f04bd170b4ada9852341778890fec40a.ppt
- Количество слайдов: 54

• This fuel is not suitable for burning directly in the diesel engine because it has some solids and water as impurity, which may cause damage to the engine parts and also has a very high viscosity, which makes it difficult for atomization of fuel in the combustion process. To make this fuel suitable for burning, it has to go through a conditioning process consisting of settling, centrifuging, boosting of pressure, filtering and heating.

Fuel Oil Systems Heavy residual fuel consists of residues left after lighter and costlier distillates fuels and gases are removed from petroleum crude oil in an oil refinery. Marine diesel engines are designed to burn heavy residual fuel blended with distillate gasoil to meet the specification of fuel oil ordered, especially viscosity and density. This is popularly known as “heavy fuel oil”.
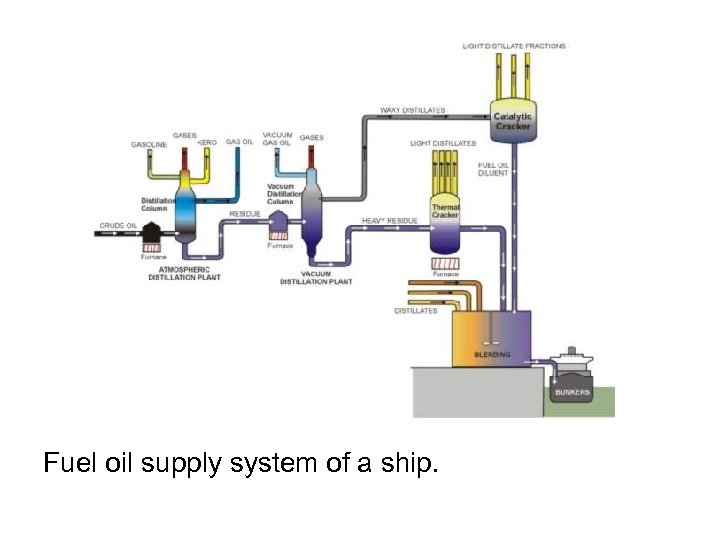
Fuel oil supply system of a ship.
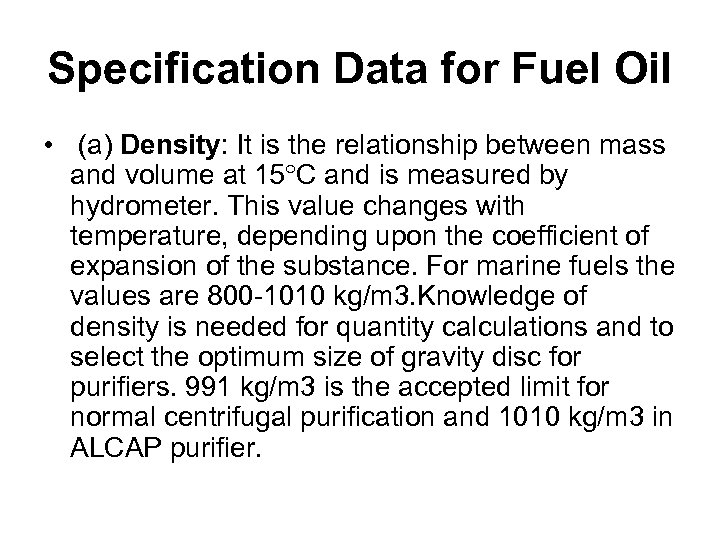
Specification Data for Fuel Oil • (a) Density: It is the relationship between mass and volume at 15 C and is measured by hydrometer. This value changes with temperature, depending upon the coefficient of expansion of the substance. For marine fuels the values are 800 -1010 kg/m 3. Knowledge of density is needed for quantity calculations and to select the optimum size of gravity disc for purifiers. 991 kg/m 3 is the accepted limit for normal centrifugal purification and 1010 kg/m 3 in ALCAP purifier.

• As already mentioned density is the ratio of the mass of a substance to its volume, but not its weight to volume ratio and therefore, density by definition is in vacuo. The term ‘density in air”, although often used, is incorrect and should be referred to as “weight factor”. This is due to the fact that a substance weighed in air is supported, to a small extent, by the buoyancy of the air acting on it. In effect therefore, the weight of a liquid in air is slightly less than its weight in vacuo.

• Viscosity : Viscosity can be termed as resistance to flow. Viscosity is measured in a viscosimeter. The kinematic viscosity is obtained by dividing dynamic viscosity by density of the fluid and its unit of measurement is stoke or centistoke and is quoted with a reference temperature. For distillate fuels the reference temperature is 40 C and for residual fuels the reference temperature is usually 50 C or 100 C. Each fuel has its own temperature viscosity relationship and although oil suppliers publish temperature/viscosity charts, it should be understood that these charts are based on average data of large number of representative fuels. Precise relationship would depend upon crude oil source and refining process. In general, for lower viscosity fuels the difference is small, but it becomes wider as viscosity of the fuel increases. A knowledge of viscosity is necessary for the determination of the heating required for a fuel for transfer purpose and the temperature range required for satisfactory injection and combustion at the fuel atomiser.

