e33710810cb049db45f842da7351d33c.ppt
- Количество слайдов: 29

Transcather Aortic Valve Replacement Using the Self-Expanding Bioprosthesis: First Report Using STS/ACC Transcatheter Valve Therapy Registry Core. Valve Data Jeffrey J. Popma, M. D. Beth Israel Deaconess Medical Center, Boston, MA

TVT Core. Valve Collaborators David Adams | Mt. Sinai, New York City Stan Chetcuti | University of MI, Ann Arbor James Hermiller | St. Vincent’s, Indianapolis Susheel Kodali | Columbia, New York City Jeffrey Popma | Beth Israel, Boston Michael Reardon | Methodist De. Bakey, Houston Paul Sorajja | Abbott Northwestern Hospital, Minneapolis Wilson Szeto | University of PA, Philadelphia *not affiliated with the STS/ACC TVT-R Research and Publications Committee 2

Funding Support and Disclaimer This research was conducted by Medtronic, independent of the STS and ACC, using a cohort of deidentified data for Core. Valve cases from the STS/ACC TVT Registry. The views expressed in this presentation represent those of the author(s).

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship • Institutional Grants Medical Advisory Board • Institutional Grants, Consultant • Institutional Grants Medical Advisory Board Company • Medtronic • Boston Scientific • Direct Flow Medical • Abbot Vascular

Background • The STS/ACC Transcatheter Valve Therapy (STS/ACC TVT) national registry was initiated in 2011 in collaboration with the FDA, the Center for Medicare and Medicaid Services and the Duke Clinical Research Institute • The STS/ACC TVT Registry™ provides: – Data repository and reporting infrastructure to monitor the safety and effectiveness of TVT devices – Real world data to confirm consistent safety and effectiveness of TAVR to compare with results from controlled clinical trials. Carroll JD, et al. JACC 2013; 62: 1026 -34.

Objectives • To report the 30 -day and 1 year clinical outcome of patients treated with the Core. Valve self-expanding bioprostheses in “real world” clinical practice • To broadly compare these findings with the baseline characteristics and clinical results obtained in the US Core. Valve Pivotal Trials
Methods • Patients with severe aortic stenosis undergoing TAVR with the Core. Valve bioprosthesis captured in the STS/ACC TVT Registry (TVT-R) • Data were collected using standardized definitions harmonized with the STS National Database and the ACC NCDR • Patient characteristics, procedural details, quality of life and clinical outcomes were collected • Study inclusion: January 2014 to March 2015 • 6160 patients treated at 214 clinical centers
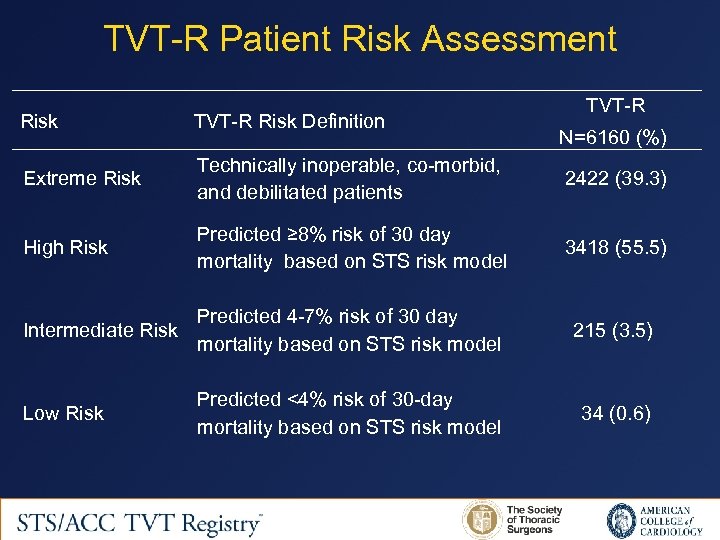
TVT-R Patient Risk Assessment TVT-R Risk Definition Extreme Risk Technically inoperable, co-morbid, and debilitated patients 2422 (39. 3) High Risk Predicted ≥ 8% risk of 30 day mortality based on STS risk model 3418 (55. 5) N=6160 (%) Predicted 4 -7% risk of 30 day Intermediate Risk mortality based on STS risk model 215 (3. 5) Predicted <4% risk of 30 -day mortality based on STS risk model 34 (0. 6) Low Risk

US Core. Valve Pivotal Trials • To broadly compare the results of TVT-R Core. Valve data with outcomes documented in the US Pivotal Trials: – Extreme Risk Patients (N=639; 62%): ≥ 50% risk for mortality or irreversible morbidity at 30 days with SAVR 1 – High Risk Patients (N=391; 38%): ≥ 15% risk for mortality and <50% risk for death or morbidity at 30 days with SAVR 2 • Because different patient populations were enrolled in the TVT-R and US Pivotal studies, no statistical comparisons were made 1 Popma J. JACC 2014; 63: 1972– 81. 2 Adams D. NEJM 2014; 370: 1790 -8.
