0f1cad4b9e041d04ddcd8f1617bddab3.ppt
- Количество слайдов: 26
 Topic 8: Energy, power and climate change 8. 5 Greenhouse Effect
Topic 8: Energy, power and climate change 8. 5 Greenhouse Effect
 The solar constant I 0 is the beam solar radiation outside the Earth's atmosphere when the sun is at its mean distance from the Earth. Its value is I 0 = 1380 W/m 2. It is defined as the amount of solar energy that falls on a area of 1 m 2 of the upper atmosphere perpendicular to the sun's rays per second. Mean distance of earth from sun approx. 1. 5 x 1011 m
The solar constant I 0 is the beam solar radiation outside the Earth's atmosphere when the sun is at its mean distance from the Earth. Its value is I 0 = 1380 W/m 2. It is defined as the amount of solar energy that falls on a area of 1 m 2 of the upper atmosphere perpendicular to the sun's rays per second. Mean distance of earth from sun approx. 1. 5 x 1011 m
 Solar constant? • • • The solar constant is not really constant ! Due to the elliptical orbit of the earth around the sun, the Earth-to-Sun distance varies by about 6%. The solar constant varies from about 1038 Wm-2 to 1398 Wm-2. The actual power that reaches an object on earth depends on many factors. Effects of clouds, atmosphere, albedo, etc. .
Solar constant? • • • The solar constant is not really constant ! Due to the elliptical orbit of the earth around the sun, the Earth-to-Sun distance varies by about 6%. The solar constant varies from about 1038 Wm-2 to 1398 Wm-2. The actual power that reaches an object on earth depends on many factors. Effects of clouds, atmosphere, albedo, etc. .
 Given that the solar radiation is 170 Wm-2 averaged over a day and night, how much radiation does one square metre of earth’s surface receive per day? • • In one day, the solar radiation would be 170 Wm-2 x 1 m 2 x 24 h = 4080 Wh
Given that the solar radiation is 170 Wm-2 averaged over a day and night, how much radiation does one square metre of earth’s surface receive per day? • • In one day, the solar radiation would be 170 Wm-2 x 1 m 2 x 24 h = 4080 Wh
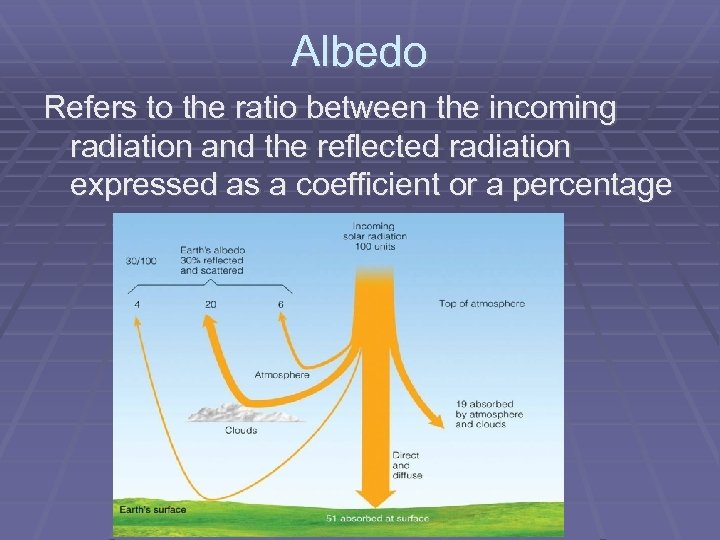 Albedo Refers to the ratio between the incoming radiation and the reflected radiation expressed as a coefficient or a percentage
Albedo Refers to the ratio between the incoming radiation and the reflected radiation expressed as a coefficient or a percentage
 Factors affecting albedo The Earth’s albedo varies daily and is dependent on season (cloud formations) and latitude. In thin clouds, the albedo varies from 30 – 40% And in thick cumulonimbus clouds, the albedo could be 90% Oceans have a low value but snow a high value. The global annual mean albedo is 0. 3 (30%) on Earth. Albedo = total scattered power/ total incident power
Factors affecting albedo The Earth’s albedo varies daily and is dependent on season (cloud formations) and latitude. In thin clouds, the albedo varies from 30 – 40% And in thick cumulonimbus clouds, the albedo could be 90% Oceans have a low value but snow a high value. The global annual mean albedo is 0. 3 (30%) on Earth. Albedo = total scattered power/ total incident power
 Factors affecting Albedo Season • Areas of ice and snow • Light colored deserts • Time of year •
Factors affecting Albedo Season • Areas of ice and snow • Light colored deserts • Time of year •
