2948585154422900d0b02f29f1a8ca22.ppt
- Количество слайдов: 53
 Trace Metals and the Ancient Earth: The Co-Evolution of Life and Earth’s Chemistry Marine (bio)inorganic chemistry as a tool for deep time studies Hello, My daughters were very happy and surprise to find a real message in a bottle. Thanks for this magic moment. we find it on the Trouville Beach (Normandy) the 10 March, 2005 Good luck for your project. Natacha Rousseau & Chloé et Emma Sbruzzi Attachment, photo taken of the bottle apparently as it was found (from the shadow it is 7 or 8 o'clock in the morning): 12. 755 L 08
Trace Metals and the Ancient Earth: The Co-Evolution of Life and Earth’s Chemistry Marine (bio)inorganic chemistry as a tool for deep time studies Hello, My daughters were very happy and surprise to find a real message in a bottle. Thanks for this magic moment. we find it on the Trouville Beach (Normandy) the 10 March, 2005 Good luck for your project. Natacha Rousseau & Chloé et Emma Sbruzzi Attachment, photo taken of the bottle apparently as it was found (from the shadow it is 7 or 8 o'clock in the morning): 12. 755 L 08
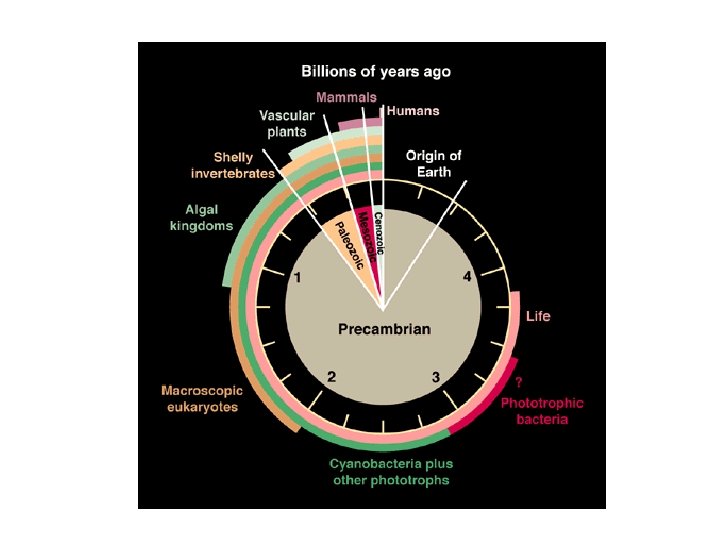
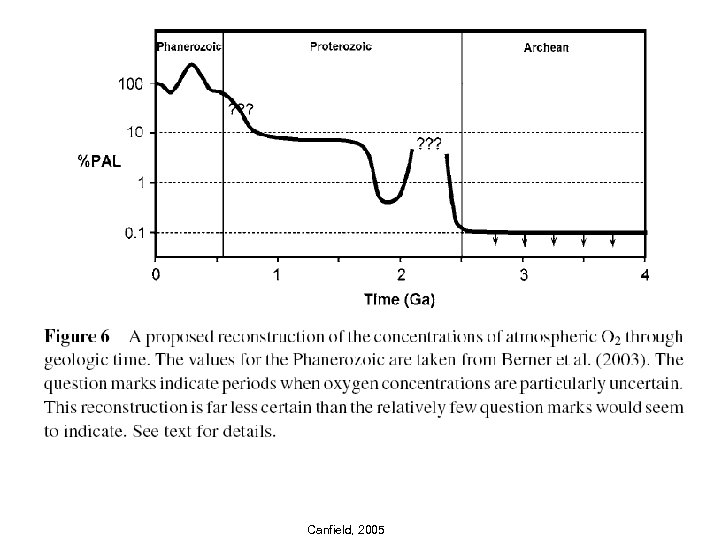 Canfield, 2005
Canfield, 2005
 Outline Some recent ways inorganic approaches have contributed to studies of the ancient Earth • • • Sulfur isotopes (Canfield, Farquar) Chemical history/modeling (Williams and Da Silva, Anbar and Knoll, Saito) Metal isotopes in ancient rocks (Anbar, Rouxel, Severmann) Metal abundances in ancient cores (Anbar) Genomic studies on extant organisms (Dupont, House, Falkowski, Wolfe-Simon, Ridge) Physiological studies on extant organisms (Sunda, Morel, Saito, Wolfe-Simon) What is this field called: Geobiology, Astrobiology, Origin of Life, Deep time, Microbial Biogeochemistry, Paleo
Outline Some recent ways inorganic approaches have contributed to studies of the ancient Earth • • • Sulfur isotopes (Canfield, Farquar) Chemical history/modeling (Williams and Da Silva, Anbar and Knoll, Saito) Metal isotopes in ancient rocks (Anbar, Rouxel, Severmann) Metal abundances in ancient cores (Anbar) Genomic studies on extant organisms (Dupont, House, Falkowski, Wolfe-Simon, Ridge) Physiological studies on extant organisms (Sunda, Morel, Saito, Wolfe-Simon) What is this field called: Geobiology, Astrobiology, Origin of Life, Deep time, Microbial Biogeochemistry, Paleo
 How do we study life and biogeochemistry of the ancient ocean? 1. 2. 3. 4. Microfossils Organic molecules: biomarkers of life Isotopes or relative abundances of elements in rocks formed in that time – Biologically influenced: Sulfur, carbon, iron – Non-biologically influenced (e. g. platinum group elements, Os, rare earth elements Nd) Genomic information ‘preserved’ in extant life The challenges in acquiring actual information about the ancient Earth: – Are microfossils really biotic in origin? – Are biomarkers really made by the organisms we think make them? – Are there abiotic processes controlling isotopic fractionation as well? – How do we decipher the information in genomes and proteomes? – Can information really be preserved in DNA over billions of years? • • • Lateral gene transfer Mutation rates “Genomic Diagenesis”
How do we study life and biogeochemistry of the ancient ocean? 1. 2. 3. 4. Microfossils Organic molecules: biomarkers of life Isotopes or relative abundances of elements in rocks formed in that time – Biologically influenced: Sulfur, carbon, iron – Non-biologically influenced (e. g. platinum group elements, Os, rare earth elements Nd) Genomic information ‘preserved’ in extant life The challenges in acquiring actual information about the ancient Earth: – Are microfossils really biotic in origin? – Are biomarkers really made by the organisms we think make them? – Are there abiotic processes controlling isotopic fractionation as well? – How do we decipher the information in genomes and proteomes? – Can information really be preserved in DNA over billions of years? • • • Lateral gene transfer Mutation rates “Genomic Diagenesis”
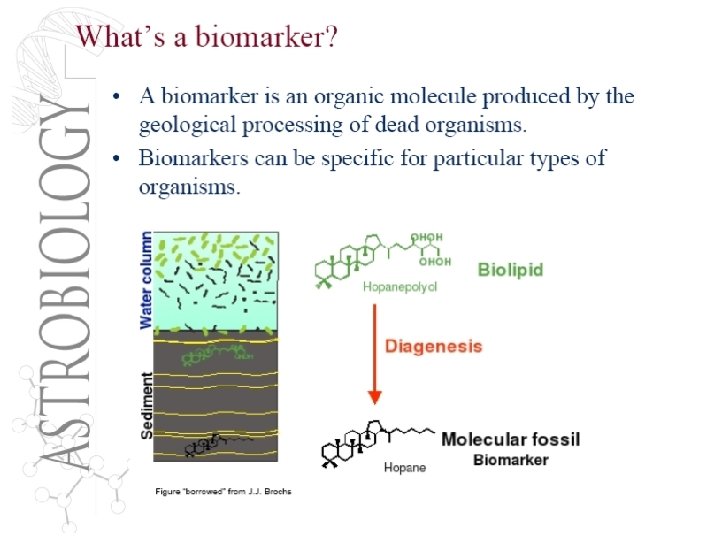

 Current major controversy: When did O 2 arise in the atmosphere? When did oxygenic photosynthesis evolve? (Is the house of cards coming down? ) • Microfossils are not reliable, may not be biological, and certainly do not give phylogenetic information • Stromatolites: do not require photosynthesis do be produced (early ocean much closer to saturation, methane oxidizers can produce them) • Sulfur isotopes, and iron isotopes • Biomarkers: – Eukaryotic specific markers: Sterols (contaminants? ) – Hopanes: 2 -methyl-bacteriohopananepolyol believed to be primarily cyanobacterial. • But synthesis genes not found in most abundant extant marine cyanobacteria: Prochlorococcus and Synechococcus • We don’t know when oxygenic photosynthesis evolved • Kirschvink’s Snowball Earth Hypothesis: much later ~2. 2 bya, oxidized methane and drove the Earth into massive ice age.
Current major controversy: When did O 2 arise in the atmosphere? When did oxygenic photosynthesis evolve? (Is the house of cards coming down? ) • Microfossils are not reliable, may not be biological, and certainly do not give phylogenetic information • Stromatolites: do not require photosynthesis do be produced (early ocean much closer to saturation, methane oxidizers can produce them) • Sulfur isotopes, and iron isotopes • Biomarkers: – Eukaryotic specific markers: Sterols (contaminants? ) – Hopanes: 2 -methyl-bacteriohopananepolyol believed to be primarily cyanobacterial. • But synthesis genes not found in most abundant extant marine cyanobacteria: Prochlorococcus and Synechococcus • We don’t know when oxygenic photosynthesis evolved • Kirschvink’s Snowball Earth Hypothesis: much later ~2. 2 bya, oxidized methane and drove the Earth into massive ice age.