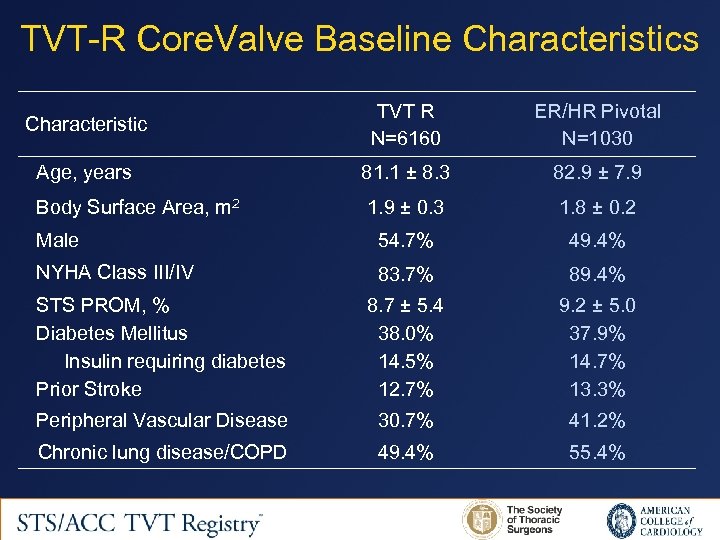
TVT-R Core. Valve Baseline Characteristics Characteristic TVT R N=6160 ER/HR Pivotal N=1030 Age, years 81. 1 ± 8. 3 82. 9 ± 7. 9 Body Surface Area, m 2 1. 9 ± 0. 3 1. 8 ± 0. 2 Male 54. 7% 49. 4% NYHA Class III/IV 83. 7% 89. 4% STS PROM, % Diabetes Mellitus Insulin requiring diabetes Prior Stroke 8. 7 ± 5. 4 38. 0% 14. 5% 12. 7% 9. 2 ± 5. 0 37. 9% 14. 7% 13. 3% Peripheral Vascular Disease 30. 7% 41. 2% Chronic lung disease/COPD 49. 4% 55. 4%
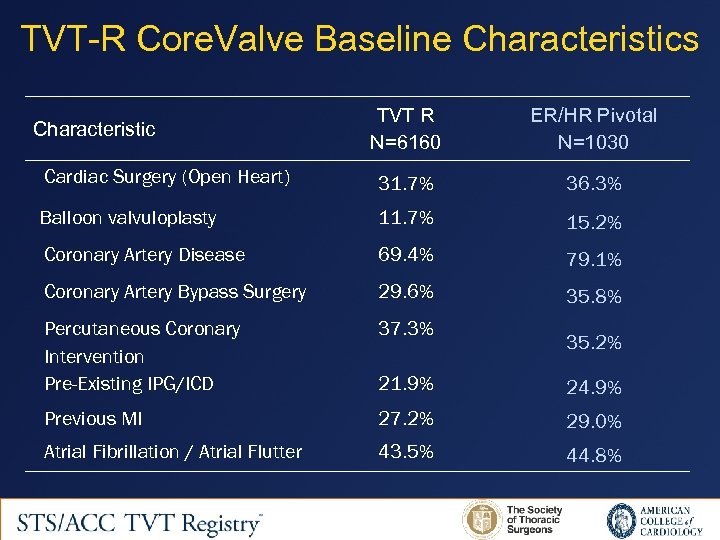
TVT-R Core. Valve Baseline Characteristics TVT R N=6160 ER/HR Pivotal N=1030 Cardiac Surgery (Open Heart) 31. 7% 36. 3% Balloon valvuloplasty 11. 7% 15. 2% Coronary Artery Disease 69. 4% 79. 1% Coronary Artery Bypass Surgery 29. 6% 35. 8% Percutaneous Coronary Intervention Pre-Existing IPG/ICD 37. 3% 21. 9% 24. 9% Previous MI 27. 2% 29. 0% Atrial Fibrillation / Atrial Flutter 43. 5% 44. 8% Characteristic 35. 2%
