486c58f4ff96c045654539ed1073f0e2.ppt
- Количество слайдов: 31
 Thermodynamics: An Engineering Approach Yunus A. Cengel, Michael A. Boles Mc. Graw-Hill© Chapter 7 ENTROPY Dr. Kagan ERYURUK Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.
Thermodynamics: An Engineering Approach Yunus A. Cengel, Michael A. Boles Mc. Graw-Hill© Chapter 7 ENTROPY Dr. Kagan ERYURUK Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.
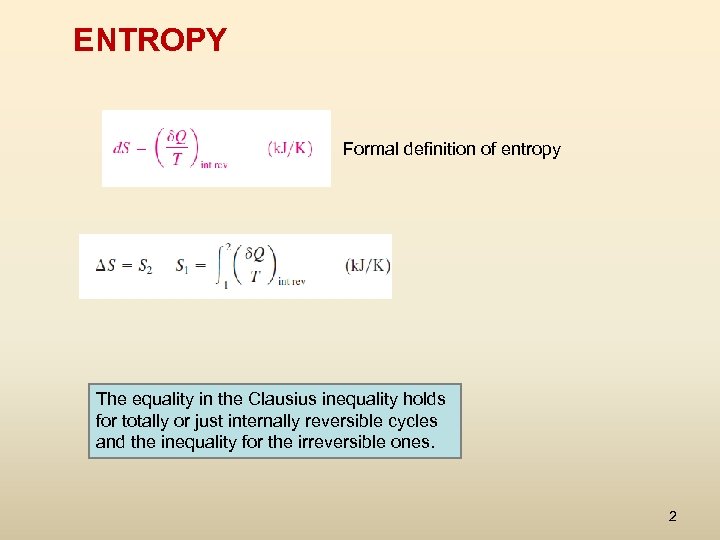 ENTROPY Formal definition of entropy The equality in the Clausius inequality holds for totally or just internally reversible cycles and the inequality for the irreversible ones. 2
ENTROPY Formal definition of entropy The equality in the Clausius inequality holds for totally or just internally reversible cycles and the inequality for the irreversible ones. 2
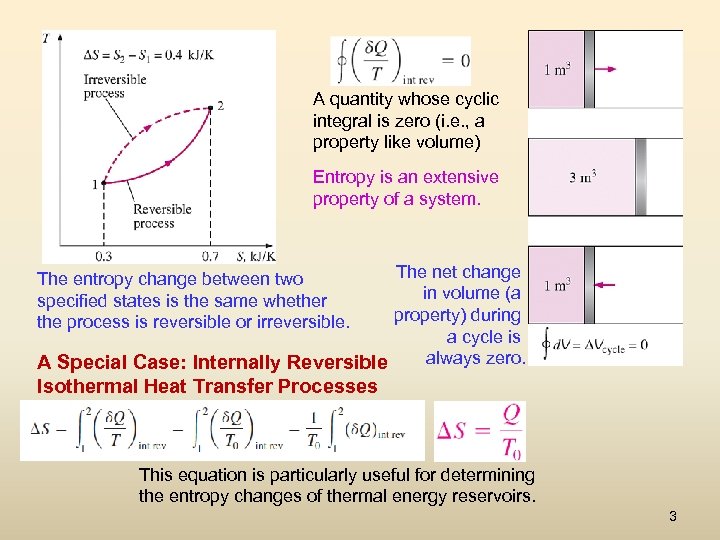 A quantity whose cyclic integral is zero (i. e. , a property like volume) Entropy is an extensive property of a system. The net change in volume (a property) during a cycle is always zero. A Special Case: Internally Reversible The entropy change between two specified states is the same whether the process is reversible or irreversible. Isothermal Heat Transfer Processes This equation is particularly useful for determining the entropy changes of thermal energy reservoirs. 3
