487fe3cd33e1f1f843903a18c3fdc805.ppt
- Количество слайдов: 84
 Three Pre-Rule Studies of Chlorpyrifos: Nolan et al. (1982) Honeycutt & De. Geare (1993) Kisicki et al. (1999) Human Studies Review Board June 24, 2009 1
Three Pre-Rule Studies of Chlorpyrifos: Nolan et al. (1982) Honeycutt & De. Geare (1993) Kisicki et al. (1999) Human Studies Review Board June 24, 2009 1
 Sequence of Presentations l Introduction and Context • Anna Lowit, Ph. D. l Science Assessments § Nolan and Kisicki Studies • John Doherty, Ph. D. , DABT § Honeycutt & Degeare Study • Wade Britton, MPH l Ethics Assessments • John Carley 2
Sequence of Presentations l Introduction and Context • Anna Lowit, Ph. D. l Science Assessments § Nolan and Kisicki Studies • John Doherty, Ph. D. , DABT § Honeycutt & Degeare Study • Wade Britton, MPH l Ethics Assessments • John Carley 2
 Chlorpyrifos Introduction and Context Anna Lowit, Ph. D. Senior Scientist Health Effects Division Office of Pesticide Programs 3
Chlorpyrifos Introduction and Context Anna Lowit, Ph. D. Senior Scientist Health Effects Division Office of Pesticide Programs 3
 Introduction l Chlorpyrifos: § Organophosphate pesticide which was first registered in 1965 § In June 2000: • The technical registrants entered into an agreement with the Agency to eliminate and phase out nearly all uses that result in residential exposures. • Human health risk assessment developed for the Interim Registration Eligibility Decision (IRED) relied on adult Ch. E data from rodents & dogs – Human studies were not used to inform point of departure or uncertainty factors – Honeycutt study used in the worker exposure assessment 4
Introduction l Chlorpyrifos: § Organophosphate pesticide which was first registered in 1965 § In June 2000: • The technical registrants entered into an agreement with the Agency to eliminate and phase out nearly all uses that result in residential exposures. • Human health risk assessment developed for the Interim Registration Eligibility Decision (IRED) relied on adult Ch. E data from rodents & dogs – Human studies were not used to inform point of departure or uncertainty factors – Honeycutt study used in the worker exposure assessment 4
 Introduction l Current regulatory activities leading to new risk assessment: § Registration review: 15 -year review cycle under FIFRA for registered pesticides • Update human health & ecological risk assessments § Petition by Natural Resources Defense Council (NRDC) and Pesticide Action Network, North America (PANNA) to revoke all tolerances and cancel all registered uses 5
Introduction l Current regulatory activities leading to new risk assessment: § Registration review: 15 -year review cycle under FIFRA for registered pesticides • Update human health & ecological risk assessments § Petition by Natural Resources Defense Council (NRDC) and Pesticide Action Network, North America (PANNA) to revoke all tolerances and cancel all registered uses 5
 Introduction l Draft Science Issue Paper reviewed by the FIFRA SAP in 2008 § Review the new science from animal & humans under the context of human health risk assessment § Focus on effects in pregnant women, fetuses, and juveniles as these groups are thought to be more susceptible to chlorpyrifos • Age-dependant metabolism • Epidemiology studies in mothers & children • Rodent studies evaluating non-cholinergic toxicities (i. e. , behavior, learning, biochemical responses) • ACh. E studies in pregnant rats, fetuses, post-natal pups 6
Introduction l Draft Science Issue Paper reviewed by the FIFRA SAP in 2008 § Review the new science from animal & humans under the context of human health risk assessment § Focus on effects in pregnant women, fetuses, and juveniles as these groups are thought to be more susceptible to chlorpyrifos • Age-dependant metabolism • Epidemiology studies in mothers & children • Rodent studies evaluating non-cholinergic toxicities (i. e. , behavior, learning, biochemical responses) • ACh. E studies in pregnant rats, fetuses, post-natal pups 6
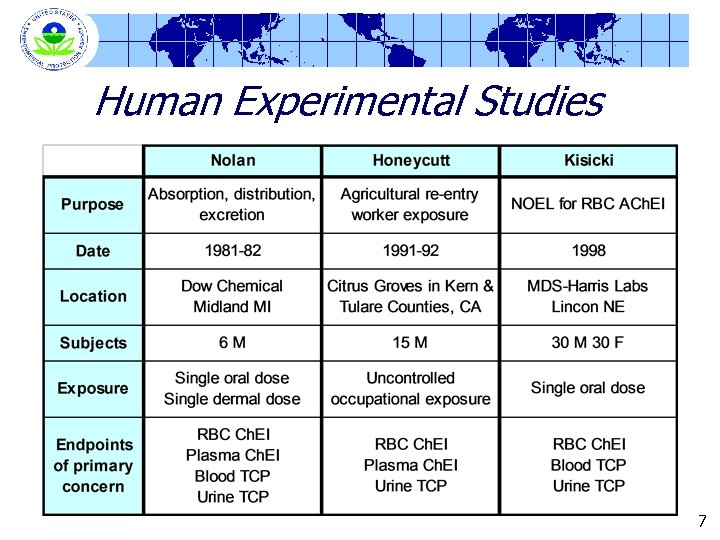 Human Experimental Studies 7
Human Experimental Studies 7
 Proposed Uses of the Human Studies l For “Bounding” Analyses § § l Comparing blood & urine data from the human experimental studies with data from animal studies & human epidemiology studies Comparing levels of ACh. E/Ch. E inhibition in humans and animals To Develop & Refine PBPK models § Physiologically-based pharmacokinetic models § Current models include Nolan et al data • Kisicki et al or Honeycutt & De. Geare not used in current model parameterization 8
Proposed Uses of the Human Studies l For “Bounding” Analyses § § l Comparing blood & urine data from the human experimental studies with data from animal studies & human epidemiology studies Comparing levels of ACh. E/Ch. E inhibition in humans and animals To Develop & Refine PBPK models § Physiologically-based pharmacokinetic models § Current models include Nolan et al data • Kisicki et al or Honeycutt & De. Geare not used in current model parameterization 8
 EPA does NOT propose to use data from the human experimental studies for a point of departure (Po. D) or to directly inform the inter-species uncertainty factor § Animal studies provide high-quality dose-response data for Ch. EI across many doses & multiple life stages § Human studies lack dose-response information • Nolan et al used only one dose level for each route of administration • Kisicki et al showed Ch. EI in only one subject • Honeycutt & De. Geare was not designed for dose response § Human studies do not address non-cholinergic toxicities • Animal data indicate susceptibility of the developing nervous system to chlorpyrifos • Epidemiology studies in children generally support the animal studies 9
EPA does NOT propose to use data from the human experimental studies for a point of departure (Po. D) or to directly inform the inter-species uncertainty factor § Animal studies provide high-quality dose-response data for Ch. EI across many doses & multiple life stages § Human studies lack dose-response information • Nolan et al used only one dose level for each route of administration • Kisicki et al showed Ch. EI in only one subject • Honeycutt & De. Geare was not designed for dose response § Human studies do not address non-cholinergic toxicities • Animal data indicate susceptibility of the developing nervous system to chlorpyrifos • Epidemiology studies in children generally support the animal studies 9
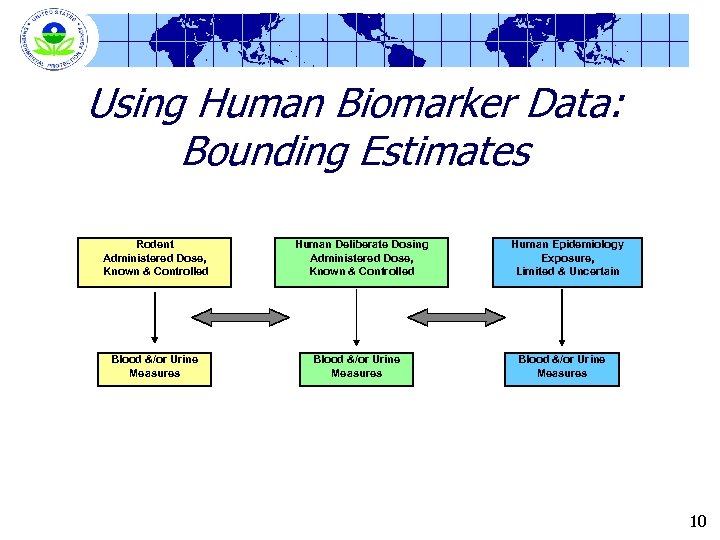 Using Human Biomarker Data: Bounding Estimates Rodent Administered Dose, Known & Controlled Blood &/or Urine Measures Human Deliberate Dosing Administered Dose, Known & Controlled Blood &/or Urine Measures Human Epidemiology Exposure, Limited & Uncertain Blood &/or Urine Measures 10
Using Human Biomarker Data: Bounding Estimates Rodent Administered Dose, Known & Controlled Blood &/or Urine Measures Human Deliberate Dosing Administered Dose, Known & Controlled Blood &/or Urine Measures Human Epidemiology Exposure, Limited & Uncertain Blood &/or Urine Measures 10
 Physiologically-Based Pharmacokinetic (PBPK) Models l Represent the anatomy & physiology of the rodent/human l Provide simulations of biological processes such as absorption, distribution, metabolism & elimination l Widely recognized as the “gold standard” in human health risk assessment l Particularly helpful in extrapolations: § § § Route to route Inter-species Across dose range 11
