d65d54113419833c42b87ec9c9e744c7.ppt
- Количество слайдов: 26
 Training in Pharmaceutical Medicine – the role of the Regulator
Training in Pharmaceutical Medicine – the role of the Regulator
 Training in Pharmaceutical Medicine – the role of the Regulator Dr John Jenkins Chair, GMC Postgraduate Board 24 May 2010
Training in Pharmaceutical Medicine – the role of the Regulator Dr John Jenkins Chair, GMC Postgraduate Board 24 May 2010
 GMC’s purpose ‘to protect, promote and maintain the health and safety of the public by ensuring proper standards in the practice of medicine’
GMC’s purpose ‘to protect, promote and maintain the health and safety of the public by ensuring proper standards in the practice of medicine’
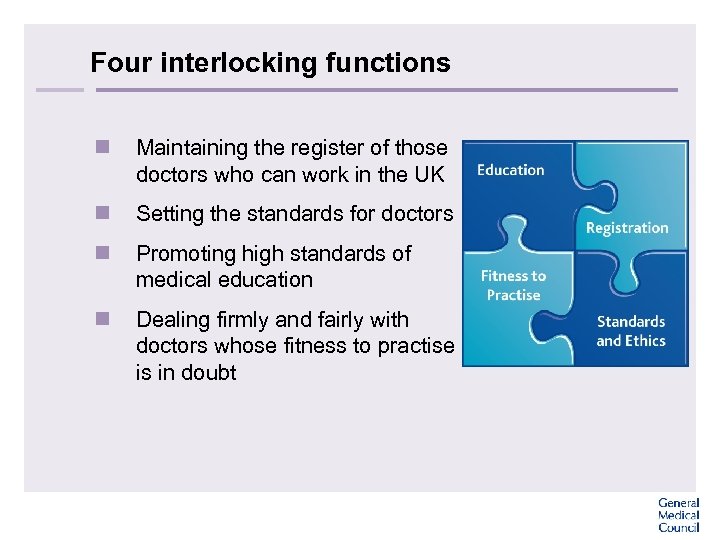 Four interlocking functions n Maintaining the register of those doctors who can work in the UK n Setting the standards for doctors n Promoting high standards of medical education n Dealing firmly and fairly with doctors whose fitness to practise is in doubt
Four interlocking functions n Maintaining the register of those doctors who can work in the UK n Setting the standards for doctors n Promoting high standards of medical education n Dealing firmly and fairly with doctors whose fitness to practise is in doubt
 The GMC’s role in medical education n n Co-ordinating all stages of medical education Promoting high standards Previously covered undergraduate education and the first year of training after graduation Quality assuring delivery of standards and outcomes: QABME and QAFP
The GMC’s role in medical education n n Co-ordinating all stages of medical education Promoting high standards Previously covered undergraduate education and the first year of training after graduation Quality assuring delivery of standards and outcomes: QABME and QAFP
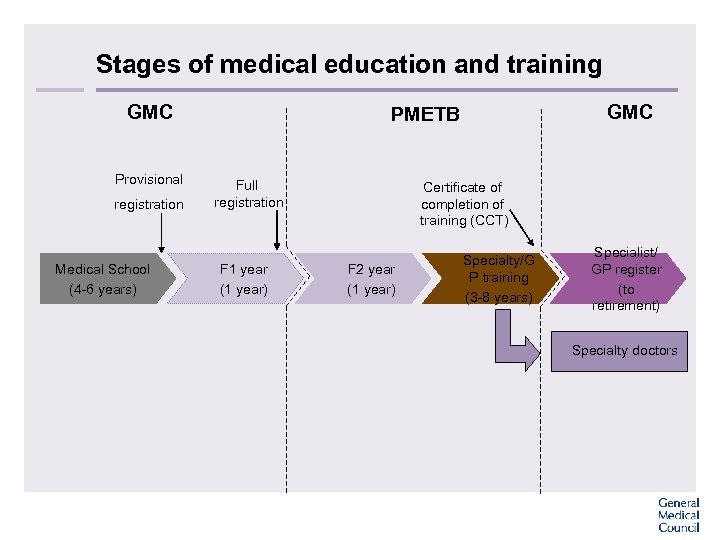 Stages of medical education and training GMC Provisional registration Medical School (4 -6 years) GMC PMETB Full registration F 1 year (1 year) Certificate of completion of training (CCT) F 2 year (1 year) Specialty/G P training (3 -8 years) Specialist/ GP register (to retirement) Specialty doctors
Stages of medical education and training GMC Provisional registration Medical School (4 -6 years) GMC PMETB Full registration F 1 year (1 year) Certificate of completion of training (CCT) F 2 year (1 year) Specialty/G P training (3 -8 years) Specialist/ GP register (to retirement) Specialty doctors
 Specialty training (3 -8 years) n n Medical royal colleges and faculties draw up curricula for specialty (including GP) training and assessments, which were approved by PMETB Competition for selection, training quality managed by postgraduate deans PMETB certified completion of training which leads to entry on GMC GP or specialist register and eligibility to work as a consultant/ GP principal PMETB quality assured specialist training
