94651d7b6afa86db0388e0d1dd7714e6.ppt
- Количество слайдов: 14
 Three-Part Small-scale Screening Platform for the Masses Ronnie Frederick Center for Eukaryotic Structural Genomics Department of Biochemistry University of Wisconsin—Madison NIGMS U 54 GM 074901, JL Markley, PI, GN Phillips, & Brian G. Fox, Co-Directors Promega/UW 133 -GT 34, BG Fox, PI http: //www. uwstructuralgenomics. org
Three-Part Small-scale Screening Platform for the Masses Ronnie Frederick Center for Eukaryotic Structural Genomics Department of Biochemistry University of Wisconsin—Madison NIGMS U 54 GM 074901, JL Markley, PI, GN Phillips, & Brian G. Fox, Co-Directors Promega/UW 133 -GT 34, BG Fox, PI http: //www. uwstructuralgenomics. org
 CESG Protein Production • Unified cell-based and cell-free expression pipelines • Expression screening in E. coli and wheat germ extract • Data captured into Sesame at each step
CESG Protein Production • Unified cell-based and cell-free expression pipelines • Expression screening in E. coli and wheat germ extract • Data captured into Sesame at each step
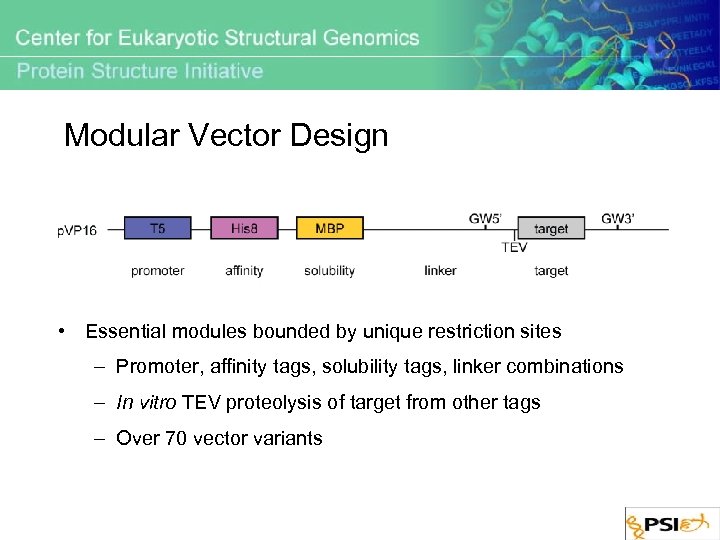 Modular Vector Design • Essential modules bounded by unique restriction sites – Promoter, affinity tags, solubility tags, linker combinations – In vitro TEV proteolysis of target from other tags – Over 70 vector variants
Modular Vector Design • Essential modules bounded by unique restriction sites – Promoter, affinity tags, solubility tags, linker combinations – In vitro TEV proteolysis of target from other tags – Over 70 vector variants
 Small-Scale Expression Testing 96 -well plasmid workgroup 96 -plate transformation 96 -well growth • Small-scale expression evaluations (Studier media) – – – SDS-PAGE in slab gels (~2 days processing and analysis) Tested for total expression, fraction soluble and fraction cleaved ~1. 5 weeks to evaluate, labor intensive, expensive ($450/ 96 targets) No reliable assessment of target status after TEV proteolysis Predicting large-scale results ~83% accurate (10% false negative and 6% false positive)
Small-Scale Expression Testing 96 -well plasmid workgroup 96 -plate transformation 96 -well growth • Small-scale expression evaluations (Studier media) – – – SDS-PAGE in slab gels (~2 days processing and analysis) Tested for total expression, fraction soluble and fraction cleaved ~1. 5 weeks to evaluate, labor intensive, expensive ($450/ 96 targets) No reliable assessment of target status after TEV proteolysis Predicting large-scale results ~83% accurate (10% false negative and 6% false positive)