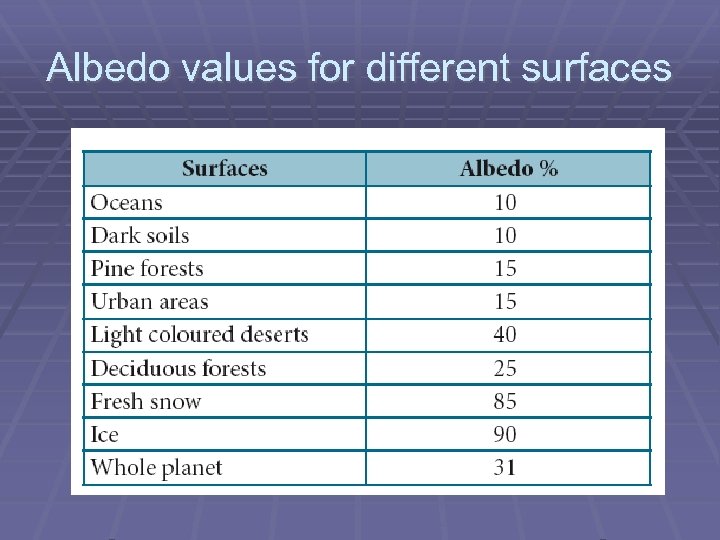 Albedo values for different surfaces
Albedo values for different surfaces
 The Greenhouse Effect The greenhouse effect refers to circumstances where the short wavelengths of visible light from the sun pass through a transparent medium and are absorbed, but the longer wavelengths of the infrared re-radiation from the heated objects are unable to pass through that medium. The trapping of the long wavelength radiation leads to more heating and a higher resultant temperature. • http: //news. bbc. co. uk/1/shared/spl/hi/sci_nat/04/climate _change/html/greenhouse. stm
The Greenhouse Effect The greenhouse effect refers to circumstances where the short wavelengths of visible light from the sun pass through a transparent medium and are absorbed, but the longer wavelengths of the infrared re-radiation from the heated objects are unable to pass through that medium. The trapping of the long wavelength radiation leads to more heating and a higher resultant temperature. • http: //news. bbc. co. uk/1/shared/spl/hi/sci_nat/04/climate _change/html/greenhouse. stm
 The main greenhouse gases • • • Carbon dioxide CO 2 Water vapour H 2 O Methane CH 4 Nitrous oxide N 2 O Sources of these gases are both natural and man-made.
The main greenhouse gases • • • Carbon dioxide CO 2 Water vapour H 2 O Methane CH 4 Nitrous oxide N 2 O Sources of these gases are both natural and man-made.
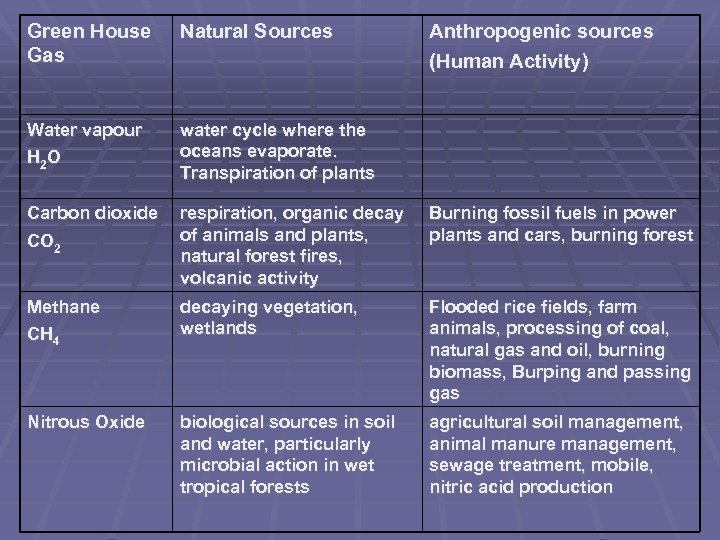 Green House Gas Natural Sources Anthropogenic sources (Human Activity) Water vapour H 2 O water cycle where the oceans evaporate. Transpiration of plants Carbon dioxide CO 2 respiration, organic decay of animals and plants, natural forest fires, volcanic activity Burning fossil fuels in power plants and cars, burning forest Methane CH 4 decaying vegetation, wetlands Flooded rice fields, farm animals, processing of coal, natural gas and oil, burning biomass, Burping and passing gas Nitrous Oxide biological sources in soil and water, particularly microbial action in wet tropical forests agricultural soil management, animal manure management, sewage treatment, mobile, nitric acid production
Green House Gas Natural Sources Anthropogenic sources (Human Activity) Water vapour H 2 O water cycle where the oceans evaporate. Transpiration of plants Carbon dioxide CO 2 respiration, organic decay of animals and plants, natural forest fires, volcanic activity Burning fossil fuels in power plants and cars, burning forest Methane CH 4 decaying vegetation, wetlands Flooded rice fields, farm animals, processing of coal, natural gas and oil, burning biomass, Burping and passing gas Nitrous Oxide biological sources in soil and water, particularly microbial action in wet tropical forests agricultural soil management, animal manure management, sewage treatment, mobile, nitric acid production
 Molecular mechanism for absorbing IR • • We already know from atomic physics that the energy of electrons within atoms is quantized (i. e. it assumes discrete values). The same effect (i. e. the existence of discrete energy values) applies to the energy of molecules due to their vibrational and rotational motion. This energy is also quantized, and there are vibrational and rotational energy levels just as there atomic energy levels.
