cfe81e906eb088246844a8ec853c7a4a.ppt
- Количество слайдов: 25

This house believes that neoadjuvant systemic therapy should be the standard of care for HER 2+/triple negative operable breast cancer > 2 cm Discussion Antonio Frassoldati Oncologia Clinica Az Ospedaliero Universitaria di Ferrara

When a therapy becomes standard? • Class I evidence of improvement in OS in respect with the previous standard • If not for OS, evidence of improvement in DFS • If not for OS and DFS, evidence of improvements in Qo. L, side effect profile or costs.
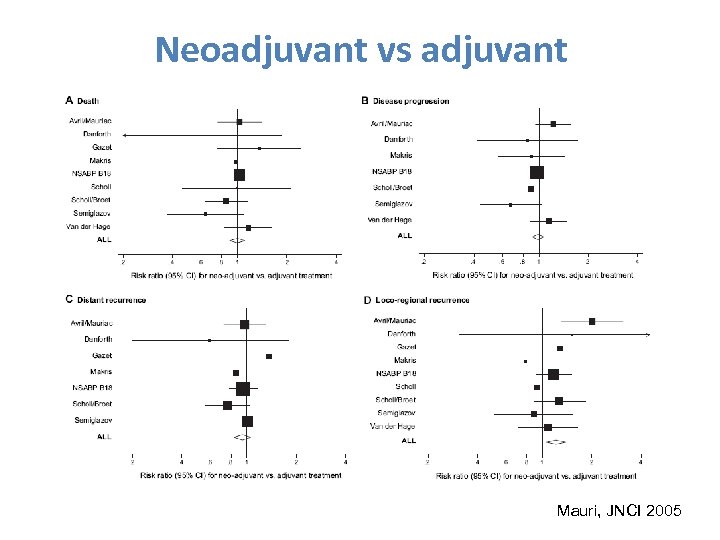
Neoadjuvant vs adjuvant Mauri, JNCI 2005
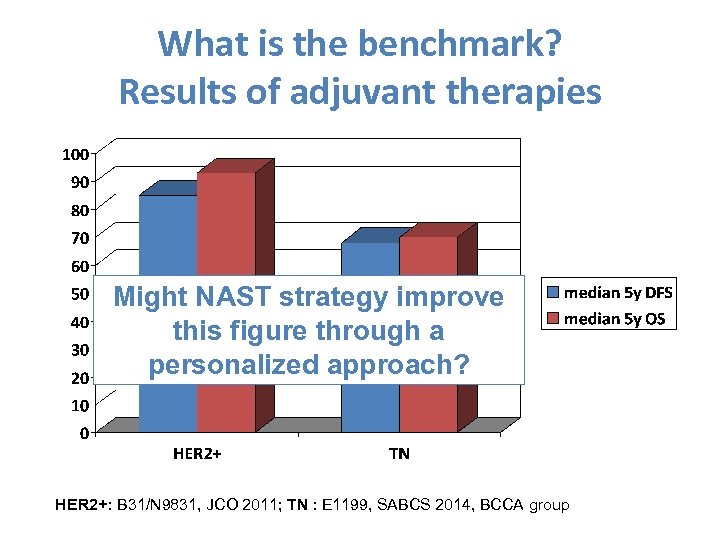
What is the benchmark? Results of adjuvant therapies Might NAST strategy improve this figure through a personalized approach? HER 2+: B 31/N 9831, JCO 2011; TN : E 1199, SABCS 2014, BCCA group