Physiologically-Based Pharmacokinetic (PBPK) Models l Represent the anatomy & physiology of the rodent/human l Provide simulations of biological processes such as absorption, distribution, metabolism & elimination l Widely recognized as the “gold standard” in human health risk assessment l Particularly helpful in extrapolations: § § § Route to route Inter-species Across dose range 11
 SAP Response l SAP was generally supportive of EPA’s preliminary conclusions, and identified areas for revision & additional analysis § “Overall, the Panel agreed that the human deliberate dosing studies contain scientifically useful information for risk assessment, but not for directly establishing Po. D or uncertainty factors. ” § “The Panel appreciated the Agency’s scientific analysis to compare the blood levels in the deliberate dosing and epidemiological studies, and considered it critically important to maximally use the information from these studies. . . as a basis to “bound” the reference doses/concentrations. . . ” § “The Panel encouraged the Agency to consider the use of a PBPK model to widen the application of these bounding data for current or potential human exposures and for the final reference dose or reference concentrations. ” 12
SAP Response l SAP was generally supportive of EPA’s preliminary conclusions, and identified areas for revision & additional analysis § “Overall, the Panel agreed that the human deliberate dosing studies contain scientifically useful information for risk assessment, but not for directly establishing Po. D or uncertainty factors. ” § “The Panel appreciated the Agency’s scientific analysis to compare the blood levels in the deliberate dosing and epidemiological studies, and considered it critically important to maximally use the information from these studies. . . as a basis to “bound” the reference doses/concentrations. . . ” § “The Panel encouraged the Agency to consider the use of a PBPK model to widen the application of these bounding data for current or potential human exposures and for the final reference dose or reference concentrations. ” 12
 Human Experimental Studies l Nolan et al (1984) § Historically used to interpret biomonitoring studies (e. g. , NHANES & worker) § Provides estimate of dermal absorption § Used in current PBPK models for inter-species scaling 13
Human Experimental Studies l Nolan et al (1984) § Historically used to interpret biomonitoring studies (e. g. , NHANES & worker) § Provides estimate of dermal absorption § Used in current PBPK models for inter-species scaling 13
 Human Experimental Studies l Kisicki et al (1999) § Lack of plasma Ch. E is a critical omission from study design decreases its utility § Possible reduced absorption of chlorpyrifos from capsule § Used in 2002 Timchalk et al PBPK paper • For purposes of model evaluation • Not used in current parameterization 14
Human Experimental Studies l Kisicki et al (1999) § Lack of plasma Ch. E is a critical omission from study design decreases its utility § Possible reduced absorption of chlorpyrifos from capsule § Used in 2002 Timchalk et al PBPK paper • For purposes of model evaluation • Not used in current parameterization 14
 Human Experimental Studies l Honeycutt & De. Geare § Will be used in combination with other available worker biomonitoring studies to evaluate range of urinary TCP concentrations for workers 15
Human Experimental Studies l Honeycutt & De. Geare § Will be used in combination with other available worker biomonitoring studies to evaluate range of urinary TCP concentrations for workers 15
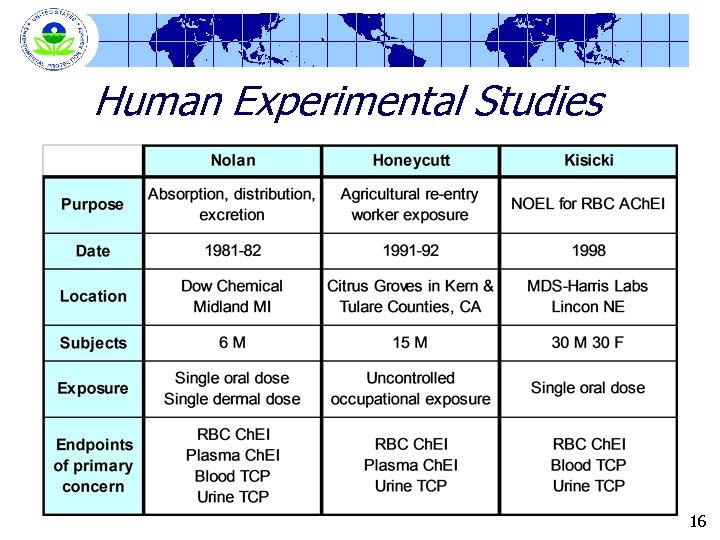 Human Experimental Studies 16
Human Experimental Studies 16
 The Nolan et al. (1982) and Kisicki et al. (1999) Chlorpyrifos Single Dose Studies in Human Volunteers: Science Assessment John Doherty, Ph. D, DABT Health Effects Division Office of Pesticide Programs 17
The Nolan et al. (1982) and Kisicki et al. (1999) Chlorpyrifos Single Dose Studies in Human Volunteers: Science Assessment John Doherty, Ph. D, DABT Health Effects Division Office of Pesticide Programs 17
 Scope of Presentation The reliability of the analytical data for chlorpyrifos and TCP* and the assessment for Ch. E and its inhibition with some emphasis on individual variability will be presented. *TCP = 3, 5, 6 -trichloro-2 -pyridinol – the principal metabolite of chlorpyrifos. 18
Scope of Presentation The reliability of the analytical data for chlorpyrifos and TCP* and the assessment for Ch. E and its inhibition with some emphasis on individual variability will be presented. *TCP = 3, 5, 6 -trichloro-2 -pyridinol – the principal metabolite of chlorpyrifos. 18
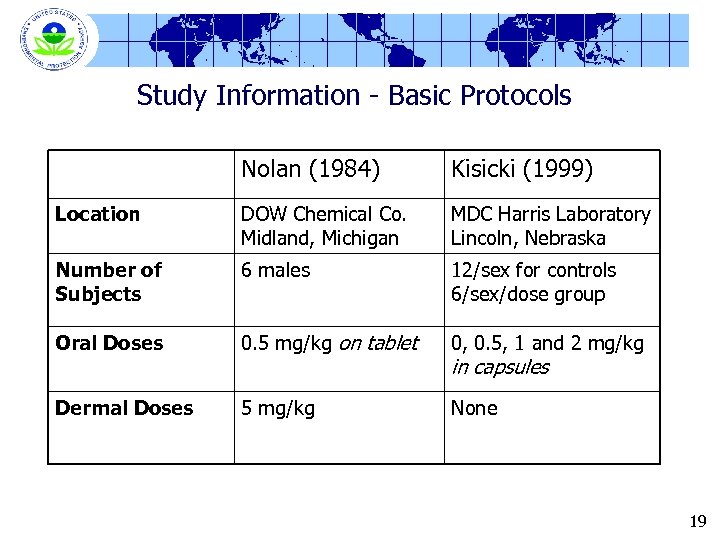 Study Information - Basic Protocols Nolan (1984) Kisicki (1999) Location DOW Chemical Co. Midland, Michigan MDC Harris Laboratory Lincoln, Nebraska Number of Subjects 6 males 12/sex for controls 6/sex/dose group Oral Doses 0. 5 mg/kg on tablet 0, 0. 5, 1 and 2 mg/kg Dermal Doses 5 mg/kg None in capsules 19
Study Information - Basic Protocols Nolan (1984) Kisicki (1999) Location DOW Chemical Co. Midland, Michigan MDC Harris Laboratory Lincoln, Nebraska Number of Subjects 6 males 12/sex for controls 6/sex/dose group Oral Doses 0. 5 mg/kg on tablet 0, 0. 5, 1 and 2 mg/kg Dermal Doses 5 mg/kg None in capsules 19
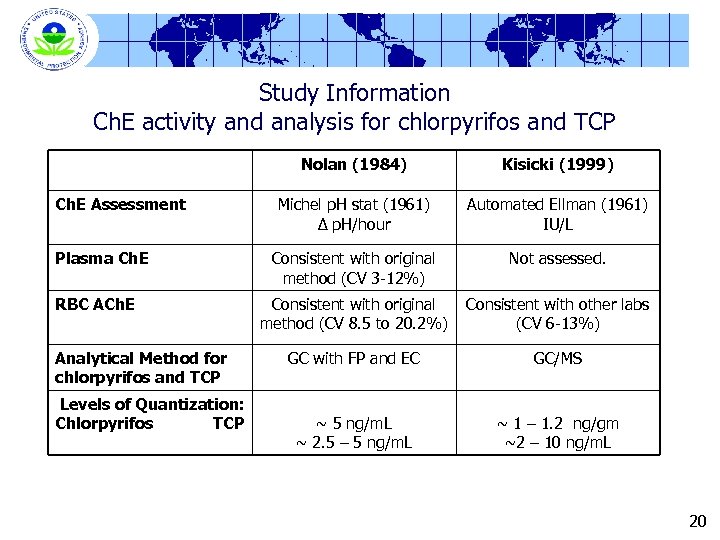 Study Information Ch. E activity and analysis for chlorpyrifos and TCP Nolan (1984) Ch. E Assessment Plasma Ch. E RBC ACh. E Analytical Method for chlorpyrifos and TCP Levels of Quantization: Chlorpyrifos TCP Kisicki (1999) Michel p. H stat (1961) ∆ p. H/hour Automated Ellman (1961) IU/L Consistent with original method (CV 3 -12%) Not assessed. Consistent with original method (CV 8. 5 to 20. 2%) Consistent with other labs (CV 6 -13%) GC with FP and EC GC/MS ~ 5 ng/m. L ~ 2. 5 – 5 ng/m. L ~ 1 – 1. 2 ng/gm ~2 – 10 ng/m. L 20
Study Information Ch. E activity and analysis for chlorpyrifos and TCP Nolan (1984) Ch. E Assessment Plasma Ch. E RBC ACh. E Analytical Method for chlorpyrifos and TCP Levels of Quantization: Chlorpyrifos TCP Kisicki (1999) Michel p. H stat (1961) ∆ p. H/hour Automated Ellman (1961) IU/L Consistent with original method (CV 3 -12%) Not assessed. Consistent with original method (CV 8. 5 to 20. 2%) Consistent with other labs (CV 6 -13%) GC with FP and EC GC/MS ~ 5 ng/m. L ~ 2. 5 – 5 ng/m. L ~ 1 – 1. 2 ng/gm ~2 – 10 ng/m. L 20
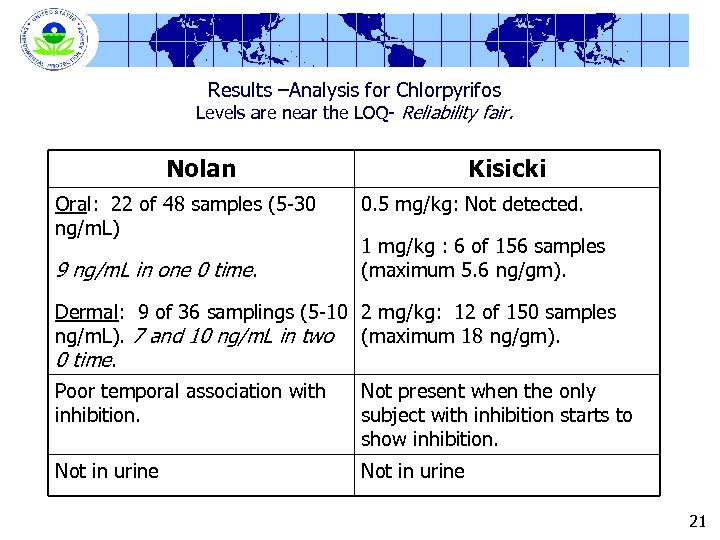 Results –Analysis for Chlorpyrifos Levels are near the LOQ- Reliability fair. Nolan Oral: 22 of 48 samples (5 -30 ng/m. L) 9 ng/m. L in one 0 time. Kisicki 0. 5 mg/kg: Not detected. 1 mg/kg : 6 of 156 samples (maximum 5. 6 ng/gm). Dermal: 9 of 36 samplings (5 -10 2 mg/kg: 12 of 150 samples ng/m. L). 7 and 10 ng/m. L in two (maximum 18 ng/gm). 0 time. Poor temporal association with inhibition. Not present when the only subject with inhibition starts to show inhibition. Not in urine 21