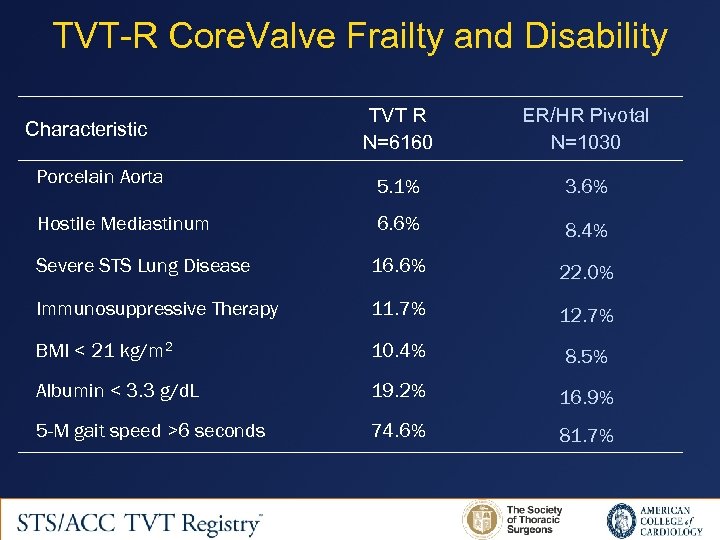
TVT-R Core. Valve Frailty and Disability TVT R N=6160 ER/HR Pivotal N=1030 Porcelain Aorta 5. 1% 3. 6% Hostile Mediastinum 6. 6% 8. 4% Severe STS Lung Disease 16. 6% 22. 0% Immunosuppressive Therapy 11. 7% 12. 7% BMI < 21 kg/m 2 10. 4% 8. 5% Albumin < 3. 3 g/d. L 19. 2% 16. 9% 5 -M gait speed >6 seconds 74. 6% 81. 7% Characteristic
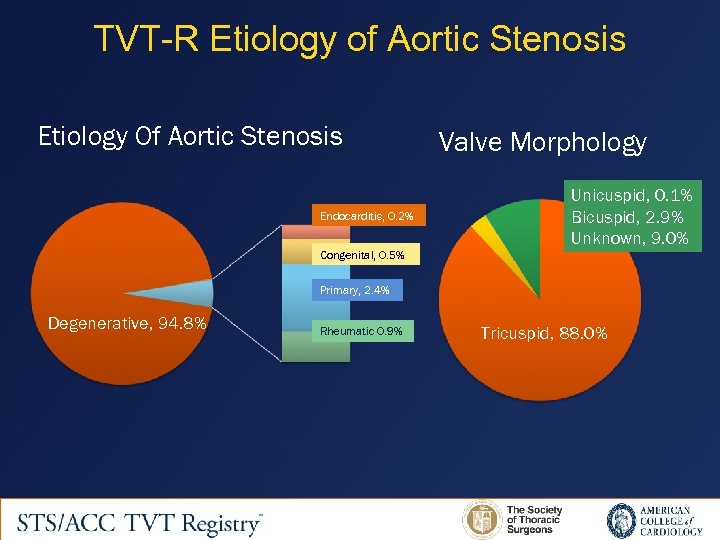
TVT-R Etiology of Aortic Stenosis Etiology Of Aortic Stenosis Endocarditis, 0. 2% Congenital, 0. 5% Valve Morphology Unicuspid, 0. 1% Bicuspid, 2. 9% Unknown, 9. 0% Primary, 2. 4% Degenerative, 94. 8% Rheumatic 0. 9% Tricuspid, 88. 0%
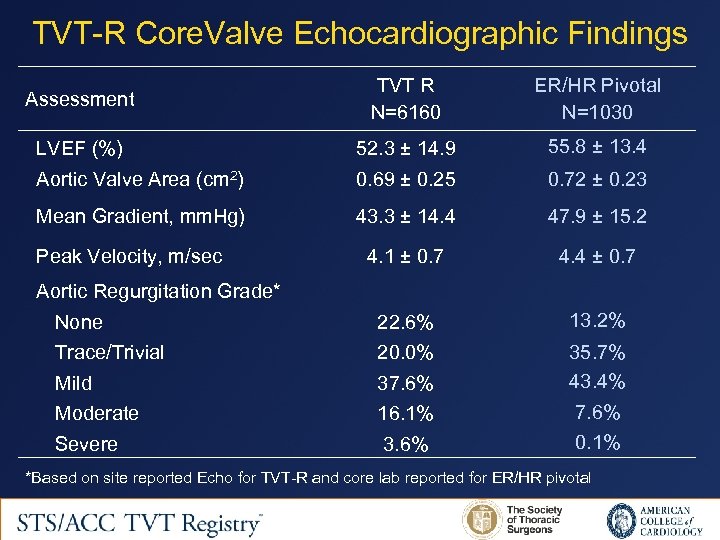
TVT-R Core. Valve Echocardiographic Findings Assessment TVT R N=6160 ER/HR Pivotal N=1030 LVEF (%) 52. 3 ± 14. 9 55. 8 ± 13. 4 Aortic Valve Area (cm 2) 0. 