cfb2f728d1590bfd2961e5e96c450aa1.ppt
- Количество слайдов: 35
Transcatheter Aortic-Valve Implantation for Degenerative Bioprosthetic Valves Results from a Global Valve-in-Valve Registry Ran Kornowski, MD, Danny Dvir, MD Rabin Medical Center, Israel On behalf of the global VIV Investigators

Ran Kornowski, MD I have no real or apparent conflicts of interest to report.
Valve in Valve Procedure - Background • Bioprosthetic valves commonly degenerate and fail within >10 years after implantation. • Transcatheter valve replacement procedure inside a degenerated bioprosthetic valve (i. e. VIV) becomes an alternative ‘off label’ TAVR approach to repeat surgery and is increasingly used among centers.
Valve in Valve Registry- Goal • To explore the safety and efficacy of the VIV technique using a large multicenter retrospective registry with predefined standardized CRFs and clinical endpoints.
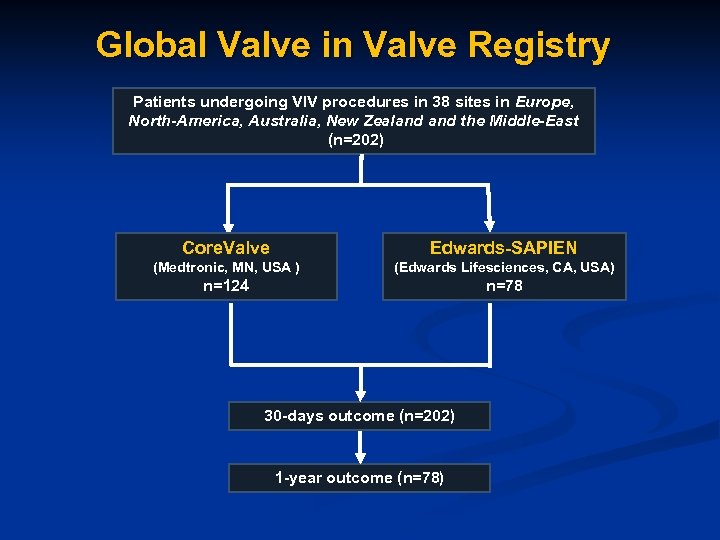
Global Valve in Valve Registry Patients undergoing VIV procedures in 38 sites in Europe, North-America, Australia, New Zealand the Middle-East (n=202) Core. Valve Edwards-SAPIEN (Medtronic, MN, USA ) (Edwards Lifesciences, CA, USA) n=124 n=78 30 -days outcome (n=202) 1 -year outcome (n=78)
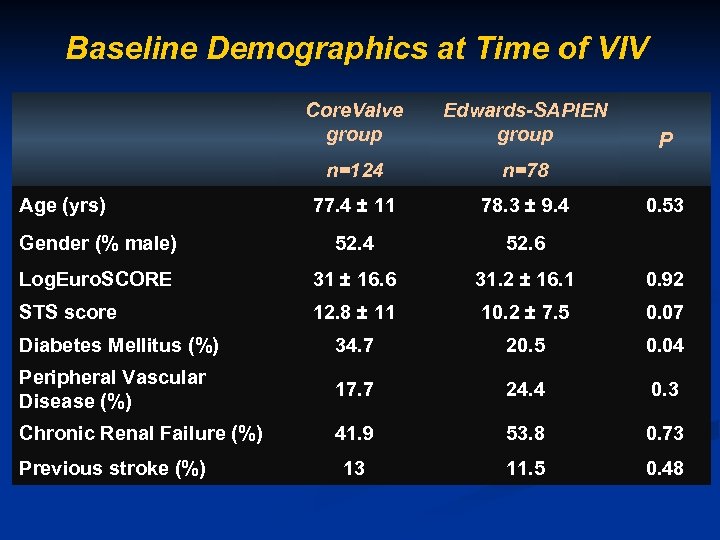
Baseline Demographics at Time of VIV Core. Valve group Edwards-SAPIEN group n=124 n=78 77. 4 ± 11 78. 3 ± 9. 4 Gender (% male) 52. 4 52. 6 Log. Euro. SCORE 31 ± 16. 6 31. 2 ± 16. 1 0. 92 STS score 12. 8 ± 11 10. 2 ± 7. 5 0. 07 Diabetes Mellitus (%) 34. 7 20. 5 0. 04 Peripheral Vascular Disease (%) 17. 7 24. 4 0. 3 Chronic Renal Failure (%) 41. 9 53. 8 0. 73 13 11. 5 0. 48 Age (yrs) Previous stroke (%) P 0. 53
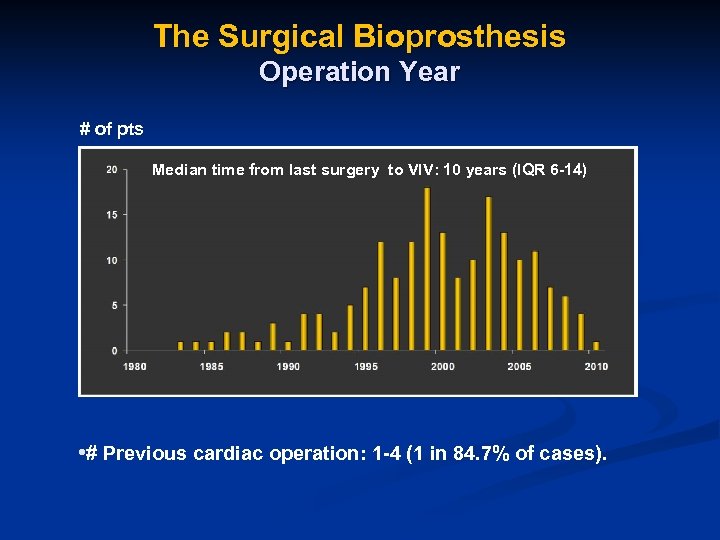
The Surgical Bioprosthesis Operation Year # of pts Median time from last surgery to VIV: 10 years (IQR 6 -14) • # Previous cardiac operation: 1 -4 (1 in 84. 7% of cases).