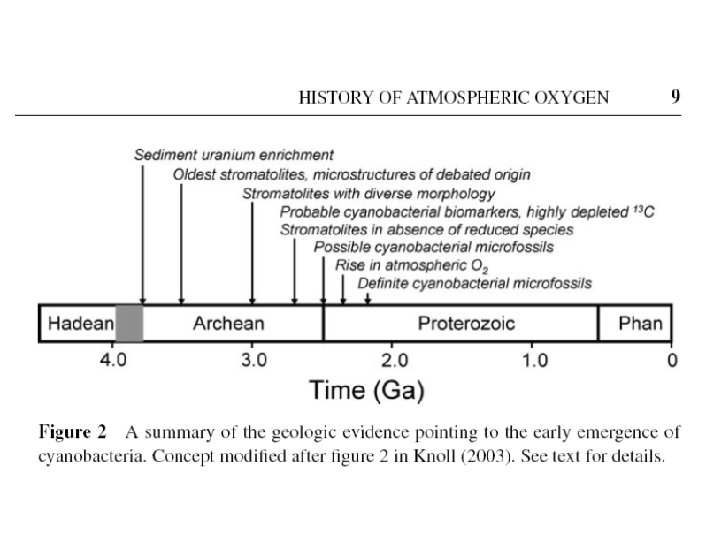
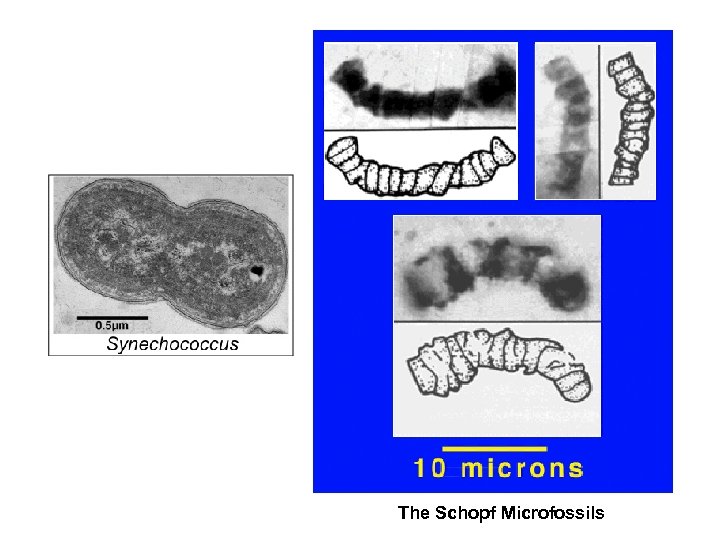 The Schopf Microfossils
The Schopf Microfossils
 Another current controversy: Early or Late evolution of oxygenic photosynthesis • • 2. 78 billion years ago (early) ~2. 3 billion years ago (late) • From Koop and Kirschvink: – “The critical piece of evidence placing the origin of cyanobacteria and locally oxic environments in the Archean is the discovery in bitumens from rocks as old as 2. 78 Ga of organic biomarkers apparently derived from lipids used by cyanobacteria and eukaryotes in the cell membranes. ” – “Raymond and Blankenship found that of 473 O 2 -dependent enzymatically catalyzed reactions…. 20 have at least one O 2 independent counterpart that performs the same reaction. [Bch. E does the same rxn as Acs. F but uses B 12 instead of O 2]. The assumption that sterol synthesis is always O 2 -dependent and always has been therefore merits close inspection”. – Conclusion, just because something requires an aerobic enzyme now, doesn’t mean there was anaerobic enzyme that is now extinct. – On the flip side, the extant cyanobacteria don’t make the biomarkers attributed to ancient cyanobacteria (Hopanes: 2 -methyl-bacteriohopananepolyol believed to be primarily cyanobacterial. But synthesis genes not found in most abundant extant marine cyanobacteria: Prochlorococcus and Synechococcus)
Another current controversy: Early or Late evolution of oxygenic photosynthesis • • 2. 78 billion years ago (early) ~2. 3 billion years ago (late) • From Koop and Kirschvink: – “The critical piece of evidence placing the origin of cyanobacteria and locally oxic environments in the Archean is the discovery in bitumens from rocks as old as 2. 78 Ga of organic biomarkers apparently derived from lipids used by cyanobacteria and eukaryotes in the cell membranes. ” – “Raymond and Blankenship found that of 473 O 2 -dependent enzymatically catalyzed reactions…. 20 have at least one O 2 independent counterpart that performs the same reaction. [Bch. E does the same rxn as Acs. F but uses B 12 instead of O 2]. The assumption that sterol synthesis is always O 2 -dependent and always has been therefore merits close inspection”. – Conclusion, just because something requires an aerobic enzyme now, doesn’t mean there was anaerobic enzyme that is now extinct. – On the flip side, the extant cyanobacteria don’t make the biomarkers attributed to ancient cyanobacteria (Hopanes: 2 -methyl-bacteriohopananepolyol believed to be primarily cyanobacterial. But synthesis genes not found in most abundant extant marine cyanobacteria: Prochlorococcus and Synechococcus)
 The oldest fossil evidence for eukaryotes and cyanobacteria therefore reverts to 1. 78– 1. 68 Gyr ago and 2. 15 Gyr ago 10, 11, respectively. Our results eliminate the evidence for oxygenic photosynthesis 2. 7 Gyr ago and exclude previous biomarker evidence for a long delay ( 300 million years) between the appearance of oxygenproducing cyanobacteria and the rise in atmospheric oxygen 2. 45– 2. 32 Gyr ago 1. Brocks Goldschmidt talk summer 2009: Reinterpretation of organic biomarker record such that Eukaryotic life evolved after the Great Oxidation Event (which occurred at ~2. 3 bya)