A quantity whose cyclic integral is zero (i. e. , a property like volume) Entropy is an extensive property of a system. The net change in volume (a property) during a cycle is always zero. A Special Case: Internally Reversible The entropy change between two specified states is the same whether the process is reversible or irreversible. Isothermal Heat Transfer Processes This equation is particularly useful for determining the entropy changes of thermal energy reservoirs. 3
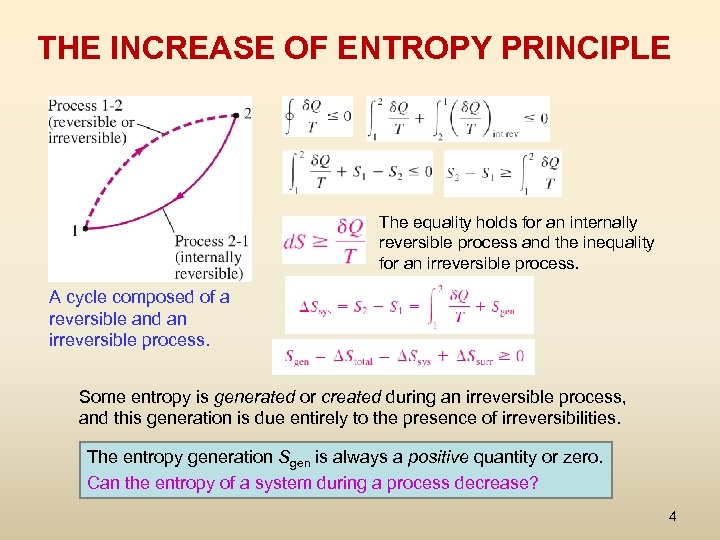 THE INCREASE OF ENTROPY PRINCIPLE The equality holds for an internally reversible process and the inequality for an irreversible process. A cycle composed of a reversible and an irreversible process. Some entropy is generated or created during an irreversible process, and this generation is due entirely to the presence of irreversibilities. The entropy generation Sgen is always a positive quantity or zero. Can the entropy of a system during a process decrease? 4
THE INCREASE OF ENTROPY PRINCIPLE The equality holds for an internally reversible process and the inequality for an irreversible process. A cycle composed of a reversible and an irreversible process. Some entropy is generated or created during an irreversible process, and this generation is due entirely to the presence of irreversibilities. The entropy generation Sgen is always a positive quantity or zero. Can the entropy of a system during a process decrease? 4
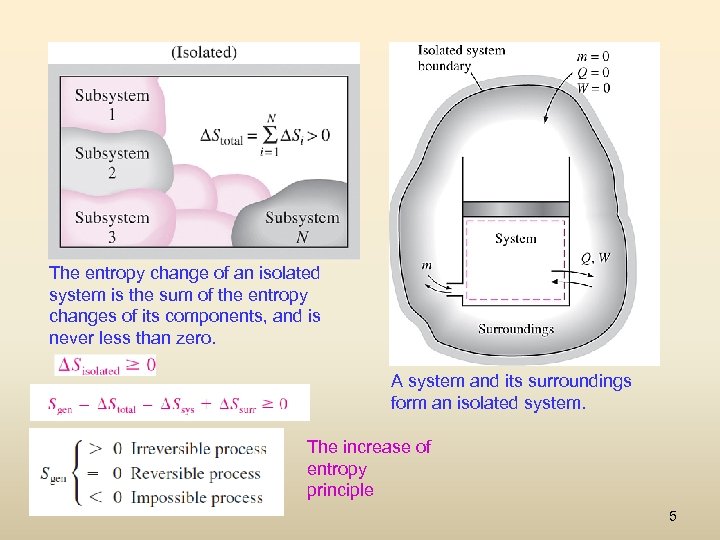 The entropy change of an isolated system is the sum of the entropy changes of its components, and is never less than zero. A system and its surroundings form an isolated system. The increase of entropy principle 5