• In order to ensure efficient atomization of the charge, when burning residual fuels it is essential to inject the fuel at the most suitable viscosity. Despite wide differences in engine and fuel system designs there is considerable agreement that the most suitable viscosity of the fuel leaving the injector nozzle lies between 12. 5 18. 0 c. St. In well designed systems, the viscosity is controlled automatically within fairly close limits by means of viscosity controllers. • Pressure/viscosity characteristics : The viscosity of hydrocarbon oils increases under pressure. The very high fuel injection pressures now employed will increase the fuel viscosity markedly. This should be allowed for when preheating the fuel.

• Cloud and pour points : The cloud point of a distillate fuel is the temperature at which wax starts to crystallise out, and this is seen when the clear fuel becomes opaque. For marine fuels this characteristic is only applicable to some light grades.
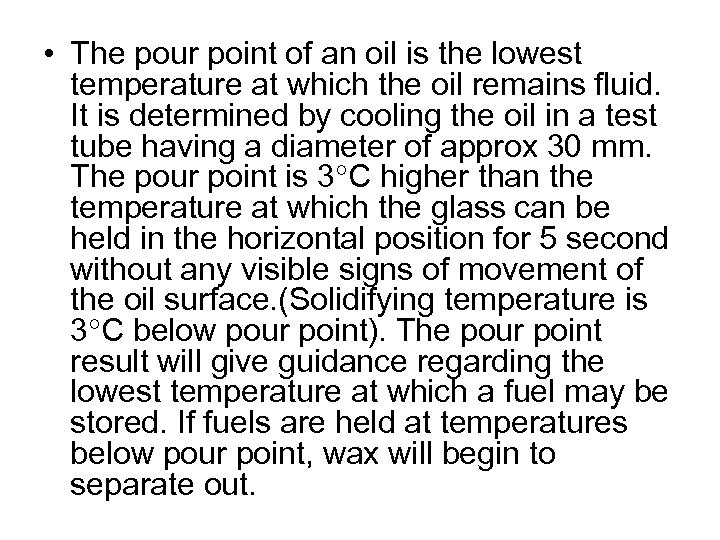
• The pour point of an oil is the lowest temperature at which the oil remains fluid. It is determined by cooling the oil in a test tube having a diameter of approx 30 mm. The pour point is 3 C higher than the temperature at which the glass can be held in the horizontal position for 5 second without any visible signs of movement of the oil surface. (Solidifying temperature is 3 C below pour point). The pour point result will give guidance regarding the lowest temperature at which a fuel may be stored. If fuels are held at temperatures below pour point, wax will begin to separate out.

• This wax may cause blocking of filters and can deposit on heat exchangers. In severe cases the wax will build up in storage tank bottom and on heating coils, which can restrict the coils from heating the fuel. When dealing with heavy marine fuels, both the pour point and the viscosity of the fuel need to be considered, if the fuel is to be maintained at a temperature to prevent wax formation and allow pumping. For efficient pumping the viscosity of the fuel should not be above approximately 600 c. St. If the suction line from the pump to the tank is very long the viscosity should be lower.
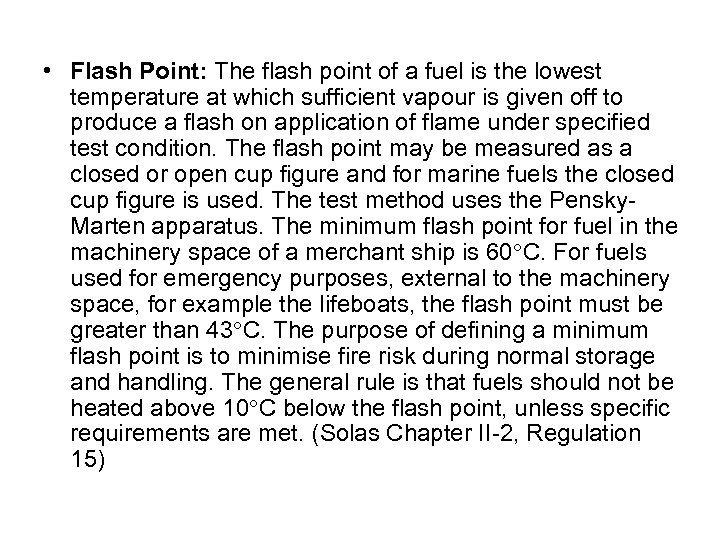
• Flash Point: The flash point of a fuel is the lowest temperature at which sufficient vapour is given off to produce a flash on application of flame under specified test condition. The flash point may be measured as a closed or open cup figure and for marine fuels the closed cup figure is used. The test method uses the Pensky. Marten apparatus. The minimum flash point for fuel in the machinery space of a merchant ship is 60 C. For fuels used for emergency purposes, external to the machinery space, for example the lifeboats, the flash point must be greater than 43 C. The purpose of defining a minimum flash point is to minimise fire risk during normal storage and handling. The general rule is that fuels should not be heated above 10 C below the flash point, unless specific requirements are met. (Solas Chapter II-2, Regulation 15)