The oldest fossil evidence for eukaryotes and cyanobacteria therefore reverts to 1. 78– 1. 68 Gyr ago and 2. 15 Gyr ago 10, 11, respectively. Our results eliminate the evidence for oxygenic photosynthesis 2. 7 Gyr ago and exclude previous biomarker evidence for a long delay ( 300 million years) between the appearance of oxygenproducing cyanobacteria and the rise in atmospheric oxygen 2. 45– 2. 32 Gyr ago 1. Brocks Goldschmidt talk summer 2009: Reinterpretation of organic biomarker record such that Eukaryotic life evolved after the Great Oxidation Event (which occurred at ~2. 3 bya)
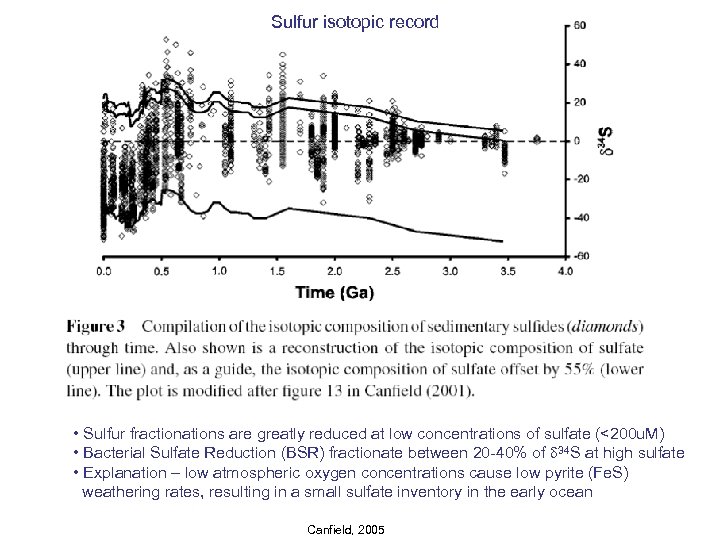 Sulfur isotopic record • Sulfur fractionations are greatly reduced at low concentrations of sulfate (<200 u. M) • Bacterial Sulfate Reduction (BSR) fractionate between 20 -40% of d 34 S at high sulfate • Explanation – low atmospheric oxygen concentrations cause low pyrite (Fe. S) weathering rates, resulting in a small sulfate inventory in the early ocean Canfield, 2005
Sulfur isotopic record • Sulfur fractionations are greatly reduced at low concentrations of sulfate (<200 u. M) • Bacterial Sulfate Reduction (BSR) fractionate between 20 -40% of d 34 S at high sulfate • Explanation – low atmospheric oxygen concentrations cause low pyrite (Fe. S) weathering rates, resulting in a small sulfate inventory in the early ocean Canfield, 2005
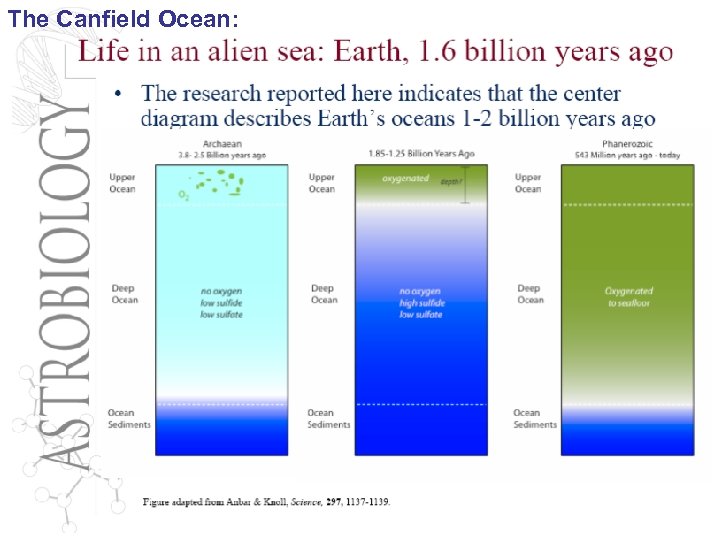 The Canfield Ocean:
The Canfield Ocean:
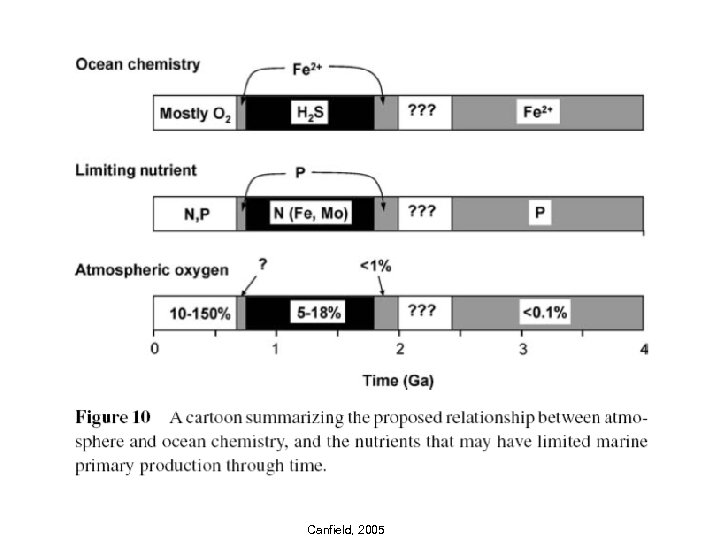 Canfield, 2005
Canfield, 2005
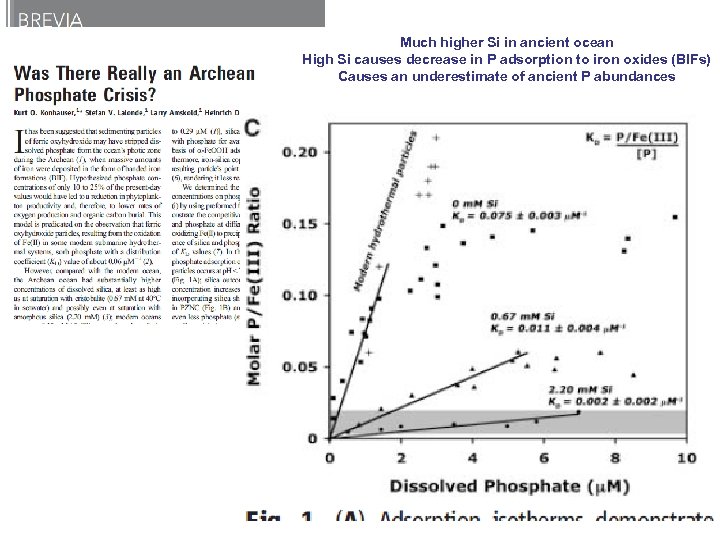 Much higher Si in ancient ocean High Si causes decrease in P adsorption to iron oxides (BIFs) Causes an underestimate of ancient P abundances
Much higher Si in ancient ocean High Si causes decrease in P adsorption to iron oxides (BIFs) Causes an underestimate of ancient P abundances
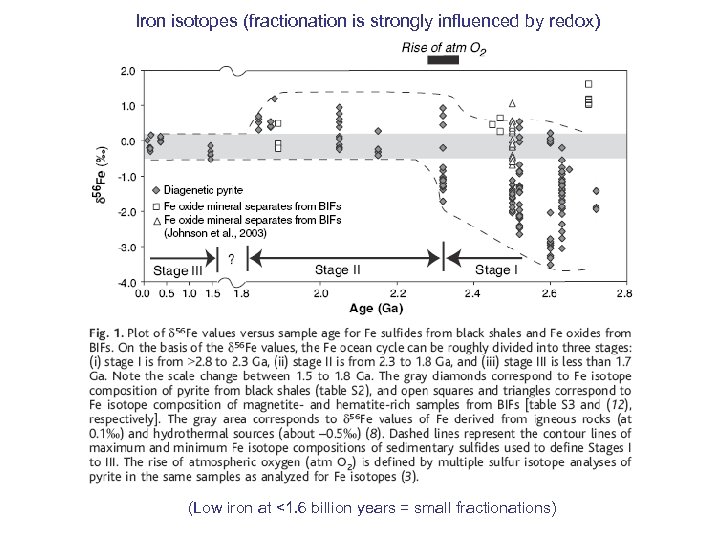 Iron isotopes (fractionation is strongly influenced by redox) (Low iron at <1. 6 billion years = small fractionations)
Iron isotopes (fractionation is strongly influenced by redox) (Low iron at <1. 6 billion years = small fractionations)
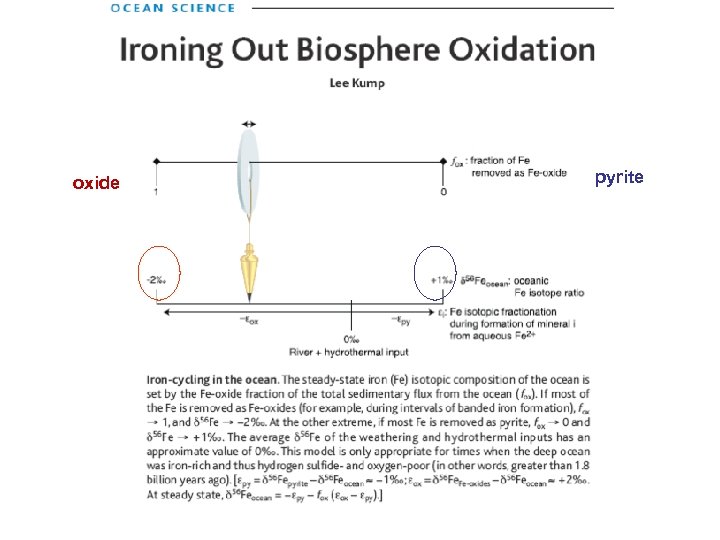 oxide pyrite
oxide pyrite
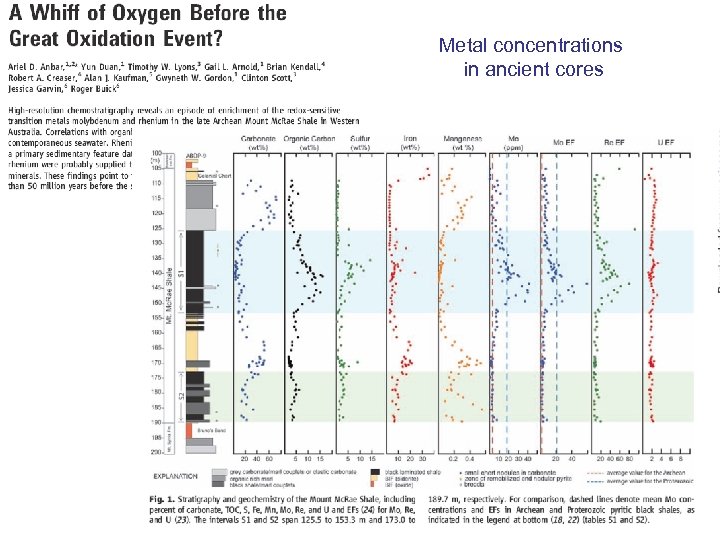 Metal concentrations in ancient cores
Metal concentrations in ancient cores
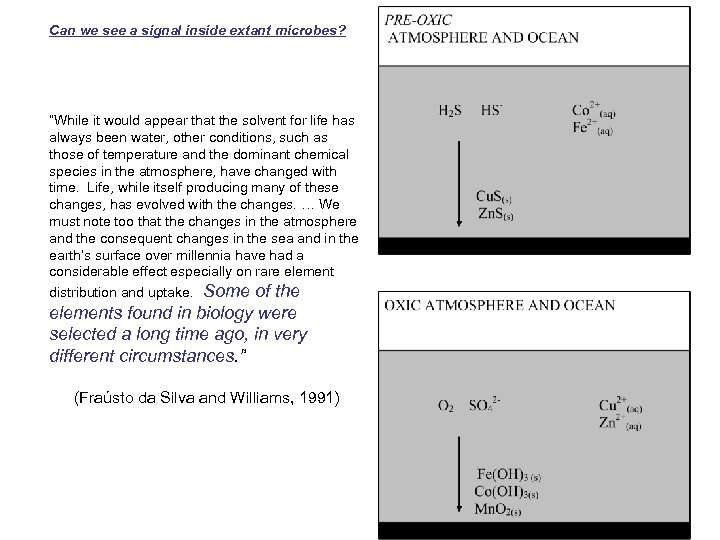 Can we see a signal inside extant microbes? “While it would appear that the solvent for life has always been water, other conditions, such as those of temperature and the dominant chemical species in the atmosphere, have changed with time. Life, while itself producing many of these changes, has evolved with the changes. … We must note too that the changes in the atmosphere and the consequent changes in the sea and in the earth’s surface over millennia have had a considerable effect especially on rare element distribution and uptake. Some of the elements found in biology were selected a long time ago, in very different circumstances. ” (Fraústo da Silva and Williams, 1991)
Can we see a signal inside extant microbes? “While it would appear that the solvent for life has always been water, other conditions, such as those of temperature and the dominant chemical species in the atmosphere, have changed with time. Life, while itself producing many of these changes, has evolved with the changes. … We must note too that the changes in the atmosphere and the consequent changes in the sea and in the earth’s surface over millennia have had a considerable effect especially on rare element distribution and uptake. Some of the elements found in biology were selected a long time ago, in very different circumstances. ” (Fraústo da Silva and Williams, 1991)