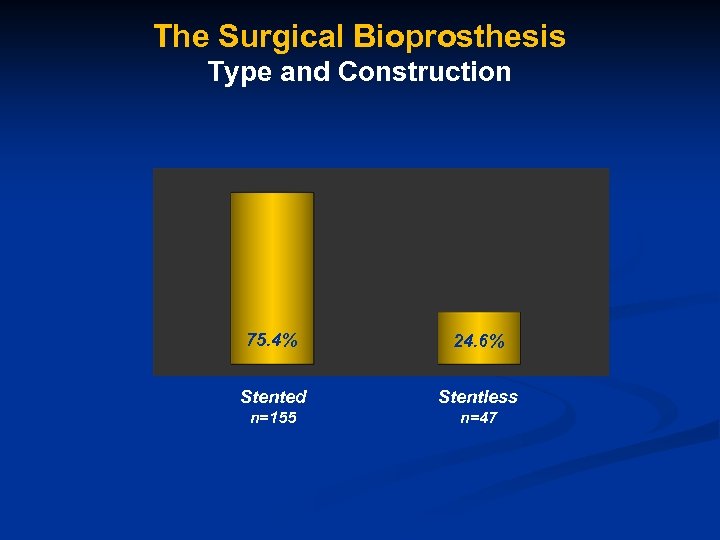
The Surgical Bioprosthesis Type and Construction 75. 4% 24. 6% Stented Stentless n=155 n=47
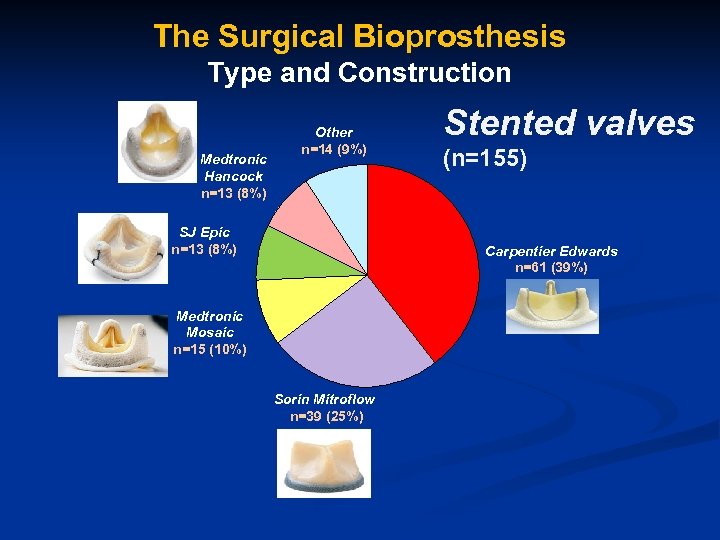
The Surgical Bioprosthesis Type and Construction Medtronic Hancock n=13 (8%) Other n=14 (9%) SJ Epic n=13 (8%) Stented valves (n=155) Carpentier Edwards n=61 (39%) Medtronic Mosaic n=15 (10%) Sorin Mitroflow n=39 (25%)
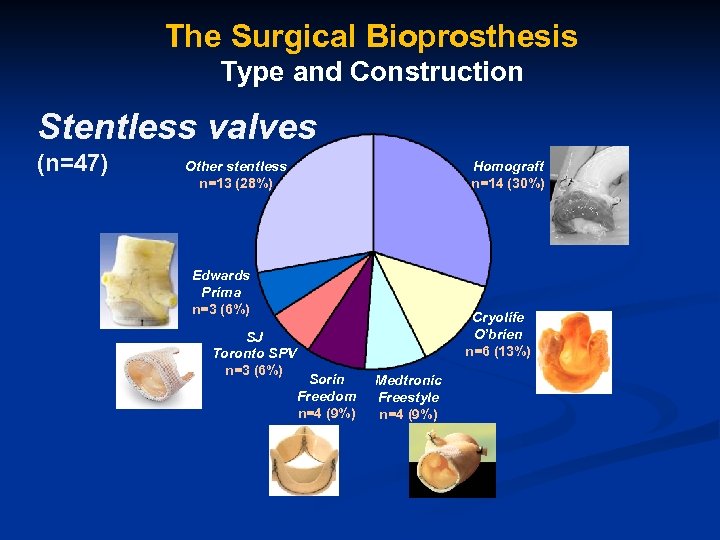
The Surgical Bioprosthesis Type and Construction Stentless valves (n=47) Other stentless n=13 (28%) Homograft n=14 (30%) Edwards Prima n=3 (6%) SJ Toronto SPV n=3 (6%) Cryolife O’brien n=6 (13%) Sorin Freedom n=4 (9%) Medtronic Freestyle n=4 (9%)
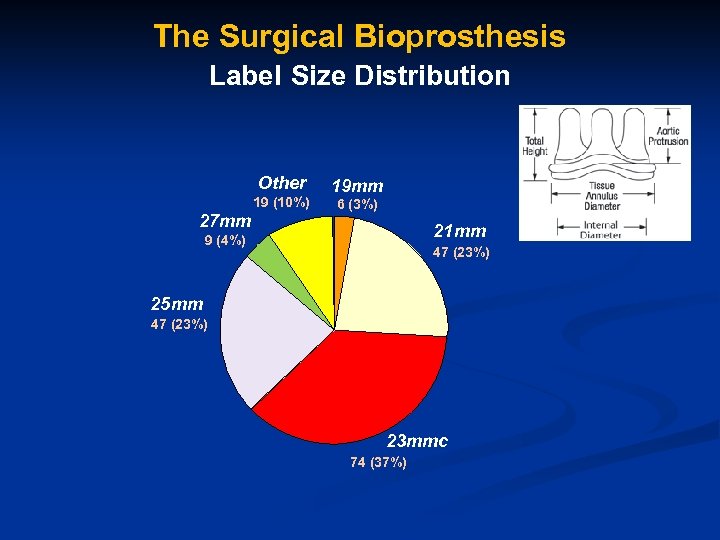
The Surgical Bioprosthesis Label Size Distribution Other 19 (10%) 27 mm 19 mm 6 (3%) 21 mm 9 (4%) 25 mm 47 (23%) 21 mm 25 mm 23 mmc 74 (37%)
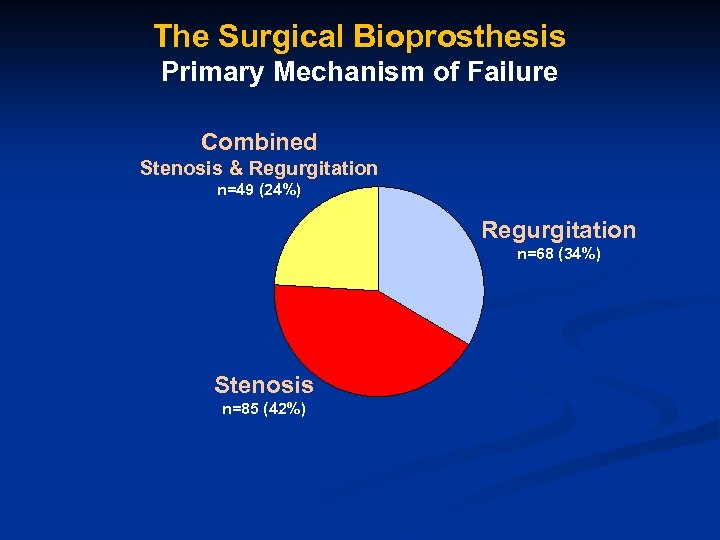
The Surgical Bioprosthesis Primary Mechanism of Failure Combined Stenosis & Regurgitation n=49 (24%) Regurgitation n=68 (34%) Stenosis n=85 (42%)
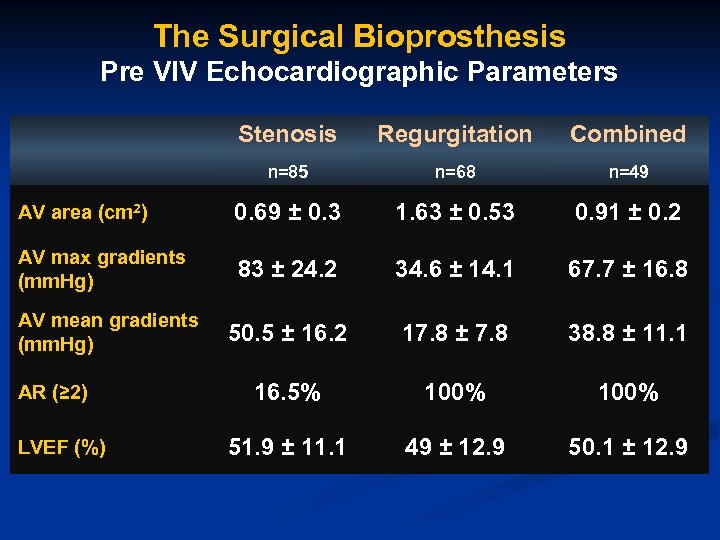