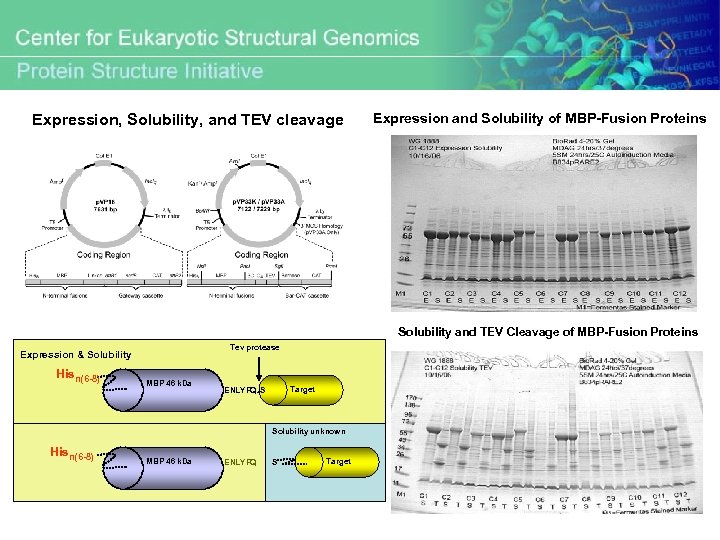 Expression, Solubility, and TEV cleavage Expression and Solubility of MBP-Fusion Proteins Solubility and TEV Cleavage of MBP-Fusion Proteins Tev protease Expression & Solubility Hisn(6 -8) MBP 46 k. Da Target ENLYFQ^S Solubility unknown Hisn(6 -8) MBP 46 k. Da ENLYFQ S Target
Expression, Solubility, and TEV cleavage Expression and Solubility of MBP-Fusion Proteins Solubility and TEV Cleavage of MBP-Fusion Proteins Tev protease Expression & Solubility Hisn(6 -8) MBP 46 k. Da Target ENLYFQ^S Solubility unknown Hisn(6 -8) MBP 46 k. Da ENLYFQ S Target
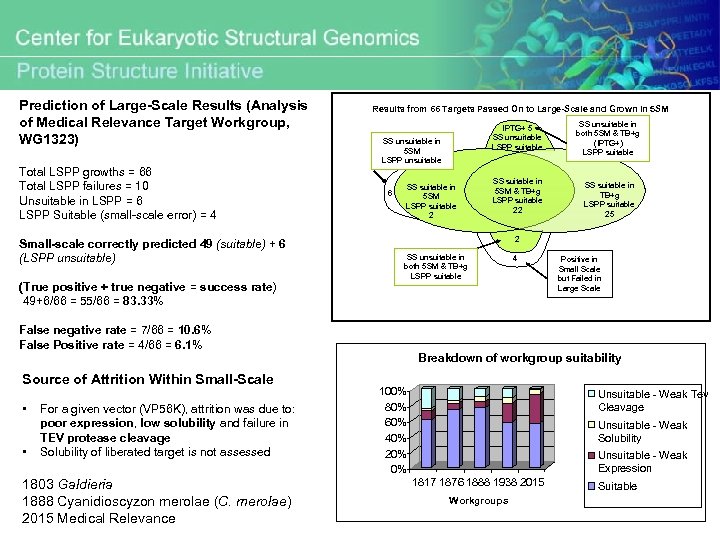 Prediction of Large-Scale Results (Analysis of Medical Relevance Target Workgroup, WG 1323) Total LSPP growths = 66 Total LSPP failures = 10 Unsuitable in LSPP = 6 LSPP Suitable (small-scale error) = 4 Small-scale correctly predicted 49 (suitable) + 6 (LSPP unsuitable) (True positive + true negative = success rate) 49+6/66 = 55/66 = 83. 33% Results from 66 Targets Passed On to Large-Scale and Grown in 5 SM IPTG+ 5 SS unsuitable LSPP suitable SS unsuitable in 5 SM LSPP unsuitable 6 SS suitable in 5 SM LSPP suitable 2 SS unsuitable in both 5 SM & TB+g (IPTG+) LSPP suitable SS suitable in 5 SM & TB+g LSPP suitable 22 SS suitable in TB+g LSPP suitable 25 2 SS unsuitable in both 5 SM & TB+g LSPP suitable 4 Positive in Small Scale but Failed in Large Scale False negative rate = 7/66 = 10. 6% False Positive rate = 4/66 = 6. 1% Breakdown of workgroup suitability Source of Attrition Within Small-Scale • • For a given vector (VP 56 K), attrition was due to: poor expression, low solubility and failure in TEV protease cleavage Solubility of liberated target is not assessed 1803 Galdieria 1888 Cyanidioscyzon merolae (C. merolae) 2015 Medical Relevance 100% 80% 60% 40% 20% 0% Unsuitable - Weak Tev Cleavage Unsuitable - Weak Solubility Unsuitable - Weak Expression 1817 1876 1888 1938 2015 Workgroups Suitable