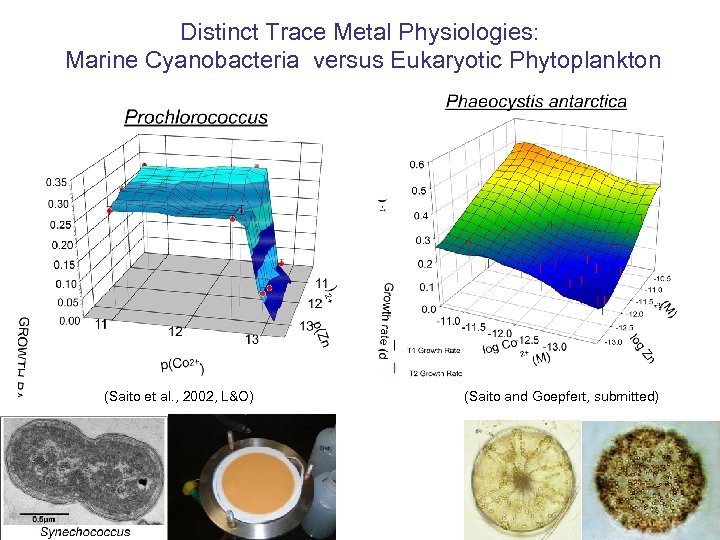 Distinct Trace Metal Physiologies: Marine Cyanobacteria versus Eukaryotic Phytoplankton (Saito et al. , 2002, L&O) (Saito and Goepfert, submitted)
Distinct Trace Metal Physiologies: Marine Cyanobacteria versus Eukaryotic Phytoplankton (Saito et al. , 2002, L&O) (Saito and Goepfert, submitted)
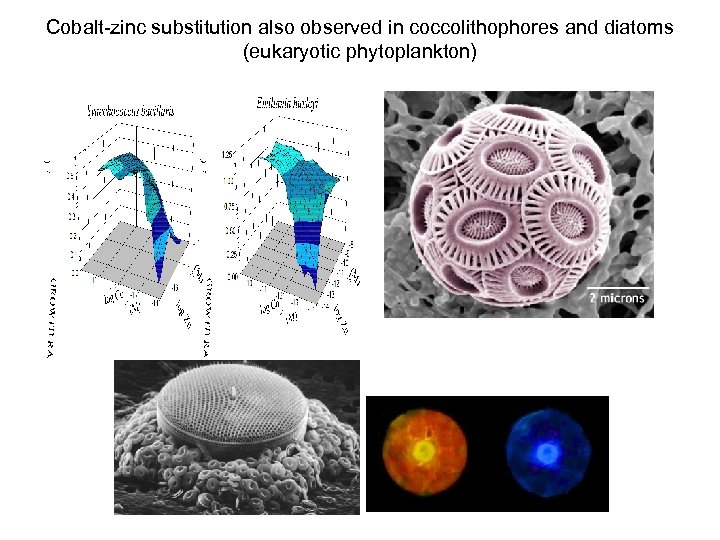 Cobalt-zinc substitution also observed in coccolithophores and diatoms (eukaryotic phytoplankton)
Cobalt-zinc substitution also observed in coccolithophores and diatoms (eukaryotic phytoplankton)
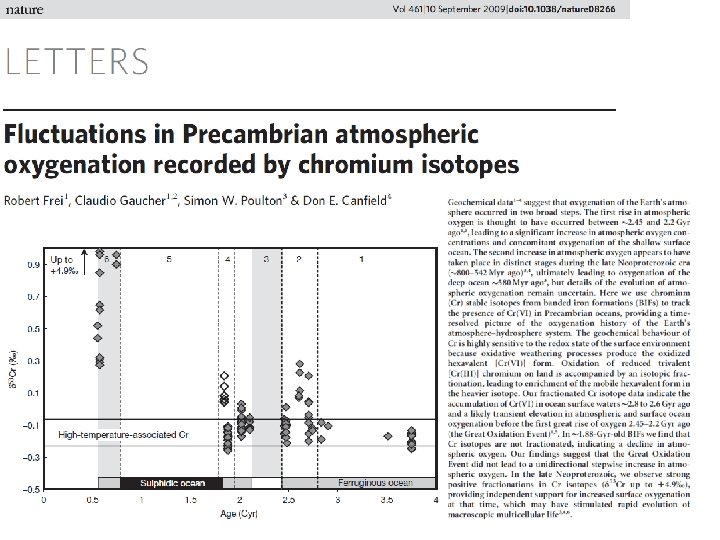
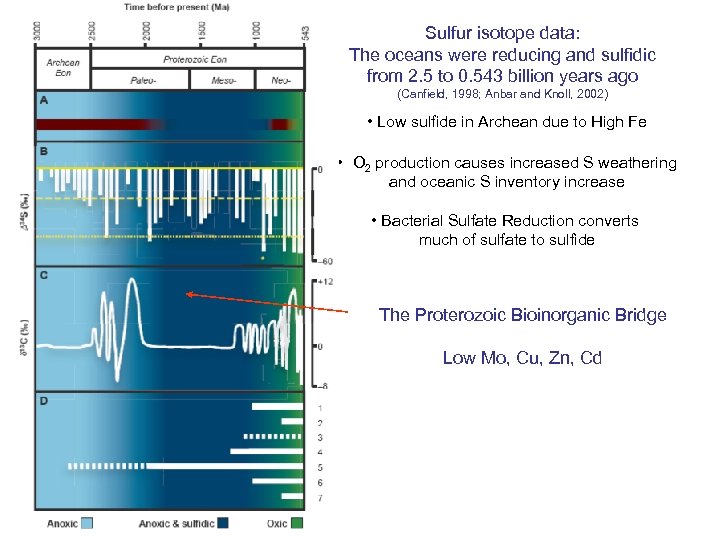 Sulfur isotope data: The oceans were reducing and sulfidic from 2. 5 to 0. 543 billion years ago (Canfield, 1998; Anbar and Knoll, 2002) • Low sulfide in Archean due to High Fe • O 2 production causes increased S weathering and oceanic S inventory increase • Bacterial Sulfate Reduction converts much of sulfate to sulfide The Proterozoic Bioinorganic Bridge Low Mo, Cu, Zn, Cd
Sulfur isotope data: The oceans were reducing and sulfidic from 2. 5 to 0. 543 billion years ago (Canfield, 1998; Anbar and Knoll, 2002) • Low sulfide in Archean due to High Fe • O 2 production causes increased S weathering and oceanic S inventory increase • Bacterial Sulfate Reduction converts much of sulfate to sulfide The Proterozoic Bioinorganic Bridge Low Mo, Cu, Zn, Cd
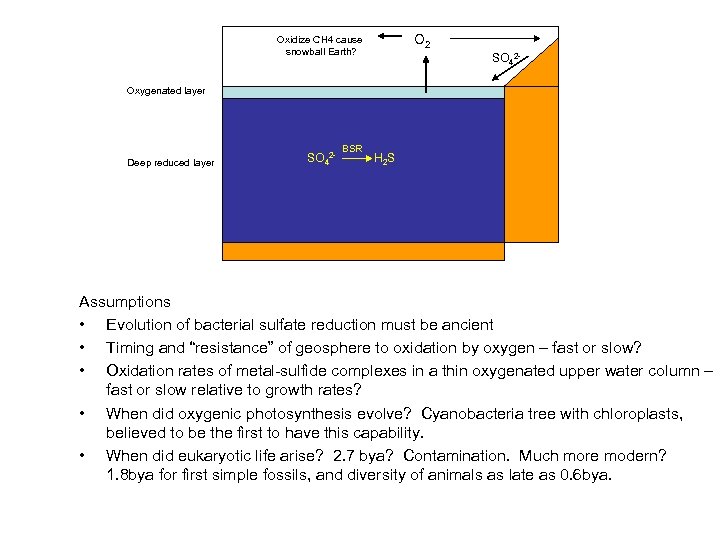 O 2 Oxidize CH 4 cause snowball Earth? SO 42 - Oxygenated layer Deep reduced layer SO 42 - BSR H 2 S Assumptions • Evolution of bacterial sulfate reduction must be ancient • Timing and “resistance” of geosphere to oxidation by oxygen – fast or slow? • Oxidation rates of metal-sulfide complexes in a thin oxygenated upper water column – fast or slow relative to growth rates? • When did oxygenic photosynthesis evolve? Cyanobacteria tree with chloroplasts, believed to be the first to have this capability. • When did eukaryotic life arise? 2. 7 bya? Contamination. Much more modern? 1. 8 bya for first simple fossils, and diversity of animals as late as 0. 6 bya.
O 2 Oxidize CH 4 cause snowball Earth? SO 42 - Oxygenated layer Deep reduced layer SO 42 - BSR H 2 S Assumptions • Evolution of bacterial sulfate reduction must be ancient • Timing and “resistance” of geosphere to oxidation by oxygen – fast or slow? • Oxidation rates of metal-sulfide complexes in a thin oxygenated upper water column – fast or slow relative to growth rates? • When did oxygenic photosynthesis evolve? Cyanobacteria tree with chloroplasts, believed to be the first to have this capability. • When did eukaryotic life arise? 2. 7 bya? Contamination. Much more modern? 1. 8 bya for first simple fossils, and diversity of animals as late as 0. 6 bya.
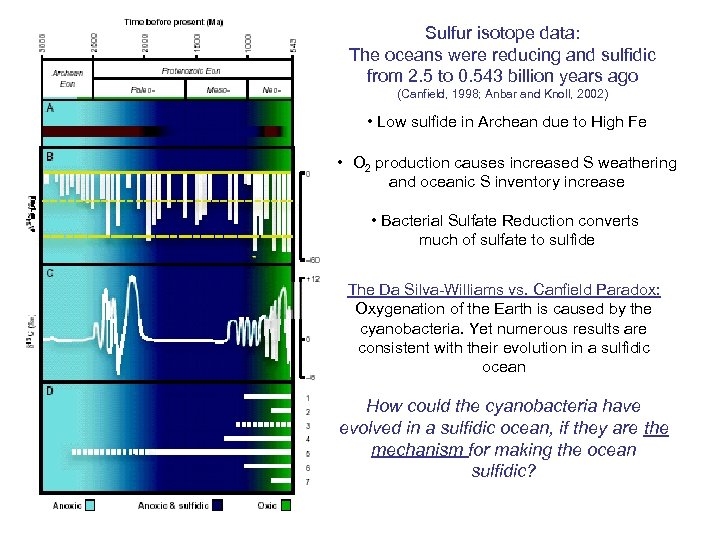 Sulfur isotope data: The oceans were reducing and sulfidic from 2. 5 to 0. 543 billion years ago (Canfield, 1998; Anbar and Knoll, 2002) • Low sulfide in Archean due to High Fe • O 2 production causes increased S weathering and oceanic S inventory increase • Bacterial Sulfate Reduction converts much of sulfate to sulfide The Da Silva-Williams vs. Canfield Paradox: Oxygenation of the Earth is caused by the cyanobacteria. Yet numerous results are consistent with their evolution in a sulfidic ocean How could the cyanobacteria have evolved in a sulfidic ocean, if they are the mechanism for making the ocean sulfidic?