Results –Analysis for Chlorpyrifos Levels are near the LOQ- Reliability fair. Nolan Oral: 22 of 48 samples (5 -30 ng/m. L) 9 ng/m. L in one 0 time. Kisicki 0. 5 mg/kg: Not detected. 1 mg/kg : 6 of 156 samples (maximum 5. 6 ng/gm). Dermal: 9 of 36 samplings (5 -10 2 mg/kg: 12 of 150 samples ng/m. L). 7 and 10 ng/m. L in two (maximum 18 ng/gm). 0 time. Poor temporal association with inhibition. Not present when the only subject with inhibition starts to show inhibition. Not in urine 21
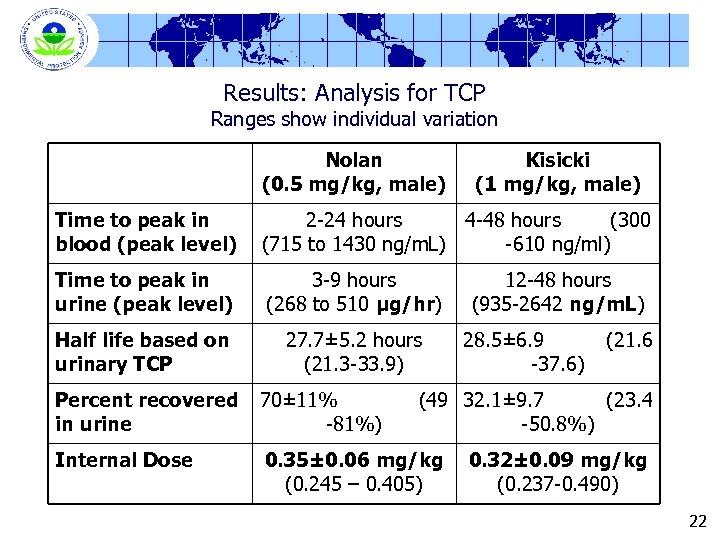 Results: Analysis for TCP Ranges show individual variation Nolan (0. 5 mg/kg, male) Kisicki (1 mg/kg, male) Time to peak in blood (peak level) 2 -24 hours 4 -48 hours (300 (715 to 1430 ng/m. L) -610 ng/ml) Time to peak in urine (peak level) 3 -9 hours (268 to 510 µg/hr) Half life based on urinary TCP 27. 7± 5. 2 hours (21. 3 -33. 9) Percent recovered in urine 70± 11% -81%) Internal Dose 0. 35± 0. 06 mg/kg (0. 245 – 0. 405) 12 -48 hours (935 -2642 ng/m. L) 28. 5± 6. 9 -37. 6) (21. 6 (49 32. 1± 9. 7 (23. 4 -50. 8%) 0. 32± 0. 09 mg/kg (0. 237 -0. 490) 22
Results: Analysis for TCP Ranges show individual variation Nolan (0. 5 mg/kg, male) Kisicki (1 mg/kg, male) Time to peak in blood (peak level) 2 -24 hours 4 -48 hours (300 (715 to 1430 ng/m. L) -610 ng/ml) Time to peak in urine (peak level) 3 -9 hours (268 to 510 µg/hr) Half life based on urinary TCP 27. 7± 5. 2 hours (21. 3 -33. 9) Percent recovered in urine 70± 11% -81%) Internal Dose 0. 35± 0. 06 mg/kg (0. 245 – 0. 405) 12 -48 hours (935 -2642 ng/m. L) 28. 5± 6. 9 -37. 6) (21. 6 (49 32. 1± 9. 7 (23. 4 -50. 8%) 0. 32± 0. 09 mg/kg (0. 237 -0. 490) 22
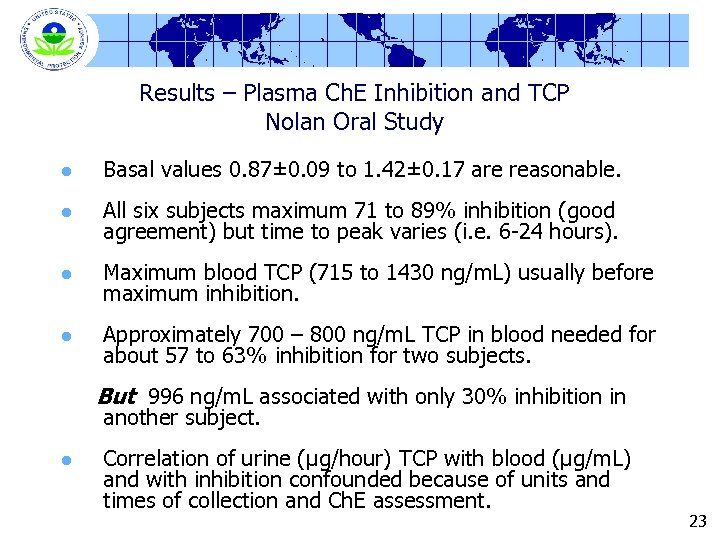 Results – Plasma Ch. E Inhibition and TCP Nolan Oral Study l Basal values 0. 87± 0. 09 to 1. 42± 0. 17 are reasonable. l All six subjects maximum 71 to 89% inhibition (good agreement) but time to peak varies (i. e. 6 -24 hours). l Maximum blood TCP (715 to 1430 ng/m. L) usually before maximum inhibition. l Approximately 700 – 800 ng/m. L TCP in blood needed for about 57 to 63% inhibition for two subjects. But 996 ng/m. L associated with only 30% inhibition in another subject. l Correlation of urine (µg/hour) TCP with blood (µg/m. L) and with inhibition confounded because of units and times of collection and Ch. E assessment. 23
Results – Plasma Ch. E Inhibition and TCP Nolan Oral Study l Basal values 0. 87± 0. 09 to 1. 42± 0. 17 are reasonable. l All six subjects maximum 71 to 89% inhibition (good agreement) but time to peak varies (i. e. 6 -24 hours). l Maximum blood TCP (715 to 1430 ng/m. L) usually before maximum inhibition. l Approximately 700 – 800 ng/m. L TCP in blood needed for about 57 to 63% inhibition for two subjects. But 996 ng/m. L associated with only 30% inhibition in another subject. l Correlation of urine (µg/hour) TCP with blood (µg/m. L) and with inhibition confounded because of units and times of collection and Ch. E assessment. 23
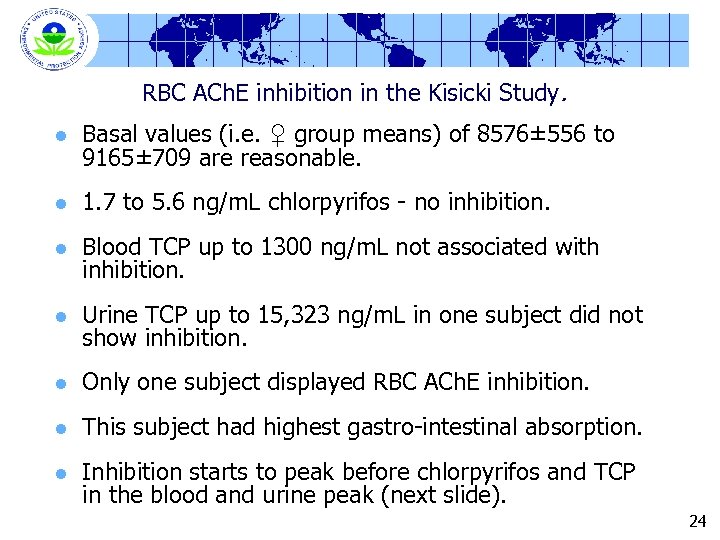 RBC ACh. E inhibition in the Kisicki Study. l Basal values (i. e. ♀ group means) of 8576± 556 to 9165± 709 are reasonable. l 1. 7 to 5. 6 ng/m. L chlorpyrifos - no inhibition. l Blood TCP up to 1300 ng/m. L not associated with inhibition. l Urine TCP up to 15, 323 ng/m. L in one subject did not show inhibition. l Only one subject displayed RBC ACh. E inhibition. l This subject had highest gastro-intestinal absorption. l Inhibition starts to peak before chlorpyrifos and TCP in the blood and urine peak (next slide). 24
RBC ACh. E inhibition in the Kisicki Study. l Basal values (i. e. ♀ group means) of 8576± 556 to 9165± 709 are reasonable. l 1. 7 to 5. 6 ng/m. L chlorpyrifos - no inhibition. l Blood TCP up to 1300 ng/m. L not associated with inhibition. l Urine TCP up to 15, 323 ng/m. L in one subject did not show inhibition. l Only one subject displayed RBC ACh. E inhibition. l This subject had highest gastro-intestinal absorption. l Inhibition starts to peak before chlorpyrifos and TCP in the blood and urine peak (next slide). 24
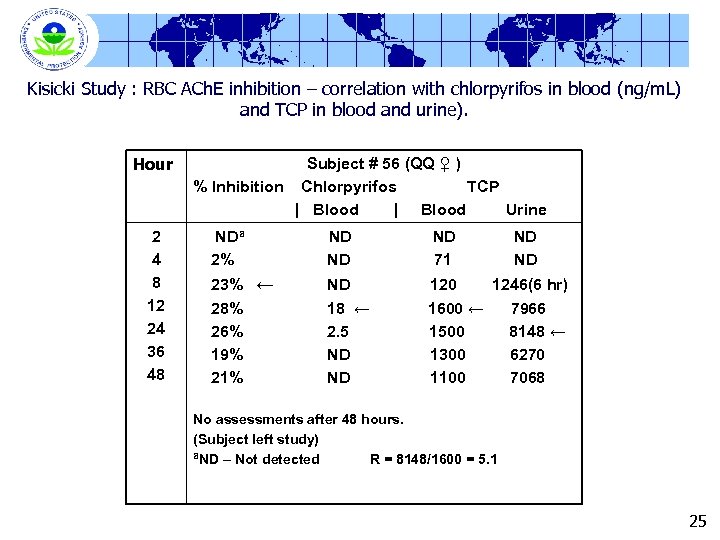 Kisicki Study : RBC ACh. E inhibition – correlation with chlorpyrifos in blood (ng/m. L) and TCP in blood and urine). Hour 2 4 8 12 24 36 48 Subject # 56 (QQ ♀ ) % Inhibition Chlorpyrifos TCP | Blood Urine NDa 2% ND ND 23% ← 28% 26% 19% 21% ND 18 ← 2. 5 ND ND ND 71 ND ND 120 1246(6 hr) 1600 ← 7966 1500 8148 ← 1300 6270 1100 7068 No assessments after 48 hours. (Subject left study) a. ND – Not detected R = 8148/1600 = 5. 1 25
Kisicki Study : RBC ACh. E inhibition – correlation with chlorpyrifos in blood (ng/m. L) and TCP in blood and urine). Hour 2 4 8 12 24 36 48 Subject # 56 (QQ ♀ ) % Inhibition Chlorpyrifos TCP | Blood Urine NDa 2% ND ND 23% ← 28% 26% 19% 21% ND 18 ← 2. 5 ND ND ND 71 ND ND 120 1246(6 hr) 1600 ← 7966 1500 8148 ← 1300 6270 1100 7068 No assessments after 48 hours. (Subject left study) a. ND – Not detected R = 8148/1600 = 5. 1 25
![Chlorpyrifos and Ch. E inhibition Chlorpyrifos ↓ [Chlorpyrifos Oxon] (Rapid irreversible inhibition of Ch. Chlorpyrifos and Ch. E inhibition Chlorpyrifos ↓ [Chlorpyrifos Oxon] (Rapid irreversible inhibition of Ch.](https://present5.com/presentation/487fe3cd33e1f1f843903a18c3fdc805/image-26.jpg) Chlorpyrifos and Ch. E inhibition Chlorpyrifos ↓ [Chlorpyrifos Oxon] (Rapid irreversible inhibition of Ch. E/ACh. E) ↓ TCP (Also from other pathways) 26
Chlorpyrifos and Ch. E inhibition Chlorpyrifos ↓ [Chlorpyrifos Oxon] (Rapid irreversible inhibition of Ch. E/ACh. E) ↓ TCP (Also from other pathways) 26
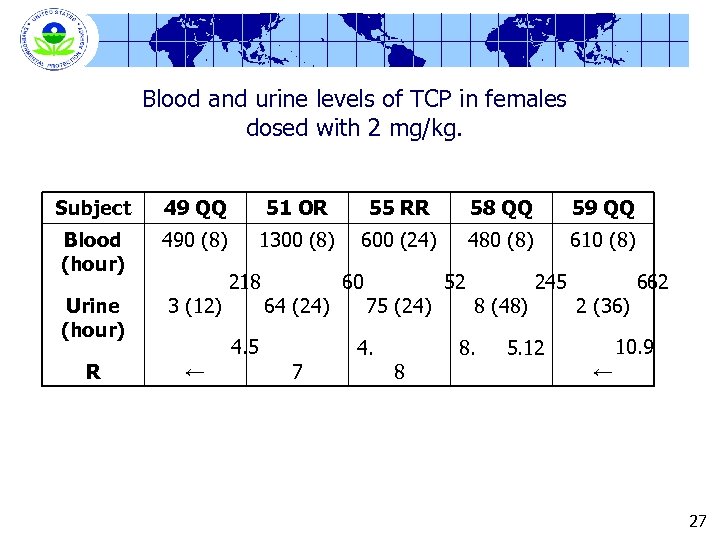 Blood and urine levels of TCP in females dosed with 2 mg/kg. Subject 49 QQ 51 OR 55 RR 58 QQ 59 QQ Blood (hour) 490 (8) 1300 (8) 600 (24) 480 (8) 610 (8) Urine (hour) 3 (12) R ← 218 64 (24) 4. 5 7 60 75 (24) 4. 8 52 8 (48) 8. 245 2 (36) 662 10. 9 5. 12 ← 27
Blood and urine levels of TCP in females dosed with 2 mg/kg. Subject 49 QQ 51 OR 55 RR 58 QQ 59 QQ Blood (hour) 490 (8) 1300 (8) 600 (24) 480 (8) 610 (8) Urine (hour) 3 (12) R ← 218 64 (24) 4. 5 7 60 75 (24) 4. 8 52 8 (48) 8. 245 2 (36) 662 10. 9 5. 12 ← 27
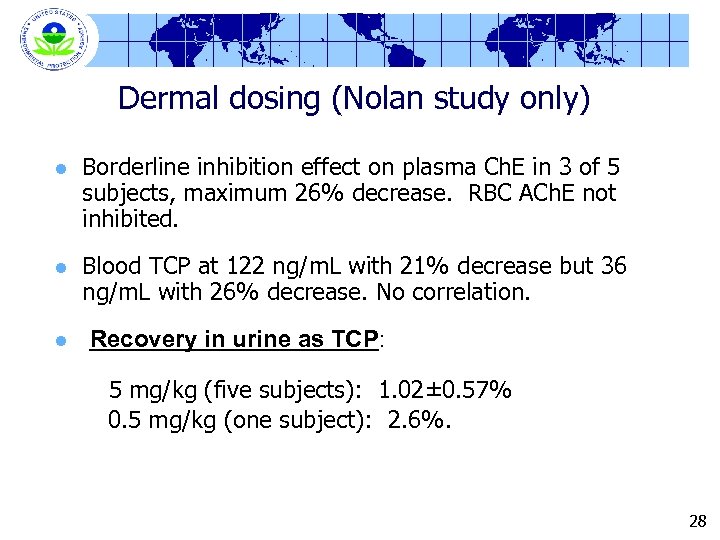 Dermal dosing (Nolan study only) l Borderline inhibition effect on plasma Ch. E in 3 of 5 subjects, maximum 26% decrease. RBC ACh. E not inhibited. l Blood TCP at 122 ng/m. L with 21% decrease but 36 ng/m. L with 26% decrease. No correlation. l Recovery in urine as TCP: 5 mg/kg (five subjects): 1. 02± 0. 57% 0. 5 mg/kg (one subject): 2. 6%. 28
Dermal dosing (Nolan study only) l Borderline inhibition effect on plasma Ch. E in 3 of 5 subjects, maximum 26% decrease. RBC ACh. E not inhibited. l Blood TCP at 122 ng/m. L with 21% decrease but 36 ng/m. L with 26% decrease. No correlation. l Recovery in urine as TCP: 5 mg/kg (five subjects): 1. 02± 0. 57% 0. 5 mg/kg (one subject): 2. 6%. 28
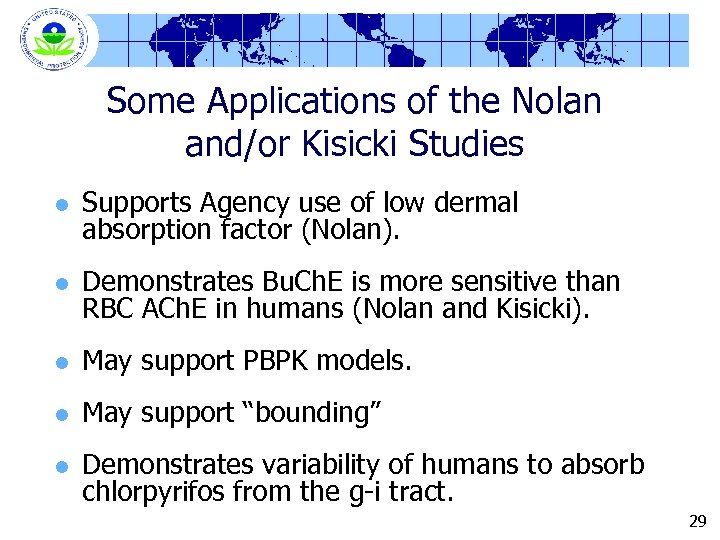 Some Applications of the Nolan and/or Kisicki Studies l Supports Agency use of low dermal absorption factor (Nolan). l Demonstrates Bu. Ch. E is more sensitive than RBC ACh. E in humans (Nolan and Kisicki). l May support PBPK models. l May support “bounding” l Demonstrates variability of humans to absorb chlorpyrifos from the g-i tract. 29
Some Applications of the Nolan and/or Kisicki Studies l Supports Agency use of low dermal absorption factor (Nolan). l Demonstrates Bu. Ch. E is more sensitive than RBC ACh. E in humans (Nolan and Kisicki). l May support PBPK models. l May support “bounding” l Demonstrates variability of humans to absorb chlorpyrifos from the g-i tract. 29
