6fd74ed61ee7250da1f9c3c473e4402b.ppt
- Количество слайдов: 17

Tolerance Free EDRF donor MOLSITON ® tab. Molsidomine 2 mg 4 mg

MOLSITON® MOLSIDOMINE 루이스 이그나로, UCLA 의대 약학과 교수 EDRF가 NO임을 밝혀내면서, 1998년 노벨의학상 공동 수상 (주)경풍약품
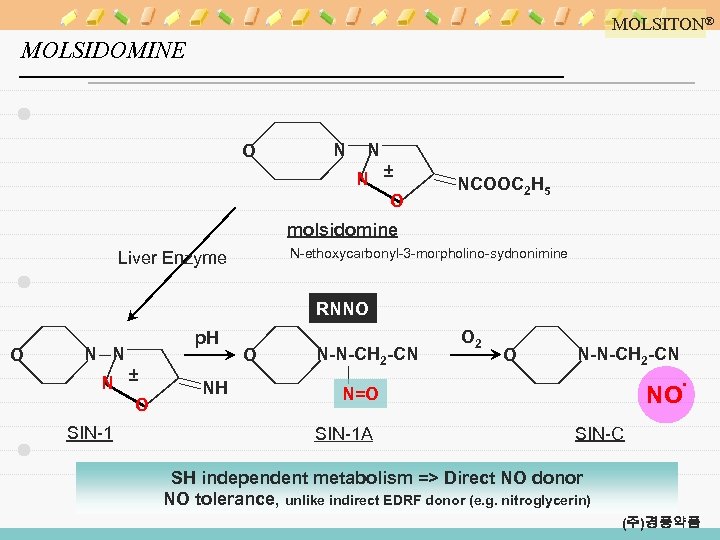
MOLSITON® MOLSIDOMINE O N N N ± O NCOOC 2 H 5 molsidomine N-ethoxycarbonyl-3 -morpholino-sydnonimine Liver Enzyme RNNO O N N N ± O SIN-1 p. H NH O N-N-CH 2 -CN O 2 O N-N-CH 2 -CN NO∙ N=O SIN-1 A SIN-C SH independent metabolism => Direct NO donor NO tolerance, unlike indirect EDRF donor (e. g. nitroglycerin) (주)경풍약품
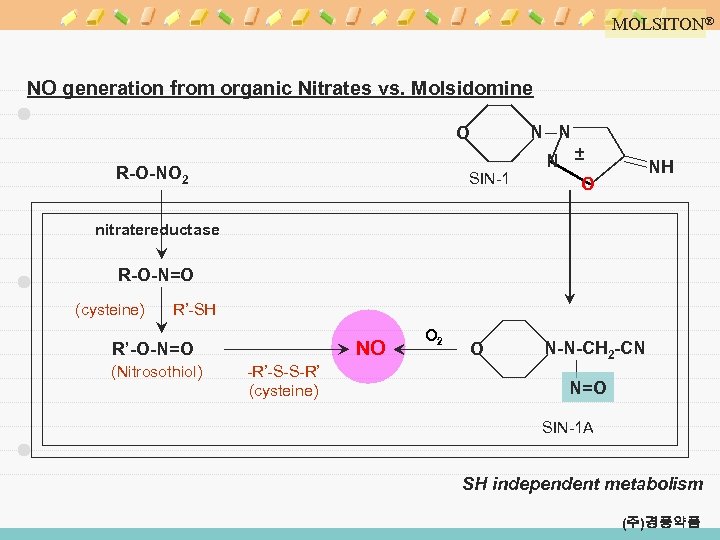
MOLSITON® NO generation from organic Nitrates vs. Molsidomine N N O SIN-1 R-O-NO 2 N ± O O N-N-CH 2 -CN NH nitratereductase R-O-N=O (cysteine) R’-SH NO R’-O-N=O (Nitrosothiol) -R’-S-S-R’ (cysteine) O 2 N=O SIN-1 A SH independent metabolism (주)경풍약품
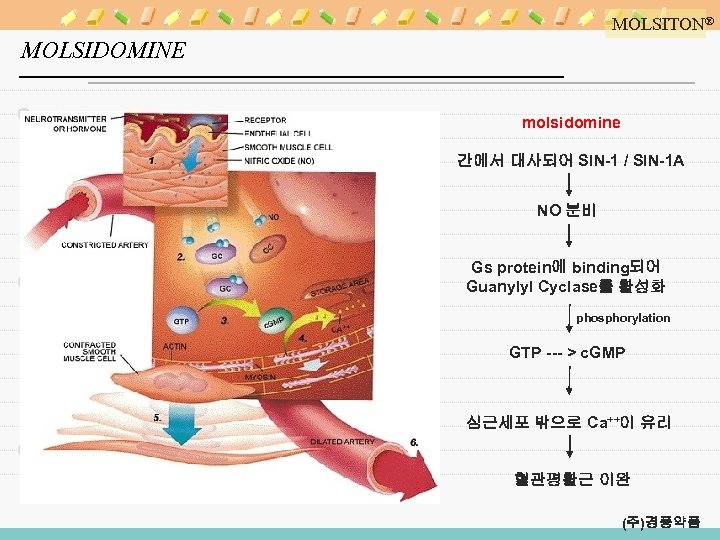
MOLSITON® MOLSIDOMINE molsidomine 간에서 대사되어 SIN-1 / SIN-1 A NO 분비 Gs protein에 binding되어 Guanylyl Cyclase를 활성화 phosphorylation GTP --- > c. GMP 심근세포 밖으로 Ca++이 유리 혈관평활근 이완 (주)경풍약품
MOLSITON® MOLSIDOMINE In Cardiovascular Drug Therapy, 2 nd edition Clinical Relevance of Nitric Oxide Release of Mechanism The Fact that antiischemic effects of molsidomine are presented in the state of established nitroglycerin tolerance confirms the clinical relevance of the differences between the mechanism of nitric oxide(NO) release. Both organic nitrates and SIN-1, through NO-mediated stimulation of GTP to c. GMP, resulting in relaxation of vascular smooth muscle. The reason molsidomine is N O T associated with tolerance development appears to be that at the molecular level only molsidomine leads directly to NO delivery, whereas organic nitrates are dependent on enzyme-catalyzed reduction processes. This difference is important because the tolerance phenomena that develop during continuous administration of organic nitrates appear attributable to depletion of sulfhydryl groups as electron donors or to other mechanisms such as inactivation of key enzymes of biotransformation (e. g. , cytochrome P-450). (주)경풍약품