Sulfur isotope data: The oceans were reducing and sulfidic from 2. 5 to 0. 543 billion years ago (Canfield, 1998; Anbar and Knoll, 2002) • Low sulfide in Archean due to High Fe • O 2 production causes increased S weathering and oceanic S inventory increase • Bacterial Sulfate Reduction converts much of sulfate to sulfide The Da Silva-Williams vs. Canfield Paradox: Oxygenation of the Earth is caused by the cyanobacteria. Yet numerous results are consistent with their evolution in a sulfidic ocean How could the cyanobacteria have evolved in a sulfidic ocean, if they are the mechanism for making the ocean sulfidic?
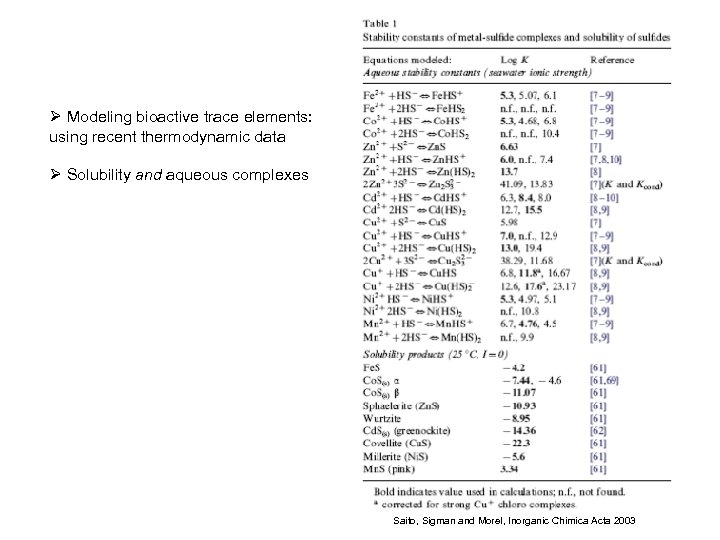 Ø Modeling bioactive trace elements: using recent thermodynamic data Ø Solubility and aqueous complexes Saito, Sigman and Morel, Inorganic Chimica Acta 2003
Ø Modeling bioactive trace elements: using recent thermodynamic data Ø Solubility and aqueous complexes Saito, Sigman and Morel, Inorganic Chimica Acta 2003
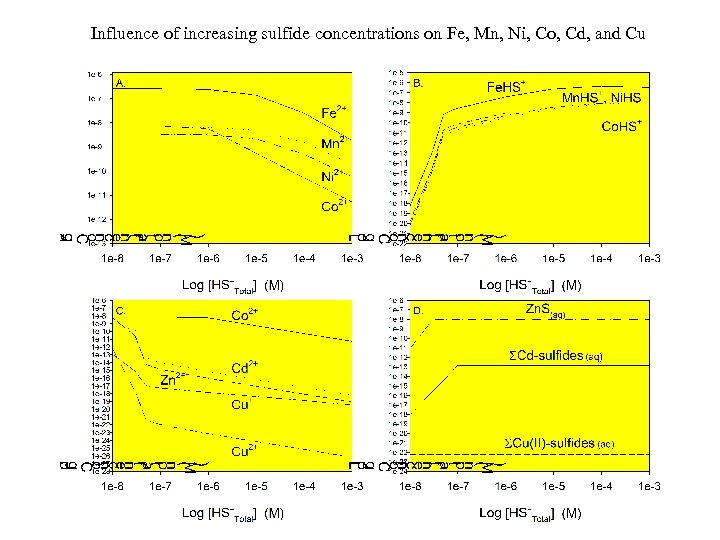 Influence of increasing sulfide concentrations on Fe, Mn, Ni, Co, Cd, and Cu
Influence of increasing sulfide concentrations on Fe, Mn, Ni, Co, Cd, and Cu
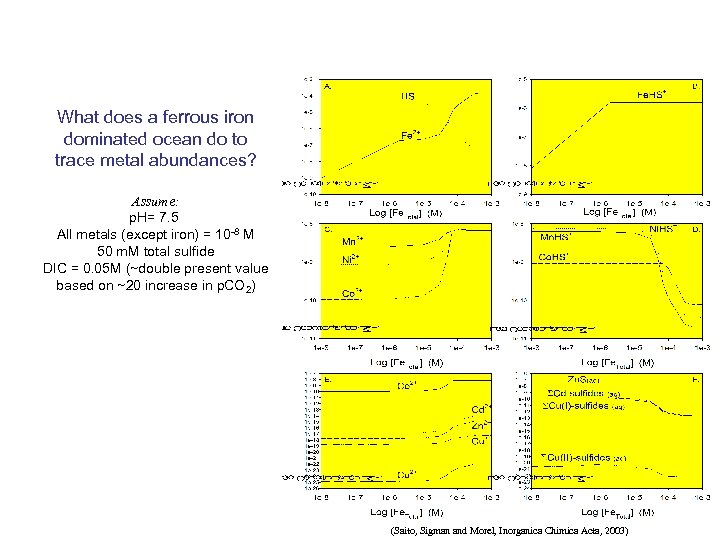 What does a ferrous iron dominated ocean do to trace metal abundances? Assume: p. H= 7. 5 All metals (except iron) = 10 -8 M 50 m. M total sulfide DIC = 0. 05 M (~double present value based on ~20 increase in p. CO 2) (Saito, Sigman and Morel, Inorganica Chimica Acta, 2003)
What does a ferrous iron dominated ocean do to trace metal abundances? Assume: p. H= 7. 5 All metals (except iron) = 10 -8 M 50 m. M total sulfide DIC = 0. 05 M (~double present value based on ~20 increase in p. CO 2) (Saito, Sigman and Morel, Inorganica Chimica Acta, 2003)
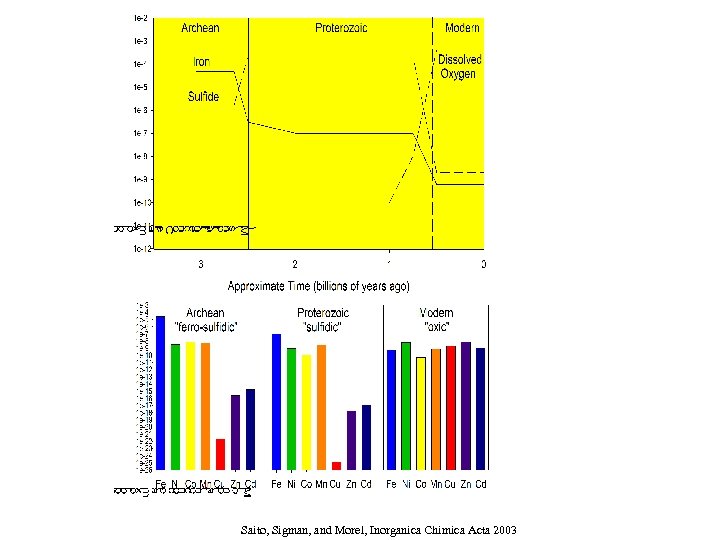 Saito, Sigman, and Morel, Inorganica Chimica Acta 2003
Saito, Sigman, and Morel, Inorganica Chimica Acta 2003
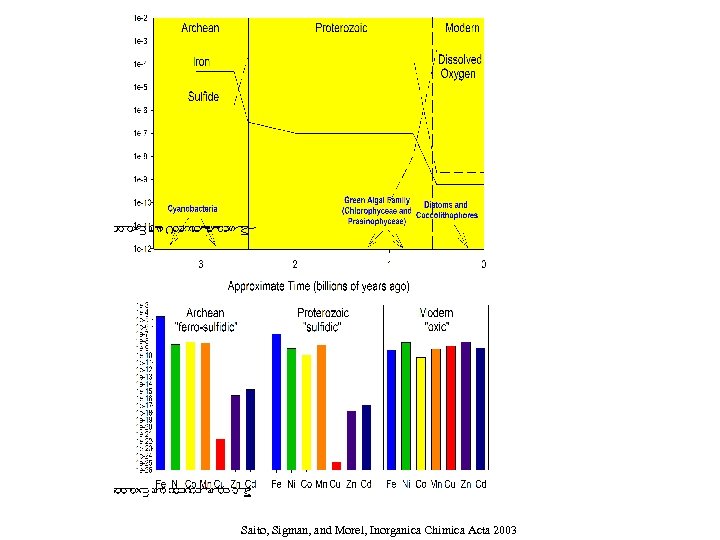 Saito, Sigman, and Morel, Inorganica Chimica Acta 2003
Saito, Sigman, and Morel, Inorganica Chimica Acta 2003
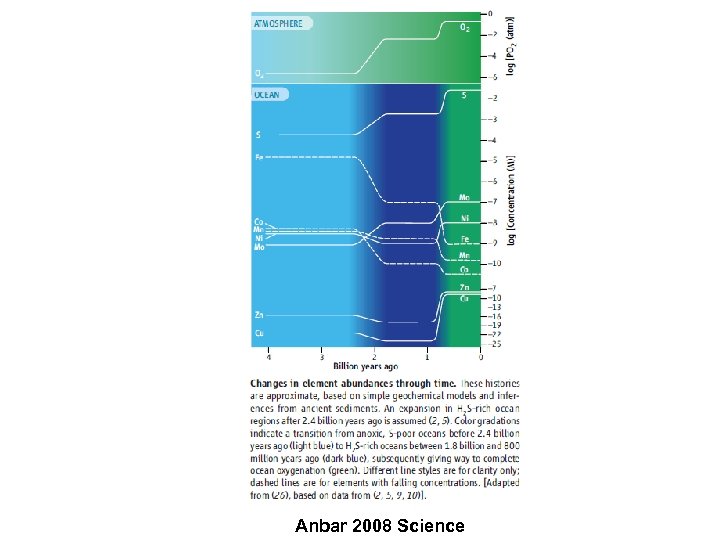 Anbar 2008 Science
Anbar 2008 Science
 Geochemical modeling (including metal-sulfide complexes): • Iron dominated Archean ocean has a similar chemistry to a Proterozoic sulfidic ocean: ~10 -6 M sulfide is enough to control speciation • Relative abundances: Mn, Co, Ni, Fe(II)>>Zn, Cd, Cu • Speciation was likely as important as solubility The trace metal physiology of marine cyanobacteria appears to be consistent with their evolution in an ancient sulfidic ocean: Absolute requirement for Co (Sunda and Huntsman, 1995; Saito et al. , 2002) Small zinc requirement relative to eukaryotic phytoplankton (ibid) Very sensitive to Cd toxicity (Saito, Sigman, Morel, 2003) High iron requirement (Raven, 1999; Berman-Frank, 2001) Some sensitivity to Cu toxicity (Brand et al. , 1986, Mann et al. , 2002) Abundant Prochlorococcus in low light suboxic zones (Goericke et al, . 2000) Abundant Nickel Superoxide Dismutase in the Synechococcus Proteome (Saito and Bertrand, unpublished data)
