0c505944800c81e2250cd38529729c68.ppt
- Количество слайдов: 23
 Total Quality Management System (TQMS) Astra. Zeneca Solid Dosage Facility, Plankstadt, Germany Ali Afnan Astra. Zeneca Pharmaceuticals Engineering Group Alderley House, Alderley Park Macclesfield, UK ali. afnan@astrazeneca. com
Total Quality Management System (TQMS) Astra. Zeneca Solid Dosage Facility, Plankstadt, Germany Ali Afnan Astra. Zeneca Pharmaceuticals Engineering Group Alderley House, Alderley Park Macclesfield, UK ali. afnan@astrazeneca. com
 Implementation of the TQM Strategy In-Process Control & Monitoring Key Process Operations Identification / specification of all incoming raw materials in the dispensaries (& the warehouse) Fluid Bed Drier end point control Continuous in-line monitoring of blending & end point control In-line monitoring of Tablet Quality parameters against registered specifications 21 CFR 11 compliant data management system Real-time continuous quality assurance The platform for parametric release Ali Afnan 2
Implementation of the TQM Strategy In-Process Control & Monitoring Key Process Operations Identification / specification of all incoming raw materials in the dispensaries (& the warehouse) Fluid Bed Drier end point control Continuous in-line monitoring of blending & end point control In-line monitoring of Tablet Quality parameters against registered specifications 21 CFR 11 compliant data management system Real-time continuous quality assurance The platform for parametric release Ali Afnan 2
 TQMS Benefits- I Enhanced product quality assurance Significant manufacturing cost reduction Reduction of product inventory The elimination of end-of-line off-line testing providing opportunity for a new way of working significant increase in supply chain velocity Reduces business risk Increases competitive advantage Allows “remote” regulatory inspection Ali Afnan 3
TQMS Benefits- I Enhanced product quality assurance Significant manufacturing cost reduction Reduction of product inventory The elimination of end-of-line off-line testing providing opportunity for a new way of working significant increase in supply chain velocity Reduces business risk Increases competitive advantage Allows “remote” regulatory inspection Ali Afnan 3
 TQMS- Dispensaries Systems approach NIR Analyser • Brimrose AOTF Multiplexed Analyser • • • Diffuse reflectance measurement (1100 -2300 nm) CIP and WIP probes Performs the analyses at every dispensing operation Allows control of recipe management Release of incoming materials by the plant Utilising • Bespoke 21 CFR 11 compliant software • Local panel PC’s • Barcode readers Ali Afnan 4
TQMS- Dispensaries Systems approach NIR Analyser • Brimrose AOTF Multiplexed Analyser • • • Diffuse reflectance measurement (1100 -2300 nm) CIP and WIP probes Performs the analyses at every dispensing operation Allows control of recipe management Release of incoming materials by the plant Utilising • Bespoke 21 CFR 11 compliant software • Local panel PC’s • Barcode readers Ali Afnan 4
 TQMS- Dispensary Operation Operator scans material barcode (Article No. & Batch No. ) Analyser enabled for operation Operator prompted through dispensing process Trigger actuated by operator Spectra collected with probe in-situ I. D. reported & confirmed Qualitative/ qualitative results reported & stored Ali Afnan 5