The entropy change of an isolated system is the sum of the entropy changes of its components, and is never less than zero. A system and its surroundings form an isolated system. The increase of entropy principle 5
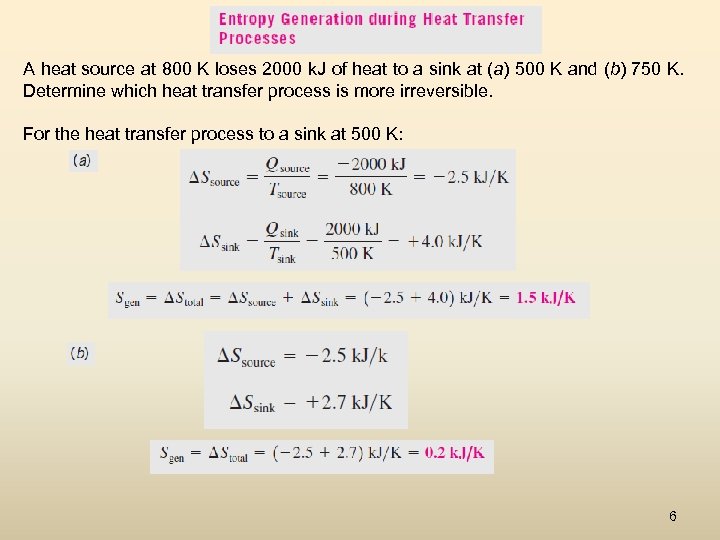 A heat source at 800 K loses 2000 k. J of heat to a sink at (a) 500 K and (b) 750 K. Determine which heat transfer process is more irreversible. For the heat transfer process to a sink at 500 K: 6
A heat source at 800 K loses 2000 k. J of heat to a sink at (a) 500 K and (b) 750 K. Determine which heat transfer process is more irreversible. For the heat transfer process to a sink at 500 K: 6
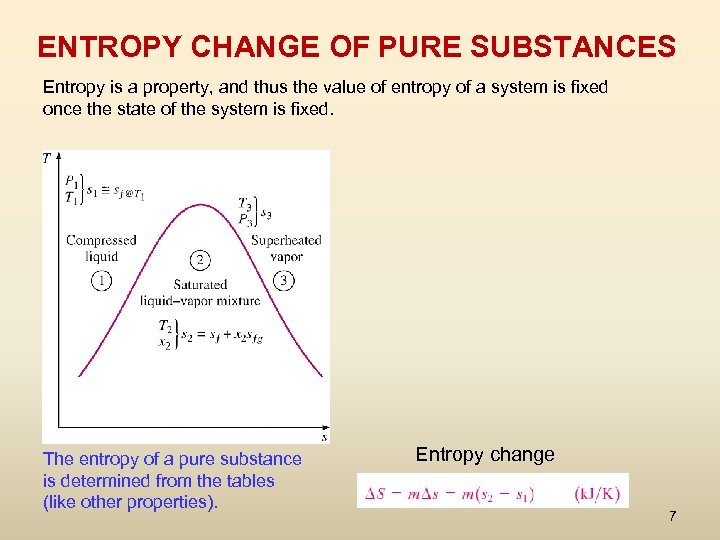 ENTROPY CHANGE OF PURE SUBSTANCES Entropy is a property, and thus the value of entropy of a system is fixed once the state of the system is fixed. The entropy of a pure substance is determined from the tables (like other properties). Entropy change 7
ENTROPY CHANGE OF PURE SUBSTANCES Entropy is a property, and thus the value of entropy of a system is fixed once the state of the system is fixed. The entropy of a pure substance is determined from the tables (like other properties). Entropy change 7
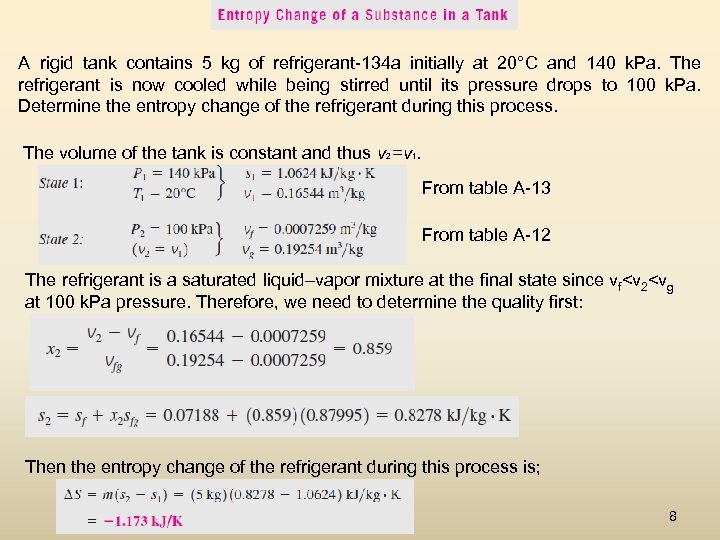 A rigid tank contains 5 kg of refrigerant-134 a initially at 20°C and 140 k. Pa. The refrigerant is now cooled while being stirred until its pressure drops to 100 k. Pa. Determine the entropy change of the refrigerant during this process. The volume of the tank is constant and thus v 2 =v 1. From table A-13 From table A-12 The refrigerant is a saturated liquid–vapor mixture at the final state since vf