Geochemical modeling (including metal-sulfide complexes): • Iron dominated Archean ocean has a similar chemistry to a Proterozoic sulfidic ocean: ~10 -6 M sulfide is enough to control speciation • Relative abundances: Mn, Co, Ni, Fe(II)>>Zn, Cd, Cu • Speciation was likely as important as solubility The trace metal physiology of marine cyanobacteria appears to be consistent with their evolution in an ancient sulfidic ocean: Absolute requirement for Co (Sunda and Huntsman, 1995; Saito et al. , 2002) Small zinc requirement relative to eukaryotic phytoplankton (ibid) Very sensitive to Cd toxicity (Saito, Sigman, Morel, 2003) High iron requirement (Raven, 1999; Berman-Frank, 2001) Some sensitivity to Cu toxicity (Brand et al. , 1986, Mann et al. , 2002) Abundant Prochlorococcus in low light suboxic zones (Goericke et al, . 2000) Abundant Nickel Superoxide Dismutase in the Synechococcus Proteome (Saito and Bertrand, unpublished data)
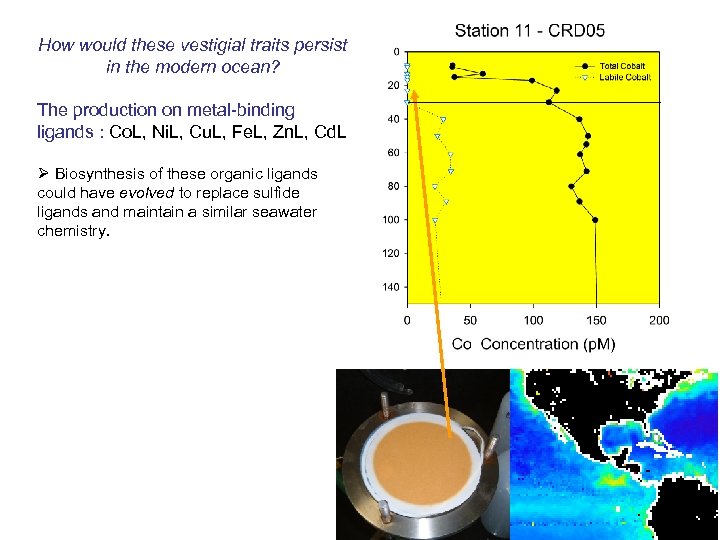 How would these vestigial traits persist in the modern ocean? The production on metal-binding ligands : Co. L, Ni. L, Cu. L, Fe. L, Zn. L, Cd. L Ø Biosynthesis of these organic ligands could have evolved to replace sulfide ligands and maintain a similar seawater chemistry.
How would these vestigial traits persist in the modern ocean? The production on metal-binding ligands : Co. L, Ni. L, Cu. L, Fe. L, Zn. L, Cd. L Ø Biosynthesis of these organic ligands could have evolved to replace sulfide ligands and maintain a similar seawater chemistry.
 Genome/Proteome Approaches to studying the Ancient Earth: • • Whole organism elemental composition analysis: – Quigg, Falkowski et al. , 2003. Ho et al. , 2003 Genomic and proteomic analyses (whole organism versus focusing on specific metalloenzymes: Whole: – Zerkle, House, Brantley. Am. J. Sci 2005 (whole) – Dupont, Palenik et al (PNAS 2007) (whole) Specific: – Gamma carbonic anhydrase (Smith and Ferry) – Superoxide Dismutase (Wolfe-Simon) – Nickel Famine of Ni requiring metalloenzymes in methanogens – Rubisco (nonmetalloenzyme) – Vitamin B 12
Genome/Proteome Approaches to studying the Ancient Earth: • • Whole organism elemental composition analysis: – Quigg, Falkowski et al. , 2003. Ho et al. , 2003 Genomic and proteomic analyses (whole organism versus focusing on specific metalloenzymes: Whole: – Zerkle, House, Brantley. Am. J. Sci 2005 (whole) – Dupont, Palenik et al (PNAS 2007) (whole) Specific: – Gamma carbonic anhydrase (Smith and Ferry) – Superoxide Dismutase (Wolfe-Simon) – Nickel Famine of Ni requiring metalloenzymes in methanogens – Rubisco (nonmetalloenzyme) – Vitamin B 12
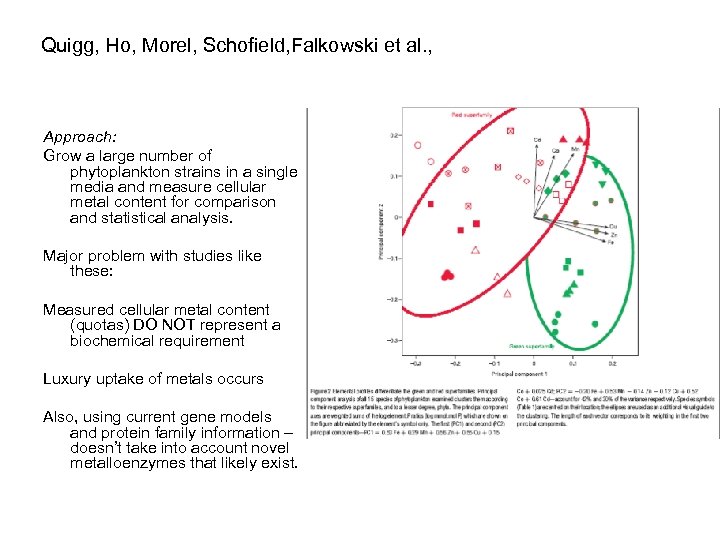 Quigg, Ho, Morel, Schofield, Falkowski et al. , Approach: Grow a large number of phytoplankton strains in a single media and measure cellular metal content for comparison and statistical analysis. Major problem with studies like these: Measured cellular metal content (quotas) DO NOT represent a biochemical requirement Luxury uptake of metals occurs Also, using current gene models and protein family information – doesn’t take into account novel metalloenzymes that likely exist.
Quigg, Ho, Morel, Schofield, Falkowski et al. , Approach: Grow a large number of phytoplankton strains in a single media and measure cellular metal content for comparison and statistical analysis. Major problem with studies like these: Measured cellular metal content (quotas) DO NOT represent a biochemical requirement Luxury uptake of metals occurs Also, using current gene models and protein family information – doesn’t take into account novel metalloenzymes that likely exist.
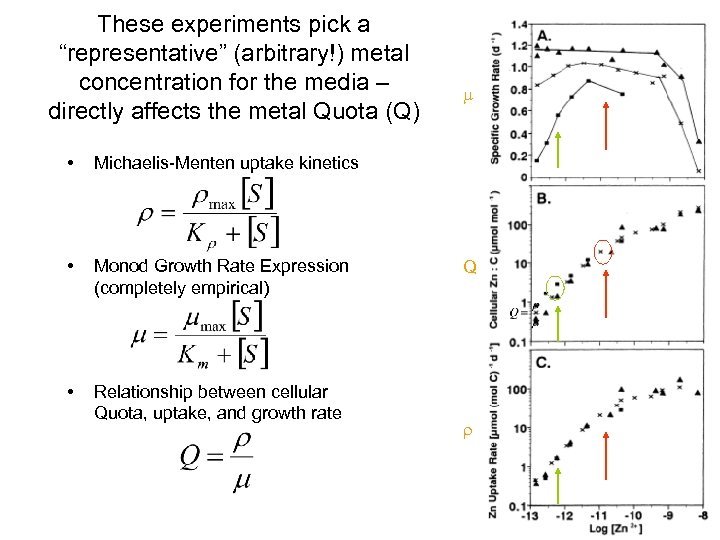 These experiments pick a “representative” (arbitrary!) metal concentration for the media – directly affects the metal Quota (Q) • Michaelis-Menten uptake kinetics • Monod Growth Rate Expression (completely empirical) • Relationship between cellular Quota, uptake, and growth rate m Q r
