16997935b77cdf45ecef2f7fadd490d2.ppt
- Количество слайдов: 31
 Toward a General Theory of Evolution Addy Pross Department of Chemistry, Ben Gurion University Be’er Sheva, Israel ILASOL - Dcember 25, 2011
Toward a General Theory of Evolution Addy Pross Department of Chemistry, Ben Gurion University Be’er Sheva, Israel ILASOL - Dcember 25, 2011
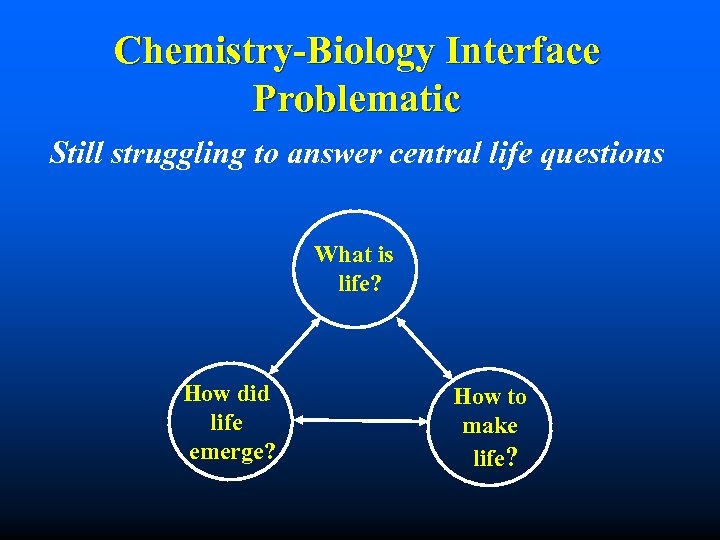 Chemistry-Biology Interface Problematic Still struggling to answer central life questions What is life? How did life emerge? How to make life?
Chemistry-Biology Interface Problematic Still struggling to answer central life questions What is life? How did life emerge? How to make life?
 General Theory of Evolution Attempts to extend and reformulate Darwinian thinking in chemical terms to help bridge between biological and chemical worlds. § Based on the unique kinetic character of the replication reaction § Identifies a stability kind associated solely with replicating entities - dynamic kinetic stability A. Pross (2003 -11)
General Theory of Evolution Attempts to extend and reformulate Darwinian thinking in chemical terms to help bridge between biological and chemical worlds. § Based on the unique kinetic character of the replication reaction § Identifies a stability kind associated solely with replicating entities - dynamic kinetic stability A. Pross (2003 -11)
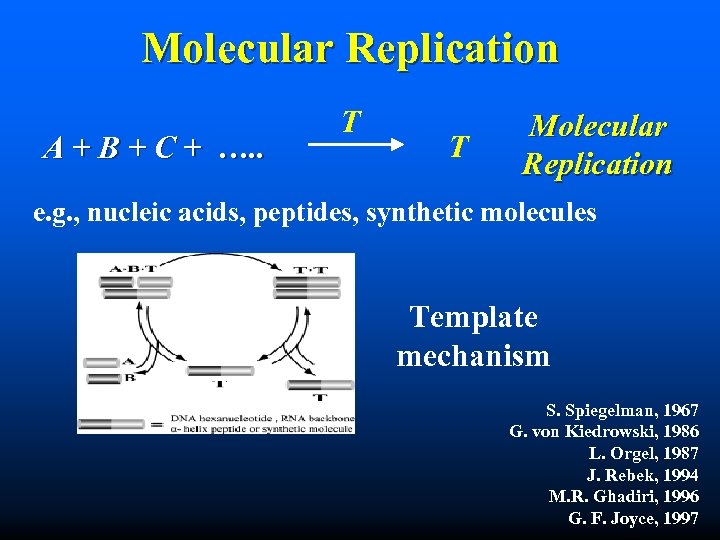 Molecular Replication A + B + C + …. . T T Molecular Replication e. g. , nucleic acids, peptides, synthetic molecules Template mechanism S. Spiegelman, 1967 G. von Kiedrowski, 1986 L. Orgel, 1987 J. Rebek, 1994 M. R. Ghadiri, 1996 G. F. Joyce, 1997
Molecular Replication A + B + C + …. . T T Molecular Replication e. g. , nucleic acids, peptides, synthetic molecules Template mechanism S. Spiegelman, 1967 G. von Kiedrowski, 1986 L. Orgel, 1987 J. Rebek, 1994 M. R. Ghadiri, 1996 G. F. Joyce, 1997
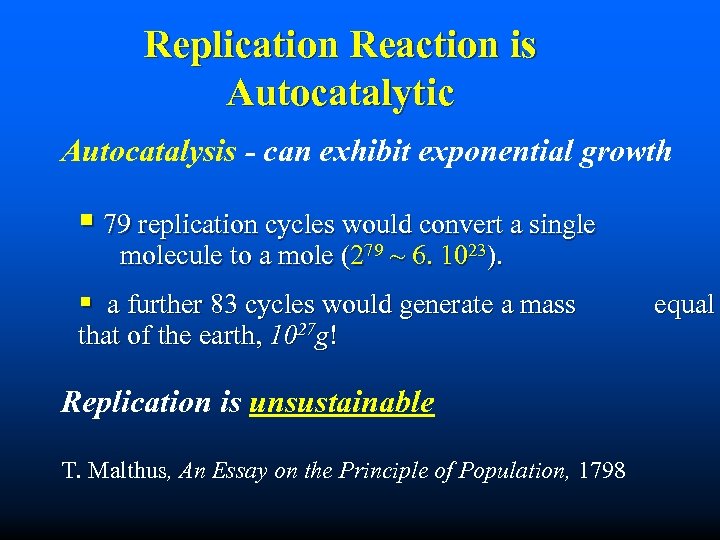 Replication Reaction is Autocatalytic Autocatalysis - can exhibit exponential growth § 79 replication cycles would convert a single molecule to a mole (279 ~ 6. 1023). § a further 83 cycles would generate a mass that of the earth, 1027 g! Replication is unsustainable T. Malthus, An Essay on the Principle of Population, 1798 equal