The Surgical Bioprosthesis Pre VIV Echocardiographic Parameters Stenosis Regurgitation Combined n=85 n=68 n=49 AV area (cm 2) 0. 69 ± 0. 3 1. 63 ± 0. 53 0. 91 ± 0. 2 AV max gradients (mm. Hg) 83 ± 24. 2 34. 6 ± 14. 1 67. 7 ± 16. 8 50. 5 ± 16. 2 17. 8 ± 7. 8 38. 8 ± 11. 1 16. 5% 100% 51. 9 ± 11. 1 49 ± 12. 9 50. 1 ± 12. 9 AV mean gradients (mm. Hg) AR (≥ 2) LVEF (%)
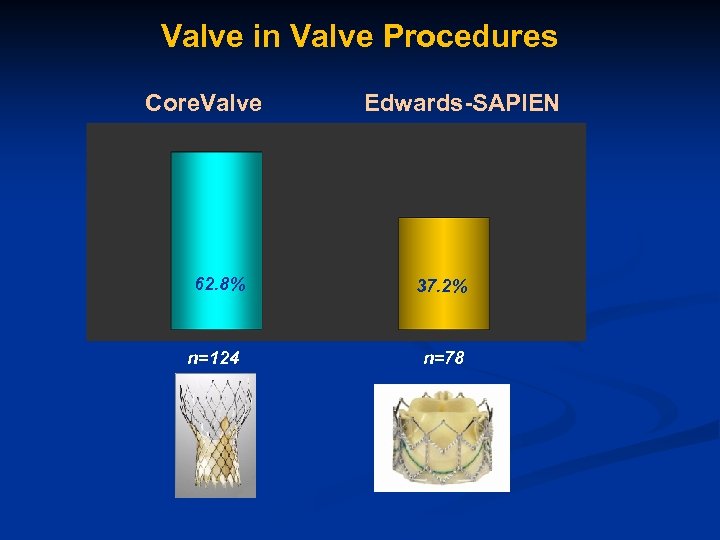
Valve in Valve Procedures Core. Valve 62. 8% n=124 Edwards-SAPIEN 37. 2% n=78
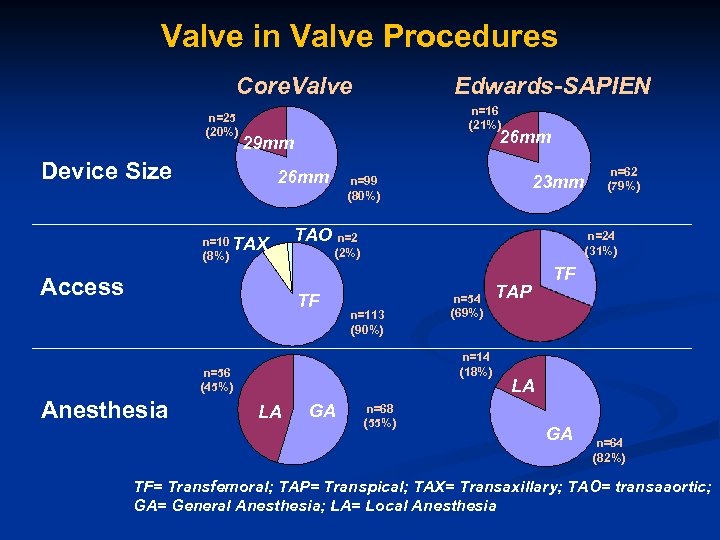
Valve in Valve Procedures Core. Valve n=25 (20%) Edwards-SAPIEN n=16 (21%) 26 mm 29 mm Device Size 26 mm n=10 TAX (8%) Access TAO n=2 n=113 (90%) n=54 (69%) n=14 (18%) n=56 (45%) LA GA n=62 (79%) n=24 (31%) (2%) TF Anesthesia 23 mm n=99 (80%) n=68 (55%) TAP TF LA GA n=64 (82%) TF= Transfemoral; TAP= Transpical; TAX= Transaxillary; TAO= transaaortic; GA= General Anesthesia; LA= Local Anesthesia
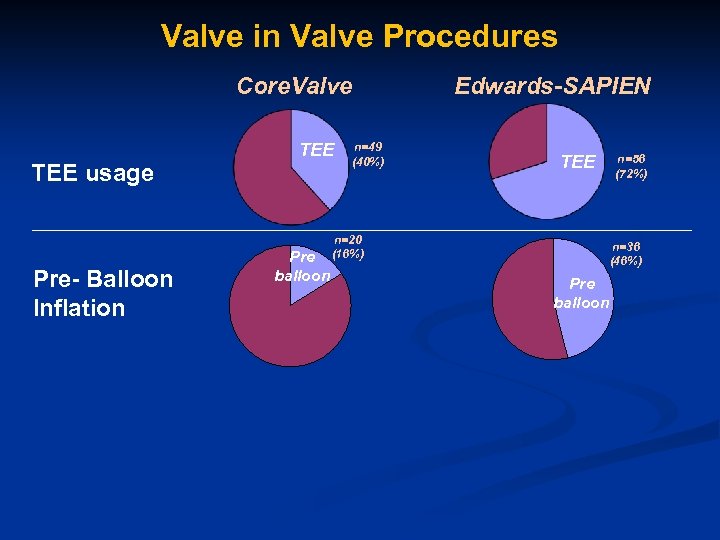
Valve in Valve Procedures Core. Valve TEE usage Pre- Balloon Inflation Pre balloon n=49 (40%) n=20 (16%) Edwards-SAPIEN TEE n=56 (72%) n=36 (46%) Pre balloon
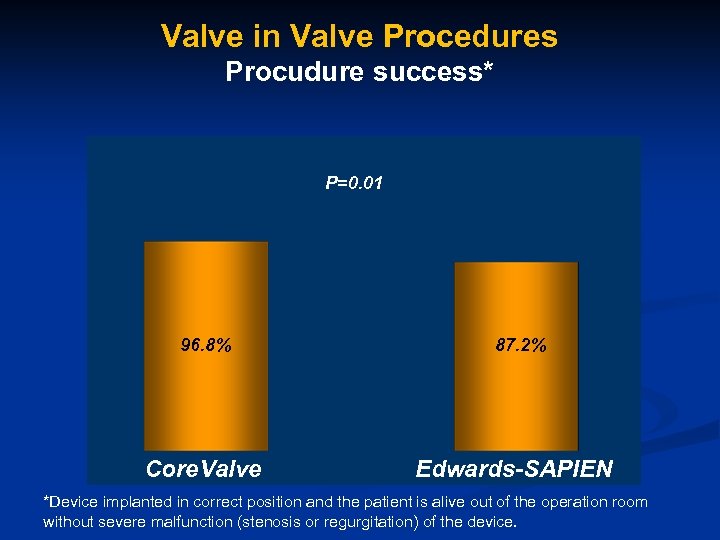