A rigid tank contains 5 kg of refrigerant-134 a initially at 20°C and 140 k. Pa. The refrigerant is now cooled while being stirred until its pressure drops to 100 k. Pa. Determine the entropy change of the refrigerant during this process. The volume of the tank is constant and thus v 2 =v 1. From table A-13 From table A-12 The refrigerant is a saturated liquid–vapor mixture at the final state since vf
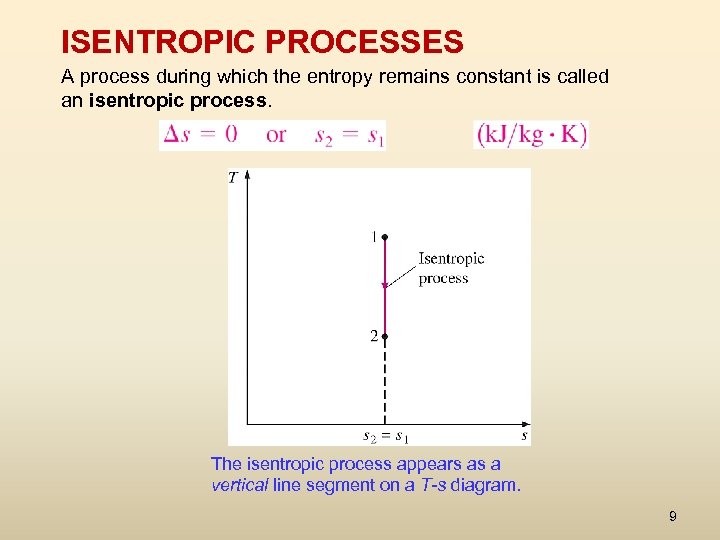 ISENTROPIC PROCESSES A process during which the entropy remains constant is called an isentropic process. The isentropic process appears as a vertical line segment on a T-s diagram. 9
ISENTROPIC PROCESSES A process during which the entropy remains constant is called an isentropic process. The isentropic process appears as a vertical line segment on a T-s diagram. 9
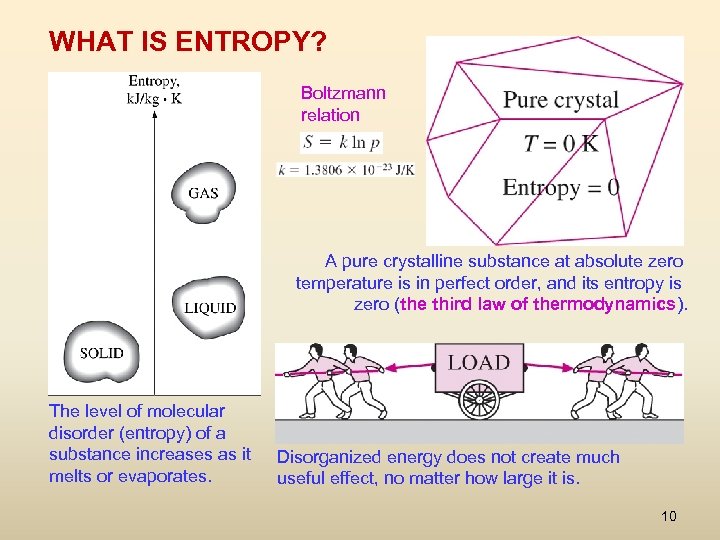 WHAT IS ENTROPY? Boltzmann relation A pure crystalline substance at absolute zero temperature is in perfect order, and its entropy is zero (the third law of thermodynamics). The level of molecular disorder (entropy) of a substance increases as it melts or evaporates. Disorganized energy does not create much useful effect, no matter how large it is. 10
WHAT IS ENTROPY? Boltzmann relation A pure crystalline substance at absolute zero temperature is in perfect order, and its entropy is zero (the third law of thermodynamics). The level of molecular disorder (entropy) of a substance increases as it melts or evaporates. Disorganized energy does not create much useful effect, no matter how large it is. 10