Replication Reaction is Autocatalytic Autocatalysis - can exhibit exponential growth § 79 replication cycles would convert a single molecule to a mole (279 ~ 6. 1023). § a further 83 cycles would generate a mass that of the earth, 1027 g! Replication is unsustainable T. Malthus, An Essay on the Principle of Population, 1798 equal
 Nature of Stability A system is stable if it is persistent, unchanging over time. Thermodynamic Stability – an inherent property of a chemical system Kinetic Stability – depends on reaction rates and barrier heights Dynamic Kinetic Stability - A stability kind associated solely with replicating entities. A. Pross, J. Syst. Chem. 2011 A. Pross, Chem. Eur. J. 2009
Nature of Stability A system is stable if it is persistent, unchanging over time. Thermodynamic Stability – an inherent property of a chemical system Kinetic Stability – depends on reaction rates and barrier heights Dynamic Kinetic Stability - A stability kind associated solely with replicating entities. A. Pross, J. Syst. Chem. 2011 A. Pross, Chem. Eur. J. 2009
 Dynamic Kinetic Stability (DKS) Replication is unsustainable, therefore for stability ~ rate of replicator formation = rate of decay d. X/dt = k. XM - g. X X = replicator conc. M = monomer conc. k, g = rate constants. Lotka, 1910 d. X/dt = 0 would define a steady state population If a replicating system is stable then its stability is of a dynamic kinetic kind
Dynamic Kinetic Stability (DKS) Replication is unsustainable, therefore for stability ~ rate of replicator formation = rate of decay d. X/dt = k. XM - g. X X = replicator conc. M = monomer conc. k, g = rate constants. Lotka, 1910 d. X/dt = 0 would define a steady state population If a replicating system is stable then its stability is of a dynamic kinetic kind
 Stability in ‘Regular’ and Replicative Worlds § ‘Regular’ chemical systems are stable because they DO NOT react. § Replicating chemical systems are stable (persistent) because they DO react – to make more of themselves! DKS would apply to all stable replicating systems, biological and chemical. A. Pross, Pure Appl. Chem. 2005