69 ± 0. 25 0. 72 ± 0. 23 Mean Gradient, mm. Hg) 43. 3 ± 14. 4 47. 9 ± 15. 2 4. 1 ± 0. 7 4. 4 ± 0. 7 None 22. 6% 13. 2% Trace/Trivial 20. 0% Mild 37. 6% 35. 7% 43. 4% Moderate 16. 1% 7. 6% Severe 3. 6% 0. 1% Peak Velocity, m/sec Aortic Regurgitation Grade* *Based on site reported Echo for TVT-R and core lab reported for ER/HR pivotal
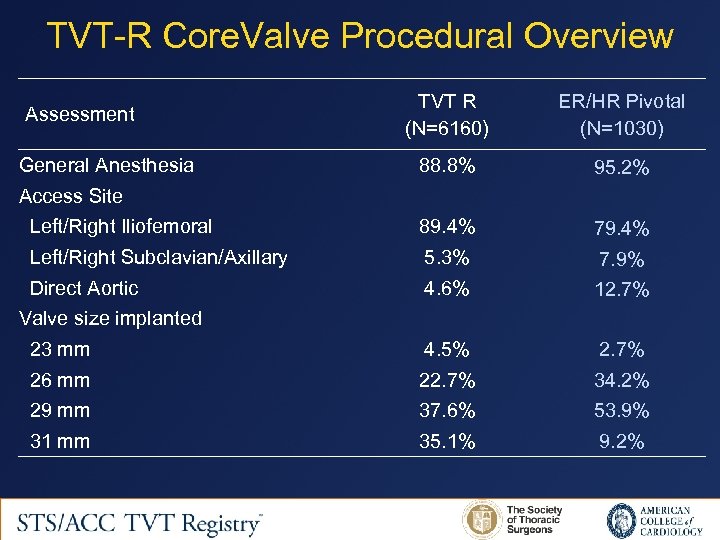
TVT-R Core. Valve Procedural Overview TVT R (N=6160) ER/HR Pivotal (N=1030) 88. 8% 95. 2% Left/Right Iliofemoral 89. 4% 79. 4% Left/Right Subclavian/Axillary 5. 3% 7. 9% Direct Aortic 4. 6% 12. 7% 23 mm 4. 5% 2. 7% 26 mm 22. 7% 34. 2% 29 mm 37. 6% 53. 9% 31 mm 35. 1% 9. 2% Assessment General Anesthesia Access Site Valve size implanted
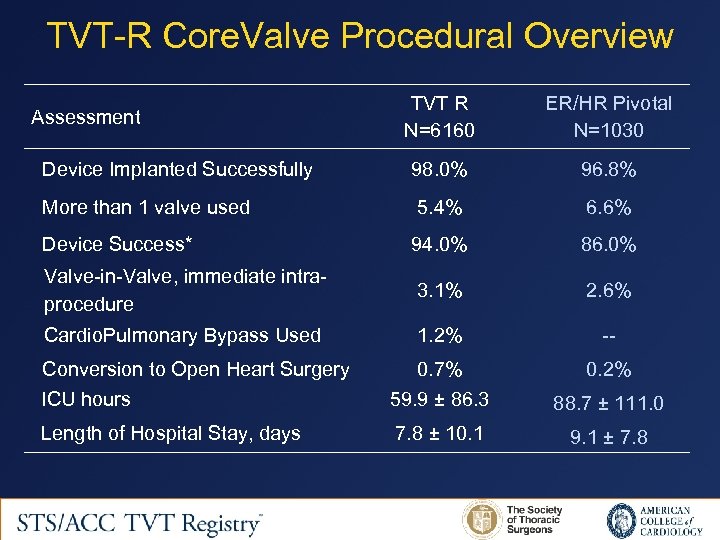
TVT-R Core. Valve Procedural Overview TVT R N=6160 ER/HR Pivotal N=1030 Device Implanted Successfully 98. 0% 96. 8% More than 1 valve used 5. 