• Fire Point : It is the lowest temperature at which vapour is generated at a rate sufficient to sustain combustion for 5 second. The same equipment which is used for determining flash point is used for this test also. • (f) Auto-ignition temperature or Self ignition temperature : It is the lowest temperature at which the generated vapour will ignite spontaneously without any source of ignition.
• Calorific Value or Heat of Combustion or Specific Energy : Heat of combustion of a fuel is the amount of heat released during combustion of a unit mass under following circumstances: • (a) The temperature of fuel before combustion and that of the combustion products after combustion is 20 C. • (b) The combustion products from carbon and sulphur are solely gaseous carbon dioxide and sulphur dioxide and no oxidation of nitrogen has occurred.

• In gross heat of combustion, the water existing before combustion as well as the water generated by the combustion process is to be found in the combustion products in liquid state. In net heat of combustion the above mentioned water is to be found in the form of vapour at 20 C. The gross heat of combustion can be determined by Berthlot-Mahler calorimeter. The net heat of combustion(hi) is calculated if the gross heat of combustion(hs) is known. hi = hs - 25 (f+w) k. J/kg where water content of fuel is f% by mass, and that w% by mass of water is generated by combustion of hydrogen in fuel. • Heat of combustion can be calculated with a degree of accuracy sufficient for normal purposes from the density of the fuel and the application of corrections for any sulphur, water and ash that are present. On a world-wide basis the heat of combustion does vary slightly, depending mainly on density and sulphur content of the fuel.

• Water - Normally the water content in the fuel oil is very low and 0. 1 -0. 2% by volume is typical. Ingress of water can come from tank condensation, tank leakage and heating coil leakage. Water is normally removed by gravitational separation in fuel oil tanks and centrifugal purification system.

• Ash : Nickel, Aluminium, Silicon, Sodium and Vanadium • The ash content is defined as the residue left after all the combustible components of the oil has been burnt. In distillate fuel this quantity is negligible. The ash constituents are concentrated in residual fuels. The ash consists generally of oxides and/or sulphates of nickel, aluminium, silicon, sodium and vanadium. The sources of these are (a) inorganic material naturally present in the crude oil, (b) Catalytic fines picked during refining process ( Catalytic fines are particles arising from the catalytic cracking process in the refinery and are in the form of complex alumino-silicates) (c) Contamination by sand, dirt, rust scale and sea water subsequent to refining process.

• Sodium and Vanadium - Fuels leaving refinery have sodium level below 50 mg/kg. If contaminated with sea water subsequently, sodium level will increase. A 1% sea water contamination represents a potential 100 mg/kg increase. Normally sea water can be removed by gravitation separation in settling tank and centrifugal separation. Vanadium is present in all crude oils in an oil soluble form and the levels found in residual fuels depends mainly on the crude oil source, with those from Venezuela and Mexico having the highest levels. The actual level is also related to the concentrating effect of the refining processes used in the production of the residual fuel. There is no economic process for removing vanadium from either the crude oil or residue.

• During combustion of the fuel, vanadium and sodium constituents form a mixture of sodium sulphate and vanadium pentoxide. This mixture has a low melting point (approx 500 -600 C) corresponding to the temperature of the exhaust valve seating. The semifluid particles of ash adhere firmly to the surfaces they touch, gradually forming a very hard, thin layer of slag which, after having reached a certain thickness, allows the hot combustion gases to leak out, the result being that the slag melts forming a narrow channel. If the layer of slag is of sufficient thickness, the channel grows and the combustion gases heat up the seating material, causing what is known as high-temperature oxidation, which in turn results in the seating material melting in the vicinity of the channel. The most critical sodium to vanadium ratio is about 1 to 3.