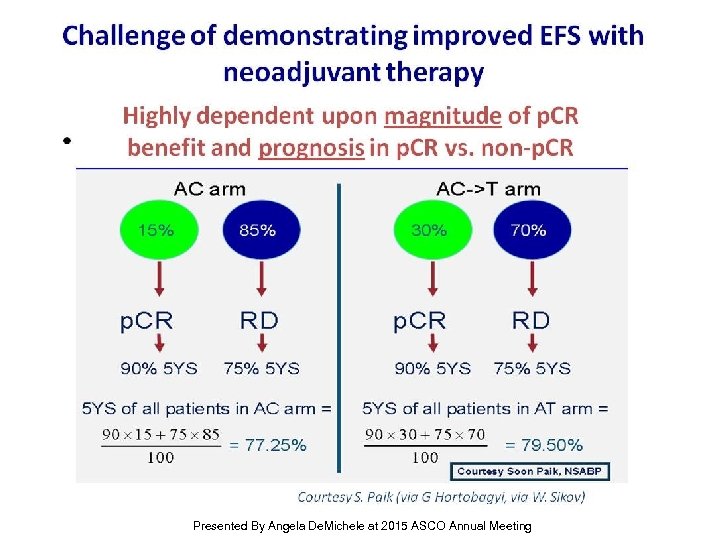
Challenge of demonstrating improved EFS with neoadjuvant therapy Presented By Angela De. Michele at 2015 ASCO Annual Meeting
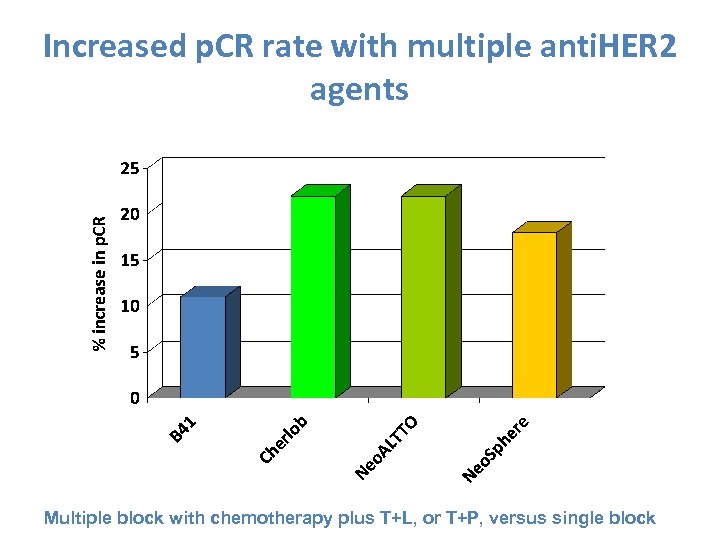
Increased p. CR rate with multiple anti. HER 2 agents Multiple block with chemotherapy plus T+L, or T+P, versus single block
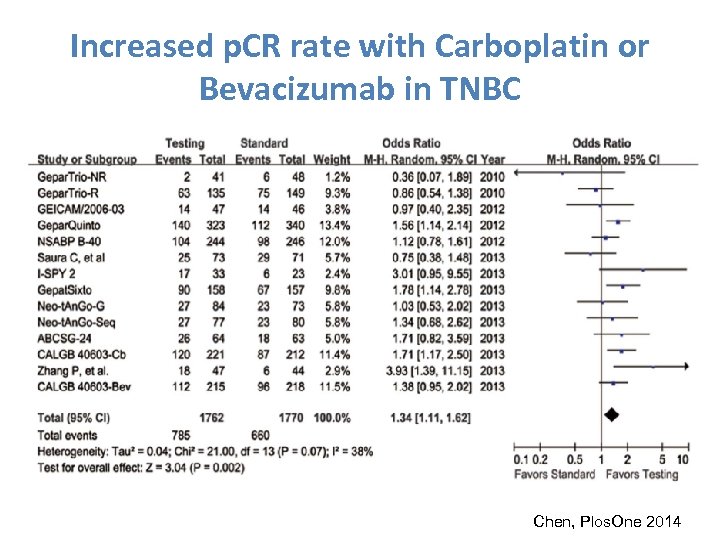
Increased p. CR rate with Carboplatin or Bevacizumab in TNBC Chen, Plos. One 2014
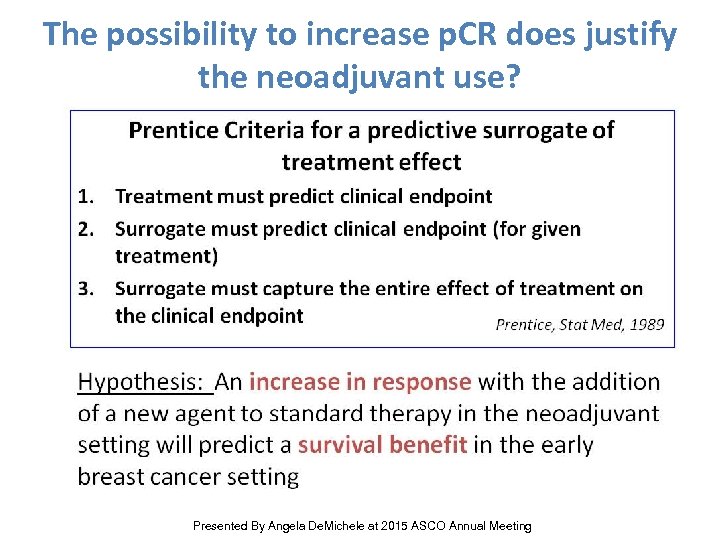
The possibility to increase p. CR does justify the neoadjuvant use? Prognostic Predictive Presented By Angela De. Michele at 2015 ASCO Annual Meeting
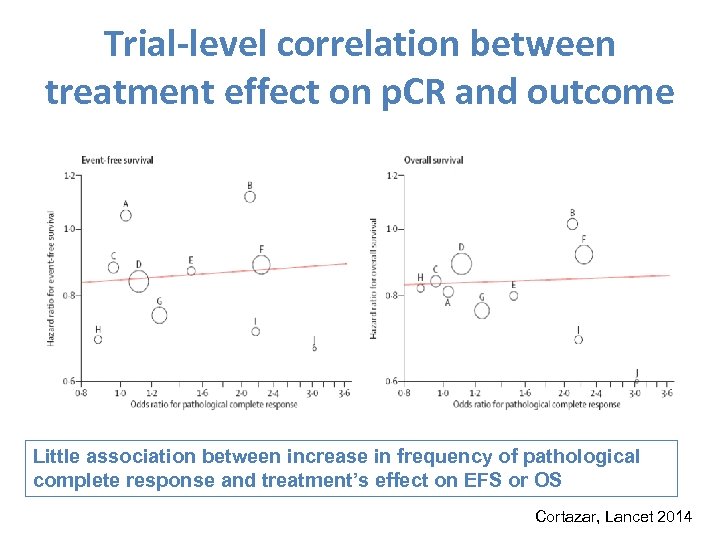