Specialty training (3 -8 years) n n Medical royal colleges and faculties draw up curricula for specialty (including GP) training and assessments, which were approved by PMETB Competition for selection, training quality managed by postgraduate deans PMETB certified completion of training which leads to entry on GMC GP or specialist register and eligibility to work as a consultant/ GP principal PMETB quality assured specialist training
 The problem Sir John Tooke: Aspiring to Excellence, January 2008: ‘The regulation of medical education from Undergraduate to CPD should be seamless but the current structure… n creates diseconomies n fails to link registration, certification and revalidation n permits the development of different cultural approaches’
The problem Sir John Tooke: Aspiring to Excellence, January 2008: ‘The regulation of medical education from Undergraduate to CPD should be seamless but the current structure… n creates diseconomies n fails to link registration, certification and revalidation n permits the development of different cultural approaches’
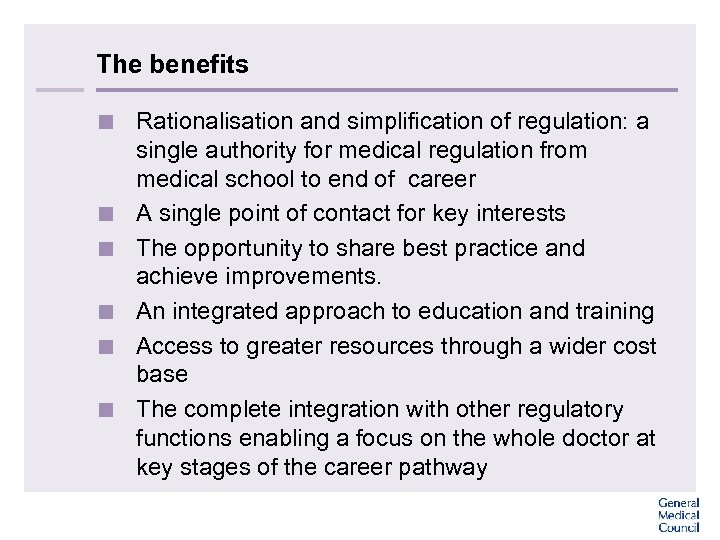 The benefits < Rationalisation and simplification of regulation: a single authority for medical regulation from medical school to end of career < A single point of contact for key interests < The opportunity to share best practice and achieve improvements. < An integrated approach to education and training < Access to greater resources through a wider cost base < The complete integration with other regulatory functions enabling a focus on the whole doctor at key stages of the career pathway
The benefits < Rationalisation and simplification of regulation: a single authority for medical regulation from medical school to end of career < A single point of contact for key interests < The opportunity to share best practice and achieve improvements. < An integrated approach to education and training < Access to greater resources through a wider cost base < The complete integration with other regulatory functions enabling a focus on the whole doctor at key stages of the career pathway
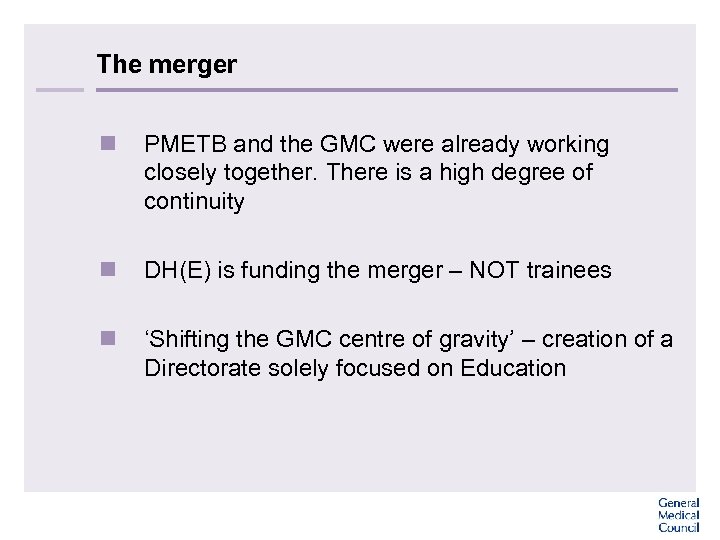 The merger n PMETB and the GMC were already working closely together. There is a high degree of continuity n DH(E) is funding the merger – NOT trainees n ‘Shifting the GMC centre of gravity’ – creation of a Directorate solely focused on Education