Molecular mechanism for absorbing IR • • We already know from atomic physics that the energy of electrons within atoms is quantized (i. e. it assumes discrete values). The same effect (i. e. the existence of discrete energy values) applies to the energy of molecules due to their vibrational and rotational motion. This energy is also quantized, and there are vibrational and rotational energy levels just as there atomic energy levels.
 The precise mechanism for photon absorption by the greenhouse gases is complex Here we will try to understand the absorption by making use of the concept of resonance.
The precise mechanism for photon absorption by the greenhouse gases is complex Here we will try to understand the absorption by making use of the concept of resonance.
 In summary • When freq of radiation = freq of vibration, resonance occurs! • It happens that the natural frequency of the molecules in greenhouse gases is in the IR region.
In summary • When freq of radiation = freq of vibration, resonance occurs! • It happens that the natural frequency of the molecules in greenhouse gases is in the IR region.
 Absorption graphs Consider infra-red radiation passing through the atmosphere. The intensity of radiation after passing through the atmosphere will be less than the incident intensity because some of the radiation will be absorbed. We may then make a transmittance curve that shows the variation with wavelength of the percentage of radiation that actually gets through the gas.
Absorption graphs Consider infra-red radiation passing through the atmosphere. The intensity of radiation after passing through the atmosphere will be less than the incident intensity because some of the radiation will be absorbed. We may then make a transmittance curve that shows the variation with wavelength of the percentage of radiation that actually gets through the gas.
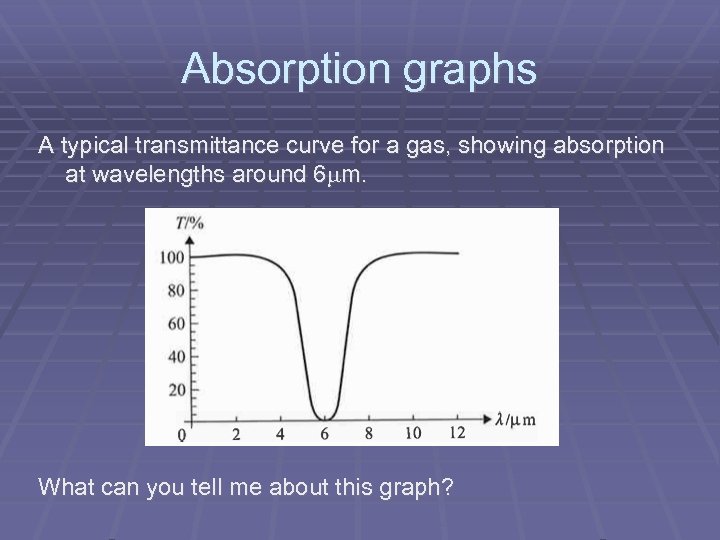 Absorption graphs A typical transmittance curve for a gas, showing absorption at wavelengths around 6 m. What can you tell me about this graph?
Absorption graphs A typical transmittance curve for a gas, showing absorption at wavelengths around 6 m. What can you tell me about this graph?