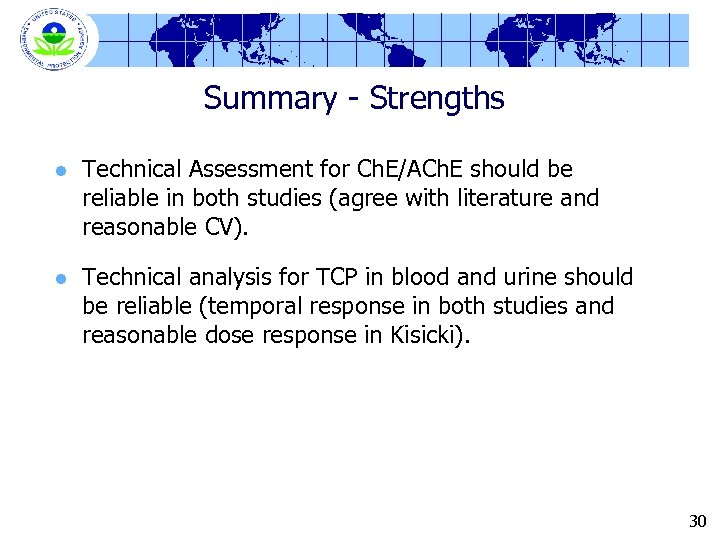 Summary - Strengths l Technical Assessment for Ch. E/ACh. E should be reliable in both studies (agree with literature and reasonable CV). l Technical analysis for TCP in blood and urine should be reliable (temporal response in both studies and reasonable dose response in Kisicki). 30
Summary - Strengths l Technical Assessment for Ch. E/ACh. E should be reliable in both studies (agree with literature and reasonable CV). l Technical analysis for TCP in blood and urine should be reliable (temporal response in both studies and reasonable dose response in Kisicki). 30
 Summary – Limitations: Both Studies l Analytical methods (nano range) are much less sensitive than the epi studies (pico range)*. l Variability in TCP analysis – humans are not equal*. *Provides challenge for interpreting epi studies. l Comparison between Nolan and Kisicki studies confounded because of tablet vs. capsule dosing. l Chlorpyrifos present near LOQ and in some 0 time samples (Nolan). Reliability only fair. 31
Summary – Limitations: Both Studies l Analytical methods (nano range) are much less sensitive than the epi studies (pico range)*. l Variability in TCP analysis – humans are not equal*. *Provides challenge for interpreting epi studies. l Comparison between Nolan and Kisicki studies confounded because of tablet vs. capsule dosing. l Chlorpyrifos present near LOQ and in some 0 time samples (Nolan). Reliability only fair. 31
 Summary – Limitations: Nolan Study l l Only one dose resulting in ~70 -89% inhibition. Does not establish NOEL. Difficult to establish minimal levels of TCP associated with inhibition. TCP is in units/hour, epi and Kisicki report units/m. L. Do not easily compare. 32
Summary – Limitations: Nolan Study l l Only one dose resulting in ~70 -89% inhibition. Does not establish NOEL. Difficult to establish minimal levels of TCP associated with inhibition. TCP is in units/hour, epi and Kisicki report units/m. L. Do not easily compare. 32
 Summary –Limitations: Kisicki Study l l Does not include plasma Ch. E assessment. Only one subject with RBC ACh. E inhibition limits usefulness. 33
Summary –Limitations: Kisicki Study l l Does not include plasma Ch. E assessment. Only one subject with RBC ACh. E inhibition limits usefulness. 33
 Honeycutt & De. Geare (1993) Science Assessment Wade Britton, MPH Health Effects Division Office of Pesticide Programs 34
Honeycutt & De. Geare (1993) Science Assessment Wade Britton, MPH Health Effects Division Office of Pesticide Programs 34
 Study Information l Agricultural postapplication workers monitored during pruning and picking activities in California citrus l Chlorpyrifos (Lorsban 4 E) applied once at each of 3 study locations (5 -6 lb ai/acre) l Study conducted between 1991/1992 35
Study Information l Agricultural postapplication workers monitored during pruning and picking activities in California citrus l Chlorpyrifos (Lorsban 4 E) applied once at each of 3 study locations (5 -6 lb ai/acre) l Study conducted between 1991/1992 35
 Sampling Strategy l 15 individuals monitored § Actual workers and typical durations l Picking § 5 individuals (5 at 1 site) § Exposure occurred 43 days after application l Pruning § 10 individuals (5 at each of 2 sites) § Exposure occurred 2 days after application 36
Sampling Strategy l 15 individuals monitored § Actual workers and typical durations l Picking § 5 individuals (5 at 1 site) § Exposure occurred 43 days after application l Pruning § 10 individuals (5 at each of 2 sites) § Exposure occurred 2 days after application 36
 Multi-faceted Approach l Biological Monitoring* § Urine collected for 4 days after exposure § Blood sampled 1 day after exposure § Pre-exposure samples collected for each l Passive Dosimetry § Dermal § Inhalation l Leaf surface residues 37
Multi-faceted Approach l Biological Monitoring* § Urine collected for 4 days after exposure § Blood sampled 1 day after exposure § Pre-exposure samples collected for each l Passive Dosimetry § Dermal § Inhalation l Leaf surface residues 37
 Biological Monitoring - Analysis l Blood analyzed for plasma and red blood cell (RBC) cholinesterase (Ch. E) levels l Urine analyzed for TCP and creatinine § TCP used to calculate chlorpyrifos body burden § Creatinine used to evaluate completeness of sample collection 38
Biological Monitoring - Analysis l Blood analyzed for plasma and red blood cell (RBC) cholinesterase (Ch. E) levels l Urine analyzed for TCP and creatinine § TCP used to calculate chlorpyrifos body burden § Creatinine used to evaluate completeness of sample collection 38
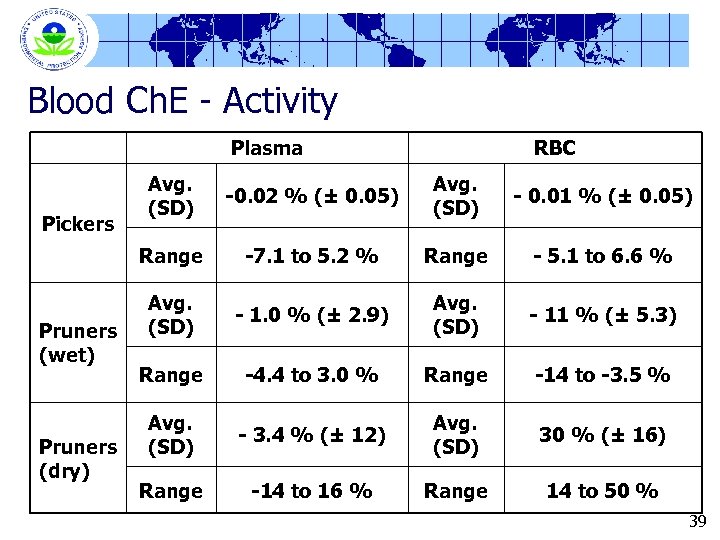 Blood Ch. E - Activity Plasma RBC Pruners (wet) Pruners (dry) -0. 02 % (± 0. 05) Avg. (SD) - 0. 01 % (± 0. 05) Range Pickers Avg. (SD) -7. 1 to 5. 2 % Range - 5. 1 to 6. 6 % Avg. (SD) - 1. 0 % (± 2. 9) Avg. (SD) - 11 % (± 5. 3) Range -4. 4 to 3. 0 % Range -14 to -3. 5 % Avg. (SD) - 3. 4 % (± 12) Avg. (SD) 30 % (± 16) Range -14 to 16 % Range 14 to 50 % 39
Blood Ch. E - Activity Plasma RBC Pruners (wet) Pruners (dry) -0. 02 % (± 0. 05) Avg. (SD) - 0. 01 % (± 0. 05) Range Pickers Avg. (SD) -7. 1 to 5. 2 % Range - 5. 1 to 6. 6 % Avg. (SD) - 1. 0 % (± 2. 9) Avg. (SD) - 11 % (± 5. 3) Range -4. 4 to 3. 0 % Range -14 to -3. 5 % Avg. (SD) - 3. 4 % (± 12) Avg. (SD) 30 % (± 16) Range -14 to 16 % Range 14 to 50 % 39
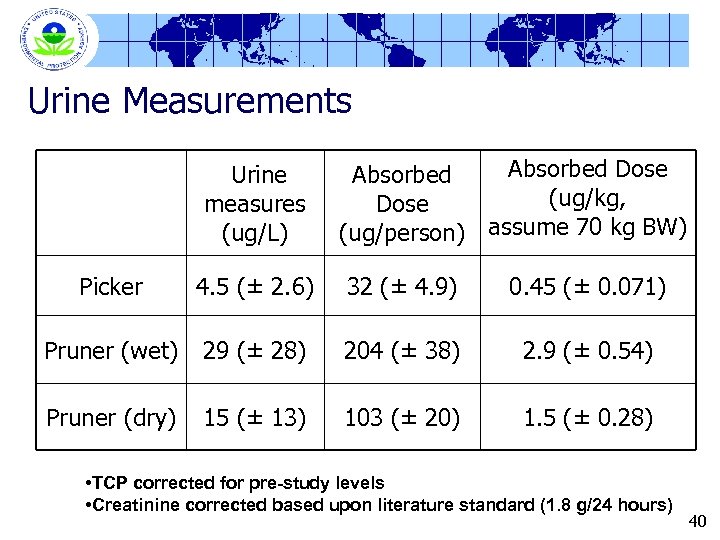 Urine Measurements Urine measures (ug/L) Absorbed Dose Absorbed (ug/kg, Dose (ug/person) assume 70 kg BW) Picker 4. 5 (± 2. 6) 32 (± 4. 9) 0. 45 (± 0. 071) Pruner (wet) 29 (± 28) 204 (± 38) 2. 9 (± 0. 54) Pruner (dry) 15 (± 13) 103 (± 20) 1. 5 (± 0. 28) • TCP corrected for pre-study levels • Creatinine corrected based upon literature standard (1. 8 g/24 hours) 40
Urine Measurements Urine measures (ug/L) Absorbed Dose Absorbed (ug/kg, Dose (ug/person) assume 70 kg BW) Picker 4. 5 (± 2. 6) 32 (± 4. 9) 0. 45 (± 0. 071) Pruner (wet) 29 (± 28) 204 (± 38) 2. 9 (± 0. 54) Pruner (dry) 15 (± 13) 103 (± 20) 1. 5 (± 0. 28) • TCP corrected for pre-study levels • Creatinine corrected based upon literature standard (1. 8 g/24 hours) 40
 Study Strengths l Monitored both urine and blood (plasma and RBC Ch. E) in all workers l Actual workers monitored while performing activities in production fields 41
Study Strengths l Monitored both urine and blood (plasma and RBC Ch. E) in all workers l Actual workers monitored while performing activities in production fields 41
 Study Limitations l l l Not statistically designed to define the relationship between TCP and Ch. E TCP exposure can occur from many sources Dosimetry possibly limited absorption of chlorpyrifos § Potential to underestimate TCP & blood Ch. E activity 42
Study Limitations l l l Not statistically designed to define the relationship between TCP and Ch. E TCP exposure can occur from many sources Dosimetry possibly limited absorption of chlorpyrifos § Potential to underestimate TCP & blood Ch. E activity 42
 Conclusions l Represents the best source for occupational worker chlorpyrifos biological monitoring l Provides urine measures and blood plasma and RBC Ch. E in the same individuals l Actual workers, activities and duration 43