TQMS- Dispensary Operation Operator scans material barcode (Article No. & Batch No. ) Analyser enabled for operation Operator prompted through dispensing process Trigger actuated by operator Spectra collected with probe in-situ I. D. reported & confirmed Qualitative/ qualitative results reported & stored Ali Afnan 5
 TQMS- FBD Probe permanently installed FBD CIP and WIP probe designed to minimise fouling Operator scans product barcode (Article No. & Batch No. ) Analyser enabled for operation Predicted moisture values sent to FBD Control System for drying end point. Ali Afnan 6
TQMS- FBD Probe permanently installed FBD CIP and WIP probe designed to minimise fouling Operator scans product barcode (Article No. & Batch No. ) Analyser enabled for operation Predicted moisture values sent to FBD Control System for drying end point. Ali Afnan 6
 The Blending Process of changing the constituent matrices Mixing (re-distribution) of particles of differing: physical characteristics / chemical properties Process of attrition & agglomeration A complex process Currently under scientific scrutiny Driving a “holistic” re-assessment of blend process understanding and means of monitoring. Ali Afnan 7
The Blending Process of changing the constituent matrices Mixing (re-distribution) of particles of differing: physical characteristics / chemical properties Process of attrition & agglomeration A complex process Currently under scientific scrutiny Driving a “holistic” re-assessment of blend process understanding and means of monitoring. Ali Afnan 7
 TQMS- The Blending Process Objectives are: To monitor the “whole” powder mix in the blending vessel Non-invasively & non-intrusively To determine completion of the blending process During every blending operation To deliver consistent quality Applicable to a range of blender types Ali Afnan 8
TQMS- The Blending Process Objectives are: To monitor the “whole” powder mix in the blending vessel Non-invasively & non-intrusively To determine completion of the blending process During every blending operation To deliver consistent quality Applicable to a range of blender types Ali Afnan 8
 TQMS NIR Blend Monitoring System IBC with Sapphire window Brimrose AOTF NIR “Free-Space” Analyser Portable Sample (Beam) size: 9 mm x 5 mm Diffuse reflectance (1100 -2300 nm) Battery & mains operation CIP and WIP analyser All solid state hardware Provides process reproducibility 21 CFR 11 compliant package Ali Afnan 9
TQMS NIR Blend Monitoring System IBC with Sapphire window Brimrose AOTF NIR “Free-Space” Analyser Portable Sample (Beam) size: 9 mm x 5 mm Diffuse reflectance (1100 -2300 nm) Battery & mains operation CIP and WIP analyser All solid state hardware Provides process reproducibility 21 CFR 11 compliant package Ali Afnan 9
 Analyser Interface Eliminates the need for optical coupling Ali Afnan 10
Analyser Interface Eliminates the need for optical coupling Ali Afnan 10
 Blending System in Position Ali Afnan 11
Blending System in Position Ali Afnan 11
 Blending & Monitoring at 10 rpm Ali Afnan 12
Blending & Monitoring at 10 rpm Ali Afnan 12
 TQMS Blender Operation Real-time blend monitoring for “end-point” control &/or other parameters (active or excipient) Stop signal transmitted to blender control unit on reaching ”end point” On returning the analyser to Docking Station, data is down-loaded to data storage system Batteries get recharged Analyser is in stand-by until next batch Ali Afnan 13
TQMS Blender Operation Real-time blend monitoring for “end-point” control &/or other parameters (active or excipient) Stop signal transmitted to blender control unit on reaching ”end point” On returning the analyser to Docking Station, data is down-loaded to data storage system Batteries get recharged Analyser is in stand-by until next batch Ali Afnan 13