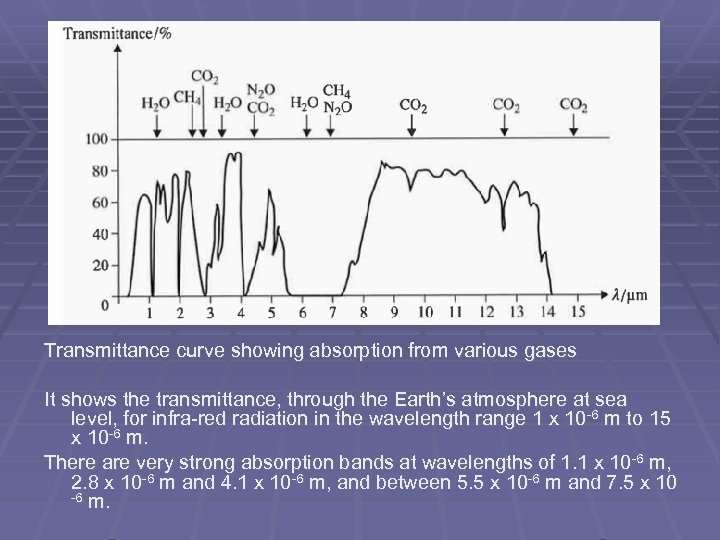 Transmittance curve showing absorption from various gases It shows the transmittance, through the Earth’s atmosphere at sea level, for infra-red radiation in the wavelength range 1 x 10 -6 m to 15 x 10 -6 m. There are very strong absorption bands at wavelengths of 1. 1 x 10 -6 m, 2. 8 x 10 -6 m and 4. 1 x 10 -6 m, and between 5. 5 x 10 -6 m and 7. 5 x 10 -6 m.
Transmittance curve showing absorption from various gases It shows the transmittance, through the Earth’s atmosphere at sea level, for infra-red radiation in the wavelength range 1 x 10 -6 m to 15 x 10 -6 m. There are very strong absorption bands at wavelengths of 1. 1 x 10 -6 m, 2. 8 x 10 -6 m and 4. 1 x 10 -6 m, and between 5. 5 x 10 -6 m and 7. 5 x 10 -6 m.
 Black body radiation Any body at any temperature above absolute zero will radiate to some extent, the intensity and frequency distribution of the radiation depending on the detailed structure of the body. The simplest possible case is an idealised body which is a perfect absorber, and therefore also a perfect emitter. For obvious reasons, this is called a “black body”.
Black body radiation Any body at any temperature above absolute zero will radiate to some extent, the intensity and frequency distribution of the radiation depending on the detailed structure of the body. The simplest possible case is an idealised body which is a perfect absorber, and therefore also a perfect emitter. For obvious reasons, this is called a “black body”.
 The “Black Body” Spectrum: a Hole in the Oven Kirchhoff’s idea : a small hole in the side of a large box is an excellent absorber, since any radiation that goes through the hole bounces around inside, a lot getting absorbed on each bounce, and has little chance of ever getting out again. So, we can do this in reverse: have an oven with a tiny hole in the side, and presumably the radiation coming out the hole is as good a representation of a perfect emitter as we’re going to find. Kirchhoff challenged theorists and experimentalists to figure out and measure (respectively) the energy/frequency curve for this “cavity radiation”, as he called it (in German, of course: hohlraumstrahlung, where hohlraum means hollow room or cavity, strahlung is radiation). In fact, it was Kirchhoff’s challenge in 1859 that led directly to quantum theory forty years later!
The “Black Body” Spectrum: a Hole in the Oven Kirchhoff’s idea : a small hole in the side of a large box is an excellent absorber, since any radiation that goes through the hole bounces around inside, a lot getting absorbed on each bounce, and has little chance of ever getting out again. So, we can do this in reverse: have an oven with a tiny hole in the side, and presumably the radiation coming out the hole is as good a representation of a perfect emitter as we’re going to find. Kirchhoff challenged theorists and experimentalists to figure out and measure (respectively) the energy/frequency curve for this “cavity radiation”, as he called it (in German, of course: hohlraumstrahlung, where hohlraum means hollow room or cavity, strahlung is radiation). In fact, it was Kirchhoff’s challenge in 1859 that led directly to quantum theory forty years later!