Trial-level correlation between treatment effect on p. CR and outcome Little association between increase in frequency of pathological complete response and treatment’s effect on EFS or OS Cortazar, Lancet 2014
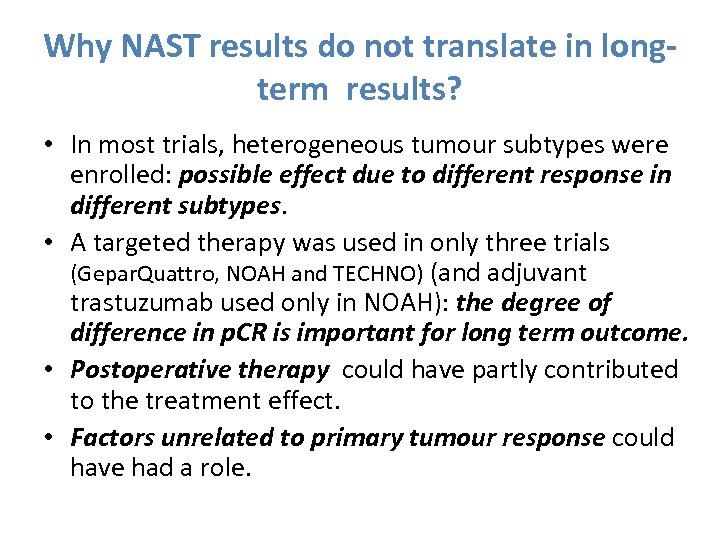
Why NAST results do not translate in longterm results? • In most trials, heterogeneous tumour subtypes were enrolled: possible effect due to different response in different subtypes. • A targeted therapy was used in only three trials (Gepar. Quattro, NOAH and TECHNO) (and adjuvant trastuzumab used only in NOAH): the degree of difference in p. CR is important for long term outcome. • Postoperative therapy could have partly contributed to the treatment effect. • Factors unrelated to primary tumour response could have had a role.