Prediction of Large-Scale Results (Analysis of Medical Relevance Target Workgroup, WG 1323) Total LSPP growths = 66 Total LSPP failures = 10 Unsuitable in LSPP = 6 LSPP Suitable (small-scale error) = 4 Small-scale correctly predicted 49 (suitable) + 6 (LSPP unsuitable) (True positive + true negative = success rate) 49+6/66 = 55/66 = 83. 33% Results from 66 Targets Passed On to Large-Scale and Grown in 5 SM IPTG+ 5 SS unsuitable LSPP suitable SS unsuitable in 5 SM LSPP unsuitable 6 SS suitable in 5 SM LSPP suitable 2 SS unsuitable in both 5 SM & TB+g (IPTG+) LSPP suitable SS suitable in 5 SM & TB+g LSPP suitable 22 SS suitable in TB+g LSPP suitable 25 2 SS unsuitable in both 5 SM & TB+g LSPP suitable 4 Positive in Small Scale but Failed in Large Scale False negative rate = 7/66 = 10. 6% False Positive rate = 4/66 = 6. 1% Breakdown of workgroup suitability Source of Attrition Within Small-Scale • • For a given vector (VP 56 K), attrition was due to: poor expression, low solubility and failure in TEV protease cleavage Solubility of liberated target is not assessed 1803 Galdieria 1888 Cyanidioscyzon merolae (C. merolae) 2015 Medical Relevance 100% 80% 60% 40% 20% 0% Unsuitable - Weak Tev Cleavage Unsuitable - Weak Solubility Unsuitable - Weak Expression 1817 1876 1888 1938 2015 Workgroups Suitable
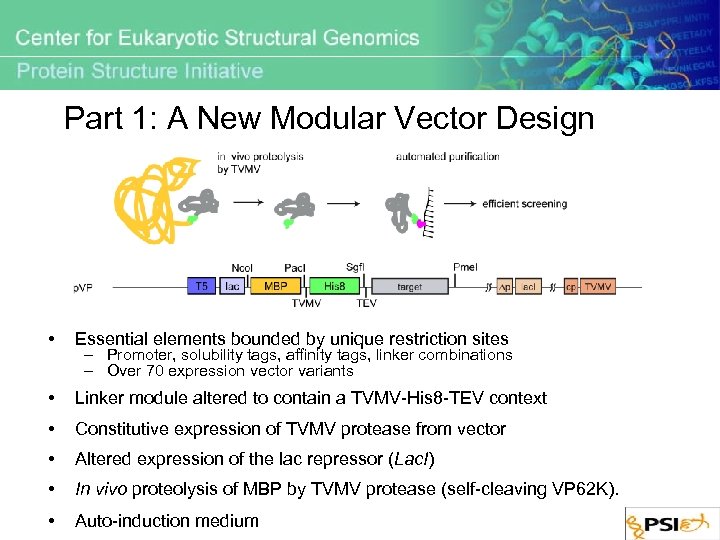 Part 1: A New Modular Vector Design • Essential elements bounded by unique restriction sites • Linker module altered to contain a TVMV-His 8 -TEV context • Constitutive expression of TVMV protease from vector • Altered expression of the lac repressor (Lac. I) • In vivo proteolysis of MBP by TVMV protease (self-cleaving VP 62 K). • Auto-induction medium – Promoter, solubility tags, affinity tags, linker combinations – Over 70 expression vector variants
Part 1: A New Modular Vector Design • Essential elements bounded by unique restriction sites • Linker module altered to contain a TVMV-His 8 -TEV context • Constitutive expression of TVMV protease from vector • Altered expression of the lac repressor (Lac. I) • In vivo proteolysis of MBP by TVMV protease (self-cleaving VP 62 K). • Auto-induction medium – Promoter, solubility tags, affinity tags, linker combinations – Over 70 expression vector variants
 Part 2: Medium Evolution • • • First pass Se-Met medium in the production pipeline Factorial design identified favored compositions that were different from original PASM (Studier’s media) Expression performance is strongly linked to the level of lac repressor (Lac. I) expressed from the backbone Reference: “Enhanced Bacterial Protein Expression During Auto-induction Obtained by Alteration of Lac Repressor Dosage and Medium Composition” Paul G. Blommel, Katie J. Becker, Petar Duvnjak, and Brian G. Fox (in press).