Conclusions l Represents the best source for occupational worker chlorpyrifos biological monitoring l Provides urine measures and blood plasma and RBC Ch. E in the same individuals l Actual workers, activities and duration 43
 Ethics Assessments of Three Pre-Rule Studies of Chlorpyrifos John M. Carley Human Research Ethics Review Officer Office of Pesticide Programs 44
Ethics Assessments of Three Pre-Rule Studies of Chlorpyrifos John M. Carley Human Research Ethics Review Officer Office of Pesticide Programs 44
 Nolan, et al. (1982) Nolan, R. ; Rick, D. ; Freshour, N. ; and Saunders, J. (1982) Chlorpyrifos: Pharmacokinetics in Human Volunteers Following Single Oral and Dermal Doses. Unpublished study prepared by the Dow Chemical Company under Protocol HEB-DR-0043 -4946 -4. 28 p. (MRID 124144) Dow Agro. Sciences (2009) Supplemental Documentation of Ethical Conduct of Nolan et al. Study. E-mail correspondence April 29 through May 8, 2009, between Kenneth Racke and Tom Myers, with attachments. 22 p. 45
Nolan, et al. (1982) Nolan, R. ; Rick, D. ; Freshour, N. ; and Saunders, J. (1982) Chlorpyrifos: Pharmacokinetics in Human Volunteers Following Single Oral and Dermal Doses. Unpublished study prepared by the Dow Chemical Company under Protocol HEB-DR-0043 -4946 -4. 28 p. (MRID 124144) Dow Agro. Sciences (2009) Supplemental Documentation of Ethical Conduct of Nolan et al. Study. E-mail correspondence April 29 through May 8, 2009, between Kenneth Racke and Tom Myers, with attachments. 22 p. 45
 Value to Society l Defines absorption, distribution, and elimination of oral and dermal doses of chlorpyrifos l Contributes to weight of evidence linking animal data and human epidemiological data 46
Value to Society l Defines absorption, distribution, and elimination of oral and dermal doses of chlorpyrifos l Contributes to weight of evidence linking animal data and human epidemiological data 46
 Subject Selection l Subject Selection § Subjects were all salaried Dow employees, recruited through in-house advertisements § 6 healthy adult males, screened by a physician not otherwise involved in the research § Women of child-bearing age excluded by IRB § Nature of endpoints and measures ruled out subject bias in reporting 47
Subject Selection l Subject Selection § Subjects were all salaried Dow employees, recruited through in-house advertisements § 6 healthy adult males, screened by a physician not otherwise involved in the research § Women of child-bearing age excluded by IRB § Nature of endpoints and measures ruled out subject bias in reporting 47
 Risks and Benefits l Risks § Doses based on earlier studies and pilot pre-test, with adequate margins of safety § Expected effects • Inhibition of plasma Ch. E but not of RBC Ch. E • No clinical signs • Effects reversible—followed until full return to baseline l Benefits § No benefits to subjects 48
Risks and Benefits l Risks § Doses based on earlier studies and pilot pre-test, with adequate margins of safety § Expected effects • Inhibition of plasma Ch. E but not of RBC Ch. E • No clinical signs • Effects reversible—followed until full return to baseline l Benefits § No benefits to subjects 48
 Ethics Oversight l l l Approved by Dow Human Health Research Review Committee Approved by University of Michigan Committee to Review Grants for Clinical Research and Investigation Involving Human Beings Approvals documented; gaps typical for research from this period 49
Ethics Oversight l l l Approved by Dow Human Health Research Review Committee Approved by University of Michigan Committee to Review Grants for Clinical Research and Investigation Involving Human Beings Approvals documented; gaps typical for research from this period 49
 Informed Consent l Subjects were given a copy of protocol to review l Subjects were briefed on § § § Study objectives Chlorpyrifos properties Pilot phase results, and study procedures Benefits, including free meals Confidential handling of data Voluntary participation and freedom to withdraw l Subjects signed consent forms reporting that they’d read the protocol and been briefed on the research l Subjects were not paid 50
Informed Consent l Subjects were given a copy of protocol to review l Subjects were briefed on § § § Study objectives Chlorpyrifos properties Pilot phase results, and study procedures Benefits, including free meals Confidential handling of data Voluntary participation and freedom to withdraw l Subjects signed consent forms reporting that they’d read the protocol and been briefed on the research l Subjects were not paid 50
 Applicable Standards l Standards of Conduct § Declaration of Helsinki (1975) § FIFRA § 12(a)(2)(P) l Standards of Acceptability § 40 CFR § 26. 1703 § 40 CFR § 26. 1704 51
Applicable Standards l Standards of Conduct § Declaration of Helsinki (1975) § FIFRA § 12(a)(2)(P) l Standards of Acceptability § 40 CFR § 26. 1703 § 40 CFR § 26. 1704 51
 Compliance with Standards l No evidence to suggest research conduct was inconsistent with Do. H (1975) l Evidence indicates compliance with FIFRA § 12(a)(2)(P) l No intentional exposure of pregnant or nursing women or of children l No clear and convincing evidence of significant deficiency relative to prevailing standards of research conduct 52
Compliance with Standards l No evidence to suggest research conduct was inconsistent with Do. H (1975) l Evidence indicates compliance with FIFRA § 12(a)(2)(P) l No intentional exposure of pregnant or nursing women or of children l No clear and convincing evidence of significant deficiency relative to prevailing standards of research conduct 52
 Conclusion l If it is deemed scientifically valid and relevant, there are no barriers in FIFRA or in 40 CFR § 26. 1703 or § 26. 1704 to EPA’s reliance on the Nolan et al. study in actions taken under FIFRA or § 408 of FFDCA 53
Conclusion l If it is deemed scientifically valid and relevant, there are no barriers in FIFRA or in 40 CFR § 26. 1703 or § 26. 1704 to EPA’s reliance on the Nolan et al. study in actions taken under FIFRA or § 408 of FFDCA 53
 Honeycutt & De. Geare (1993) Honeycutt, R. , and M. De. Geare (1993) Worker Reentry Exposure to Chlorpyrifos in Citrus Treated with Lorsban® 4 E Insecticide. Unpublished study prepared by H. E. R. A. C. , Inc. under study numbers 91 -102 HE and DECO-HEH 2. 2 -1 -182(125)B. 950 p. (MRID 43062701) Dow Agro. Sciences (2009) Supplemental Documentation of Ethical Conduct of Honeycutt and De. Geare Study. E-mail submission of May 22, 2009 from Kenneth Racke to Tom Myers. 27 p. 54
Honeycutt & De. Geare (1993) Honeycutt, R. , and M. De. Geare (1993) Worker Reentry Exposure to Chlorpyrifos in Citrus Treated with Lorsban® 4 E Insecticide. Unpublished study prepared by H. E. R. A. C. , Inc. under study numbers 91 -102 HE and DECO-HEH 2. 2 -1 -182(125)B. 950 p. (MRID 43062701) Dow Agro. Sciences (2009) Supplemental Documentation of Ethical Conduct of Honeycutt and De. Geare Study. E-mail submission of May 22, 2009 from Kenneth Racke to Tom Myers. 27 p. 54
 Value to Society l Conducted in response to EPA requirement l Part of larger project to monitor agricultural worker exposure to chlorpyrifos l Determined Ch. E activity and TCP residues for workers re-entering treated citrus groves § § l Orange pickers re-entering 43 days after treatment Lemon pruners re-entering 2 days after treatment Contributes to weight of evidence linking animal data and human epidemiological data 55