These experiments pick a “representative” (arbitrary!) metal concentration for the media – directly affects the metal Quota (Q) • Michaelis-Menten uptake kinetics • Monod Growth Rate Expression (completely empirical) • Relationship between cellular Quota, uptake, and growth rate m Q r
 Similar Genome/Proteome approaches: • Elemental composition analysis: – Quigg, Falkowski et al. , 2003. • Genomic and proteomic analyses: – Zerkle, House, Brantley. Am. J. Sci 2005 • “Model metallomes” – “We calculated model metallomes for 52 prokaryotes based on the number of atoms of trace metals required to express one molecule of each metallo-enzyme coded for in the corresponding genomes. Our results suggest that the use of metals in prokaryotes as a group generally follows the hierarchy: Fe >>Zn>Mn>>Mo, Cu, >> Ni, > W, V – Dupont, Palenik et al (PNAS in press)
Similar Genome/Proteome approaches: • Elemental composition analysis: – Quigg, Falkowski et al. , 2003. • Genomic and proteomic analyses: – Zerkle, House, Brantley. Am. J. Sci 2005 • “Model metallomes” – “We calculated model metallomes for 52 prokaryotes based on the number of atoms of trace metals required to express one molecule of each metallo-enzyme coded for in the corresponding genomes. Our results suggest that the use of metals in prokaryotes as a group generally follows the hierarchy: Fe >>Zn>Mn>>Mo, Cu, >> Ni, > W, V – Dupont, Palenik et al (PNAS in press)
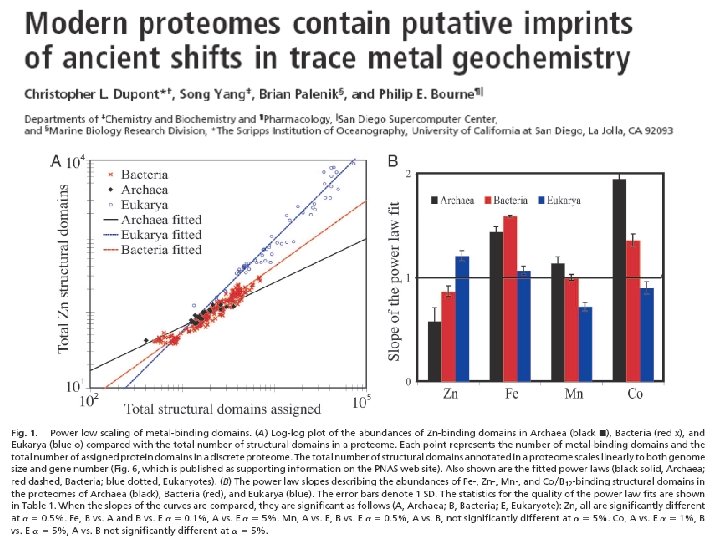
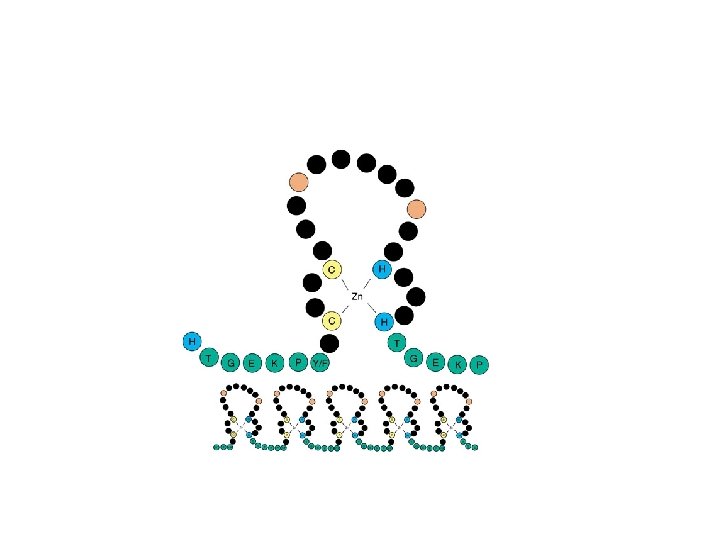
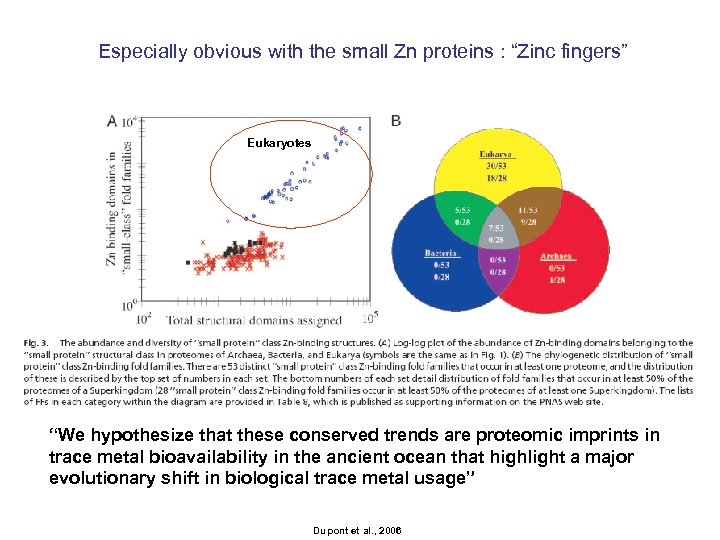 Especially obvious with the small Zn proteins : “Zinc fingers” Eukaryotes “We hypothesize that these conserved trends are proteomic imprints in trace metal bioavailability in the ancient ocean that highlight a major evolutionary shift in biological trace metal usage” Dupont et al. , 2006
Especially obvious with the small Zn proteins : “Zinc fingers” Eukaryotes “We hypothesize that these conserved trends are proteomic imprints in trace metal bioavailability in the ancient ocean that highlight a major evolutionary shift in biological trace metal usage” Dupont et al. , 2006
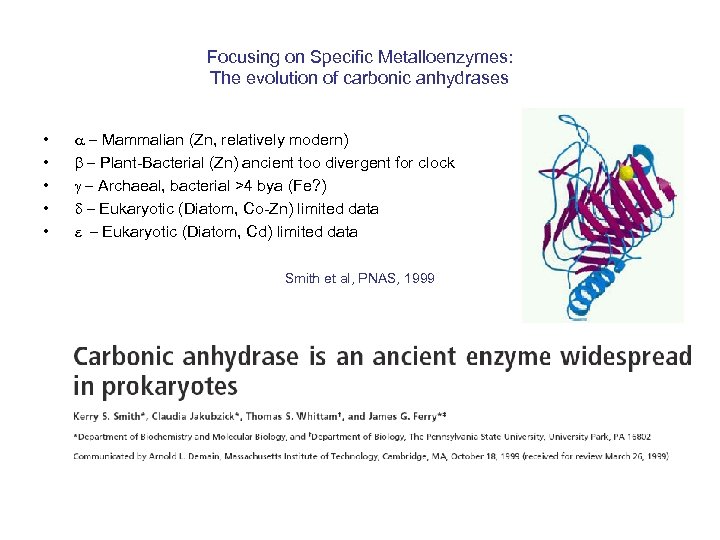 Focusing on Specific Metalloenzymes: The evolution of carbonic anhydrases • • • a - Mammalian (Zn, relatively modern) b - Plant-Bacterial (Zn) ancient too divergent for clock g - Archaeal, bacterial >4 bya (Fe? ) d - Eukaryotic (Diatom, Co-Zn) limited data e - Eukaryotic (Diatom, Cd) limited data Smith et al, PNAS, 1999
Focusing on Specific Metalloenzymes: The evolution of carbonic anhydrases • • • a - Mammalian (Zn, relatively modern) b - Plant-Bacterial (Zn) ancient too divergent for clock g - Archaeal, bacterial >4 bya (Fe? ) d - Eukaryotic (Diatom, Co-Zn) limited data e - Eukaryotic (Diatom, Cd) limited data Smith et al, PNAS, 1999
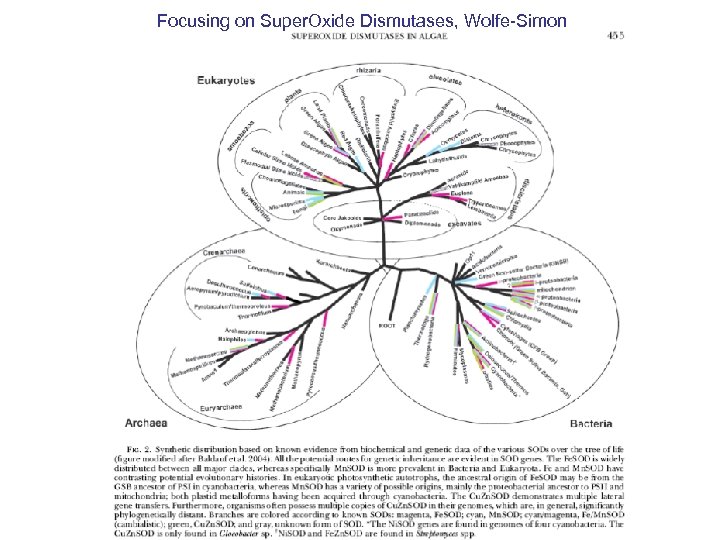 Focusing on Super. Oxide Dismutases, Wolfe-Simon
Focusing on Super. Oxide Dismutases, Wolfe-Simon
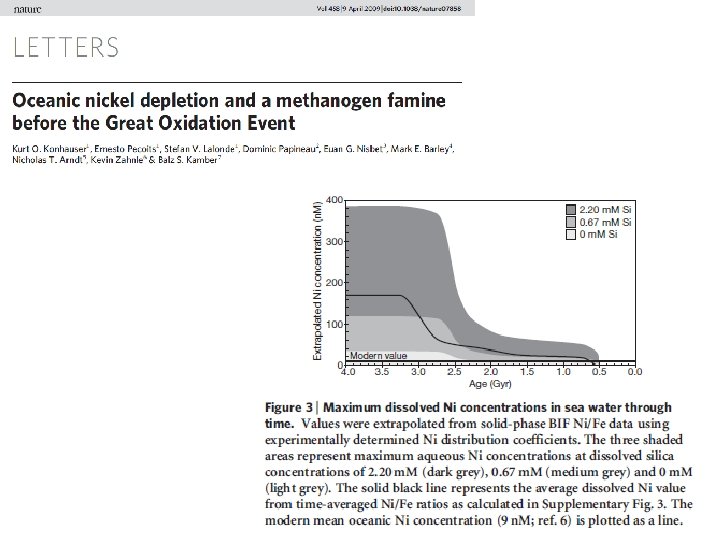
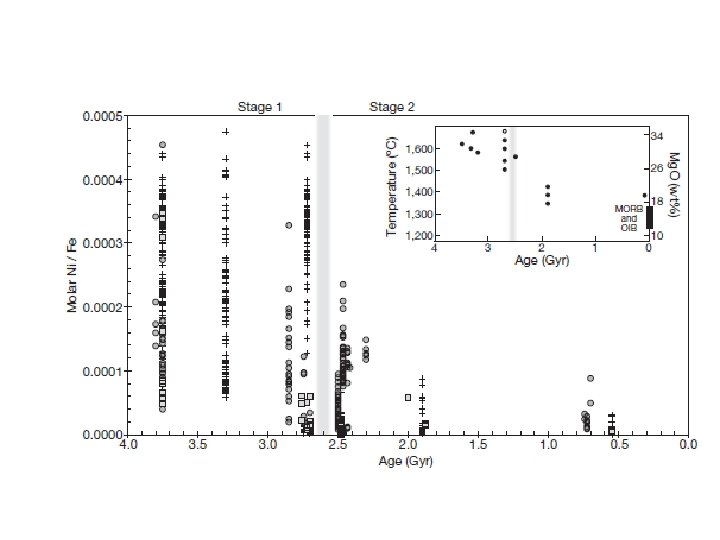
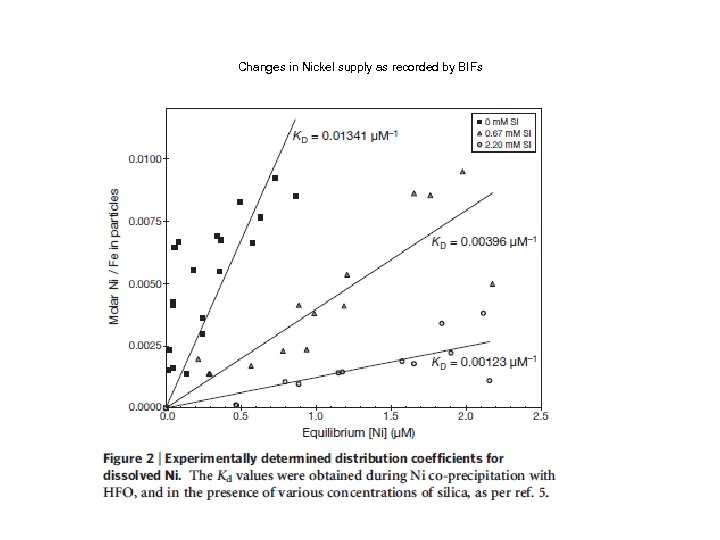 Changes in Nickel supply as recorded by BIFs
Changes in Nickel supply as recorded by BIFs