 During a heat transfer process, the net entropy increases. (The increase in the entropy of the cold body more than offsets the decrease in the entropy of the hot body. ) 11
During a heat transfer process, the net entropy increases. (The increase in the entropy of the cold body more than offsets the decrease in the entropy of the hot body. ) 11
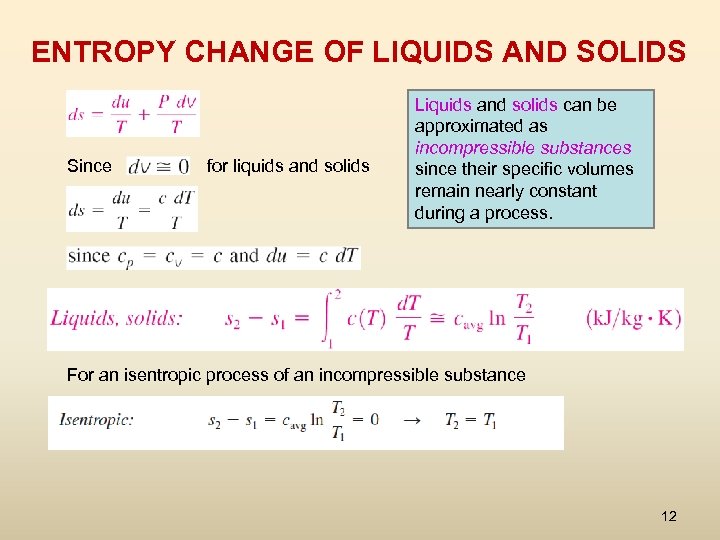 ENTROPY CHANGE OF LIQUIDS AND SOLIDS Since for liquids and solids Liquids and solids can be approximated as incompressible substances since their specific volumes remain nearly constant during a process. For an isentropic process of an incompressible substance 12
ENTROPY CHANGE OF LIQUIDS AND SOLIDS Since for liquids and solids Liquids and solids can be approximated as incompressible substances since their specific volumes remain nearly constant during a process. For an isentropic process of an incompressible substance 12
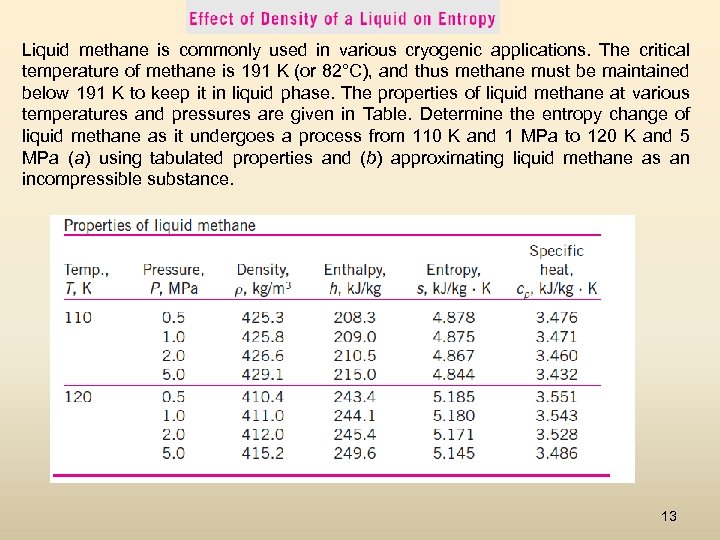 Liquid methane is commonly used in various cryogenic applications. The critical temperature of methane is 191 K (or 82°C), and thus methane must be maintained below 191 K to keep it in liquid phase. The properties of liquid methane at various temperatures and pressures are given in Table. Determine the entropy change of liquid methane as it undergoes a process from 110 K and 1 MPa to 120 K and 5 MPa (a) using tabulated properties and (b) approximating liquid methane as an incompressible substance. 13