Value to Society l Conducted in response to EPA requirement l Part of larger project to monitor agricultural worker exposure to chlorpyrifos l Determined Ch. E activity and TCP residues for workers re-entering treated citrus groves § § l Orange pickers re-entering 43 days after treatment Lemon pruners re-entering 2 days after treatment Contributes to weight of evidence linking animal data and human epidemiological data 55
 Subject Selection l Subjects were experienced citrus workers l Recruitment was through labor contractor, who may have influenced subject choice to participate l Difficulty reported in finding qualified and willing subjects l Mingling of subjects in this and companion study of chlorpyrifos handlers 56
Subject Selection l Subjects were experienced citrus workers l Recruitment was through labor contractor, who may have influenced subject choice to participate l Difficulty reported in finding qualified and willing subjects l Mingling of subjects in this and companion study of chlorpyrifos handlers 56
 Risks and Benefits l Risks § “No increased health risk as I will be doing my job wearing normal protective clothing” § Unaddressed risks: • • l Heat stress from wearing WBD Differential risks for pickers and pruners Benefits § § No benefits to subjects Benefits likely to Dow, EPA, and CDFA 57
Risks and Benefits l Risks § “No increased health risk as I will be doing my job wearing normal protective clothing” § Unaddressed risks: • • l Heat stress from wearing WBD Differential risks for pickers and pruners Benefits § § No benefits to subjects Benefits likely to Dow, EPA, and CDFA 57
 Ethics Oversight l Protocol review by UCSF Committee on Human Research, brokered by CDFA/CDPR, as was thenstandard practice in California l Revised CF approved by IRB before use l Some amendments affecting subjects may not have been reviewed by IRB l Ethics oversight was closer and is better documented than is typical for worker exposure studies from this period 58
Ethics Oversight l Protocol review by UCSF Committee on Human Research, brokered by CDFA/CDPR, as was thenstandard practice in California l Revised CF approved by IRB before use l Some amendments affecting subjects may not have been reviewed by IRB l Ethics oversight was closer and is better documented than is typical for worker exposure studies from this period 58
 Informed Consent l l Subjects were briefed in Spanish or English Consent forms used were approved by IRB, included all required elements, but retained erroneous content from companion handler study CF discussion of MOE should have been revised for pruners Process and form were above average for exposure studies in 1991 -92 59
Informed Consent l l Subjects were briefed in Spanish or English Consent forms used were approved by IRB, included all required elements, but retained erroneous content from companion handler study CF discussion of MOE should have been revised for pruners Process and form were above average for exposure studies in 1991 -92 59
 Applicable Standards l Standards of Conduct § CCR Title 3 § 6710 (26 Sep 1988) • Health of subjects will not be endangered • Participants informed of potential risks • Medical supervision • Incorporation of recommendations by Human Study Committee of California Health and Welfare Agency § FIFRA § 12(a)(2)(P) l Standards of Acceptability § 40 CFR § 26. 1703 § 40 CFR § 26. 1704 60
Applicable Standards l Standards of Conduct § CCR Title 3 § 6710 (26 Sep 1988) • Health of subjects will not be endangered • Participants informed of potential risks • Medical supervision • Incorporation of recommendations by Human Study Committee of California Health and Welfare Agency § FIFRA § 12(a)(2)(P) l Standards of Acceptability § 40 CFR § 26. 1703 § 40 CFR § 26. 1704 60
 Compliance with Standards of Conduct l Evidence indicates substantial compliance with California rule and with FIFRA § 12(a)(2)(P) § § § l Active CDPR oversight and approval IRB review and approval Voluntary and informed consent Some protocol amendments should have led to further revisions to the consent form 61
Compliance with Standards of Conduct l Evidence indicates substantial compliance with California rule and with FIFRA § 12(a)(2)(P) § § § l Active CDPR oversight and approval IRB review and approval Voluntary and informed consent Some protocol amendments should have led to further revisions to the consent form 61
 Compliance with Acceptance Standards l 40 CFR § 26. 1703 § No intentional exposure of pregnant or nursing women or of children l 40 CFR § 26. 1704 § Not fundamentally unethical § No clear and convincing evidence of significant deficiency relative to prevailing standards 62
Compliance with Acceptance Standards l 40 CFR § 26. 1703 § No intentional exposure of pregnant or nursing women or of children l 40 CFR § 26. 1704 § Not fundamentally unethical § No clear and convincing evidence of significant deficiency relative to prevailing standards 62
 Conclusion l If it is deemed scientifically valid and relevant, there are no barriers in FIFRA or in 40 CFR § 26. 1703 or § 26. 1704 to EPA’s reliance on the Honeycutt & De. Geare study in actions taken under FIFRA or § 408 of FFDCA 63
Conclusion l If it is deemed scientifically valid and relevant, there are no barriers in FIFRA or in 40 CFR § 26. 1703 or § 26. 1704 to EPA’s reliance on the Honeycutt & De. Geare study in actions taken under FIFRA or § 408 of FFDCA 63
 Kisicki, et al. (1999) Kisicki, J. ; Seip, C. ; Combs, M. (1999) A Rising Dose Toxicological Study to Determine the No-Observable-Effect-Levels (NOEL) For Erythrocyte Acetylcholinesterase (ACh. E) Inhibition and Cholinergic Signs and Symptoms of Chlorpyrifos at Three Dose Levels. Unpublished study prepared by MDS Harris Laboratories under Project No. 21438 and Dow Agro. Sciences Study No. DR K -0044793 -284. 578 p. (MRID 44811002) Juberg, D. ; Mattsson, J. (2008) Dow Agro. Sciences Response to EPA Query Regarding Two Toxicology Reports. Unpublished document prepared by Dow Agro. Sciences LLC under Study ID DRJ 05142008. 20 p. (MRID 47429401) Juberg, D. ; Mattsson, J. (2008) Updated Dow Agro. Sciences Response to EPA Query Regarding Two Toxicology Reports. Unpublished document prepared by Dow Agro. Sciences LLC under Study ID DRJ 05282008. 27 p. (MRID 47436401) 64
Kisicki, et al. (1999) Kisicki, J. ; Seip, C. ; Combs, M. (1999) A Rising Dose Toxicological Study to Determine the No-Observable-Effect-Levels (NOEL) For Erythrocyte Acetylcholinesterase (ACh. E) Inhibition and Cholinergic Signs and Symptoms of Chlorpyrifos at Three Dose Levels. Unpublished study prepared by MDS Harris Laboratories under Project No. 21438 and Dow Agro. Sciences Study No. DR K -0044793 -284. 578 p. (MRID 44811002) Juberg, D. ; Mattsson, J. (2008) Dow Agro. Sciences Response to EPA Query Regarding Two Toxicology Reports. Unpublished document prepared by Dow Agro. Sciences LLC under Study ID DRJ 05142008. 20 p. (MRID 47429401) Juberg, D. ; Mattsson, J. (2008) Updated Dow Agro. Sciences Response to EPA Query Regarding Two Toxicology Reports. Unpublished document prepared by Dow Agro. Sciences LLC under Study ID DRJ 05282008. 27 p. (MRID 47436401) 64