• Silicon and aluminium : Silicon may be present in the fuel in form of sand aluminium may also be present in very small quantities, having been picked up by the crude oil in sub-surface rocks. However presence of aluminium and silicon is mainly due to catalytic fines discussed earlier. Catalyst is an expensive material for the oil refiner and stringent methods are taken for its retention but some still find their way in residual fuel. Excessive catalytic fines can lead to high wear of piston rings and liners, fuel pump barrels and plungers, and fuel injector nozzle needle and guide. The level of catalytic fines in delivered fuels can be significantly reduced by efficient centrifugal purification prior to combustion in the engine.

• Carbon Residue: The carbon residue of a fuel is the tendency to form carbon deposits under high temperature conditions in an inert atmosphere, and may be expressed as either Ramsbottom carbon residue, Conradson carbon residue (CCR) or micro carbon residue (MCR). This parameter is considered by some to give an approximate indication of the combustibility/deposit forming tendency of the fuel.

• Sulphur: Sulphur is naturally occurring element in crude oil which is concentrated in the residual component. The amount of sulphur in fuel oil depends mainly on the source of crude oil and to a lesser extent on the refining process. Sulphur content is typically 1. 5 -4% wt in residual fuel world wide. In the combustion process in a diesel engine the presence of sulphur in the fuel can give rise to corrosive wear. This can be minimised by suitable operating conditions, and suitable lubrication of the cylinder liner with alkaline lubricant. MARPOL Annex VI limits the sulphur content of marine oil to reduce atmospheric pollution, in the form of sulphur dioxide, from international shipping.

Amendment to Marpol Annex VI • Regulation 14 of MARPOL Annex VI has been significantly revised. For the Global Cap, the sulphur content limits are as follows: • 4. 5% prior to 1 January 2012 • 3. 50% on and after 1 January 2012 • 0. 50% on and after 1 January 2020

Special Emission Control Areas • For the Special Emission Control Areas, the sulphur content will be as follows: • 1. 50% prior to 1 March 2010 • 1. 00% on and after 1 March 2010 • 0. 10% on and after 1 January 2015 • The existing Emission Control Areas (ECAs) are the North Sea and the Baltic Sea.

Review provision • The amended Regulation 14 has a “review provision” which requires the IMO to complete by 2018 a review of the availability of the 0. 50% sulphur content fuel. Based on the results of such a review, the Parties to MARPOL Annex VI will decide whether the global cap of 0. 50% can be enforced from 1 January 2020. If not, the 0. 50% sulphur global cap will be enforced on 1 January 2025 without any additional review.
• Ignition Quality : Cetane number - The cetane number for any fuel is a measure of the oil’s readiness to ignite, under conditions prevailing in the diesel engine. This number is determined by comparing the oil with a mixture of cetane and heptamethylnanone. Cetane, which has a very high spontaneous combustion ability is rated at 100 and the corresponding cetane number for heptamethylnonane is 15. The oil for which cetane number is to be determined is used as fuel in a so-called CFR (Co-operation Fuel Research) engine, which is a single cylinder diesel engine with a variable and controllable compression ratio.
• Fuel injection and combustion timing are controlled by electronic equipment. When these have been determined then engine is run with different mixtures of of cetane and heptamethylnanone until a mixture gives same results. Cetane number = a -0. 15 x b, where a is the volume % of cetane and b the volume % of heptamethylnanone. For high speed diesel engines, a cetane number of over 50 is desirable. Medium speed diesel engines require a fuel with a cetane number of around 40 -50. Large, slow speed diesel engines operate satisfactorily with fuels having a cetane number of approx 30. However slow speed engines are said to be not so sensitive to with regard to the cetane number and it is not normally specified for these engine types. • (b) Calculated Ignition Index (CII) and Calculated Carbon Aromatic Index(CCAI) : These are calculated by empirical equations , where use is made of the density and viscosity of the residual fuel.

Standards of Fuels - Need for quality control in bunker fuel • The cost of bunker fuel is one of the most significant components of a ships operating cost. Ship owners in their effort to limit this cost have preferentially turned to the use of heavier and thus less expensive bunker fuels. Technology developments in petroleum refining, such as in vacuum distillation, catalytic cracking etc, often result in a deterioration of the characteristics of heavy fuels as lesser volumes of residues are left after petroleum refining.