Valve in Valve Procedures Procudure success* P=0. 01 96. 8% Core. Valve 87. 2% Edwards-SAPIEN *Device implanted in correct position and the patient is alive out of the operation room without severe malfunction (stenosis or regurgitation) of the device.
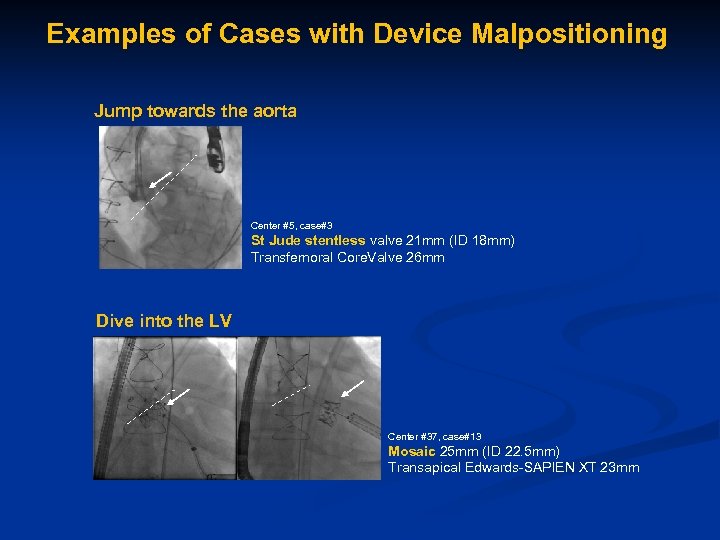
Examples of Cases with Device Malpositioning Jump towards the aorta Center #5, case#3 St Jude stentless valve 21 mm (ID 18 mm) Transfemoral Core. Valve 26 mm Dive into the LV Center #37, case#13 Mosaic 25 mm (ID 22. 5 mm) Transapical Edwards-SAPIEN XT 23 mm
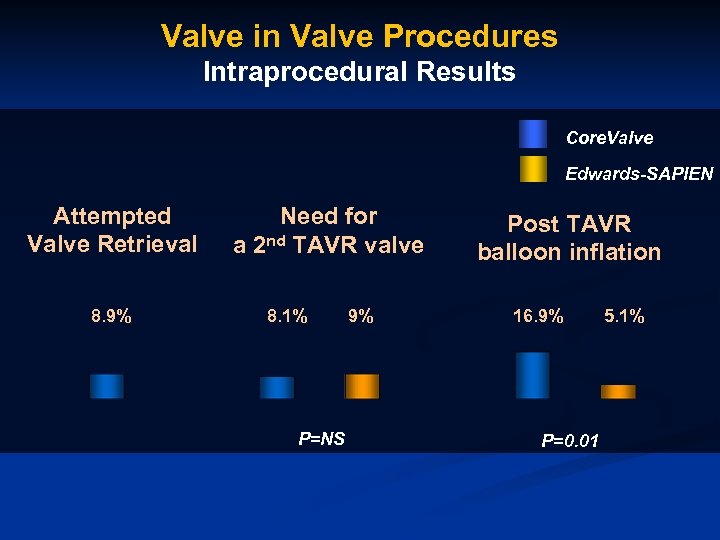
Valve in Valve Procedures Intraprocedural Results Core. Valve Edwards-SAPIEN Attempted Valve Retrieval 8. 9% Need for a 2 nd TAVR valve 8. 1% P=NS 9% Post TAVR balloon inflation 16. 9% P=0. 01 5. 1%
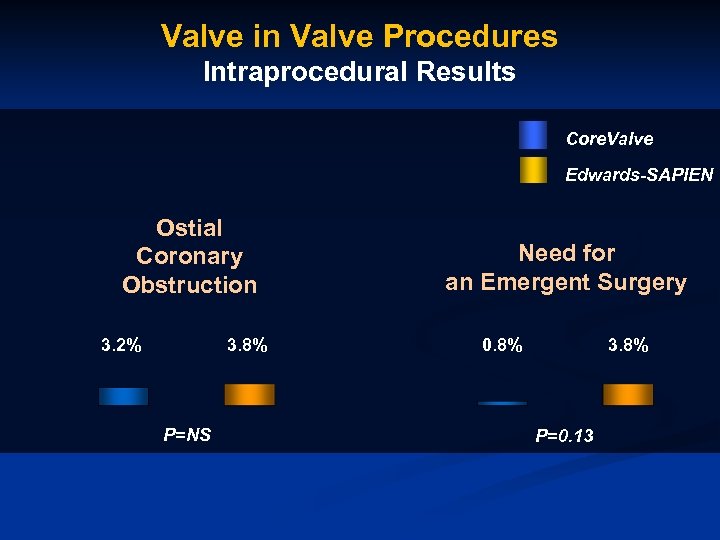
Valve in Valve Procedures Intraprocedural Results Core. Valve Edwards-SAPIEN Ostial Coronary Obstruction 3. 2% 3. 8% P=NS Need for an Emergent Surgery 0. 8% 3. 8% P=0. 13
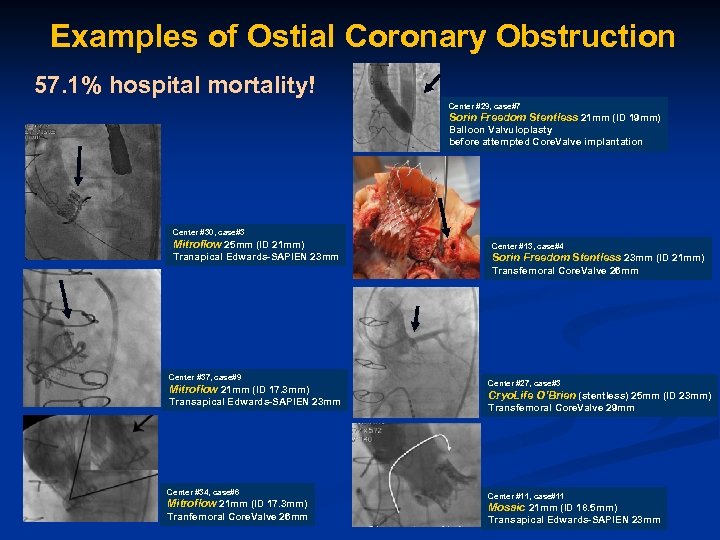
Examples of Ostial Coronary Obstruction 57. 