 Value to Society l l Objective was NOEL for RBC Ch. EI following single oral dose Research undertaken by Dow at their own initiative Determined RBC Ch. EI and chlorpyrifos and TCP residues in blood and urine May contribute to weight of evidence linking animal data and human epidemiological data 65
Value to Society l l Objective was NOEL for RBC Ch. EI following single oral dose Research undertaken by Dow at their own initiative Determined RBC Ch. EI and chlorpyrifos and TCP residues in blood and urine May contribute to weight of evidence linking animal data and human epidemiological data 65
 Subject Selection l Subjects were “non-institutionalized subjects consisting of college students and members of the community at large” l More than the reported 60 were involved § § 60 candidates were enrolled as primary subjects and 22 more as alternates § l 140 candidates who responded to a “standard advertisement” were screened After extensive substitution at the time of “check-in”, 30 males and 30 females served as treated or control subjects An alternate replacing a primary subject was identified by the same subject number as the person replaced 66
Subject Selection l Subjects were “non-institutionalized subjects consisting of college students and members of the community at large” l More than the reported 60 were involved § § 60 candidates were enrolled as primary subjects and 22 more as alternates § l 140 candidates who responded to a “standard advertisement” were screened After extensive substitution at the time of “check-in”, 30 males and 30 females served as treated or control subjects An alternate replacing a primary subject was identified by the same subject number as the person replaced 66
 Subject Selection— 2 l Age range 19 -54, mean age 31 l Inclusion criteria few; exclusion criteria extensive l Candidates rejected in screening mainly for drugs or blood chemistry l Enrollees were replaced by alternates mainly because of not showing up at check-in 67
Subject Selection— 2 l Age range 19 -54, mean age 31 l Inclusion criteria few; exclusion criteria extensive l Candidates rejected in screening mainly for drugs or blood chemistry l Enrollees were replaced by alternates mainly because of not showing up at check-in 67
 Dose Selection l Low dose (0. 5 mg/kg) for overlap with Nolan, et al. l Because 0. 5 mg/kg showed no RBC Ch. EI in Nolan and no signs, low- and mid-doses (0. 5 and 1. 0 mg/kg) were administered concurrently in Phase 1 l Pause to confirm no effects at 1. 0 mg/kg before escalating dose to 2. 0 mg/kg in Phase 2 68
Dose Selection l Low dose (0. 5 mg/kg) for overlap with Nolan, et al. l Because 0. 5 mg/kg showed no RBC Ch. EI in Nolan and no signs, low- and mid-doses (0. 5 and 1. 0 mg/kg) were administered concurrently in Phase 1 l Pause to confirm no effects at 1. 0 mg/kg before escalating dose to 2. 0 mg/kg in Phase 2 68
 Risks to Subjects l Not discussed in protocol l Consent Form: § “Potential side effects include. . . improved performance on numerous tests of mental function” § § “No adverse effects are anticipated” § § “It may be very unsafe for me to leave the clinic. . . ” “Animal studies indicate little or no risk in humans” “There are specific and effective antidotes available” “In all but exceptional cases, persons seriously poisoned. . . recover rapidly leaving no long term effects” “Risks involved in drawing blood. . . ” 69
Risks to Subjects l Not discussed in protocol l Consent Form: § “Potential side effects include. . . improved performance on numerous tests of mental function” § § “No adverse effects are anticipated” § § “It may be very unsafe for me to leave the clinic. . . ” “Animal studies indicate little or no risk in humans” “There are specific and effective antidotes available” “In all but exceptional cases, persons seriously poisoned. . . recover rapidly leaving no long term effects” “Risks involved in drawing blood. . . ” 69
 Risks to Subjects— 2 § “This procedure may be associated with undesirable effects, some of which are not predictable. However, I understand that in the opinion of MDS Harris’ medical consultants, those risks are not great enough to keep me from participating” 70
Risks to Subjects— 2 § “This procedure may be associated with undesirable effects, some of which are not predictable. However, I understand that in the opinion of MDS Harris’ medical consultants, those risks are not great enough to keep me from participating” 70
 Risk Minimization l Vital signs taken periodically l Subjects were asked open-ended questions about how they felt l Physicians were on call during subject confinement l Antidotes described were not required by protocol to be available during test 71
Risk Minimization l Vital signs taken periodically l Subjects were asked open-ended questions about how they felt l Physicians were on call during subject confinement l Antidotes described were not required by protocol to be available during test 71
 Risk: Benefit Relation l Consent Form states subjects would receive “no direct medical benefit”, but that the information developed “may provide potential benefit to others” l Protocol is silent concerning societal value of information expected to be gained l Relation of risks and benefits not addressed in protocol or by IRB 72
Risk: Benefit Relation l Consent Form states subjects would receive “no direct medical benefit”, but that the information developed “may provide potential benefit to others” l Protocol is silent concerning societal value of information expected to be gained l Relation of risks and benefits not addressed in protocol or by IRB 72
 Ethics Oversight l Protocol, MSDS, Consent Form, payment, and recruiting advertisement were reviewed and approved by MDS-Harris in-house IRB l “Additional changes” were reported to have been submitted to IRB direct from sponsor l Amendment 1 and revised CF also approved by IRB l MDS-Harris IRB holds a Federal-Wide Assurance from OHRP 73
Ethics Oversight l Protocol, MSDS, Consent Form, payment, and recruiting advertisement were reviewed and approved by MDS-Harris in-house IRB l “Additional changes” were reported to have been submitted to IRB direct from sponsor l Amendment 1 and revised CF also approved by IRB l MDS-Harris IRB holds a Federal-Wide Assurance from OHRP 73
 Informed Consent Process l Explanation of research and signature of consent form occurred during “check-in” the evening before treatment l Hectic circumstances at check-in are unlikely to have provided the prospective subject sufficient opportunity to consider whether or not to participate l All enrolled primary and alternate subjects had provided blood and urine samples for screening and baseline before receiving an explanation of the research or signing the consent form 74
Informed Consent Process l Explanation of research and signature of consent form occurred during “check-in” the evening before treatment l Hectic circumstances at check-in are unlikely to have provided the prospective subject sufficient opportunity to consider whether or not to participate l All enrolled primary and alternate subjects had provided blood and urine samples for screening and baseline before receiving an explanation of the research or signing the consent form 74
