days at screening) & LV dysfunction (EF 25 -45%) ¨ Randomized (2: 1), controlled, dose-escalation safety and preliminary efficacy study (MRI for scar mass, viable mass, volumes, & function) ¨ Two centers (Cedars-Sinai Heart Institute; Johns Hopkins) ¨ Endomyocardial biopsies; CDCs manufactured at Cedars-Sinai Heart Institute ¨ Intracoronary infusions of autologous CDCs
CADUCEUS Design ¨ Post-MI (<30 days at screening) & LV dysfunction (EF 25 -45%) ¨ Randomized (2: 1), controlled, dose-escalation safety and preliminary efficacy study (MRI for scar mass, viable mass, volumes, & function) ¨ Two centers (Cedars-Sinai Heart Institute; Johns Hopkins) ¨ Endomyocardial biopsies; CDCs manufactured at Cedars-Sinai Heart Institute ¨ Intracoronary infusions of autologous CDCs
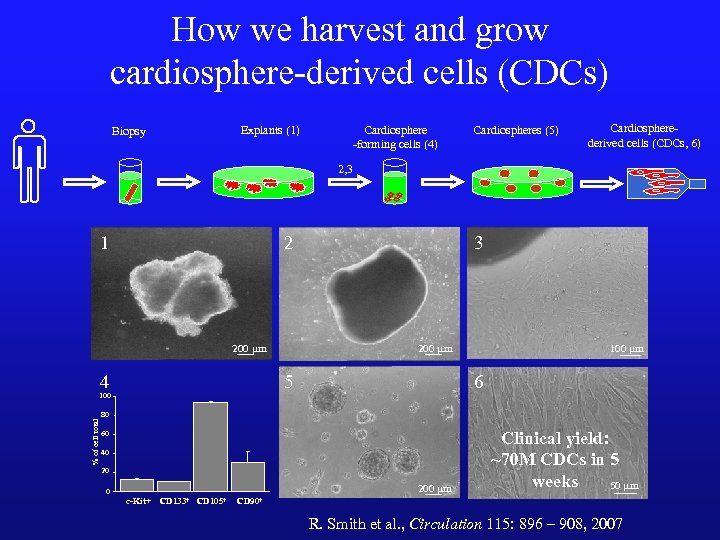 How we harvest and grow cardiosphere-derived cells (CDCs) Explants (1) Biopsy Cardiosphere -forming cells (4) Cardiospheres (5) Cardiospherederived cells (CDCs, 6) 2, 3 1 2 200 mm 4 5 100 % of cell total 3 100 mm 6 80 60 40 20 200 mm 0 c-Kit+ CD 133+ CD 105+ Clinical yield: ~70 M CDCs in 5 weeks 50 mm CD 90+ R. Smith et al. , Circulation 115: 896 – 908, 2007