1% hospital mortality! Center #29, case#7 Sorin Freedom Stentless 21 mm (ID 19 mm) Balloon Valvuloplasty before attempted Core. Valve implantation Center #30, case#3 Mitroflow 25 mm (ID 21 mm) Center #13, case#4 Tranapical Edwards-SAPIEN 23 mm Sorin Freedom Stentless 23 mm (ID 21 mm) Transfemoral Core. Valve 26 mm Center #37, case#9 Mitroflow 21 mm (ID 17. 3 mm) Transapical Edwards-SAPIEN 23 mm Center #34, case#6 Mitroflow 21 mm (ID 17. 3 mm) Tranfemoral Core. Valve 26 mm Center #27, case#3 Cryo. Life O’Brien (stentless) 25 mm (ID 23 mm) Transfemoral Core. Valve 29 mm Center #11, case#11 Mosaic 21 mm (ID 18. 5 mm) Transapical Edwards-SAPIEN 23 mm
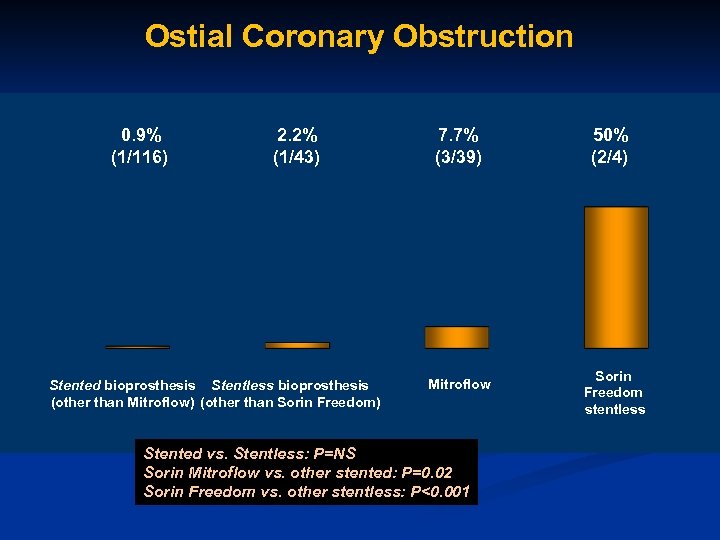
Ostial Coronary Obstruction 0. 9% (1/116) 2. 2% (1/43) Stented bioprosthesis Stentless bioprosthesis (other than Mitroflow) (other than Sorin Freedom) 7. 7% (3/39) Mitroflow Stented vs. Stentless: P=NS Sorin Mitroflow vs. other stented: P=0. 02 Sorin Freedom vs. other stentless: P<0. 001 50% (2/4) Sorin Freedom stentless
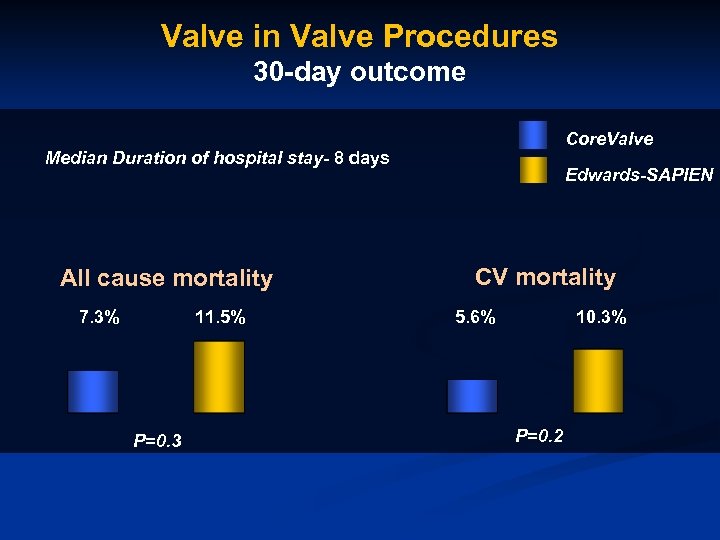
Valve in Valve Procedures 30 -day outcome Core. Valve Median Duration of hospital stay- 8 days All cause mortality 7. 3% 11. 5% P=0. 3 Edwards-SAPIEN CV mortality 5. 6% 10. 3% P=0. 2
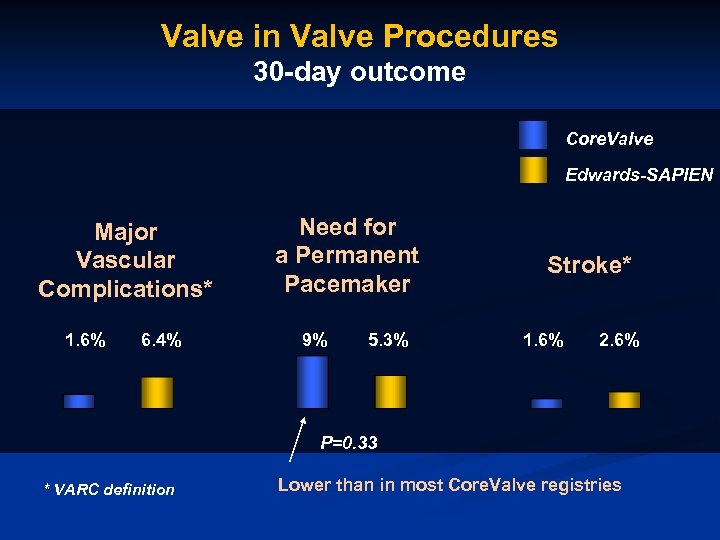