• These residuals may contain elevated levels of undesirable constituents such as Aluminium and Silicon, compounds that could result to significant engine wear and damage. In addition to the above the supply of marine bunker fuels is nowadays often the result of a complex sequence of buying, selling and mixing of fuels of different origins. The use of poor quality fuel is known to result to the serious damage of boilers, fuel pumps springs, pistons and cylinders.
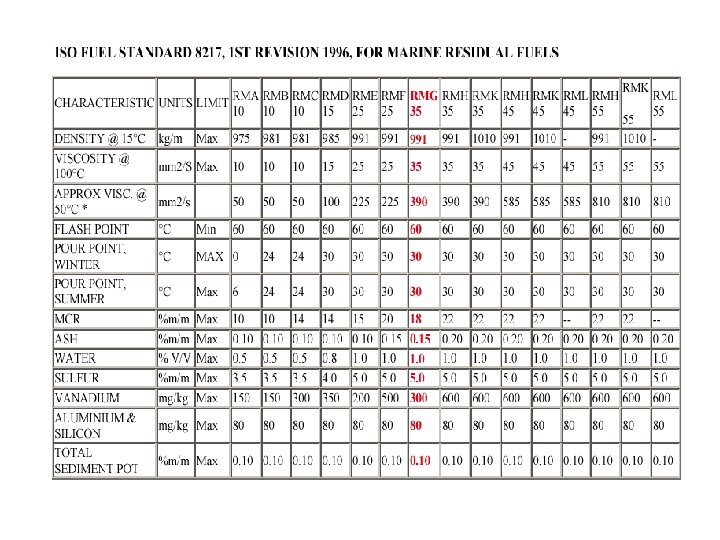
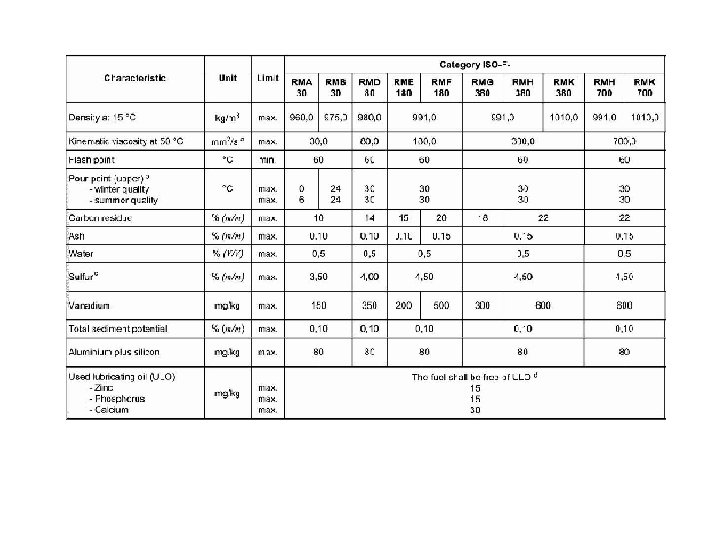
ISO 8217 • During 2005 and 2006 the set of regulations included in MARPOL Annex VI that relate to the use of the marine bunker fuels came into effect. The sampling of the bunkered fuels became mandatory, following a detailed list of requirements listed in the above document and in the MEPC. 96(47) IMO document. • To obviate dispute between ship owners and bunker suppliers and also to meet MARPOL Annex VI requirements, International Organisation for Standards published the first edition of International fuel specification ISO 8217 known as “Petroleum products Fuels (class F) - Specifications of marine fuels” in 1987. It was revised in 1996 and again in 2005. ISO 8217 -2005 defines four distillate grades (DMX, DMA, DMB, DMC) and ten residual grades.
• Distillate grades remain same and the main changes are in marine residual fuels: • Reduction of residual fuel grades from 15 to 10 - With the viscosity classification of residual fuel grades being measured at 50 °C (instead of 100°C as under ISO 8217 -1996), the names of the 10 residual fuel grades have been changed as follows – RMA 30, RMB 30, RMD 80, RME 180, RMF 180, RMG 380 RMH 380, RMK 380, RMH 700 AND RMK 700. • Maximum sulphur limit reduced to 4. 5% - for all the residual fuel grades with viscosity higher than that of RMD 80. For RMA, RMB and RMD grades the previous lower sulphur limits have been retained. • Fuel to be free of ULO (used lubricating oil) • Reduced water content - from 1. 0%v to 0. 5%v. • Reduced ash content
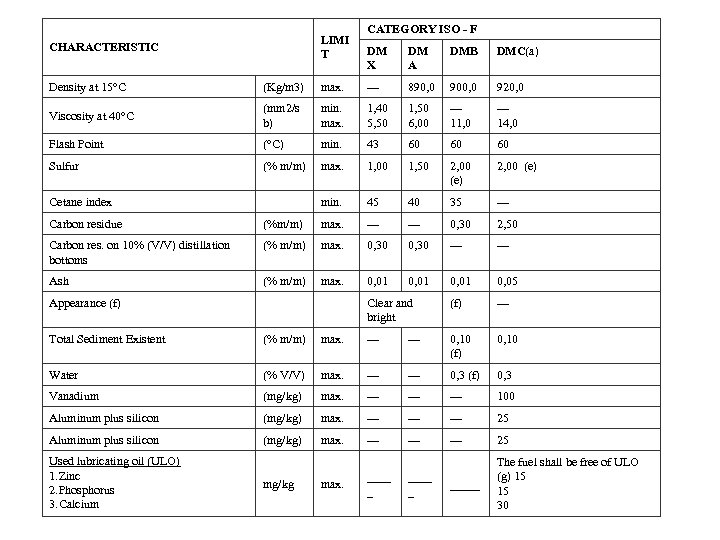
LIMI T CHARACTERISTIC CATEGORY ISO - F DM X DM A DMB DMC(a) Density at 15°C (Kg/m 3) max. --- 890, 0 900, 0 920, 0 Viscosity at 40°C (mm 2/s b) min. max. 1, 40 5, 50 1, 50 6, 00 --- 11, 0 --- 14, 0 Flash Point (°C) min. 43 60 60 60 Sulfur (% m/m) max. 1, 00 1, 50 2, 00 (e) Cetane index min. 45 40 35 --- Carbon residue (%m/m) max. --- 0, 30 2, 50 Carbon res. on 10% (V/V) distillation bottoms (% m/m) max. 0, 30 --- Ash (% m/m) max. 0, 01 0, 05 Appearance (f) Clear and bright (f) --- Total Sediment Existent (% m/m) max. --- 0, 10 (f) 0, 10 Water (% V/V) max. --- 0, 3 (f) 0, 3 Vanadium (mg/kg) max. --- --- 100 Aluminum plus silicon (mg/kg) max. --- --- --- 25 _____ The fuel shall be free of ULO (g) 15 15 30 Used lubricating oil (ULO) 1. Zinc 2. Phosphorus 3. Calcium mg/kg max. ____ _
• Fuel Testing: Analysis of particular characteristics of the fuel delivered may be carried out by some independent shore based laboratory or by tests carried out on board. Testing of fuel on board may range from one or two tests to fully automated online monitors where direct read out of viscosity, density and elemental analysis (e. g. sulphur, silicon, vanadium) as well as derived parameters such as ‘ignition index’ expressed as CII or CCAI are available.