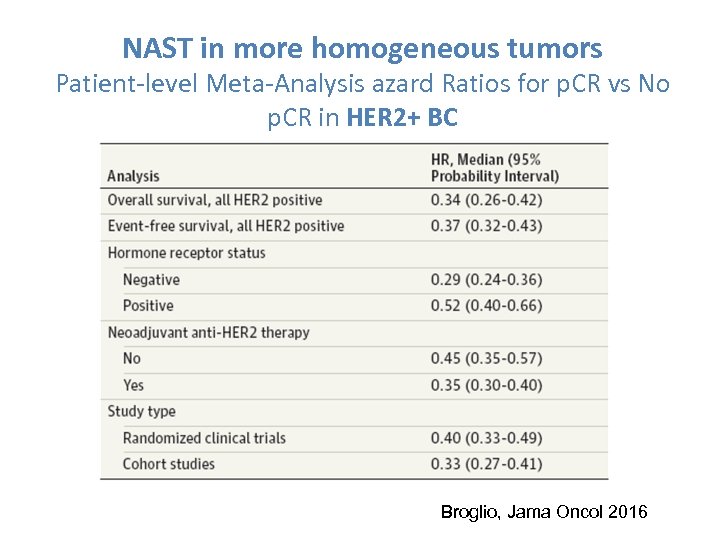
NAST in more homogeneous tumors Patient-level Meta-Analysis azard Ratios for p. CR vs No p. CR in HER 2+ BC Broglio, Jama Oncol 2016
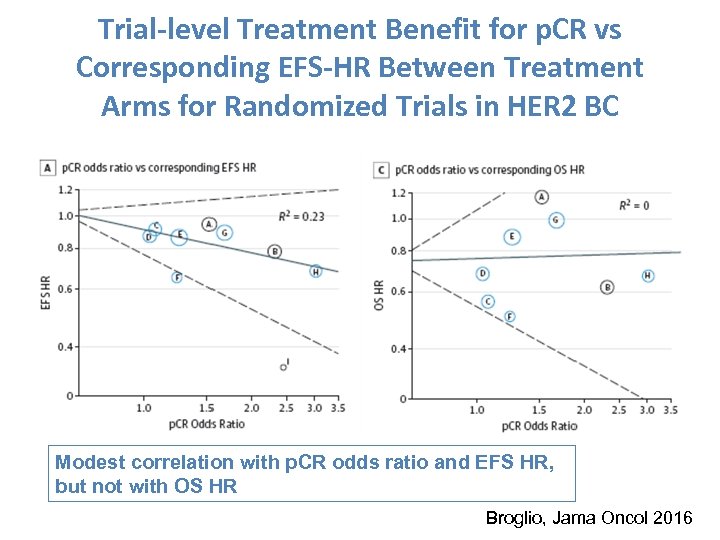
Trial-level Treatment Benefit for p. CR vs Corresponding EFS-HR Between Treatment Arms for Randomized Trials in HER 2 BC Modest correlation with p. CR odds ratio and EFS HR, but not with OS HR Broglio, Jama Oncol 2016

Why NAST should be the standard? • Increase in the rate of breast conservation • Improvement in axillary surgery (avoiding axillary clearence in c. N 1 pts) • Increase in the disease outcome (DFS, DDFS, OS)? • Increase the kwnoledge of the disease behavior (prognostic; useful for adjuvant phase) • Increase in the possibility of tailoring therapies (to increase the p. CR or the long term outcomes)
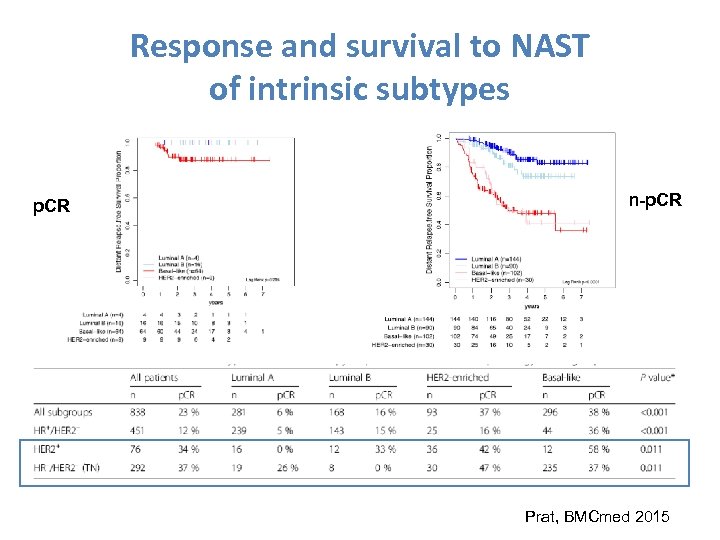