Stability in ‘Regular’ and Replicative Worlds § ‘Regular’ chemical systems are stable because they DO NOT react. § Replicating chemical systems are stable (persistent) because they DO react – to make more of themselves! DKS would apply to all stable replicating systems, biological and chemical. A. Pross, Pure Appl. Chem. 2005
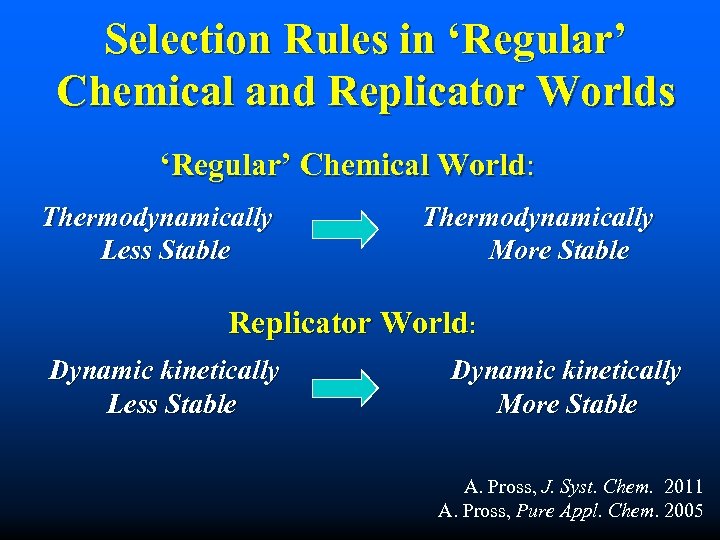 Selection Rules in ‘Regular’ Chemical and Replicator Worlds ‘Regular’ Chemical World: Thermodynamically Less Stable Thermodynamically More Stable Replicator World: Dynamic kinetically Less Stable Dynamic kinetically More Stable A. Pross, J. Syst. Chem. 2011 A. Pross, Pure Appl. Chem. 2005
Selection Rules in ‘Regular’ Chemical and Replicator Worlds ‘Regular’ Chemical World: Thermodynamically Less Stable Thermodynamically More Stable Replicator World: Dynamic kinetically Less Stable Dynamic kinetically More Stable A. Pross, J. Syst. Chem. 2011 A. Pross, Pure Appl. Chem. 2005
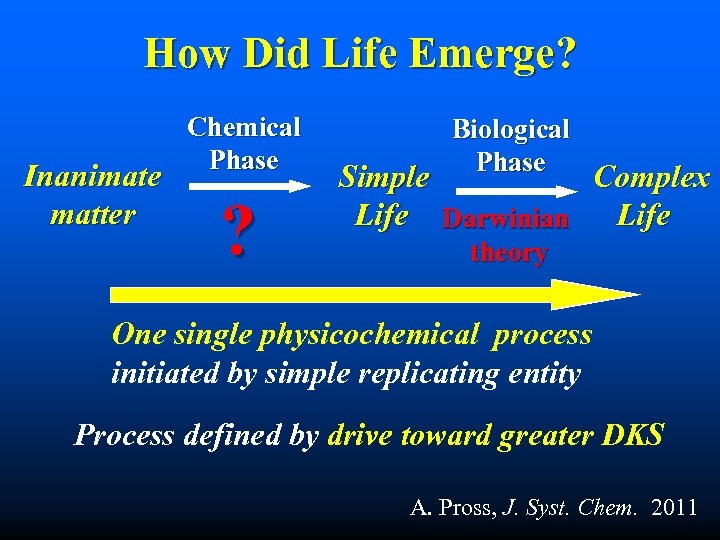 How Did Life Emerge? Inanimate matter Chemical Phase ? Biological Phase Simple Complex Life Darwinian Life theory One single physicochemical process initiated by simple replicating entity Process defined by drive toward greater DKS A. Pross, J. Syst. Chem. 2011
How Did Life Emerge? Inanimate matter Chemical Phase ? Biological Phase Simple Complex Life Darwinian Life theory One single physicochemical process initiated by simple replicating entity Process defined by drive toward greater DKS A. Pross, J. Syst. Chem. 2011
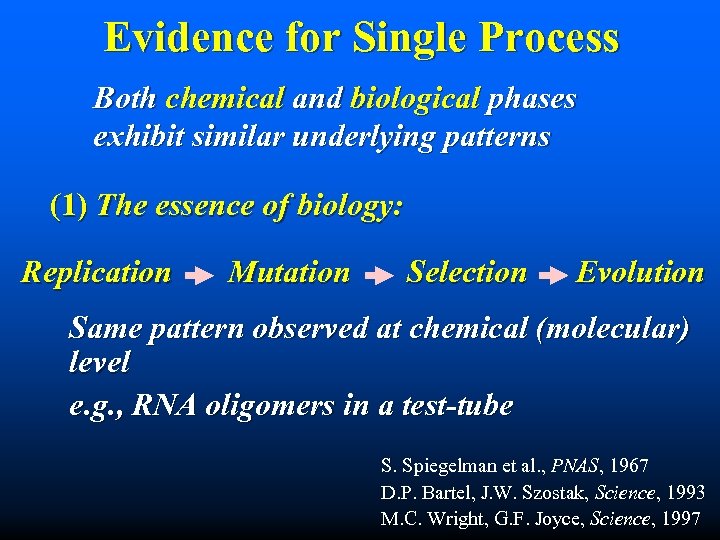 Evidence for Single Process Both chemical and biological phases exhibit similar underlying patterns (1) The essence of biology: Replication Mutation Selection Evolution Same pattern observed at chemical (molecular) level e. g. , RNA oligomers in a test-tube S. Spiegelman et al. , PNAS, 1967 D. P. Bartel, J. W. Szostak, Science, 1993 M. C. Wright, G. F. Joyce, Science, 1997