• Storage and Transfer : The pump for fuel transfer is of the positive displacement type and are usually of screw or gear design. The temperature of fuel in the storage should be maintained 5 C above its pour point otherwise there is a possibility of wax formation and in case of high wax content, if left to cool, it may be difficult to reheat the fuel to a temperature above the pour point. Also the temperature has to be raised for higher viscosity fuel to 45 C to bring it below 500 c. St for pumping it. Fuel oil is heated in storage tanks by low pressure steam, but in some ships thermal fluid heating is used.
STORAGE • Bunkering: Marine heavy fuel oils are blends of viscous residues from various refinery operations, cut back with distillate cutter stock. The growing trend is towards cracked residues of a highly aromatic/asphaltic nature to be cut back to an acceptable viscosity with cracked aromatic distillates. Both components have a high carbon/hydrogen ratio, cracked distillates having good solvency properties for large-asphaltene hydrocarbons. In a stable fuel the asphaltenes are carried in a colloidal dispersion in the lighter phase. If the equilibrium between the two phases is disturbed the asphaltene will agglomerate to a size which can no longer be maintained in suspension, and they will tend to separate out as ‘sludge’. If sludge deposition does occur this is made worse, not better, by the addition of more distillate,

• This is particularly true if a high-quality straight-run paraffinic distillate is added to a cracked, high asphaltenic content, residual fuel. It is possible that two residual fuels, each stable by themselves, when mixed together can prove to be incompatible and throw down objectionable sludge or sediment. If compatibility tests have not been carried out beforehand, when bunkering, every effort should be made to segregate bunkers from different source in different tanks to avoid potential problems of incompatibility. In such a case an unstable blend may occur in the ship’s tanks, which could result in precipitation of asphaltenic deposits as sludge in the tanks, pipes, filters and centrifuges.

TREATMENT OF FUEL OIL • Before the fuel is burnt in diesel engine or a boiler, a shipboard treatment takes place. Distillate fuels are generally filtered through a coalescer type filter to remove water and solid impurities. For boilers burning residual fuels, in addition to settling tanks, cold and hot filters are installed in the system prior to boiler. In case of diesel engines burning residual fuel, in addition to settling tanks and filters, centrifuges are installed to clean the fuel to take account of the fine clearances which exist in fuel system of diesel engine.

• Treatment of High Density Fuel: As the density of fuel oil increases and exceeds 991 kg/m 3, the density difference between the fuel oil and fresh water is so small that any change in oil temperature, viscosity or flow rate will cause the oil/water interface to fluctuate leading to a potential failure of water seal. For residual fuel having density above 991 kg/m 3, alternative arrangements to traditional purifier are used. One such arrangement called ALCAP system is used, where fuels of density upto 1010 kg/m 3 can be treated. The centrifuge operates as a clarifier and clean oil is continuously discharged from clean oil outlet, and any free water and separated sludge accumulate at the periphery of the bowl.