The merger n PMETB and the GMC were already working closely together. There is a high degree of continuity n DH(E) is funding the merger – NOT trainees n ‘Shifting the GMC centre of gravity’ – creation of a Directorate solely focused on Education
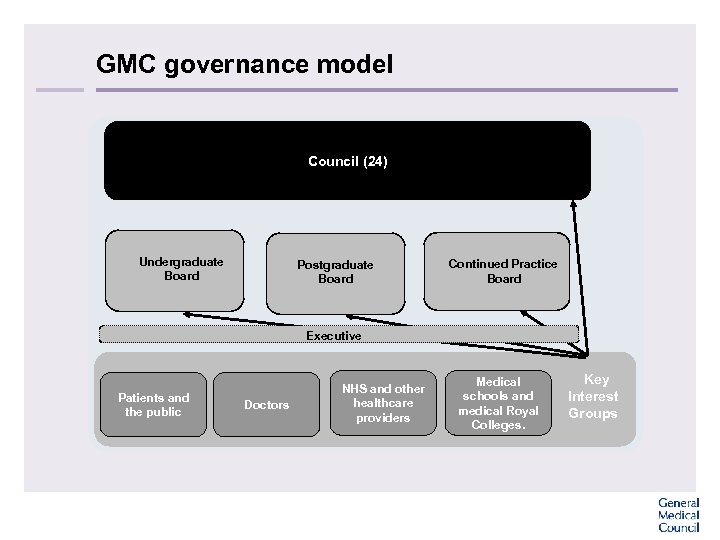 GMC governance model Council (24) Undergraduate Board Postgraduate Board Continued Practice Board Executive Patients and the public Doctors NHS and other healthcare providers Medical schools and medical Royal Colleges. Key Interest Groups
GMC governance model Council (24) Undergraduate Board Postgraduate Board Continued Practice Board Executive Patients and the public Doctors NHS and other healthcare providers Medical schools and medical Royal Colleges. Key Interest Groups
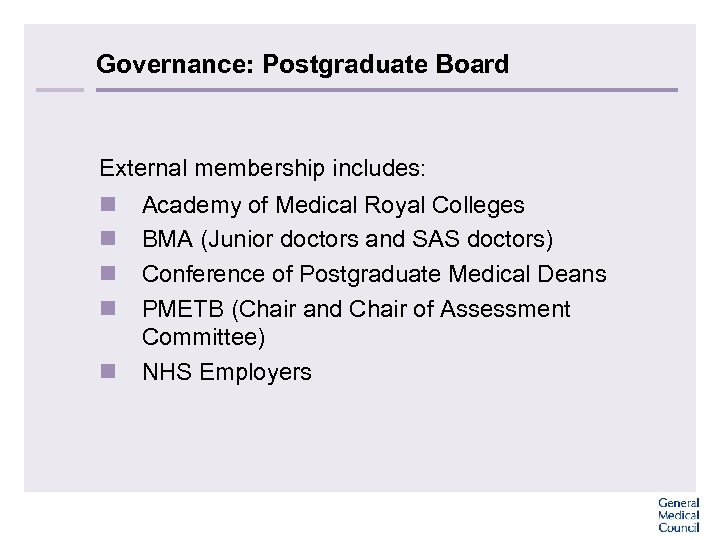 Governance: Postgraduate Board External membership includes: n n n Academy of Medical Royal Colleges BMA (Junior doctors and SAS doctors) Conference of Postgraduate Medical Deans PMETB (Chair and Chair of Assessment Committee) NHS Employers
Governance: Postgraduate Board External membership includes: n n n Academy of Medical Royal Colleges BMA (Junior doctors and SAS doctors) Conference of Postgraduate Medical Deans PMETB (Chair and Chair of Assessment Committee) NHS Employers
 Quality and Certification work n How trainees are assessed for CCT/CESR/CEGPR certificates has transferred to the GMC since April 2010 n GMC has agreed to maintain 2010/2011 fees at 2009/2010 rates n National Surveys of Trainees and Trainers will continue ¡ 4 th year for Trainees ¡ 3 rd year for Trainers
Quality and Certification work n How trainees are assessed for CCT/CESR/CEGPR certificates has transferred to the GMC since April 2010 n GMC has agreed to maintain 2010/2011 fees at 2009/2010 rates n National Surveys of Trainees and Trainers will continue ¡ 4 th year for Trainees ¡ 3 rd year for Trainers
 Training in Pharmaceutical Medicine
Training in Pharmaceutical Medicine
 Training in Pharmaceutical Medicine is: n n n Outside of the NHS Within industry, policy, research Unlike most medical training within the UK, as it is not organised within the geographical Deanery structure (but not unique - occupational medicine and medical oncology) Quality assured by GMC according to its Quality Framework for specialty (including GP) training Virtual deanery and faculty are accountable to GMC and need to reflect the Standards for Deaneries. Training must meet the Generic standards for training