Evidence for Single Process Both chemical and biological phases exhibit similar underlying patterns (1) The essence of biology: Replication Mutation Selection Evolution Same pattern observed at chemical (molecular) level e. g. , RNA oligomers in a test-tube S. Spiegelman et al. , PNAS, 1967 D. P. Bartel, J. W. Szostak, Science, 1993 M. C. Wright, G. F. Joyce, Science, 1997
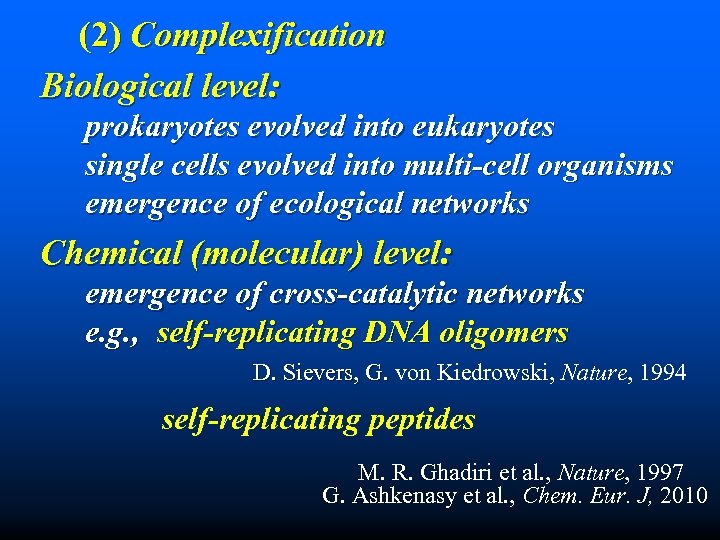 (2) Complexification Biological level: prokaryotes evolved into eukaryotes single cells evolved into multi-cell organisms emergence of ecological networks Chemical (molecular) level: emergence of cross-catalytic networks e. g. , self-replicating DNA oligomers D. Sievers, G. von Kiedrowski, Nature, 1994 self-replicating peptides M. R. Ghadiri et al. , Nature, 1997 G. Ashkenasy et al. , Chem. Eur. J, 2010
(2) Complexification Biological level: prokaryotes evolved into eukaryotes single cells evolved into multi-cell organisms emergence of ecological networks Chemical (molecular) level: emergence of cross-catalytic networks e. g. , self-replicating DNA oligomers D. Sievers, G. von Kiedrowski, Nature, 1994 self-replicating peptides M. R. Ghadiri et al. , Nature, 1997 G. Ashkenasy et al. , Chem. Eur. J, 2010
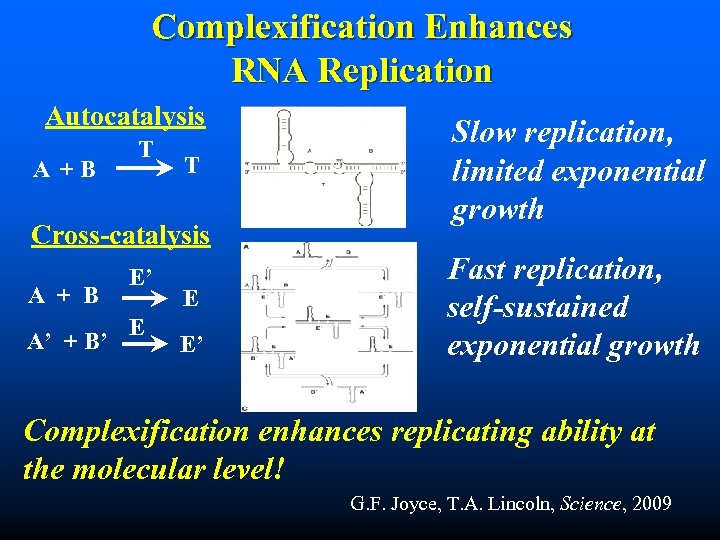 Complexification Enhances RNA Replication Autocatalysis A +B T T Cross-catalysis A + B A’ + B’ E’ E E E’ Slow replication, limited exponential growth Fast replication, self-sustained exponential growth Complexification enhances replicating ability at the molecular level! G. F. Joyce, T. A. Lincoln, Science, 2009
Complexification Enhances RNA Replication Autocatalysis A +B T T Cross-catalysis A + B A’ + B’ E’ E E E’ Slow replication, limited exponential growth Fast replication, self-sustained exponential growth Complexification enhances replicating ability at the molecular level! G. F. Joyce, T. A. Lincoln, Science, 2009
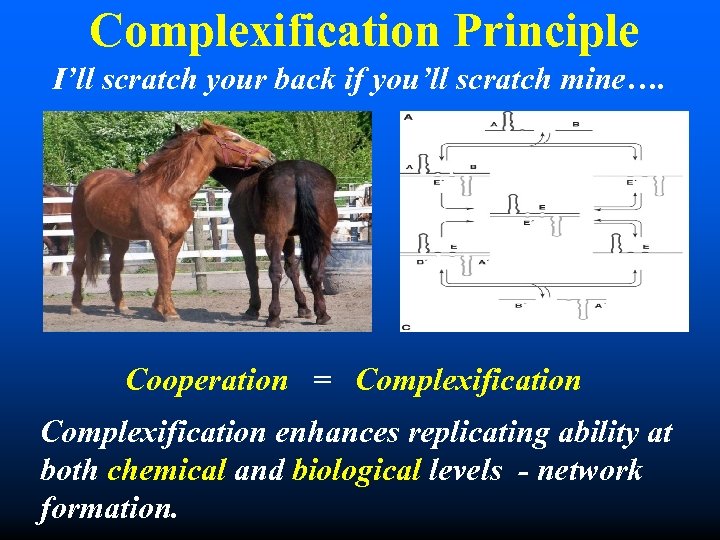 Complexification Principle I’ll scratch your back if you’ll scratch mine…. Cooperation = Complexification enhances replicating ability at both chemical and biological levels - network formation.