Response and survival to NAST of intrinsic subtypes p. CR n-p. CR Prat, BMCmed 2015
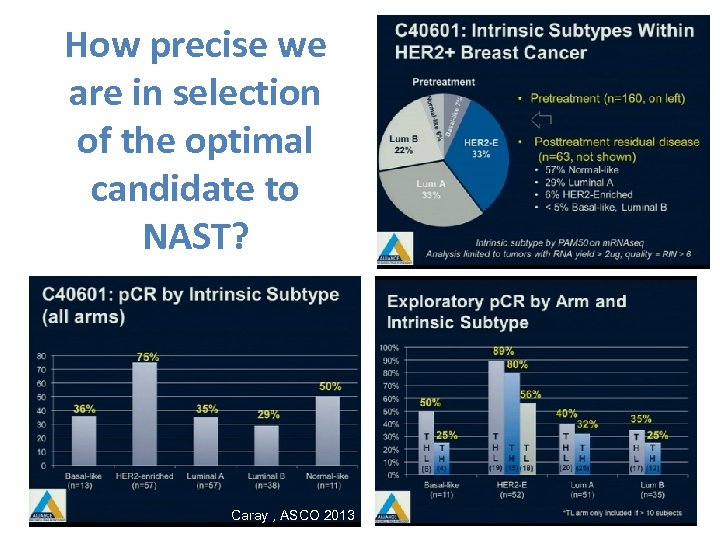
How precise we are in selection of the optimal candidate to NAST? Caray , ASCO 2013
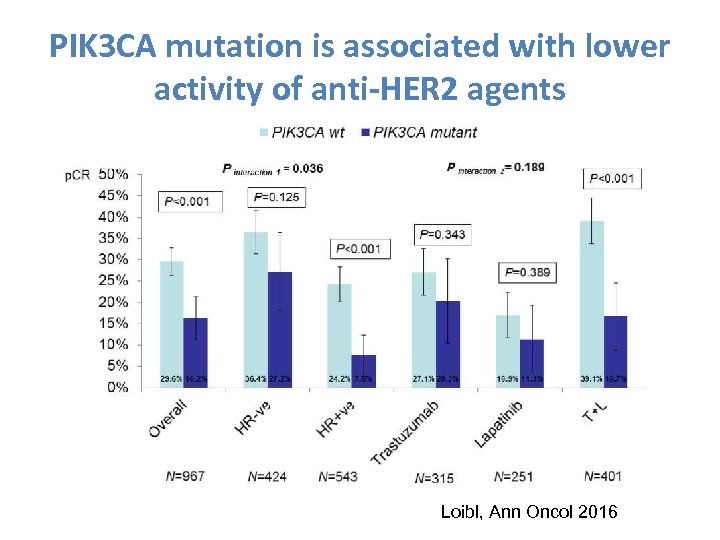
PIK 3 CA mutation is associated with lower activity of anti-HER 2 agents Loibl, Ann Oncol 2016
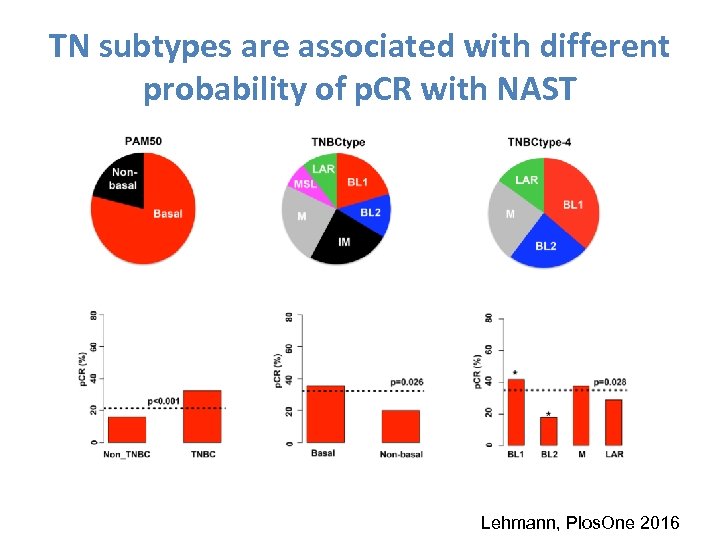
TN subtypes are associated with different probability of p. CR with NAST Lehmann, Plos. One 2016
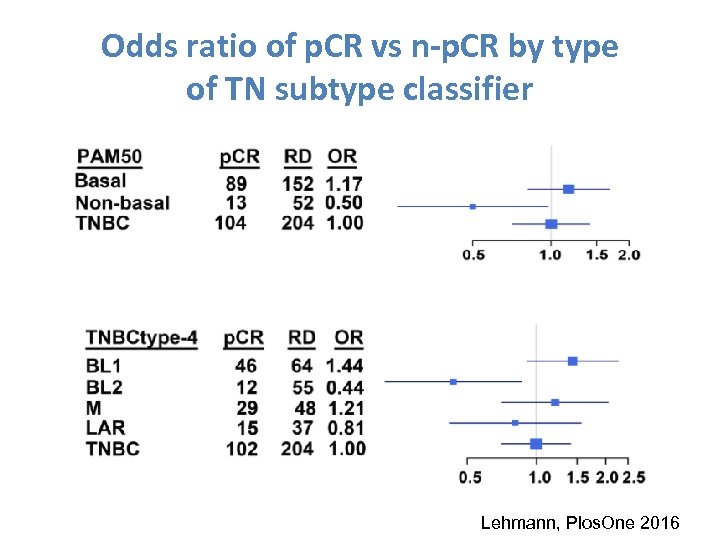
Odds ratio of p. CR vs n-p. CR by type of TN subtype classifier Lehmann, Plos. One 2016
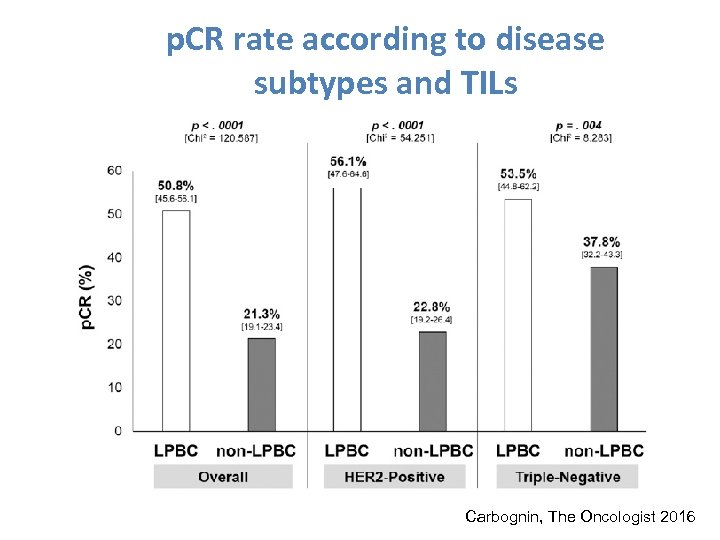
p. CR rate according to disease subtypes and TILs Carbognin, The Oncologist 2016