4% 6. 6% Device Success* 94. 0% 86. 0% Valve-in-Valve, immediate intraprocedure 3. 1% 2. 6% Cardio. Pulmonary Bypass Used 1. 2% -- Conversion to Open Heart Surgery 0. 7% 0. 2% ICU hours 59. 9 ± 86. 3 88. 7 ± 111. 0 Length of Hospital Stay, days 7. 8 ± 10. 1 9. 1 ± 7. 8 Assessment
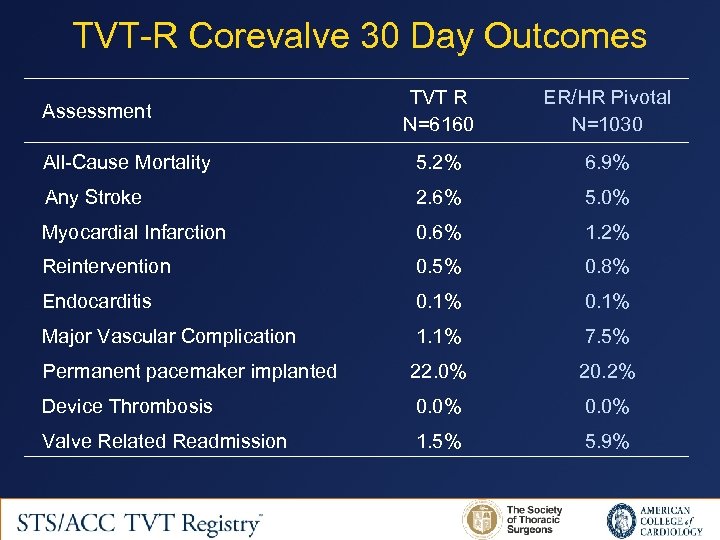
TVT-R Corevalve 30 Day Outcomes TVT R N=6160 ER/HR Pivotal N=1030 All-Cause Mortality 5. 2% 6. 9% Any Stroke 2. 6% 5. 0% Myocardial Infarction 0. 6% 1. 2% Reintervention 0. 5% 0. 8% Endocarditis 0. 1% Major Vascular Complication 1. 1% 7. 5% Permanent pacemaker implanted 22. 0% 20. 2% Device Thrombosis 0. 0% Valve Related Readmission 1. 5% 5. 9% Assessment
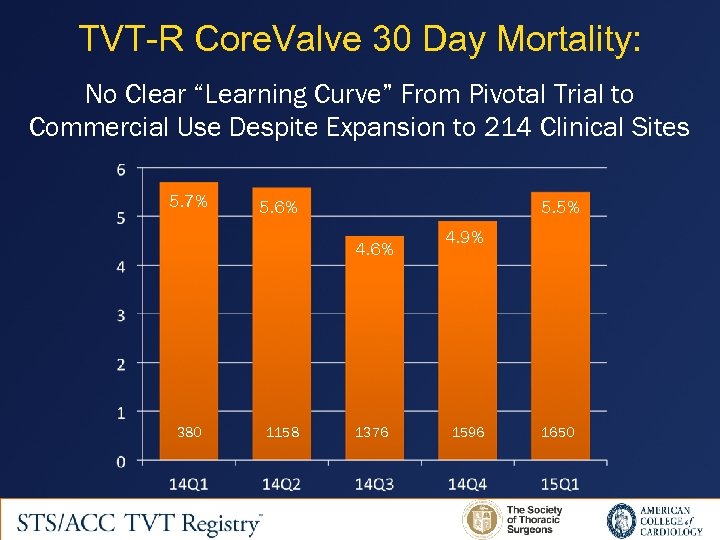
TVT-R Core. Valve 30 Day Mortality: No Clear “Learning Curve” From Pivotal Trial to Commercial Use Despite Expansion to 214 Clinical Sites 5. 7% 5. 6% 5. 5% 4. 6% 380 1158 1376 4. 9% 1596 1650