Complexification Principle I’ll scratch your back if you’ll scratch mine…. Cooperation = Complexification enhances replicating ability at both chemical and biological levels - network formation.
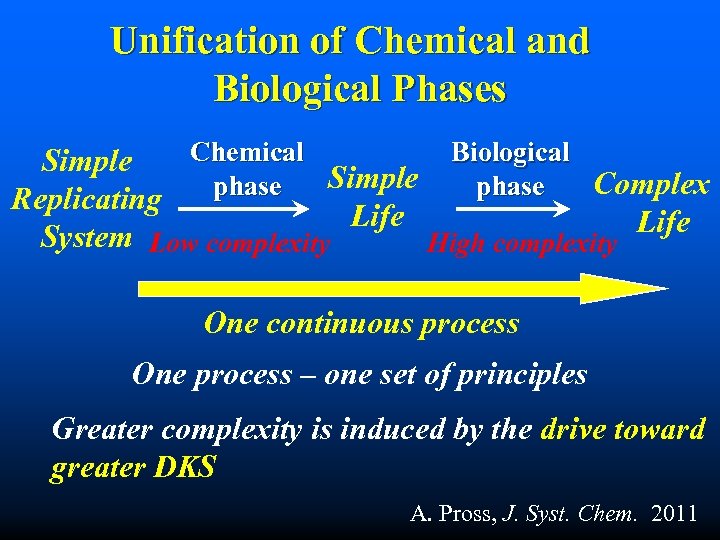 Unification of Chemical and Biological Phases Chemical Biological Simple Complex phase Replicating Life System Low complexity High complexity One continuous process One process – one set of principles Greater complexity is induced by the drive toward greater DKS A. Pross, J. Syst. Chem. 2011
Unification of Chemical and Biological Phases Chemical Biological Simple Complex phase Replicating Life System Low complexity High complexity One continuous process One process – one set of principles Greater complexity is induced by the drive toward greater DKS A. Pross, J. Syst. Chem. 2011
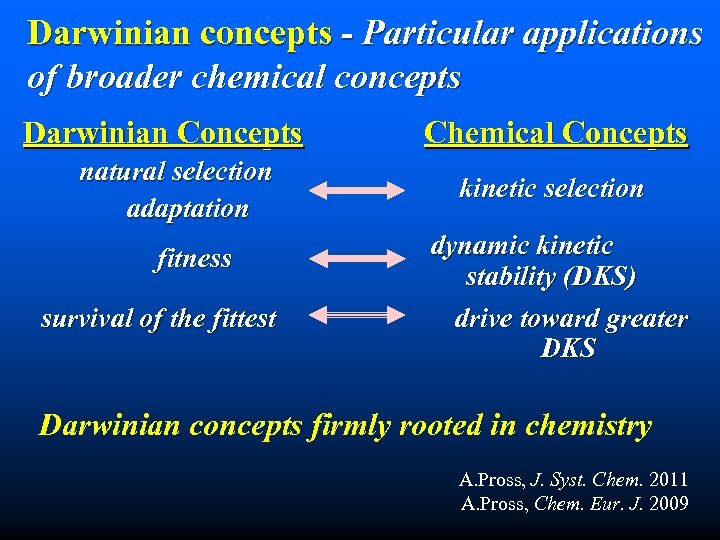 Darwinian concepts - Particular applications of broader chemical concepts Darwinian Concepts natural selection adaptation fitness survival of the fittest Chemical Concepts kinetic selection dynamic kinetic stability (DKS) drive toward greater DKS Darwinian concepts firmly rooted in chemistry A. Pross, J. Syst. Chem. 2011 A. Pross, Chem. Eur. J. 2009
Darwinian concepts - Particular applications of broader chemical concepts Darwinian Concepts natural selection adaptation fitness survival of the fittest Chemical Concepts kinetic selection dynamic kinetic stability (DKS) drive toward greater DKS Darwinian concepts firmly rooted in chemistry A. Pross, J. Syst. Chem. 2011 A. Pross, Chem. Eur. J. 2009
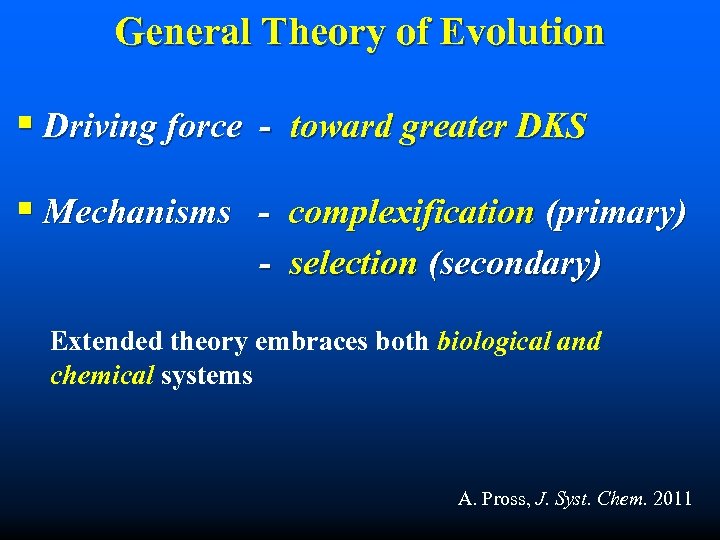 General Theory of Evolution § Driving force - toward greater DKS § Mechanisms - complexification (primary) - selection (secondary) Extended theory embraces both biological and chemical systems A. Pross, J. Syst. Chem. 2011
General Theory of Evolution § Driving force - toward greater DKS § Mechanisms - complexification (primary) - selection (secondary) Extended theory embraces both biological and chemical systems A. Pross, J. Syst. Chem. 2011
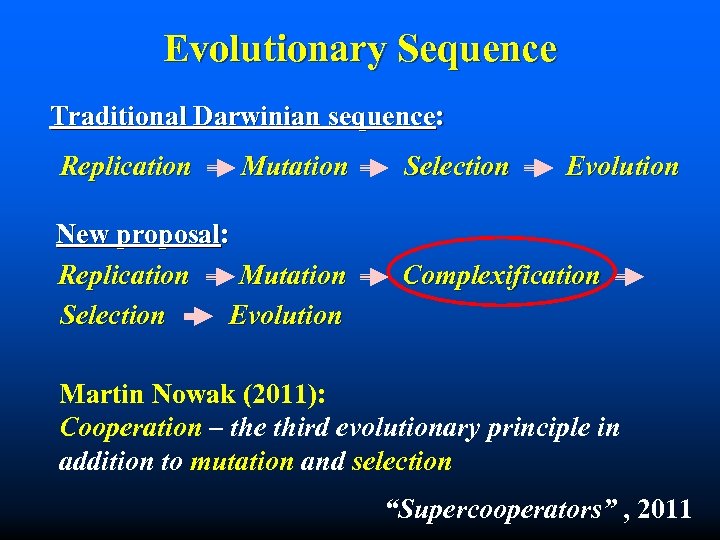 Evolutionary Sequence Traditional Darwinian sequence: Replication Mutation New proposal: Replication Mutation Selection Evolution Complexification Martin Nowak (2011): Cooperation – the third evolutionary principle in addition to mutation and selection “Supercooperators” , 2011