Valve in Valve Procedures 30 -day outcome Core. Valve Edwards-SAPIEN Major Vascular Complications* 1. 6% 6. 4% Need for a Permanent Pacemaker 9% 5. 3% Stroke* 1. 6% 2. 6% P=0. 33 * VARC definition Lower than in most Core. Valve registries
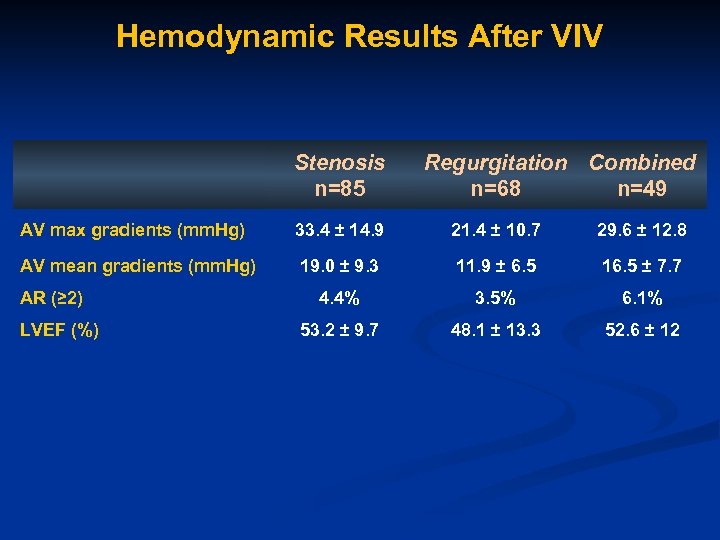
Hemodynamic Results After VIV Stenosis n=85 Regurgitation Combined n=68 n=49 AV max gradients (mm. Hg) 33. 4 ± 14. 9 21. 4 ± 10. 7 29. 6 ± 12. 8 AV mean gradients (mm. Hg) 19. 0 ± 9. 3 11. 9 ± 6. 5 16. 5 ± 7. 7 4. 4% 3. 5% 6. 1% 53. 2 ± 9. 7 48. 1 ± 13. 3 52. 6 ± 12 AR (≥ 2) LVEF (%)
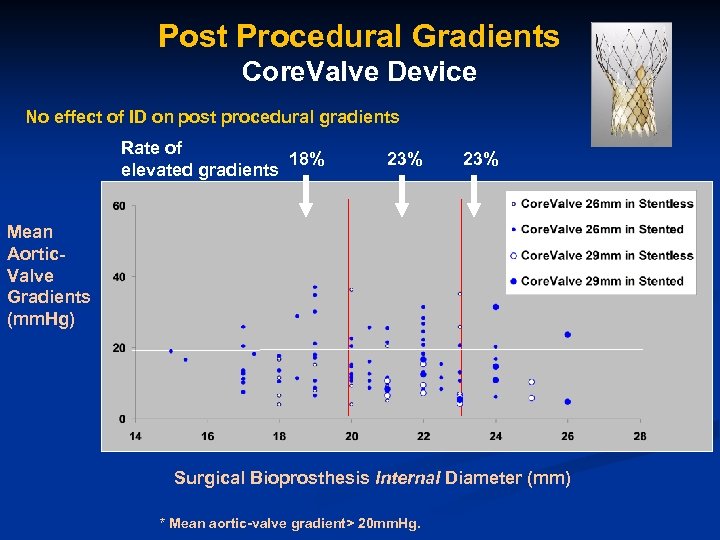
Post Procedural Gradients Core. Valve Device No effect of ID on post procedural gradients Rate of 18% elevated gradients 23% Mean Aortic. Valve Gradients (mm. Hg) Surgical Bioprosthesis Internal Diameter (mm) * Mean aortic-valve gradient> 20 mm. Hg.
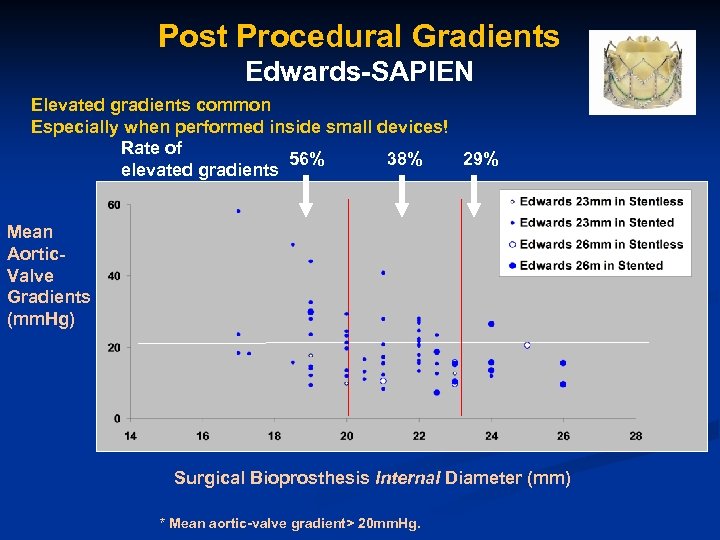
Post Procedural Gradients Edwards-SAPIEN Elevated gradients common Especially when performed inside small devices! Rate of 56% 38% 29% elevated gradients Mean Aortic. Valve Gradients (mm. Hg) Surgical Bioprosthesis Internal Diameter (mm) * Mean aortic-valve gradient> 20 mm. Hg.