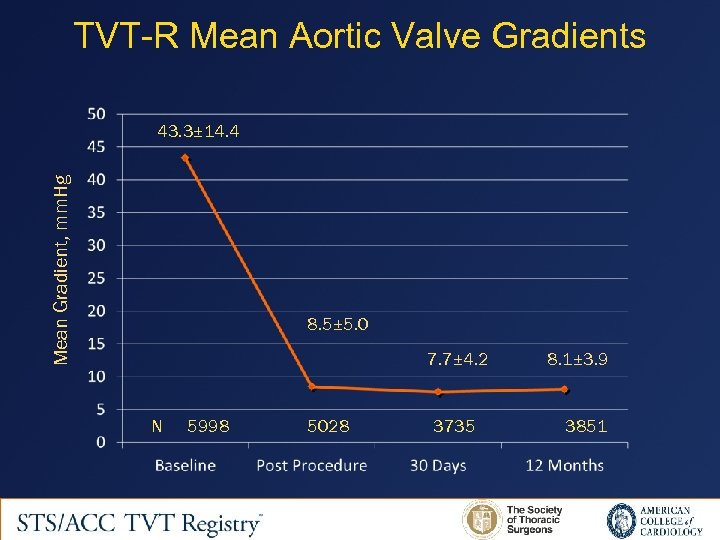
TVT-R Mean Aortic Valve Gradients Mean Gradient, mm. Hg 43. 3± 14. 4 8. 5± 5. 0 7. 7± 4. 2 N 5998 5028 3735 8. 1± 3. 9 3851
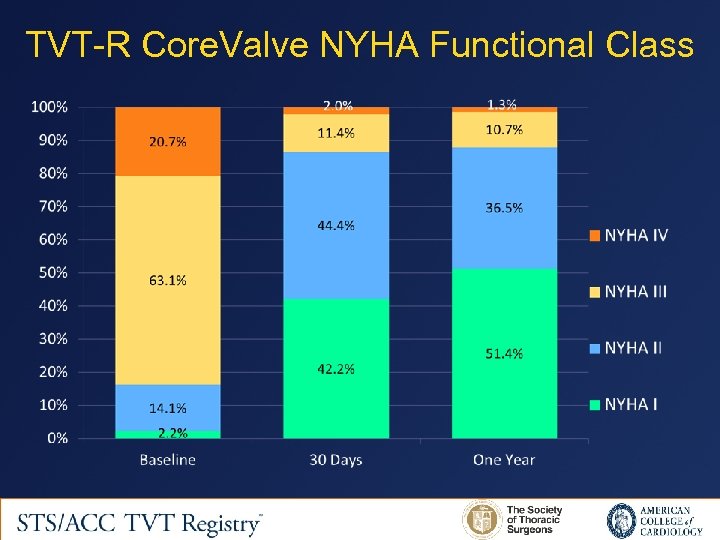
TVT-R Core. Valve NYHA Functional Class
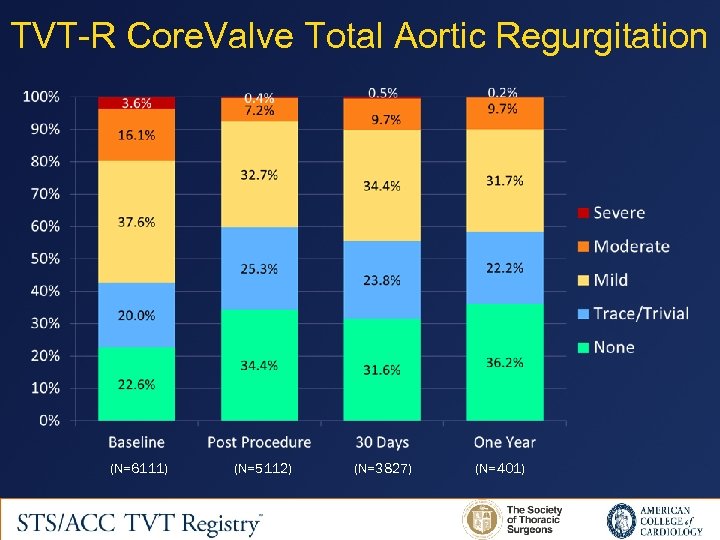
TVT-R Core. Valve Total Aortic Regurgitation (N=6111) (N=5112) (N=3827) (N=401)
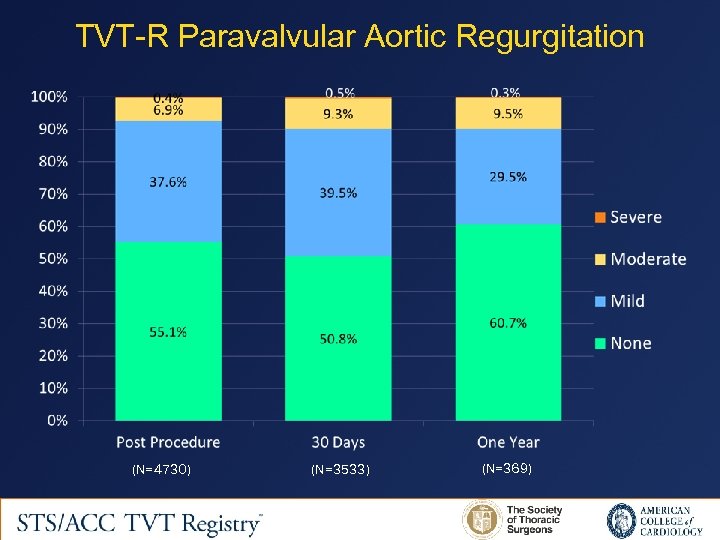
TVT-R Paravalvular Aortic Regurgitation (N=4730) (N=3533) (N=369)
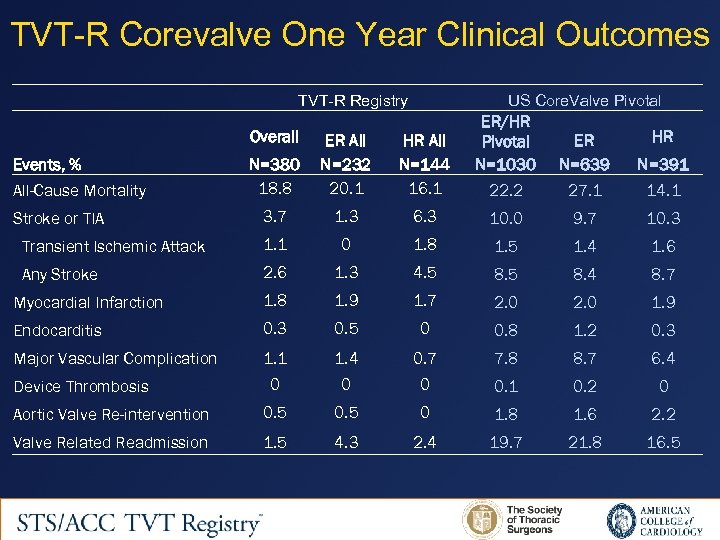
TVT-R Corevalve One Year Clinical Outcomes TVT-R Registry US Core. Valve Pivotal N=380 18. 8 ER All N=232 20. 1 HR All N=144 16. 1 ER/HR Pivotal N=1030 22. 2 3. 7 1. 3 6. 3 10. 0 9. 7 10. 3 Transient Ischemic Attack 1. 1 0 1. 8 1. 5 1. 4 1. 6 Any Stroke 2. 6 1. 3 4. 5 8. 4 8. 7 Myocardial Infarction 1. 8 1. 9 1. 7 2. 0 1. 9 Endocarditis 0. 3 0. 5 0 0. 8 1. 