• When the sludge space is filled up, the separated water approaches the disc and traces water start to escape with clean oil. Increased water content in clean oil is sensed by the water transducer in the clean oil outlet side. The electrical signal from water transducer are continuously transmitted to and interpreted by the control unit. When the water content in clean oil reaches a specific ‘trigger’ point, the control unit determines, based on the time elapsed since the last sludge sequence, which of the two methods it will use to empty the bowl. This can either be through a water drain valve or with the sludge through the sludge ports at the periphery of the bowl.

• Fuel Heating : Residual fuels have to be heated to reduce the viscosity to that required for atomisation. In case of boilers this is in the range of 15 -65 c. St, whilst for diesel engines the injection viscosity is usually 12. 5 -18 c. St. Fuel heaters may be operated by low pressure saturated steam, a thermal fluid or electrical elements. It is important to maintain correct viscosity range under all conditions. Local overheating may cause cracking of fuel, which may lay down deposits on the heating surface, impairing efficient operation of the heater.

• Viscosity Controller : A viscosity controller is often installed downstream of a fuel oil heater so that a constant injection viscosity can be maintained. There are various types of these. One of these measures the differential pressure resulting from laminar flow through a capillary tube and compares this value to a set point, generating a signal to control the temperature of fuel oil heater.

• Additives : There are two types of additives: (a) Which reduce problem in pre-combustion phase • (b)Which react during post combustion phase • (a)With normal fuel handling procedures, with respect to correct heating, and avoidance of mixing of fuels from different bunkering, no problem should occur. In the event of problems, an effective additive should contribute as follows : • (1) Dispersion of possible sludge in fuel oil tanks. • (2)Promotion of separation of any dispersed water. • (3) Prevention of sludge formation. • (b)An additive which has the effect of an ash modifier (ability to raise the melting temperature of ash) may be beneficial. Slagging and high temperature corrosion occurs when molten ash adheres to the metal surface. By increasing the melting temperature the ash is not in molten form and less likely to stick to metal surfaces.
Combustion In Diesel Engine • For combustion of fuel in a diesel engine, the air charge is highly compressed to a temperature well above the spontaneous ignition temperature (SIT) of the fuel. As the piston approaches TDC fuel is injected at high pressure and suitable viscosity. This continues for 14 -28 degrees of crankshaft rotation, depending upon engine speed and design. The fuel passes through the following phases : 1. A delay period between the commencement of injection of the very finely divided fuel droplets and the commencement of ignition. 2. Rapid combustion of the fuel accumulated in the cylinder during the initial delay period, accompanied by a rise in pressure. 3. Steady combustion of the remainder of the fuel charge as it is mixed. 4. An after burning period during which remaining unburnt fuel finds oxygen and combustion is completed.
Factors Influencing ignition 1. Exactly when ignition commences is dependent upon several factors, the most important being: 2. The size of the droplets injected into the cylinder; 3. The pressure of the fuel at the injector tip; 4. The velocity of the droplets entering the dense air mass; 5. The air pressure and temperature in the cylinder; 6. The air turbulence in the cylinder; 7. The ignition delay properties of the fuel; 8. The surface tension of the fuel; 9. The chemical composition of the fuel; 10. The engine design.
Droplet formation and size • The size of the droplets in the injected fuel spray is controlled primarily by the size, shape and number of holes in the injector tip, their position and the fuel injection pressure and the viscosity of the fuel leaving the injector. The higher the viscosity, the larger will be the droplet size. As the fuel leaves the small injector orifices at pressures in modern pressure-charged engines of upto 1500 bar the pressure falls sharply as it enters the cylinder, in which the charge-air pressure is much lower. The pressure energy is converted into kinetic energy, so that there is a sharp rise in velocity. Both the fall in pressure and the shearing action as the fuel passes through the dense air charge at high velocity break up the liquid stream, while its viscosity and surface tension form the mechanically disrupted liquid into small droplets.
• The droplets sprayed into the cylinder are of varying sizes; the higher the injection pressure, the higher the percentage of small droplets. With current trend towards much higher injection pressures fuel droplet sizes will be reduced correspondingly. The droplet size decreases as the compression pressure increases. The increased density of the air charge helps to break up the spray into smaller droplets. This is beneficial, as the smaller the droplets, the quicker they will vaporize as there is a greater overall area of the oil charge exposed to the hot compressed air. This reduces the ignition delay period, measured either in milliseconds or degrees of crank angle.