MOLSITON® 해외 임상 시험 자료 Molsidomine in the treatment of patients with angina pectoris NEJM, 1980 January 3, Vol. 302 Jan P. Roos, MD, Ph D. etc, the Department of Cardiology, Free University Hospital, Netherland Abstract 中 In a double-blind crossover comparison with a placebo, molsidomine (2 mg three times daily) reduced the frequency of anginal attacks and the consumption of nitroglycerin tablets in 14 patients. During exercise testing on a treadmill a statistically significant reduction in ST-segment depression lasted for up to six hours nitroglycerin but is effects last longer. We conclude that molsidomine is effective in preventing the symptoms of angina pectoris. (주)경풍약품

MOLSITON® 해외 임상 시험 자료 Treatment of Stable Angina HEART 2007; 93: 868 -874 Itsik Ben-Dor, Alexander Battler, Department of Cardiology, Rabin Medical Center, Israel Molsidomine is a nitric oxide donating vasodilator. When compared with placebo, it reduced the incidence of anginal attacks and use of sublingual nitrates, and increased exercise capacity, in patients with stable angina. Higher dose provided better protection from angina, although hypotension was a side effect. (주)경풍약품

MOLSITON® 해외 임상 시험 자료 Influence of a combination Treatment with Isosorbide dinitrate and Molsidomine on the Frequency of Angina Pectoris with Coronary Heart Disease PRAXIS 1997; 86: 1849 -1853 Prof. Dr. O. M. Hess, the Department of Internal Medicine, Cardiology, Universitatsspital, Zurich Summary Patients and methods • 15 patinets with severe coronary heart disease • After 2 weeks wash-out period, 4 weeks treatment phase with 100 mg ISDN in the morning as well as 8 mg SR molsidomine at 6 p. m. was followed. Discussion : Combination treatment with ISDN with molsidomine in a SR form(in the free interval) showed a distinct improvement in patients with angina pectoris refractory to treatment with reduction of complaints. The effect of the combination is possibly based on a prolonged vasodilation reduction of filling pressure (reduction of preload). (주)경풍약품