Training in Pharmaceutical Medicine is: n n n Outside of the NHS Within industry, policy, research Unlike most medical training within the UK, as it is not organised within the geographical Deanery structure (but not unique - occupational medicine and medical oncology) Quality assured by GMC according to its Quality Framework for specialty (including GP) training Virtual deanery and faculty are accountable to GMC and need to reflect the Standards for Deaneries. Training must meet the Generic standards for training
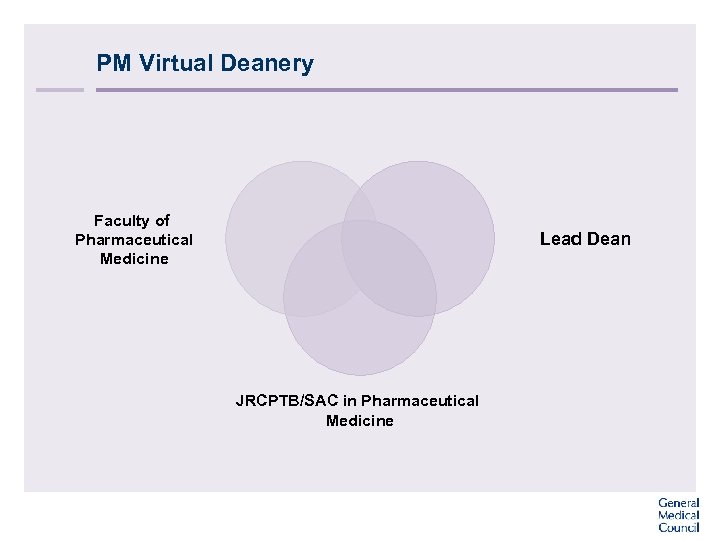 PM Virtual Deanery Faculty of Pharmaceutical Medicine Lead Dean JRCPTB/SAC in Pharmaceutical Medicine
PM Virtual Deanery Faculty of Pharmaceutical Medicine Lead Dean JRCPTB/SAC in Pharmaceutical Medicine
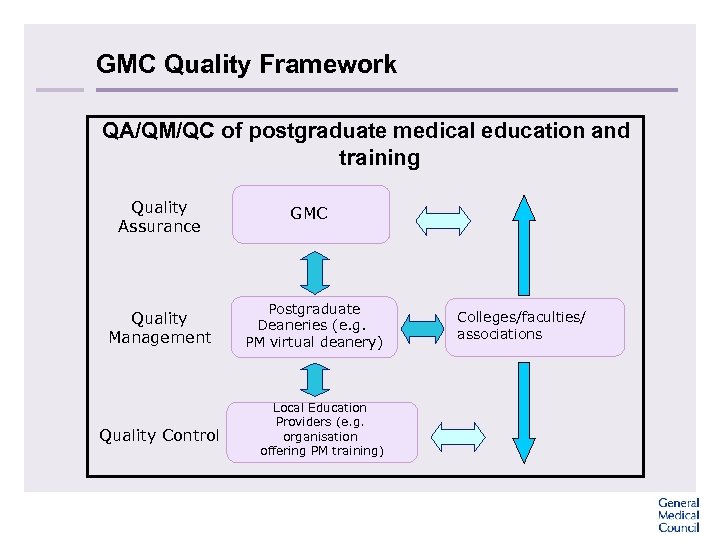 GMC Quality Framework QA/QM/QC of postgraduate medical education and training Quality Assurance Quality Management Quality Control GMC Postgraduate Deaneries (e. g. PM virtual deanery) Local Education Providers (e. g. organisation offering PM training) Colleges/faculties/ associations
GMC Quality Framework QA/QM/QC of postgraduate medical education and training Quality Assurance Quality Management Quality Control GMC Postgraduate Deaneries (e. g. PM virtual deanery) Local Education Providers (e. g. organisation offering PM training) Colleges/faculties/ associations
 GMC Approval of Pharmaceutical Medicine Includes: n One programme n Capacity to train 200 trainees n 96 training locations in UK and Europe Retained via: n Annual Deanery Report n Visit to Deanery
GMC Approval of Pharmaceutical Medicine Includes: n One programme n Capacity to train 200 trainees n 96 training locations in UK and Europe Retained via: n Annual Deanery Report n Visit to Deanery
 Visit to Pharmaceutical Medicine “Thematic QA is a term used to describe ways that the GMC might work with colleges/faculties and other organisations to explore, assure and develop specialty including GP training beyond the deanery confines” (2007 onwards) GMC Quality Framework for specialty including GP training (paragraphs 86 – 88) Pharmaceutical medicine has been the first to experience of thematic QA
Visit to Pharmaceutical Medicine “Thematic QA is a term used to describe ways that the GMC might work with colleges/faculties and other organisations to explore, assure and develop specialty including GP training beyond the deanery confines” (2007 onwards) GMC Quality Framework for specialty including GP training (paragraphs 86 – 88) Pharmaceutical medicine has been the first to experience of thematic QA
 Visit to Pharmaceutical Medicine n n n Thematic visit took place in December 2009 Pharmaceutical Medicine specialist amongst visitors Considered QM at virtual deanery level and QC at organisational level, sampling training at these locations: 1. 2. 3. 4. 5. Astra. Zeneca Roche Bayer Schering Sanofi-Aventis Boehringer and training at small research firms and MHRA n Visit Report available at: www. gmc-uk. org/Pharmaceutical_Medicine_ Visit_To_Deanery_Report_December_2009. pdf_31583 898. pdf