Importance of high fuel pressure • If the droplets leaving the injector have a diameter of about 20 -40 m, there is minimum delay in combustion. Conversely, if the droplet diameter exceeds some 100120 m, the combustion period is so long that even a slow-speed, two-stroke engine runs the risk of some particles remaining unburnt when the exhaust ports or valves open. Below 20 m droplet size there is insufficient kinetic energy in the tiny droplet to penetrate the dense air mass in the cylinder, resulting in poor fuel/air admixture. In order to ensure the required fine droplet size, an injection pressure exceeding 1200 bar is being used by some engine manufacturers.
The effect of air temperature • The temperature of the air compressed in the cylinder has a major effect upon ignition delay. The higher the temperature, the shorter the delay period, everything else being equal. Several factors determine the air compression temperature, the main ones being the engine compression pressure, which, in turn, is determined by the charge air pressure, the compression ratio and the volumetric clearance, the temperature of the induction air entering the cylinder, and the temperature of the cylinder head, liner and piston crown. In turn, the combustion chamber and piston temperatures are controlled by the temperature of the cooling water, or oil, and by the design of the combustion chamber and piston components. Compression temperatures in normally aspirated engines are in the order of 500 -600 C, but in modern highly pressurecharged medium and large output engines, they may be as high as 700 C.

Compression Pressure • Increased compression pressure (or densities), which are now as high as 90110 bar in modern crosshead and trunk piston engines, not only promote the formation of more, smaller fuel droplets but, equally important, reduce the spontaneous ignition temperature of the fuel appreciably.
Air Turbulence • Turbulence or swirl, in the compressed air charge promotes efficient distribution of the fuel spray droplets throughout the combustion chamber, ensuring thorough mixing of the fuel and clean air (increasing the rate of heating and vaporization) thus tending to reduce ignition delay and assisting in efficient burning of the fuel charge.

Droplet combustion process • If an individual fuel droplet is considered it will be found to be very small, the size depending upon factors discussed previously but, as compared with the physical size of individual hydrocarbon molecules which form the droplet, they are relatively large. Even the smallest droplets in the fuel spray contain thousands of hydrocarbon molecules having widely different chemical structures. This is particularly true of heavy residual fuels with high carbon-numbers. The molecules vary appreciably in their volatility, ignition temperature, rate of burning, the completeness of burning and their tendency to release carbon and associated organometallic compounds. The heating of the spherical droplet occurs from the outer surface inwards to the centre, so that evaporation and subsequent ignition commences at the surface. The more volatile constituents with the lowest ignition temperatures burn first, leaving the less combustible hydrocarbon constituents to find clean air and burn slowly.
Advanced injection timing • During a long ignition delay, injection of fuel into the cylinder continues, so that the longer the delay, the greater is the amount injected before ignition commences. When ignition finally occurs, the accumulated fuel ignites violently with a very rapid, highpressure rise. The resultant high pressure causes shock loading on the piston and running-gear bearings. With a poor equivalent Cetane Number residual fuel, within fairly narrow limits one way of reducing this harmful effect is to advance the ignition timing. In case of low-speed crosshead engines, upto 2 degrees crank angle may be adequate, with a somewhat greater advance for mediumspeed engines - possibly 3 to 6 degrees, depending upon engine design and, in particular, engine speed. Advancing the injection timing enables ignition to occur at maximum compression pressure and temperature and smooth combustion to be completed earlier in the stroke. The manufacturer's maximum firing pressure, related to load conditions, should be maintained.
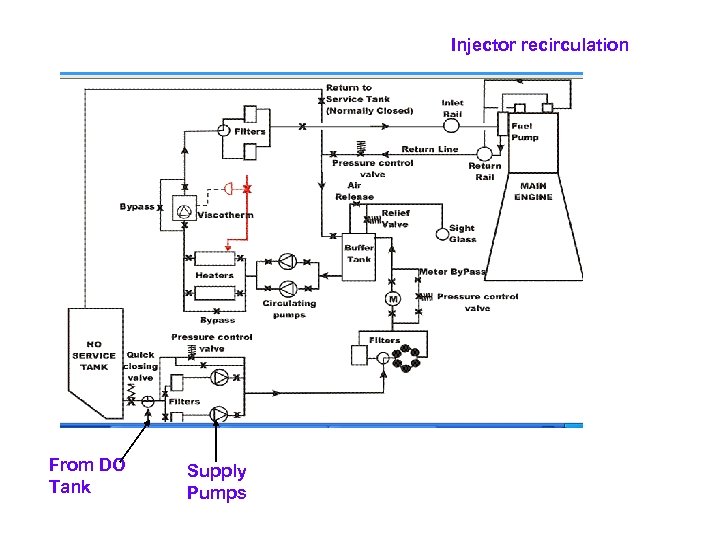
Injector recirculation From DO Tank Supply Pumps
f04bd170b4ada9852341778890fec40a.ppt