MOLSITON® 해외 임상 시험 자료 Nicorandil associated anal ulceration Lancet 2002 Dec 14; 360(9349) Terry O’Kelly, Department of Gastroenterology and Surgery, Aberdeen Royal Infirmary, UK (주)경풍약품
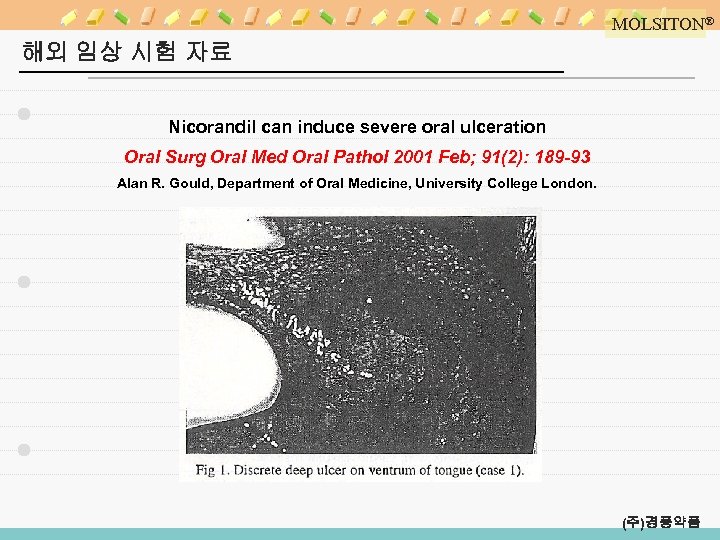
MOLSITON® 해외 임상 시험 자료 Nicorandil can induce severe oral ulceration Oral Surg Oral Med Oral Pathol 2001 Feb; 91(2): 189 -93 Alan R. Gould, Department of Oral Medicine, University College London. (주)경풍약품
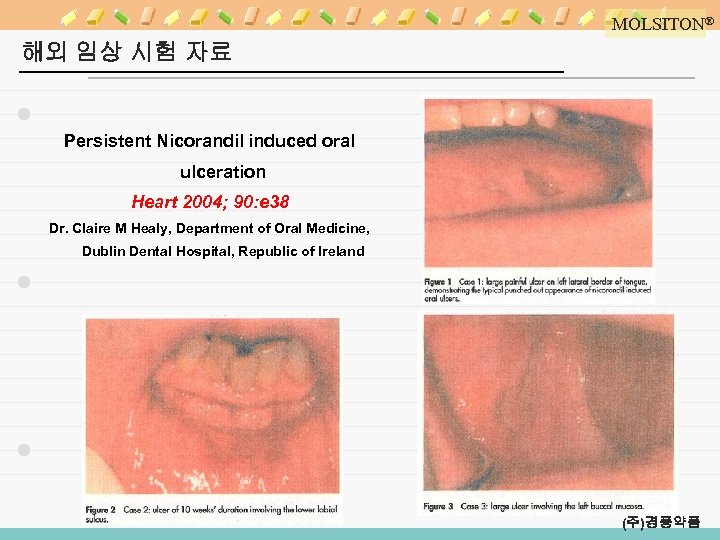
MOLSITON® 해외 임상 시험 자료 Persistent Nicorandil induced oral ulceration Heart 2004; 90: e 38 Dr. Claire M Healy, Department of Oral Medicine, Dublin Dental Hospital, Republic of Ireland (주)경풍약품
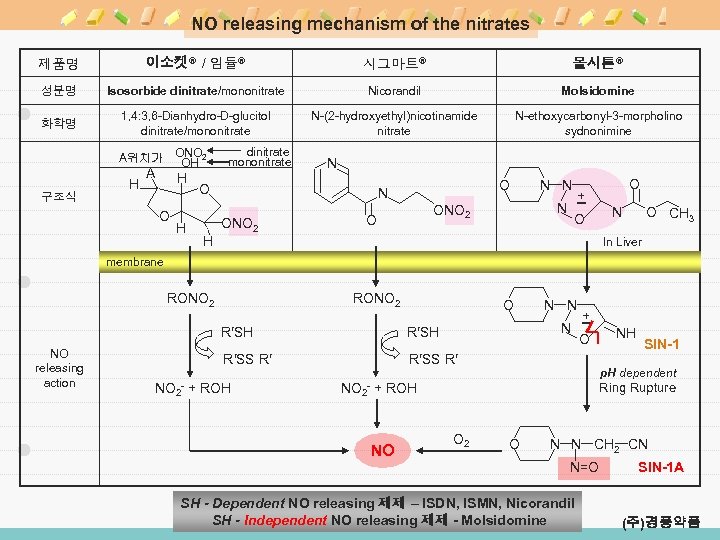
NO releasing mechanism of the nitrates 제품명 이소켓® / 임듈® 시그마트® 몰시톤® 성분명 Isosorbide dinitrate/mononitrate Nicorandil Molsidomine 화학명 1, 4: 3, 6 -Dianhydro-D-glucitol dinitrate/mononitrate N-(2 -hydroxyethyl)nicotinamide nitrate N-ethoxycarbonyl-3 -morpholino sydnonimine ONO 2 OH A위치가 구조식 H A H O H dinitrate mononitrate N O H O N ONO 2 O N N + O O N O CH 3 In Liver membrane RONO 2 O N R′SH NO releasing action R′SH R′SS R′ NO 2 - N N + R′SS R′ + ROH NO 2 - O SIN-1 p. H dependent + ROH NO NH Ring Rupture O 2 O N N CH 2 CN N=O SH - Dependent NO releasing 제제 – ISDN, ISMN, Nicorandil SH - Independent NO releasing 제제 - Molsidomine SIN-1 A (주)경풍약품
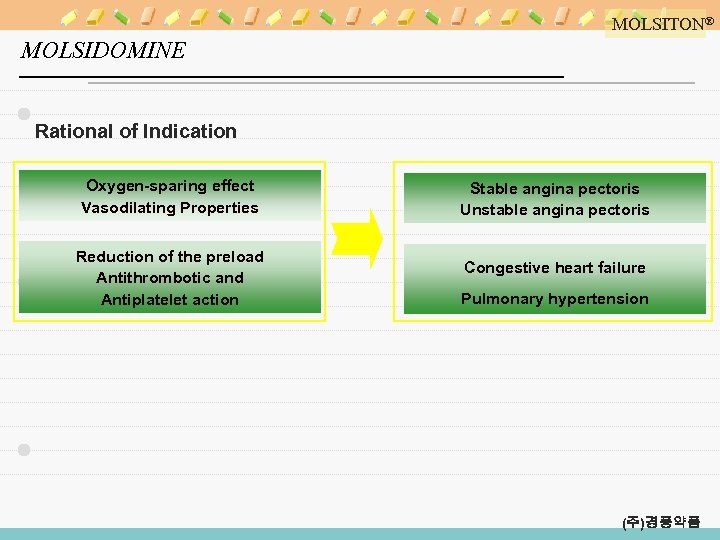
MOLSITON® MOLSIDOMINE Rational of Indication Oxygen-sparing effect Vasodilating Properties Reduction of the preload Antithrombotic and Antiplatelet action Stable angina pectoris Unstable angina pectoris Congestive heart failure Pulmonary hypertension (주)경풍약품