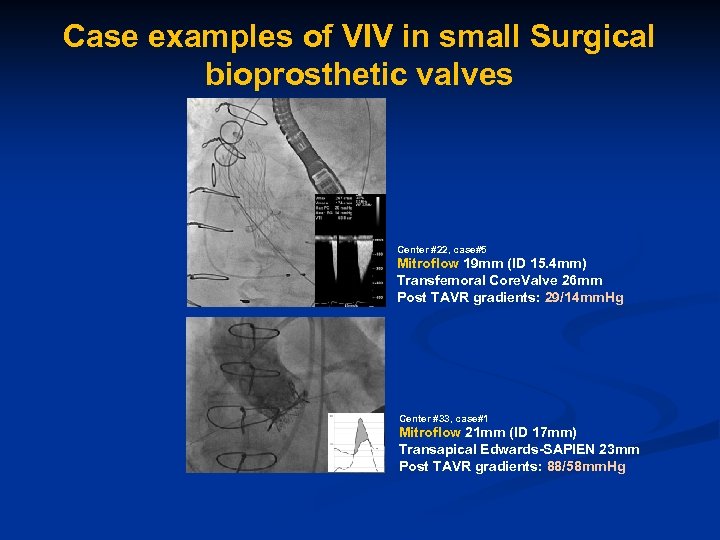
Case examples of VIV in small Surgical bioprosthetic valves Center #22, case#5 Mitroflow 19 mm (ID 15. 4 mm) Transfemoral Core. Valve 26 mm Post TAVR gradients: 29/14 mm. Hg Center #33, case#1 Mitroflow 21 mm (ID 17 mm) Transapical Edwards-SAPIEN 23 mm Post TAVR gradients: 88/58 mm. Hg
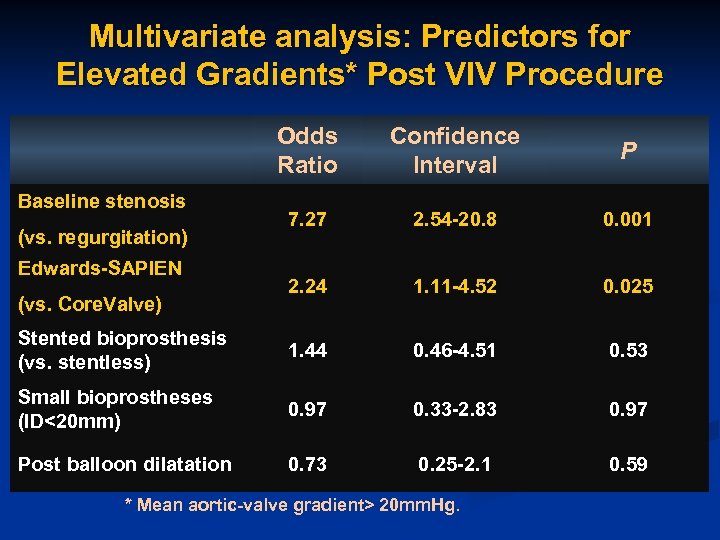
Multivariate analysis: Predictors for Elevated Gradients* Post VIV Procedure Odds Ratio Confidence Interval P 7. 27 2. 54 -20. 8 0. 001 2. 24 1. 11 -4. 52 0. 025 Stented bioprosthesis (vs. stentless) 1. 44 0. 46 -4. 51 0. 53 Small bioprostheses (ID<20 mm) 0. 97 0. 33 -2. 83 0. 97 Post balloon dilatation 0. 73 0. 25 -2. 1 0. 59 Baseline stenosis (vs. regurgitation) Edwards-SAPIEN (vs. Core. Valve) * Mean aortic-valve gradient> 20 mm. Hg.
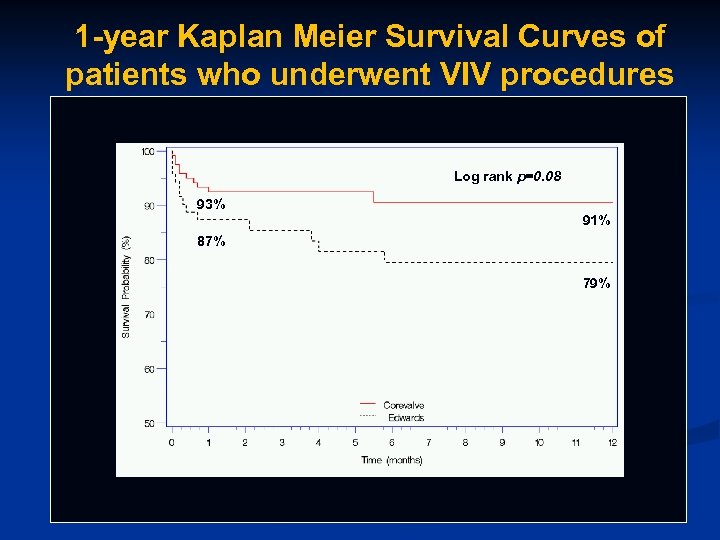
1 -year Kaplan Meier Survival Curves of patients who underwent VIV procedures Log rank p=0. 08 93% 91% 87% 79%
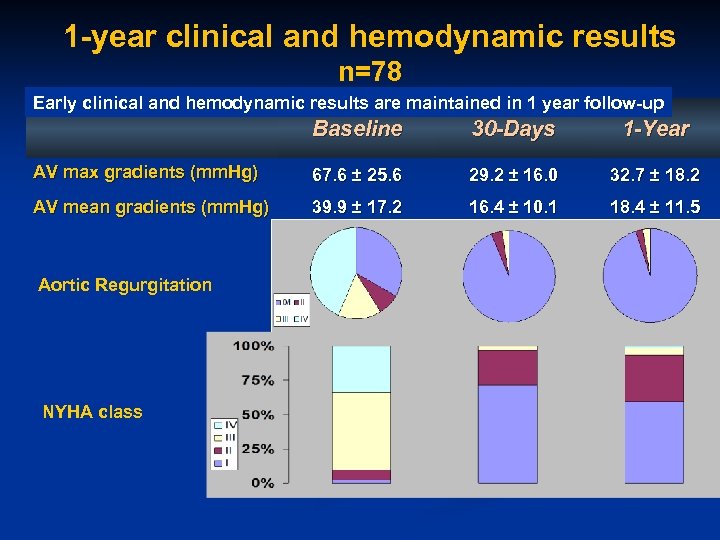
1 -year clinical and hemodynamic results n=78 Early clinical and hemodynamic results are maintained in 1 year follow-up Baseline 30 -Days 1 -Year AV max gradients (mm. Hg) 67. 6 ± 25. 6 29. 2 ± 16. 0 32. 7 ± 18. 2 AV mean gradients (mm. Hg) 39. 9 ± 17. 2 16. 4 ± 10. 1 18. 4 ± 11. 5 Aortic Regurgitation NYHA class
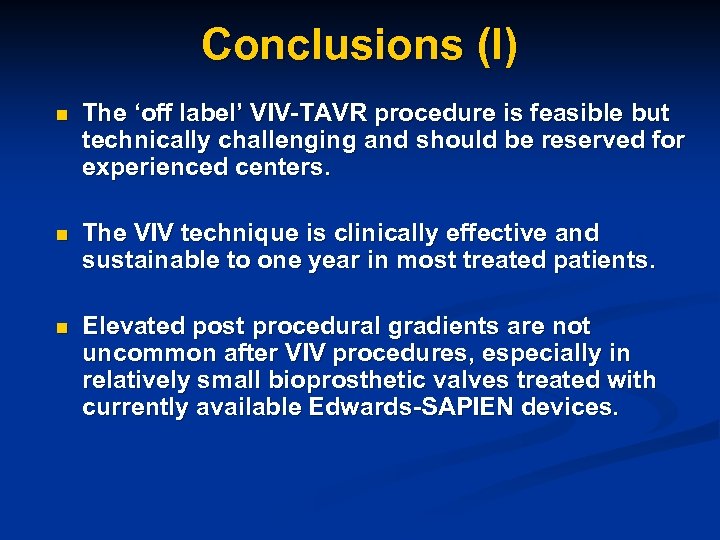
Conclusions (I) n The ‘off label’ VIV-TAVR procedure is feasible but technically challenging and should be reserved for experienced centers. n The VIV technique is clinically effective and sustainable to one year in most treated patients. n Elevated post procedural gradients are not uncommon after VIV procedures, especially in relatively small bioprosthetic valves treated with currently available Edwards-SAPIEN devices.