Liquid methane is commonly used in various cryogenic applications. The critical temperature of methane is 191 K (or 82°C), and thus methane must be maintained below 191 K to keep it in liquid phase. The properties of liquid methane at various temperatures and pressures are given in Table. Determine the entropy change of liquid methane as it undergoes a process from 110 K and 1 MPa to 120 K and 5 MPa (a) using tabulated properties and (b) approximating liquid methane as an incompressible substance. 13
 14
14
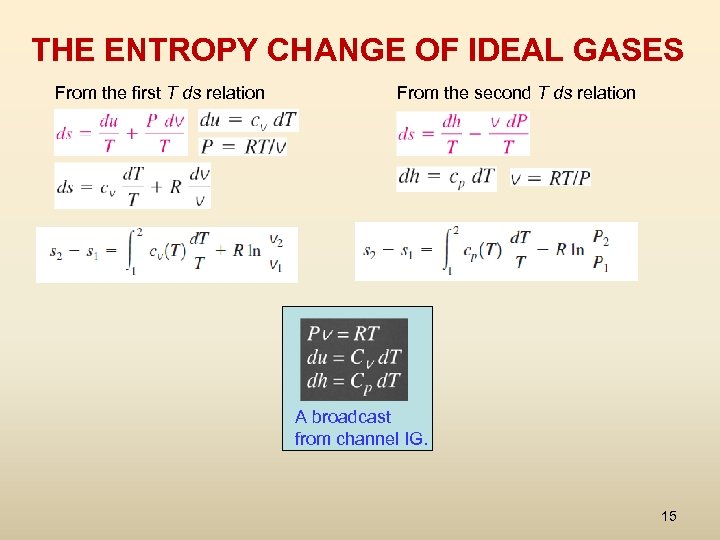 THE ENTROPY CHANGE OF IDEAL GASES From the first T ds relation From the second T ds relation A broadcast from channel IG. 15
THE ENTROPY CHANGE OF IDEAL GASES From the first T ds relation From the second T ds relation A broadcast from channel IG. 15
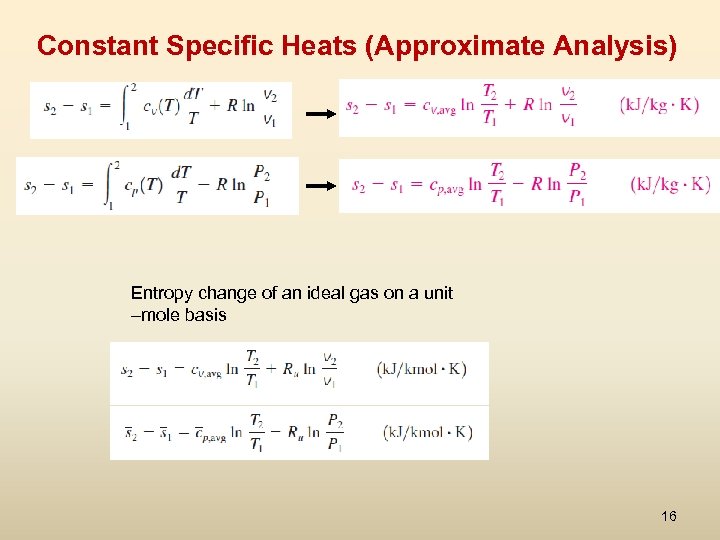 Constant Specific Heats (Approximate Analysis) Entropy change of an ideal gas on a unit –mole basis 16
Constant Specific Heats (Approximate Analysis) Entropy change of an ideal gas on a unit –mole basis 16
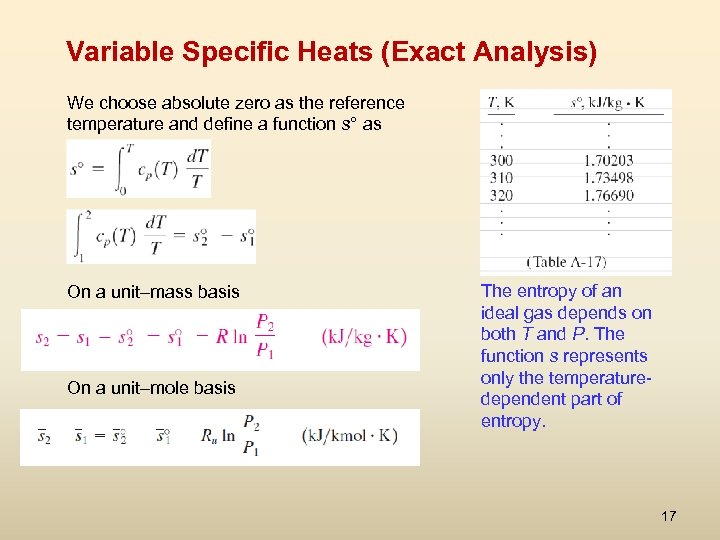 Variable Specific Heats (Exact Analysis) We choose absolute zero as the reference temperature and define a function s° as On a unit–mass basis On a unit–mole basis The entropy of an ideal gas depends on both T and P. The function s represents only the temperaturedependent part of entropy. 17