Evolutionary Sequence Traditional Darwinian sequence: Replication Mutation New proposal: Replication Mutation Selection Evolution Complexification Martin Nowak (2011): Cooperation – the third evolutionary principle in addition to mutation and selection “Supercooperators” , 2011
 Global Characteristics of Living Systems § Extraordinary complexity § Dynamic character § Far-from-equilibrium state § Teleonomy (purposeful nature) § Homochiral character § Diversity Can be understood through the DKS concept A. Pross, J. Sys. Chem. 2011
Global Characteristics of Living Systems § Extraordinary complexity § Dynamic character § Far-from-equilibrium state § Teleonomy (purposeful nature) § Homochiral character § Diversity Can be understood through the DKS concept A. Pross, J. Sys. Chem. 2011
 Dynamic Kinetic Stability (DKS)
Dynamic Kinetic Stability (DKS)
 Dynamic Steady States Exist at Various Levels of Complexity § For molecular replicators there is just one level of turnover § At cell level two levels of turnover Protein degradation and re-synthesis is a tightly regulated process. intracellular protein t 1/2 = 11 mins - 48 hrs Hershko, Ciechanover & Rose (Nobel Prize, 2004) § At the organismic level three levels of turnover
Dynamic Steady States Exist at Various Levels of Complexity § For molecular replicators there is just one level of turnover § At cell level two levels of turnover Protein degradation and re-synthesis is a tightly regulated process. intracellular protein t 1/2 = 11 mins - 48 hrs Hershko, Ciechanover & Rose (Nobel Prize, 2004) § At the organismic level three levels of turnover
 Global Characteristics of Living Systems § Extraordinary complexity § Dynamic character § Far-from-equilibrium state § Teleonomy (purposeful nature) § Homochiral character § Diversity Can be understood through the DKS concept A. Pross, J. Sys. Chem. 2011
Global Characteristics of Living Systems § Extraordinary complexity § Dynamic character § Far-from-equilibrium state § Teleonomy (purposeful nature) § Homochiral character § Diversity Can be understood through the DKS concept A. Pross, J. Sys. Chem. 2011
 Q: How could the evolutionary process lead to the formation of thermodynamically unstable systems? A: In replicative world the stability that counts is dynamic kinetic stability (DKS). How can high stability of one kind lead to low stability of another kind?