How we harvest and grow cardiosphere-derived cells (CDCs) Explants (1) Biopsy Cardiosphere -forming cells (4) Cardiospheres (5) Cardiospherederived cells (CDCs, 6) 2, 3 1 2 200 mm 4 5 100 % of cell total 3 100 mm 6 80 60 40 20 200 mm 0 c-Kit+ CD 133+ CD 105+ Clinical yield: ~70 M CDCs in 5 weeks 50 mm CD 90+ R. Smith et al. , Circulation 115: 896 – 908, 2007
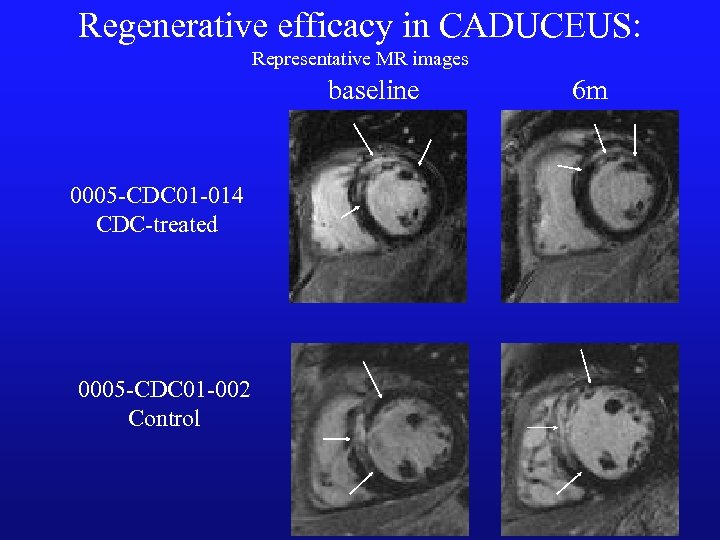 Regenerative efficacy in CADUCEUS: Representative MR images baseline 0005 -CDC 01 -014 CDC-treated 0005 -CDC 01 -002 Control 6 m
Regenerative efficacy in CADUCEUS: Representative MR images baseline 0005 -CDC 01 -014 CDC-treated 0005 -CDC 01 -002 Control 6 m
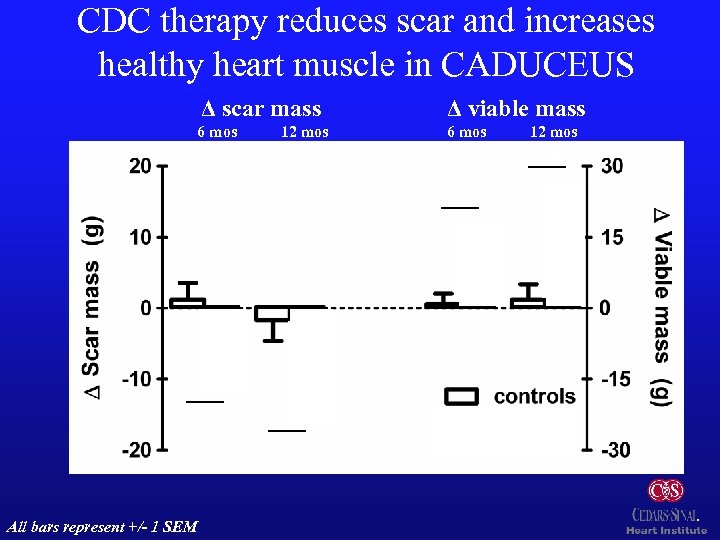 CDC therapy reduces scar and increases healthy heart muscle in CADUCEUS Δ scar mass Δ viable mass 6 mos 12 mos p=0. 001 p: 0. 01 p=0. 001 p: 0. 02 n=8 All bars represent +/- 1 SEM n=15 n=7 n=8
CDC therapy reduces scar and increases healthy heart muscle in CADUCEUS Δ scar mass Δ viable mass 6 mos 12 mos p=0. 001 p: 0. 01 p=0. 001 p: 0. 02 n=8 All bars represent +/- 1 SEM n=15 n=7 n=8
 SCIPIO ¨ Post-MI LV dysfunction undergoing CABG ¨ ¨ (EF<40%) Autologous cardiac stem cells (c-kit+) obtained at CABG from right atrium. Open label, “block randomization due to most patients wanting CSCs”? Cells expanded in Boston and delivered in Ky. IC infusion (133 days): up to 1 M cells Bolli, Lancet 2011