Variable Specific Heats (Exact Analysis) We choose absolute zero as the reference temperature and define a function s° as On a unit–mass basis On a unit–mole basis The entropy of an ideal gas depends on both T and P. The function s represents only the temperaturedependent part of entropy. 17
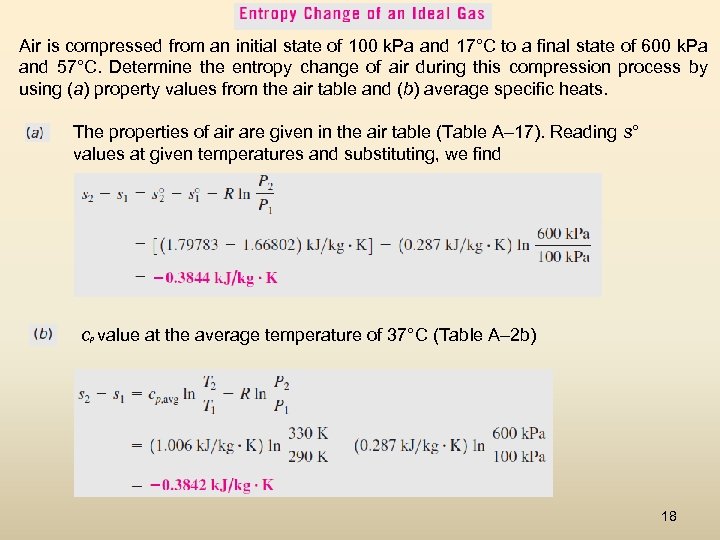 Air is compressed from an initial state of 100 k. Pa and 17°C to a final state of 600 k. Pa and 57°C. Determine the entropy change of air during this compression process by using (a) property values from the air table and (b) average specific heats. The properties of air are given in the air table (Table A– 17). Reading s° values at given temperatures and substituting, we find cp value at the average temperature of 37°C (Table A– 2 b) 18
Air is compressed from an initial state of 100 k. Pa and 17°C to a final state of 600 k. Pa and 57°C. Determine the entropy change of air during this compression process by using (a) property values from the air table and (b) average specific heats. The properties of air are given in the air table (Table A– 17). Reading s° values at given temperatures and substituting, we find cp value at the average temperature of 37°C (Table A– 2 b) 18
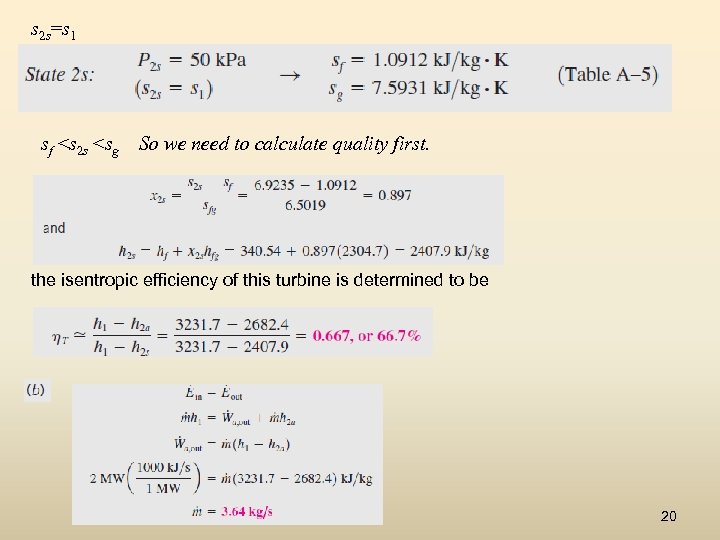
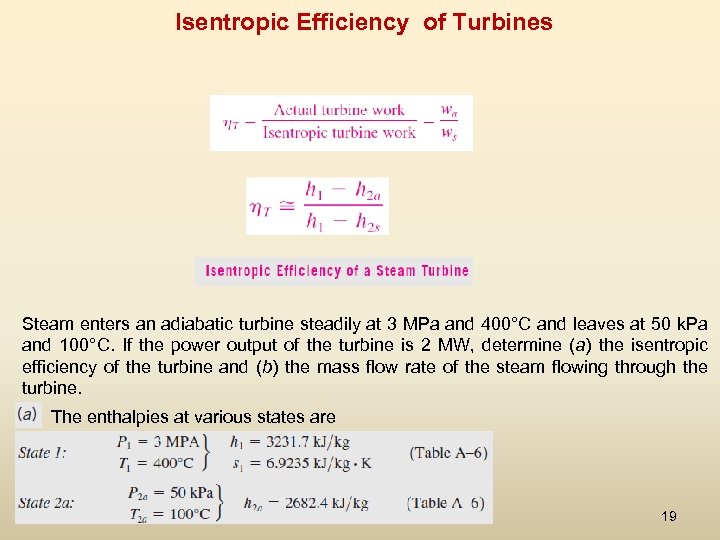 Isentropic Efficiency of Turbines Steam enters an adiabatic turbine steadily at 3 MPa and 400°C and leaves at 50 k. Pa and 100°C. If the power output of the turbine is 2 MW, determine (a) the isentropic efficiency of the turbine and (b) the mass flow rate of the steam flowing through the turbine. The enthalpies at various states are 19