NAST: already standard or still a model for reasearch? • Are the endpoints of therapy the same if therapy is given before or after surgery? – Some of them can be influenced by the timing of therapy – The tumor biology may not be the same for primary or micrometastatic tumor cells
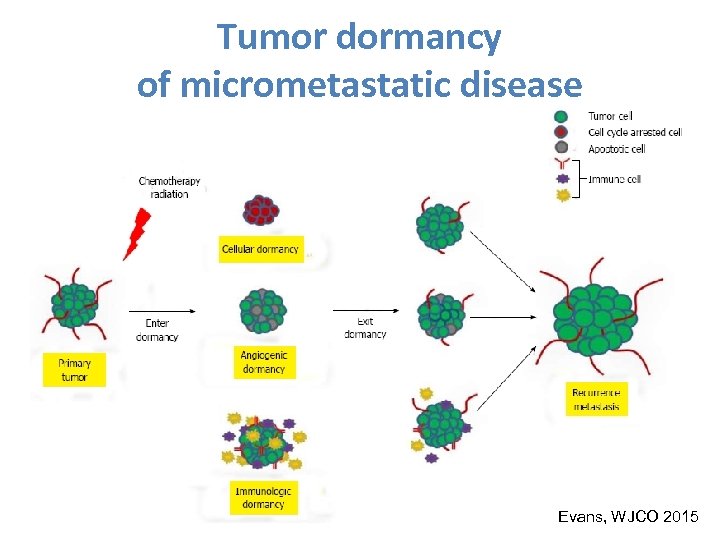
Tumor dormancy of micrometastatic disease Evans, WJCO 2015
Clinical and Prognostic information How we practically use them (EBM guided)? • • • Change the type of surgery Reassure the patient if p. CR Change the adjuvant strategy Change the duration of treatments Change the type of radiation Change the type of controls during the followup

If NAST is standard, which standard after NAST? • For HER 2+ BC, trastuzumab up to one year can be considered standard (even if p. CR? ) • What to do if HER 2 status changes? (stop or go trastuzumab? ) • No established guideline for Chemo in tumors with residual disease at surgery (other chemo? which chemo? ) • No established radiotherapy strategy for c. N+ becoming yp. N 0, nor for large tumor obtaining good PR but undergoing to mastectomy. (RT on axilla? RT after mastectomy? • Maybe more questions than answers generated by NAST

Which lessons from the NAST story? • Tumor selection is the first point – we are still imprecise • Patient involvement in the decision is the second point – an honest discussion about the endpoints, the potential true benefits and the limits of NAST must be done • NAST remains the best model to study the activity of new drugs and to evaluate the “in vivo” effects of them, even if it is not automatically the same in other tumor settings.

NAST in HER 2+ & TNBC NAST is NOT the standard, but is one of the possible standards (to be carefully selected for the right situation)
cfe81e906eb088246844a8ec853c7a4a.ppt