2 0. 3 Major Vascular Complication 1. 1 1. 4 0. 7 7. 8 8. 7 6. 4 0 0. 1 0. 2 0 Aortic Valve Re-intervention 0. 5 0 1. 8 1. 6 2. 2 Valve Related Readmission 1. 5 4. 3 2. 4 19. 7 21. 8 16. 5 Overall Events, % All-Cause Mortality Stroke or TIA Device Thrombosis HR ER N=639 N=391 27. 1 14. 1
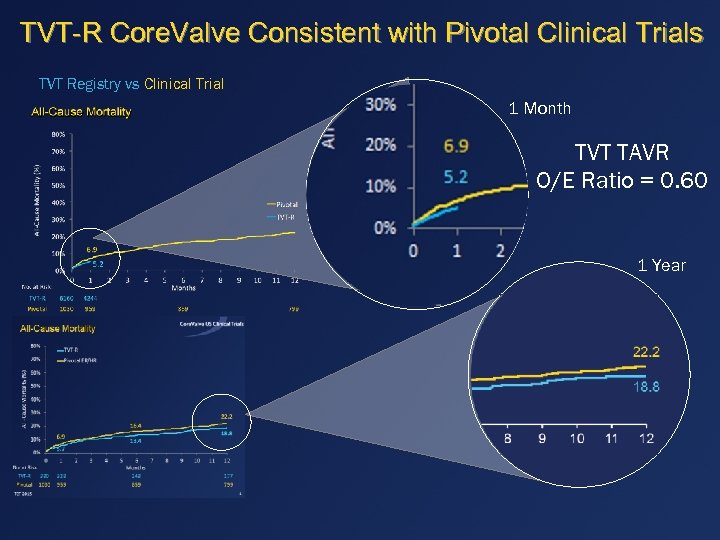
TVT-R Core. Valve Consistent with Pivotal Clinical Trials TVT Registry vs Clinical Trial 1 Month TVT TAVR O/E Ratio = 0. 60 1 Year
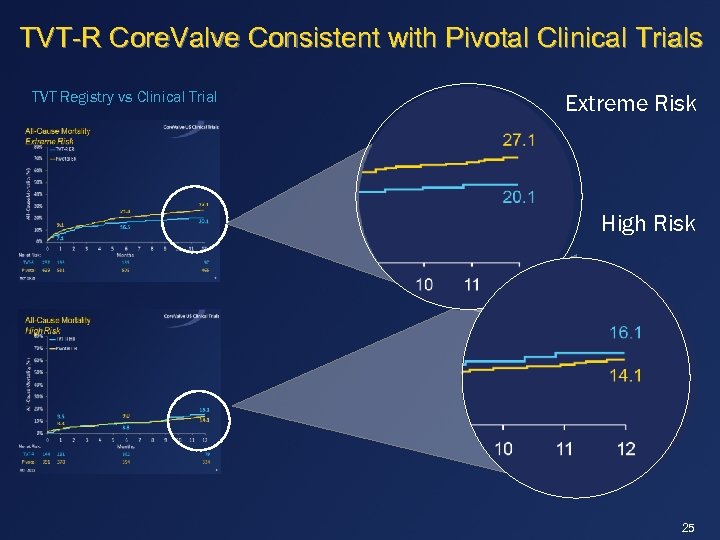
TVT-R Core. Valve Consistent with Pivotal Clinical Trials TVT Registry vs Clinical Trial Extreme Risk High Risk 25
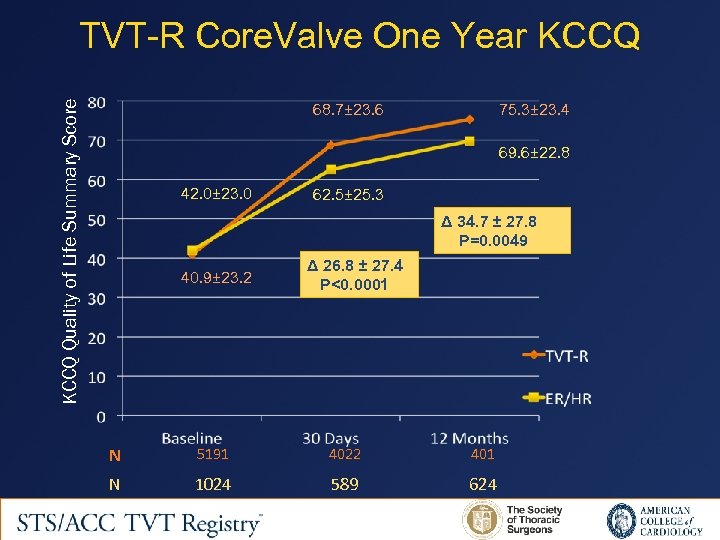
KCCQ Quality of Life Summary Score TVT-R Core. Valve One Year KCCQ 68. 7± 23. 6 75. 3± 23. 4 69. 6± 22. 8 42. 0± 23. 0 62. 5± 25. 3 Δ 34. 7 ± 27. 8 P=0. 0049 40. 9± 23. 2 Δ 26. 8 ± 27. 4 P<0. 0001 N 5191 4022 401 N 1024 589 624

Limitations • Risk categories “Risk creep” not detected by conventional factors • Potential underreporting of events • Clinical site echocardiographic reporting • Data compliance

Summary • First report of 6160 patient treated with Core. Valve selfexpanding TAVR documenting “real world” commercial use in patients with symptomatic aortic stenosis • Baseline characteristics confirm the high surgical risk of the TVT registry patients with aortic stenosis • Early (30 day) and late (one year) clinically outcomes are broadly comparable to those patients enrolled in the Pivotal studies • No detectable learning curve or safety issues were identified with commercial expansion

Conclusion • The results of this study support the safety and efficacy of the self-expanding Core. Valve bioprosthesis in “real world” commercial use in patients with severe aortic stenosis
e33710810cb049db45f842da7351d33c.ppt