Q: How could the evolutionary process lead to the formation of thermodynamically unstable systems? A: In replicative world the stability that counts is dynamic kinetic stability (DKS). How can high stability of one kind lead to low stability of another kind?
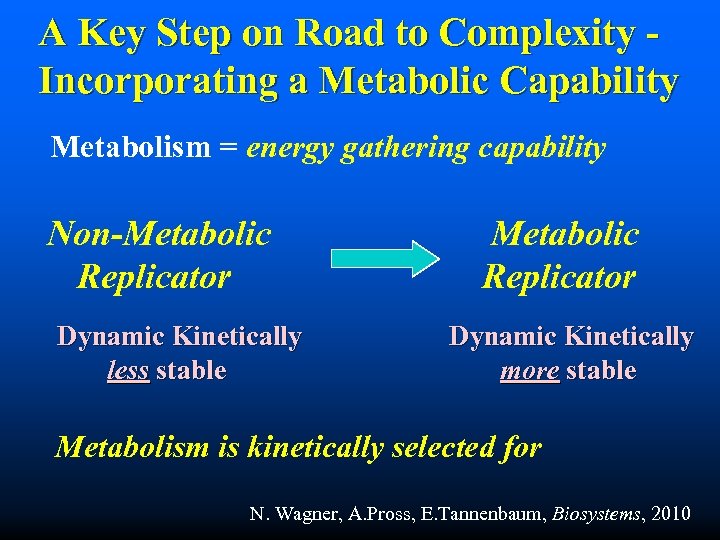 A Key Step on Road to Complexity Incorporating a Metabolic Capability Metabolism = energy gathering capability Non-Metabolic Replicator Dynamic Kinetically less stable Metabolic Replicator Dynamic Kinetically more stable Metabolism is kinetically selected for N. Wagner, A. Pross, E. Tannenbaum, Biosystems, 2010
A Key Step on Road to Complexity Incorporating a Metabolic Capability Metabolism = energy gathering capability Non-Metabolic Replicator Dynamic Kinetically less stable Metabolic Replicator Dynamic Kinetically more stable Metabolism is kinetically selected for N. Wagner, A. Pross, E. Tannenbaum, Biosystems, 2010
 Consequences of Metabolism § Metabolism (energy gathering) frees the replicator from thermodynamic constraints. § The result: Thermodynamically unstable but dynamic kinetically stable replicating entities § With thermodynamic constraints eliminated, primary directive for chemical change becomes kinetic rather than thermodynamic. The moment life began… § Death – reversion to thermodynamic world
Consequences of Metabolism § Metabolism (energy gathering) frees the replicator from thermodynamic constraints. § The result: Thermodynamically unstable but dynamic kinetically stable replicating entities § With thermodynamic constraints eliminated, primary directive for chemical change becomes kinetic rather than thermodynamic. The moment life began… § Death – reversion to thermodynamic world
 Global Characteristics of Living Systems § Extraordinary complexity § Dynamic character § Far-from-equilibrium state § Teleonomy (purposeful nature) § Homochiral character § Diversity Can be understood through the DKS concept A. Pross, J. Sys. Chem. 2011
Global Characteristics of Living Systems § Extraordinary complexity § Dynamic character § Far-from-equilibrium state § Teleonomy (purposeful nature) § Homochiral character § Diversity Can be understood through the DKS concept A. Pross, J. Sys. Chem. 2011