MOLSITON® MOLSIDOMINE 약가 상품명 적응증 용법/용량 몰시톤 정 협심증의 예방과 유지 일반적으로 1일 4 mg bid 2 mg 174 (1일 4 mg tid까지 보험인정) 4 mg 243 2 & 4 mg 국내 허가일 : 1997년. 신약으로 등록됨. (주)경풍약품
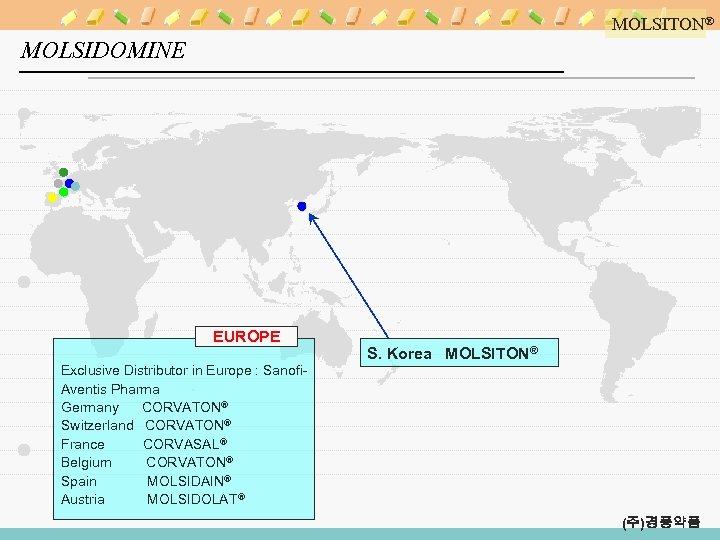
MOLSITON® MOLSIDOMINE EUROPE S. Korea MOLSITON® Exclusive Distributor in Europe : Sanofi. Aventis Pharma Germany CORVATON® Switzerland CORVATON® France CORVASAL® Belgium CORVATON® Spain MOLSIDAIN® Austria MOLSIDOLAT® (주)경풍약품

감 사 합 니 다. (주)경풍약품
6fd74ed61ee7250da1f9c3c473e4402b.ppt