 TQMS- Operator Interface I Ali Afnan 14
TQMS- Operator Interface I Ali Afnan 14
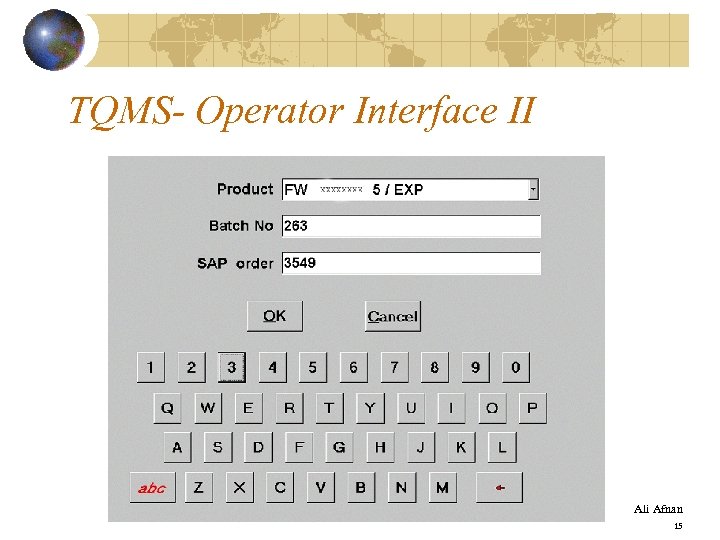 TQMS- Operator Interface II Ali Afnan 15
TQMS- Operator Interface II Ali Afnan 15
 TQMS- Operator Interface VII Ali Afnan 16
TQMS- Operator Interface VII Ali Afnan 16
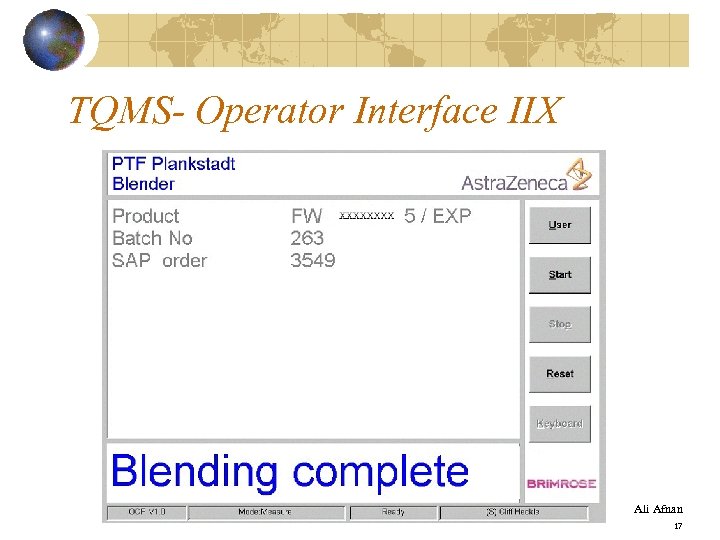 TQMS- Operator Interface IIX Ali Afnan 17
TQMS- Operator Interface IIX Ali Afnan 17
 TQMS Tablet Analyser Unique, novel, purpose-built Brimrose AOTF analyser Transmission (800 -1300 nm) and, Diffuse reflectance (1100 -2300 nm) Non-destructive, real-time tablets analysis of tablets ex press prior to check weighing system IP 65 instrument CIP and WIP analyser Vibration resistant Up to 30 tablets per minute, different tablet size and shapes Ali Afnan 18
TQMS Tablet Analyser Unique, novel, purpose-built Brimrose AOTF analyser Transmission (800 -1300 nm) and, Diffuse reflectance (1100 -2300 nm) Non-destructive, real-time tablets analysis of tablets ex press prior to check weighing system IP 65 instrument CIP and WIP analyser Vibration resistant Up to 30 tablets per minute, different tablet size and shapes Ali Afnan 18
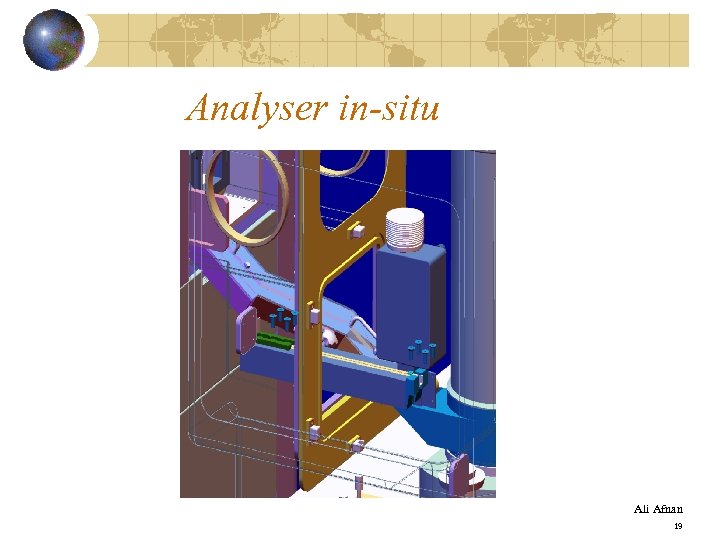 Analyser in-situ Ali Afnan 19
Analyser in-situ Ali Afnan 19
 Tablet Analyser- Off-line Ali Afnan 20
Tablet Analyser- Off-line Ali Afnan 20
 TQMS Tablet Analyses For monitoring: Moisture I. D. of active (& excipient) Concentration of active (& excipient) Dissolution Disintegration Hardness Density Ali Afnan 21
TQMS Tablet Analyses For monitoring: Moisture I. D. of active (& excipient) Concentration of active (& excipient) Dissolution Disintegration Hardness Density Ali Afnan 21
 TQM System Extensive testing & Analyses per manufacturing process @ every manufacturing process @ Lower cost than existing practices Ø Compliance @ point of use Ø Quality Control @ time of manufacture Ø Statistical process control Manufacturing Operations responsible for Quality Assurance Ali Afnan 22
TQM System Extensive testing & Analyses per manufacturing process @ every manufacturing process @ Lower cost than existing practices Ø Compliance @ point of use Ø Quality Control @ time of manufacture Ø Statistical process control Manufacturing Operations responsible for Quality Assurance Ali Afnan 22
 Acknowledgements The NIR Team Mr Robert Chisholm, Tech. Dev. Section Manager, PEG Mr Simon Blackett, Tech. Dev. Section, PEG Mr Ray Bolton, Tech. Dev. Section, PEG Dr Johann Mueller- Plant Manager, PTF, Plankstadt Dr Hubert Gotta- QA Manager, Plankstadt Dr Christina Gerhaeusser- QA, Plankstadt Dr Thorsten Herkert- QA, Plankstadt Mr Peter Marshall- Process Engineer, Tech. Dev. Section, PEG Mr Peter Jewitt- PTF Project Manager, PEG Mr Daniel Frick, Development IS, Molndal Mr Bengt Boissier, Development IS, Molndal Ali Afnan 23
Acknowledgements The NIR Team Mr Robert Chisholm, Tech. Dev. Section Manager, PEG Mr Simon Blackett, Tech. Dev. Section, PEG Mr Ray Bolton, Tech. Dev. Section, PEG Dr Johann Mueller- Plant Manager, PTF, Plankstadt Dr Hubert Gotta- QA Manager, Plankstadt Dr Christina Gerhaeusser- QA, Plankstadt Dr Thorsten Herkert- QA, Plankstadt Mr Peter Marshall- Process Engineer, Tech. Dev. Section, PEG Mr Peter Jewitt- PTF Project Manager, PEG Mr Daniel Frick, Development IS, Molndal Mr Bengt Boissier, Development IS, Molndal Ali Afnan 23