Part 2: Medium Evolution • • • First pass Se-Met medium in the production pipeline Factorial design identified favored compositions that were different from original PASM (Studier’s media) Expression performance is strongly linked to the level of lac repressor (Lac. I) expressed from the backbone Reference: “Enhanced Bacterial Protein Expression During Auto-induction Obtained by Alteration of Lac Repressor Dosage and Medium Composition” Paul G. Blommel, Katie J. Becker, Petar Duvnjak, and Brian G. Fox (in press).
 Part 3: Simple robotics for the masses Automated Purification System • • Couple the designed vector and the improved expression medium with the new robotics • E. coli pipeline: Auto-induction • www. promega. com/paguide/images Can the simple Maxwell robotics contribute to our pipeline? Use it to obtain predictive information of protein behavior in purification, NMR and X-ray structure determination in vivo cleavage automated Maxwell purification
Part 3: Simple robotics for the masses Automated Purification System • • Couple the designed vector and the improved expression medium with the new robotics • E. coli pipeline: Auto-induction • www. promega. com/paguide/images Can the simple Maxwell robotics contribute to our pipeline? Use it to obtain predictive information of protein behavior in purification, NMR and X-ray structure determination in vivo cleavage automated Maxwell purification
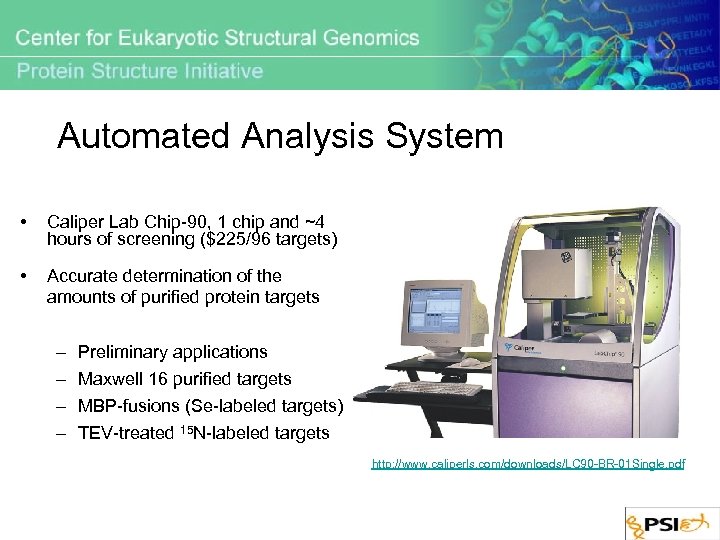 Automated Analysis System • Caliper Lab Chip-90, 1 chip and ~4 hours of screening ($225/96 targets) • Accurate determination of the amounts of purified protein targets – – Preliminary applications Maxwell 16 purified targets MBP-fusions (Se-labeled targets) TEV-treated 15 N-labeled targets http: //www. caliperls. com/downloads/LC 90 -BR-01 Single. pdf
Automated Analysis System • Caliper Lab Chip-90, 1 chip and ~4 hours of screening ($225/96 targets) • Accurate determination of the amounts of purified protein targets – – Preliminary applications Maxwell 16 purified targets MBP-fusions (Se-labeled targets) TEV-treated 15 N-labeled targets http: //www. caliperls. com/downloads/LC 90 -BR-01 Single. pdf
 LC 90 Analysis of Maxwell Screen • • • Various structural genomics targets or controls – A mix of the good, the bad and the ugly Target protein detected for all 24 targets In vivo cleavage with GFP as a control worked
LC 90 Analysis of Maxwell Screen • • • Various structural genomics targets or controls – A mix of the good, the bad and the ugly Target protein detected for all 24 targets In vivo cleavage with GFP as a control worked