Conclusions (II) n The potential impact of elevated gradients on valve durability should be further examined. n Aorto-ostial coronary obstruction during VIV procedures is a concern, especially when performed in specific bioprosthetic valves. n New TAVR devices dedicated for the VIV procedures should be developed, to enable the treatment of small bioprosthetic valves with improved efficacy and safety.
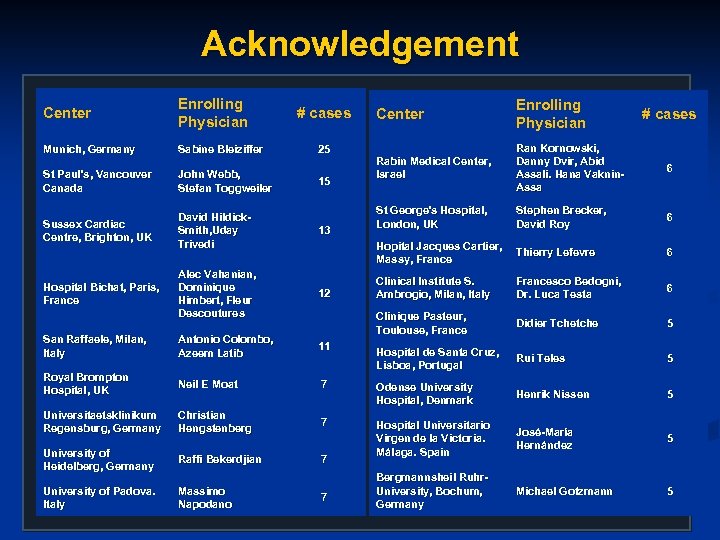
Acknowledgement Center Enrolling Physician Munich, Germany Sabine Bleiziffer St Paul’s, Vancouver Canada John Webb, Stefan Toggweiler Sussex Cardiac Centre, Brighton, UK David Hildick. Smith, Uday Trivedi 13 Alec Vahanian, Dominique Himbert, Fleur Descoutures 12 San Raffaele, Milan, Italy Antonio Colombo, Azeem Latib 11 Royal Brompton Hospital, UK Neil E Moat Universitaetsklinikum Regensburg, Germany Center Enrolling Physician Rabin Medical Center, Israel Ran Kornowski, Danny Dvir, Abid Assali. Hana Vaknin. Assa 6 St George's Hospital, London, UK Stephen Brecker, David Roy 6 Hopital Jacques Cartier, Massy, France Thierry Lefevre 6 Clinical Institute S. Ambrogio, Milan, Italy Francesco Bedogni, Dr. Luca Testa 6 Clinique Pasteur, Toulouse, France Didier Tchetche 5 Hospital de Santa Cruz, Lisboa, Portugal Rui Teles 5 7 Odense University Hospital, Denmark Henrik Nissen 5 Christian Hengstenberg 7 José-María Hernández 5 University of Heidelberg, Germany Raffi Bekerdjian 7 Hospital Universitario Virgen de la Victoria. Málaga. Spain University of Padova. Italy Massimo Napodano Bergmannsheil Ruhr. University, Bochum, Germany Michael Gotzmann 5 Hospital Bichat, Paris, France # cases 25 15 7 # cases
Acknowledgement # cases Ospedal Niguarda Ca'Granda-Milan, Italy Federico De Marco, Silvio Klugmann 4 Spedali Civili Brescia, Spain Ettori Federica, Claudia Fiorina 4 Bristol heart Institute, UK Andreas Baumbach, Ali Khavandi, Mark Turner 4 Sheba Medical Center, Israel Victor Guetta, Amit Segev 4 Rangueil University Hospital, Toulouse, France Nicolas Dumonteil Segeberger Kliniken Gmb. H, Bad Segeberg Germany Mohamed Abdel. Wahab 4 4 Enrolling Physician ICLAS Rapallo, Italy Paolo Pantaleo 2 Azienda Ospedaliero. Universitaria di Bologna Policlinico Sant'Orsola Malpighi, Bologna, Italy Antonio Marzocchi 2 Hamilton hospital WDHB, New Zealand Sanjeevan Pasupati 2 Pisa, Italy Anna Sonia Petronio 2 Medizinische Klinil II, Universitatsklinikum Bonn, Germany Georg Nickenig, Jan-Malte Sinning 2 Michael Grund 1 Ayred Hospital Melbourn, Australia Antony Walton 1 Blackpool, UK Enrolling Physician Center AKH Linz, Austria Center David H Roberts 1 # cases Sahlgrenska University Hospital, Gothenburg, Sweden Dan Ioanes 3 Cardiocentre Praha Czech rep Viktor Kocka 1 University Hospital Duesseldorf, Germany Marc. W. Merx, M. Kelm 3 University Hospital of Geneva, Switzerland Stephane Noble 1 Azienda Policlinico Vittorio Emanuele, Catania, Italy Marco Barbanti, Gian Paolo Ussia 3 Silesian Center for Heart Diseases in Zabrze, Poland Piotr Chodor 1
cfb2f728d1590bfd2961e5e96c450aa1.ppt