Visit to Pharmaceutical Medicine n n n Thematic visit took place in December 2009 Pharmaceutical Medicine specialist amongst visitors Considered QM at virtual deanery level and QC at organisational level, sampling training at these locations: 1. 2. 3. 4. 5. Astra. Zeneca Roche Bayer Schering Sanofi-Aventis Boehringer and training at small research firms and MHRA n Visit Report available at: www. gmc-uk. org/Pharmaceutical_Medicine_ Visit_To_Deanery_Report_December_2009. pdf_31583 898. pdf
 Revalidation 1. Consultation running until 4 th June ‘Revalidation: The Way Ahead’ – covers four main themes: n n How revalidation will work What doctors, employers and contractors of doctors’ services will need to do Patient and public involvement in revalidation How and when revalidation will work < A significant change is that the current proposals are based on a single revalidation process – rather than the parallel processes of relicensing and recertification that were previously proposed
Revalidation 1. Consultation running until 4 th June ‘Revalidation: The Way Ahead’ – covers four main themes: n n How revalidation will work What doctors, employers and contractors of doctors’ services will need to do Patient and public involvement in revalidation How and when revalidation will work < A significant change is that the current proposals are based on a single revalidation process – rather than the parallel processes of relicensing and recertification that were previously proposed
 Proposals – Responsible officer Under the draft Responsible Officer regulations, the Faculty of Pharmaceutical Medicine is one of the designated organisations that will appoint a Responsible Officer In the longer term, some pharmaceutical companies may also appoint Responsible Officers
Proposals – Responsible officer Under the draft Responsible Officer regulations, the Faculty of Pharmaceutical Medicine is one of the designated organisations that will appoint a Responsible Officer In the longer term, some pharmaceutical companies may also appoint Responsible Officers
 Appraisal < For doctors working in the pharmaceutical industry, revalidation will be based on appraisal, normally through the doctor’s employing organisation < Appraisal will be based on what doctors do in practice; the specialty framework developed by the Faculty will help doctors demonstrate that they meet the standards relevant to their practice < Appraisers will normally be a senior physician or have had the appropriate training and experience