 What was observed • Stefan - Boltzmann Law (1879): the total power P radiated per unit area is proportional to the fourth power of the temperature T of the black surface. P = σAT 4 where σ= 5. 67 x 10 -8 Wm-2 K-4 This formula applies to a perfectly black body that absorbs and emits all of the energy that is incident on it
What was observed • Stefan - Boltzmann Law (1879): the total power P radiated per unit area is proportional to the fourth power of the temperature T of the black surface. P = σAT 4 where σ= 5. 67 x 10 -8 Wm-2 K-4 This formula applies to a perfectly black body that absorbs and emits all of the energy that is incident on it
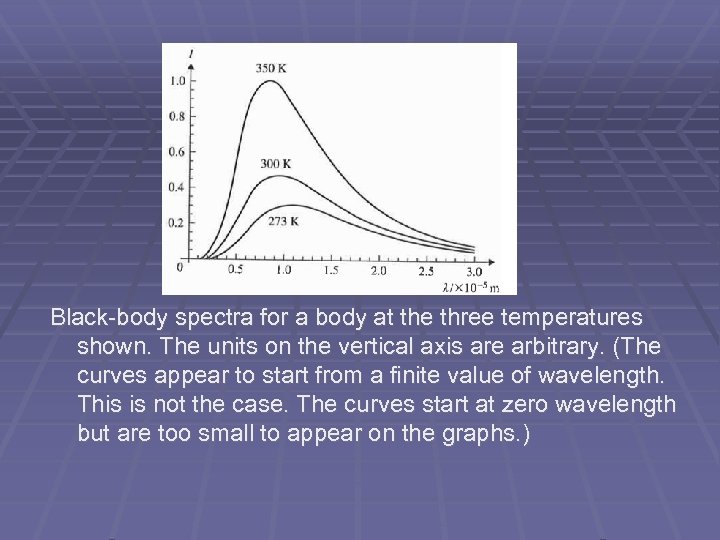 Black-body spectra for a body at the three temperatures shown. The units on the vertical axis are arbitrary. (The curves appear to start from a finite value of wavelength. This is not the case. The curves start at zero wavelength but are too small to appear on the graphs. )
Black-body spectra for a body at the three temperatures shown. The units on the vertical axis are arbitrary. (The curves appear to start from a finite value of wavelength. This is not the case. The curves start at zero wavelength but are too small to appear on the graphs. )
 Emissivity Not everything is a perfect black body so to make the formula more applicable to real life situations we use another variable called the emissivity (e). The emissivity is a number between 0 and 1. Black surfaces will have an emissivity close to 1 and shiny surfaces will have an emissivity close to 0. material emissivity material Emissivity Mercury 0. 05 – 0. 15 Snow 0. 9 Tungsten 0. 1 – 0. 6 Ice 0. 98 Rusted iron 0. 6 – 0. 9 Plate glass 0. 85 Water 0. 6 – 0. 7 Coal 0. 95 Soil 0. 4 – 0. 95 Black paint 0. 92
Emissivity Not everything is a perfect black body so to make the formula more applicable to real life situations we use another variable called the emissivity (e). The emissivity is a number between 0 and 1. Black surfaces will have an emissivity close to 1 and shiny surfaces will have an emissivity close to 0. material emissivity material Emissivity Mercury 0. 05 – 0. 15 Snow 0. 9 Tungsten 0. 1 – 0. 6 Ice 0. 98 Rusted iron 0. 6 – 0. 9 Plate glass 0. 85 Water 0. 6 – 0. 7 Coal 0. 95 Soil 0. 4 – 0. 95 Black paint 0. 92
 Emissivity The formula now becomes P = eσAT 4 where σ= 5. 67 x 10 -8 Wm-2 K-4
Emissivity The formula now becomes P = eσAT 4 where σ= 5. 67 x 10 -8 Wm-2 K-4
 Surface Heat Capacity We define CS, the surface heat capacity of the body, to be the energy required to increase the temperature of 1 m 2 of the surface by 1 K. The concept is useful in the context of bodies radiating and absorbing energy, since it is the surface that is responsible for the energy lost and gained. The units of CS are Jm-2 K-1. Thus, for a surface of surface heat capacity CS and area A, the amount of thermal energy needed to increase its temperature by T is given by Q = ACS T
Surface Heat Capacity We define CS, the surface heat capacity of the body, to be the energy required to increase the temperature of 1 m 2 of the surface by 1 K. The concept is useful in the context of bodies radiating and absorbing energy, since it is the surface that is responsible for the energy lost and gained. The units of CS are Jm-2 K-1. Thus, for a surface of surface heat capacity CS and area A, the amount of thermal energy needed to increase its temperature by T is given by Q = ACS T
 Show that the surface heat capacity is related to the ordinary specific heat capacity c, through CS = hc, where is the density of the material and h is the depth of the surface. We have Q = ACS T and Q = mc T, so ACS T = mc T. But m = V = Ah, and hence ACS T = Ahc T CS = hc (shown)
Show that the surface heat capacity is related to the ordinary specific heat capacity c, through CS = hc, where is the density of the material and h is the depth of the surface. We have Q = ACS T and Q = mc T, so ACS T = mc T. But m = V = Ah, and hence ACS T = Ahc T CS = hc (shown)
 Simple Energy Climate Model Slides from climateprediction. net Excel worksheet on the simple climate prediction model
Simple Energy Climate Model Slides from climateprediction. net Excel worksheet on the simple climate prediction model