 Informed Consent Form l Inappropriate technical language in CF—reading grade level for first full paragraph was 17. 7 l Poor organization, pronoun shifts, mix of dire warnings and soothing reassurance made it difficult to follow or understand l Discussion of risks incomplete and misleading l Escalation rule was not explained to subjects, nor were results of Phase 1 incorporated into Consent Form for Phase 2 75
Informed Consent Form l Inappropriate technical language in CF—reading grade level for first full paragraph was 17. 7 l Poor organization, pronoun shifts, mix of dire warnings and soothing reassurance made it difficult to follow or understand l Discussion of risks incomplete and misleading l Escalation rule was not explained to subjects, nor were results of Phase 1 incorporated into Consent Form for Phase 2 75
 Respect for Subjects l l Subjects were free to withdraw Subject privacy was not compromised in reports Subjects were compensated for participation Recruiting and screening processes were needlessly intrusive 76
Respect for Subjects l l Subjects were free to withdraw Subject privacy was not compromised in reports Subjects were compensated for participation Recruiting and screening processes were needlessly intrusive 76
 Unreported Protocol Deviation l Protocol: Adverse events, whether serious or non-serious, will be followed to resolution regardless of whether the subject is still participating in the study l l The only subject with significant Ch. EI was lost to follow-up 48 h post treatment This was not acknowledged to be a deviation from the protocol 77
Unreported Protocol Deviation l Protocol: Adverse events, whether serious or non-serious, will be followed to resolution regardless of whether the subject is still participating in the study l l The only subject with significant Ch. EI was lost to follow-up 48 h post treatment This was not acknowledged to be a deviation from the protocol 77
 Applicable Standards l Standards of Conduct § 21 CFR parts 50, 56, and 321 § Declaration of Helsinki (1996) § FIFRA § 12(a)(2)(P) l Standards of Acceptability § 40 CFR § 26. 1703 § 40 CFR § 26. 1704 78
Applicable Standards l Standards of Conduct § 21 CFR parts 50, 56, and 321 § Declaration of Helsinki (1996) § FIFRA § 12(a)(2)(P) l Standards of Acceptability § 40 CFR § 26. 1703 § 40 CFR § 26. 1704 78
 Compliance with Standards l 21 CFR 50 and 56, like the Common Rule, require § § § IRB oversight and prior approval Risk minimization Favorable risk: benefit balance Acceptable informed consent process and consent form Equitable subject selection Fully voluntary participation by subjects l Review and approval by the MDS-Harris IRB did not show concern for or ensure compliance with these standards l Deficiencies in consent process made conduct non-compliant with FIFRA § 12(a)(2)(P) 79
Compliance with Standards l 21 CFR 50 and 56, like the Common Rule, require § § § IRB oversight and prior approval Risk minimization Favorable risk: benefit balance Acceptable informed consent process and consent form Equitable subject selection Fully voluntary participation by subjects l Review and approval by the MDS-Harris IRB did not show concern for or ensure compliance with these standards l Deficiencies in consent process made conduct non-compliant with FIFRA § 12(a)(2)(P) 79
 Conclusions l No intentional exposure of pregnant or nursing women or of children l No clear and convincing evidence that research was fundamentally unethical l In spite of some gaps in the record, there is clear and convincing evidence that conduct of the Kisicki study was significantly deficient relative to the standards of 21 CFR parts 50 and 56, cited by investigators as governing this work l Except under the provisions of 40 CFR § 26. 1706, EPA is forbidden to rely on this study in actions under FIFRA or FFDCA 80
Conclusions l No intentional exposure of pregnant or nursing women or of children l No clear and convincing evidence that research was fundamentally unethical l In spite of some gaps in the record, there is clear and convincing evidence that conduct of the Kisicki study was significantly deficient relative to the standards of 21 CFR parts 50 and 56, cited by investigators as governing this work l Except under the provisions of 40 CFR § 26. 1706, EPA is forbidden to rely on this study in actions under FIFRA or FFDCA 80
 Charge Questions to the HSRB: Three Pre-Rule Studies of Chlorpyrifos 81
Charge Questions to the HSRB: Three Pre-Rule Studies of Chlorpyrifos 81
 Nolan et al. (1982) 1 Are the blood and urine measurements of chlorpyrifos and/or TCP from the Nolan et al. oral and dermal studies reliable? 2 Are the measurements of cholinesterase activity/inhibition from the Nolan et al. oral and dermal studies reliable? 3 Is there clear and convincing evidence that the conduct of the Nolan et al. study was fundamentally unethical, or significantly deficient relative to the standards of ethical research conduct prevailing when it was conducted? 82
Nolan et al. (1982) 1 Are the blood and urine measurements of chlorpyrifos and/or TCP from the Nolan et al. oral and dermal studies reliable? 2 Are the measurements of cholinesterase activity/inhibition from the Nolan et al. oral and dermal studies reliable? 3 Is there clear and convincing evidence that the conduct of the Nolan et al. study was fundamentally unethical, or significantly deficient relative to the standards of ethical research conduct prevailing when it was conducted? 82
 Honeycutt & De. Geare (1993) 1 Are the blood and urine measurements of chlorpyrifos and/or TCP from the Honeycutt & De. Geare worker biomonitoring study reliable? 2 Are the measurements of cholinesterase activity/inhibition from the Honeycutt & De. Geare worker biomonitoring study reliable? 3 Is there clear and convincing evidence that the conduct of the Honeycutt & De. Geare study was fundamentally unethical, or significantly deficient relative to the standards of ethical research conduct prevailing when it was conducted? 83
Honeycutt & De. Geare (1993) 1 Are the blood and urine measurements of chlorpyrifos and/or TCP from the Honeycutt & De. Geare worker biomonitoring study reliable? 2 Are the measurements of cholinesterase activity/inhibition from the Honeycutt & De. Geare worker biomonitoring study reliable? 3 Is there clear and convincing evidence that the conduct of the Honeycutt & De. Geare study was fundamentally unethical, or significantly deficient relative to the standards of ethical research conduct prevailing when it was conducted? 83
 Kisicki et al. (1999) 1 Are the blood and urine measurements of chlorpyrifos and/or TCP from the Kisicki et al. oral study reliable? 2 Are the measurements of cholinesterase activity/inhibition from the Kisicki et al. oral study reliable? 3 Is there clear and convincing evidence that the conduct of the Kisicki et al. study was fundamentally unethical, or significantly deficient relative to the standards of ethical research conduct prevailing when it was conducted? 84
Kisicki et al. (1999) 1 Are the blood and urine measurements of chlorpyrifos and/or TCP from the Kisicki et al. oral study reliable? 2 Are the measurements of cholinesterase activity/inhibition from the Kisicki et al. oral study reliable? 3 Is there clear and convincing evidence that the conduct of the Kisicki et al. study was fundamentally unethical, or significantly deficient relative to the standards of ethical research conduct prevailing when it was conducted? 84