Appraisal < For doctors working in the pharmaceutical industry, revalidation will be based on appraisal, normally through the doctor’s employing organisation < Appraisal will be based on what doctors do in practice; the specialty framework developed by the Faculty will help doctors demonstrate that they meet the standards relevant to their practice < Appraisers will normally be a senior physician or have had the appropriate training and experience
 Timelines < Pilots – 2009 and 2010 < Progressive roll out – 2011 onwards < The shared aim is to put in place arrangements that delivered necessary assurance, but which to the maximum extent possible build on valued systems already in place and are sufficiently flexible to avoid imposing unreasonable burdens
Timelines < Pilots – 2009 and 2010 < Progressive roll out – 2011 onwards < The shared aim is to put in place arrangements that delivered necessary assurance, but which to the maximum extent possible build on valued systems already in place and are sufficiently flexible to avoid imposing unreasonable burdens
 A Unique Opportunity Regulating Medical Education and Training: Policy Review Lord Naren Patel n n Personal regulation Team based regulation Workplace regulation National regulation To form conclusions on what is an appropriate, modern approach to the regulation of education and training within an independent framework of four interlocking regulatory functions and four regulatory layers
A Unique Opportunity Regulating Medical Education and Training: Policy Review Lord Naren Patel n n Personal regulation Team based regulation Workplace regulation National regulation To form conclusions on what is an appropriate, modern approach to the regulation of education and training within an independent framework of four interlocking regulatory functions and four regulatory layers
 Conclusions n New editions of Tomorrow’s Doctors and curricula for Foundation and Specialty training n Introduction of revalidation n Merger of the GMC and PMETB n A once in a lifetime opportunity to work together to develop the continuum of medical education and training as an essential element of the protection and promotion of the safety and quality of heath care throughout the UK, with professionalism at its heart
Conclusions n New editions of Tomorrow’s Doctors and curricula for Foundation and Specialty training n Introduction of revalidation n Merger of the GMC and PMETB n A once in a lifetime opportunity to work together to develop the continuum of medical education and training as an essential element of the protection and promotion of the safety and quality of heath care throughout the UK, with professionalism at its heart