SCIPIO ¨ Post-MI LV dysfunction undergoing CABG ¨ ¨ (EF<40%) Autologous cardiac stem cells (c-kit+) obtained at CABG from right atrium. Open label, “block randomization due to most patients wanting CSCs”? Cells expanded in Boston and delivered in Ky. IC infusion (133 days): up to 1 M cells Bolli, Lancet 2011
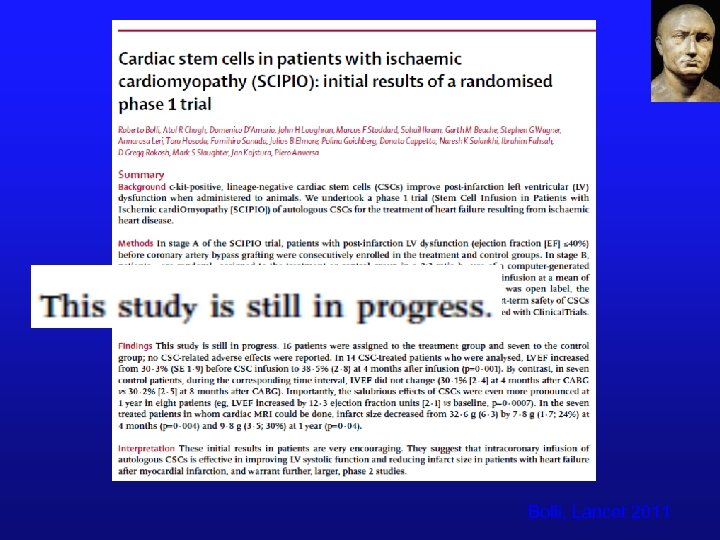 Bolli, Lancet 2011
Bolli, Lancet 2011
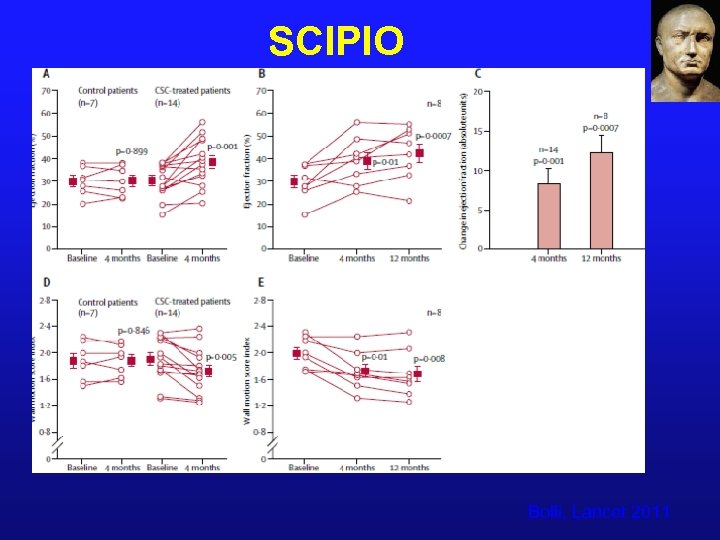 SCIPIO Bolli, Lancet 2011
SCIPIO Bolli, Lancet 2011
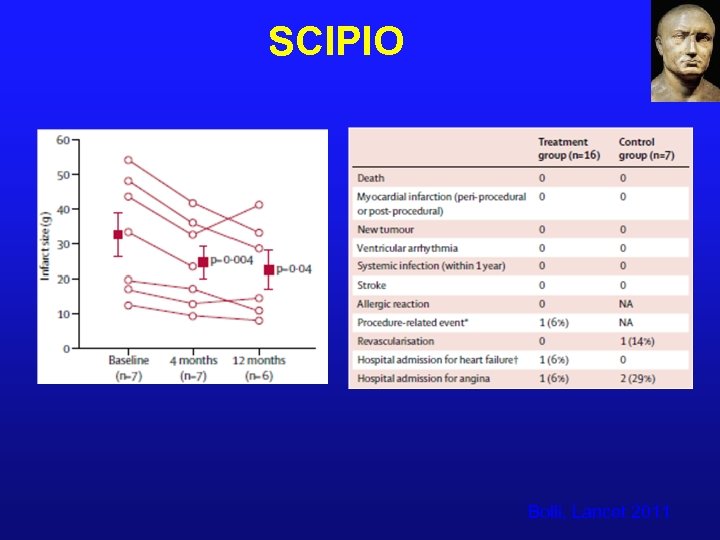 SCIPIO Bolli, Lancet 2011
SCIPIO Bolli, Lancet 2011
 So, where are we headed? ¨ BMCs-AMI ¨ Phase 3 BAMI in Europe-5 years ¨ TIME in 2012 ¨ BMCs-CHF ¨ FOCUS ACC 2012 ¨ CD 34+ for ischemia- ACT 34 ¨ MSCs- Ongoing and planned larger studies including C-Cure approach ¨ Cardiac derived cells- ALLSTAR ¨ CCTRN 2 - range of cells and targets
So, where are we headed? ¨ BMCs-AMI ¨ Phase 3 BAMI in Europe-5 years ¨ TIME in 2012 ¨ BMCs-CHF ¨ FOCUS ACC 2012 ¨ CD 34+ for ischemia- ACT 34 ¨ MSCs- Ongoing and planned larger studies including C-Cure approach ¨ Cardiac derived cells- ALLSTAR ¨ CCTRN 2 - range of cells and targets
 We still need better Options!! Will it be Cell Therapy? ?
We still need better Options!! Will it be Cell Therapy? ?