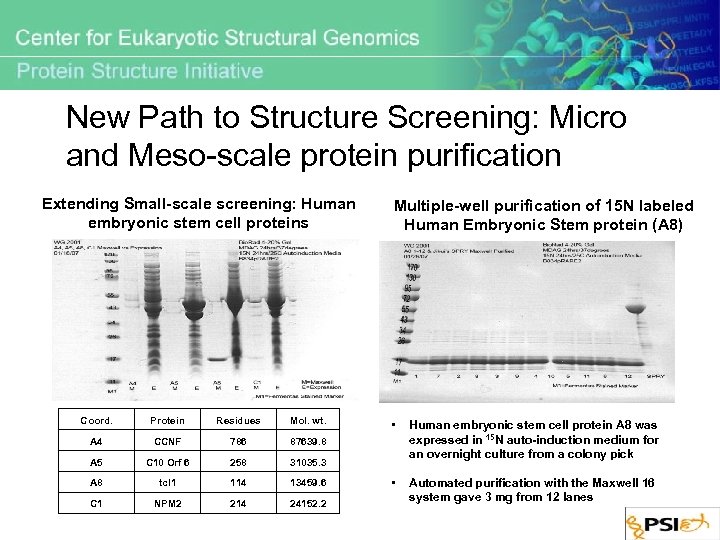 New Path to Structure Screening: Micro and Meso-scale protein purification Extending Small-scale screening: Human embryonic stem cell proteins Coord. Protein Residues Mol. wt. A 4 CCNF 786 87639. 8 A 5 C 10 Orf 6 258 31035. 3 A 8 tcl 1 114 13459. 6 C 1 NPM 2 214 24152. 2 Multiple-well purification of 15 N labeled Human Embryonic Stem protein (A 8) • Human embryonic stem cell protein A 8 was expressed in 15 N auto-induction medium for an overnight culture from a colony pick • Automated purification with the Maxwell 16 system gave 3 mg from 12 lanes
New Path to Structure Screening: Micro and Meso-scale protein purification Extending Small-scale screening: Human embryonic stem cell proteins Coord. Protein Residues Mol. wt. A 4 CCNF 786 87639. 8 A 5 C 10 Orf 6 258 31035. 3 A 8 tcl 1 114 13459. 6 C 1 NPM 2 214 24152. 2 Multiple-well purification of 15 N labeled Human Embryonic Stem protein (A 8) • Human embryonic stem cell protein A 8 was expressed in 15 N auto-induction medium for an overnight culture from a colony pick • Automated purification with the Maxwell 16 system gave 3 mg from 12 lanes
 Meso-Scale Protein Production and Purification for HSQC NMR Analysis • 15 N HSQC NMR of His 8 -GFP expressed in selfcleaving vector p. VP 62 K at 35 ˚C. The NMR time required was 1 h • Cost is $50 (labeled medium and purification cartridges). 40 minute run (1. 5 mg from 8 m. L). • NMR measurements by Dr. Jikui Song • 15 N HSQC NMR of human embryonic stem cell protein A 8 expressed in self-cleaving vector p. VP 62 K. • The NMR time required was 8 h • Crystals of Se. Met-labeled GFP
Meso-Scale Protein Production and Purification for HSQC NMR Analysis • 15 N HSQC NMR of His 8 -GFP expressed in selfcleaving vector p. VP 62 K at 35 ˚C. The NMR time required was 1 h • Cost is $50 (labeled medium and purification cartridges). 40 minute run (1. 5 mg from 8 m. L). • NMR measurements by Dr. Jikui Song • 15 N HSQC NMR of human embryonic stem cell protein A 8 expressed in self-cleaving vector p. VP 62 K. • The NMR time required was 8 h • Crystals of Se. Met-labeled GFP
 Acknowledgements Professor Brian G. Fox (section supervisor) Vector Design and Flexi. Vector Cloning Paul Blommel, Michael Goren, Peter Martin, Kory Seder, Eric Steffan and Russell Wrobel Factorial Evolution of Auto-Induction Media Paul Blommel, Katy Becker and Petar Duvnak CESG Small-scale screening team Lai Bergeman, Mike Cassidy, Ah-Young Lim and Jung (James) Whan Yoon, Jason Bunge (High school student) & Dr Jikui Song (NMR analysis) Past members John Kunert, Megan Riters, Nick Dillion, Rachel Schiesher & Jay Juhjung Chin
Acknowledgements Professor Brian G. Fox (section supervisor) Vector Design and Flexi. Vector Cloning Paul Blommel, Michael Goren, Peter Martin, Kory Seder, Eric Steffan and Russell Wrobel Factorial Evolution of Auto-Induction Media Paul Blommel, Katy Becker and Petar Duvnak CESG Small-scale screening team Lai Bergeman, Mike Cassidy, Ah-Young Lim and Jung (James) Whan Yoon, Jason Bunge (High school student) & Dr Jikui Song (NMR analysis) Past members John Kunert, Megan Riters, Nick Dillion, Rachel Schiesher & Jay Juhjung Chin