 Darwin’s Two Principles Principle of Natural Selection Principle of Divergence
Darwin’s Two Principles Principle of Natural Selection Principle of Divergence
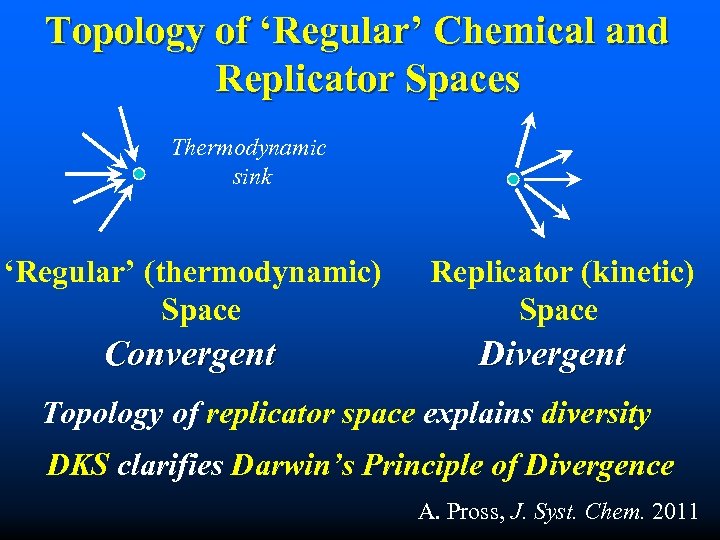 Topology of ‘Regular’ Chemical and Replicator Spaces Thermodynamic sink ‘Regular’ (thermodynamic) Space Convergent Replicator (kinetic) Space Divergent Topology of replicator space explains diversity DKS clarifies Darwin’s Principle of Divergence A. Pross, J. Syst. Chem. 2011
Topology of ‘Regular’ Chemical and Replicator Spaces Thermodynamic sink ‘Regular’ (thermodynamic) Space Convergent Replicator (kinetic) Space Divergent Topology of replicator space explains diversity DKS clarifies Darwin’s Principle of Divergence A. Pross, J. Syst. Chem. 2011
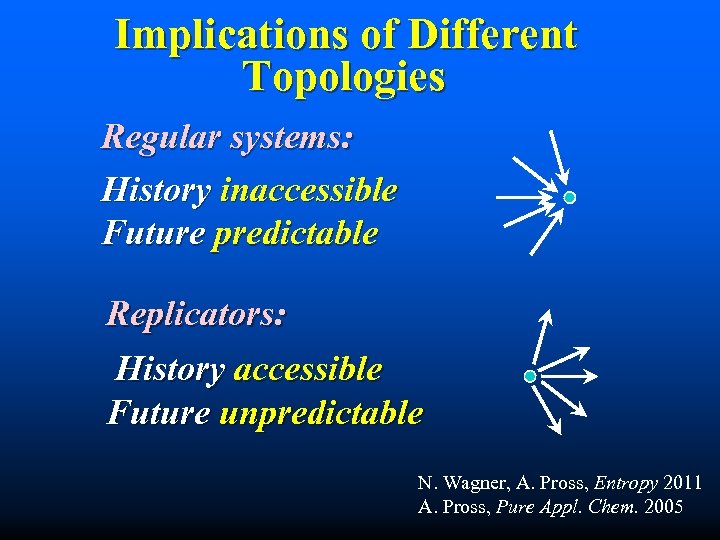 Implications of Different Topologies Regular systems: History inaccessible Future predictable Replicators: History accessible Future unpredictable N. Wagner, A. Pross, Entropy 2011 A. Pross, Pure Appl. Chem. 2005
Implications of Different Topologies Regular systems: History inaccessible Future predictable Replicators: History accessible Future unpredictable N. Wagner, A. Pross, Entropy 2011 A. Pross, Pure Appl. Chem. 2005
 Key Conclusions DKS - the conceptual bridge between Chemistry and Biology. • Unifies abiogenesis and biological evolution • Integrates Darwinian theory into general chemical theory • DKS – the driving force for evolution • Explains life’s unusual characteristics Life - an ever expanding dynamic network of chemical reactions derived from the replication 30 reaction.
Key Conclusions DKS - the conceptual bridge between Chemistry and Biology. • Unifies abiogenesis and biological evolution • Integrates Darwinian theory into general chemical theory • DKS – the driving force for evolution • Explains life’s unusual characteristics Life - an ever expanding dynamic network of chemical reactions derived from the replication 30 reaction.
 Acknowledgements Prof. Emmanuel Tannenbaum – BGU Dr. Nathaniel Wagner – BGU Dr. Nella Pross - BGU
Acknowledgements Prof. Emmanuel Tannenbaum – BGU Dr. Nathaniel Wagner – BGU Dr. Nella Pross - BGU