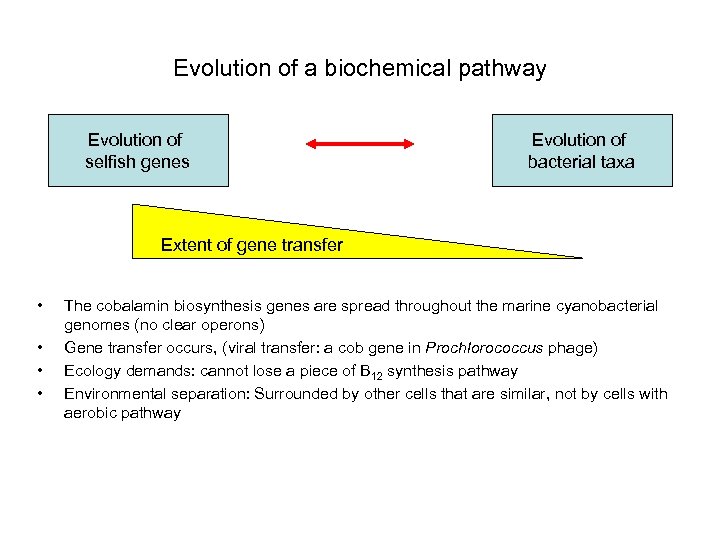 Evolution of a biochemical pathway Evolution of selfish genes Evolution of bacterial taxa Extent of gene transfer • • The cobalamin biosynthesis genes are spread throughout the marine cyanobacterial genomes (no clear operons) Gene transfer occurs, (viral transfer: a cob gene in Prochlorococcus phage) Ecology demands: cannot lose a piece of B 12 synthesis pathway Environmental separation: Surrounded by other cells that are similar, not by cells with aerobic pathway
Evolution of a biochemical pathway Evolution of selfish genes Evolution of bacterial taxa Extent of gene transfer • • The cobalamin biosynthesis genes are spread throughout the marine cyanobacterial genomes (no clear operons) Gene transfer occurs, (viral transfer: a cob gene in Prochlorococcus phage) Ecology demands: cannot lose a piece of B 12 synthesis pathway Environmental separation: Surrounded by other cells that are similar, not by cells with aerobic pathway
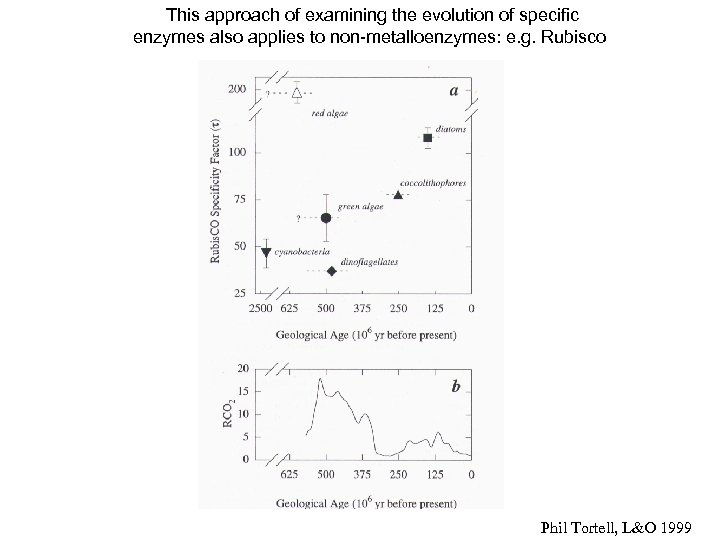 This approach of examining the evolution of specific enzymes also applies to non-metalloenzymes: e. g. Rubisco Phil Tortell, L&O 1999
This approach of examining the evolution of specific enzymes also applies to non-metalloenzymes: e. g. Rubisco Phil Tortell, L&O 1999
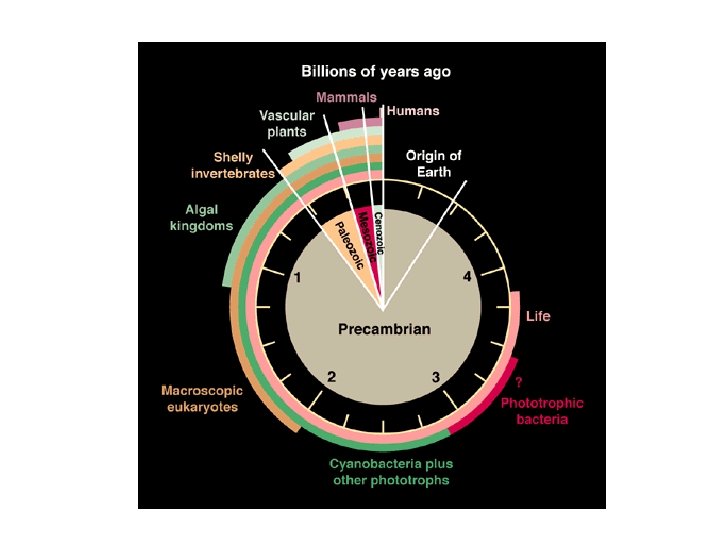
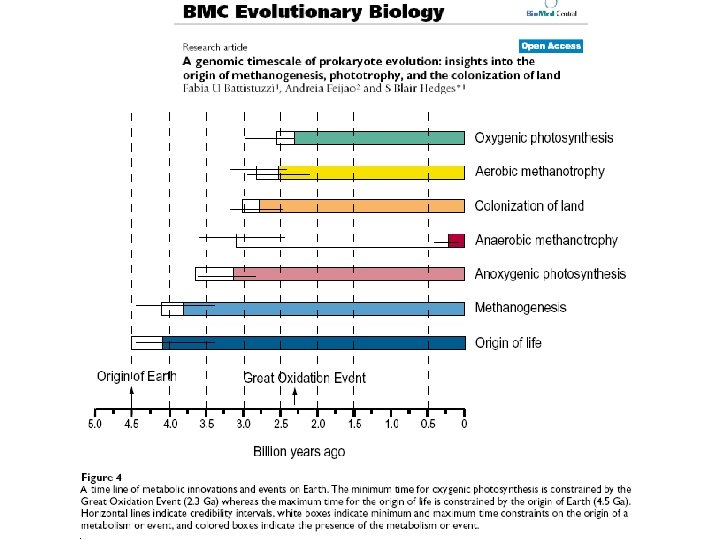
 Another problem with genomic approach: extinction of pathways (!? )
Another problem with genomic approach: extinction of pathways (!? )
 Conclusions: the potential for Marine Bioinorganic Chemistry to contribute to deep-time studies of the Earth Approaches: • Isotopic records (S, Fe, Mo, Cr, others? ) • Chemical records (Fe, Mo, Ni) • Chemical modeling based on 1) geochemical records of major controls or 2) on genomes and protein coordination chemistry • Physiological studies of extant organisms • Genomic studies of metalloenzymes • Studies of evolution of specific metalloenzymes • Combining multiple approaches to piece together a story Some Observations: • Evidence of changes in ocean chemistry/co-evolution of life and biogeochemistry? Geochemical modeling solves disagreement between S isotopic and phytoplankton physiological data. • Physiological characteristics of the Cyanobacteria are consistent with their evolution in an ancient ferro-sulfide-influenced ocean • A nickel famine of methanogenesis Overall • There is potential for utilizing information “stored” in modern organisms to understand the evolution of biogeochemical cycles, despite the potential for “Genomic Diagensis”
Conclusions: the potential for Marine Bioinorganic Chemistry to contribute to deep-time studies of the Earth Approaches: • Isotopic records (S, Fe, Mo, Cr, others? ) • Chemical records (Fe, Mo, Ni) • Chemical modeling based on 1) geochemical records of major controls or 2) on genomes and protein coordination chemistry • Physiological studies of extant organisms • Genomic studies of metalloenzymes • Studies of evolution of specific metalloenzymes • Combining multiple approaches to piece together a story Some Observations: • Evidence of changes in ocean chemistry/co-evolution of life and biogeochemistry? Geochemical modeling solves disagreement between S isotopic and phytoplankton physiological data. • Physiological characteristics of the Cyanobacteria are consistent with their evolution in an ancient ferro-sulfide-influenced ocean • A nickel famine of methanogenesis Overall • There is potential for utilizing information “stored” in modern organisms to understand the evolution of biogeochemical cycles, despite the potential for “Genomic Diagensis”