Isentropic Efficiency of Turbines Steam enters an adiabatic turbine steadily at 3 MPa and 400°C and leaves at 50 k. Pa and 100°C. If the power output of the turbine is 2 MW, determine (a) the isentropic efficiency of the turbine and (b) the mass flow rate of the steam flowing through the turbine. The enthalpies at various states are 19
 s 2 s=s 1 sf
s 2 s=s 1 sf
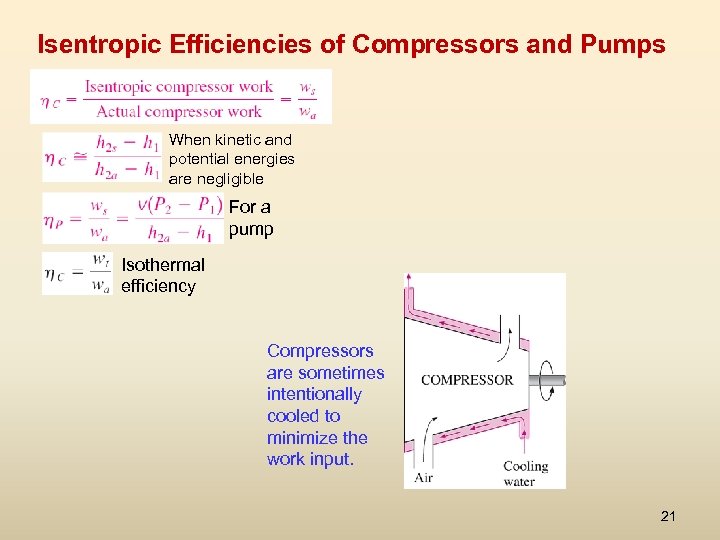 Isentropic Efficiencies of Compressors and Pumps When kinetic and potential energies are negligible For a pump Isothermal efficiency Compressors are sometimes intentionally cooled to minimize the work input. 21
Isentropic Efficiencies of Compressors and Pumps When kinetic and potential energies are negligible For a pump Isothermal efficiency Compressors are sometimes intentionally cooled to minimize the work input. 21
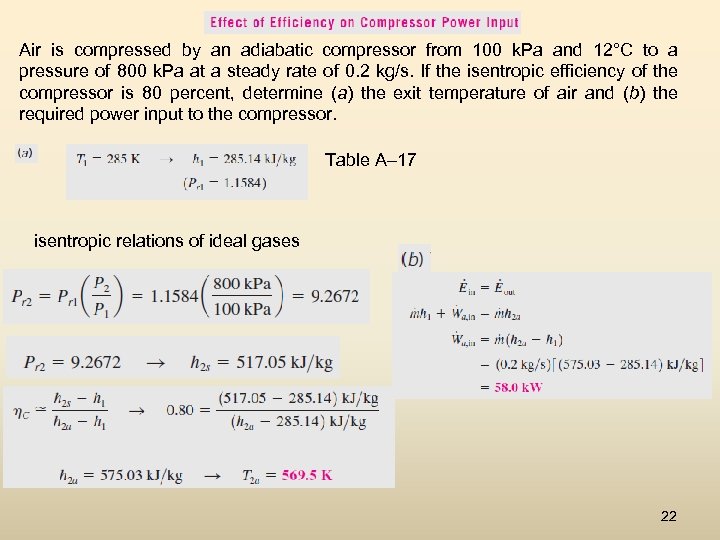 Air is compressed by an adiabatic compressor from 100 k. Pa and 12°C to a pressure of 800 k. Pa at a steady rate of 0. 2 kg/s. If the isentropic efficiency of the compressor is 80 percent, determine (a) the exit temperature of air and (b) the required power input to the compressor. Table A– 17 isentropic relations of ideal gases 22
Air is compressed by an adiabatic compressor from 100 k. Pa and 12°C to a pressure of 800 k. Pa at a steady rate of 0. 2 kg/s. If the isentropic efficiency of the compressor is 80 percent, determine (a) the exit temperature of air and (b) the required power input to the compressor. Table A– 17 isentropic relations of ideal gases 22
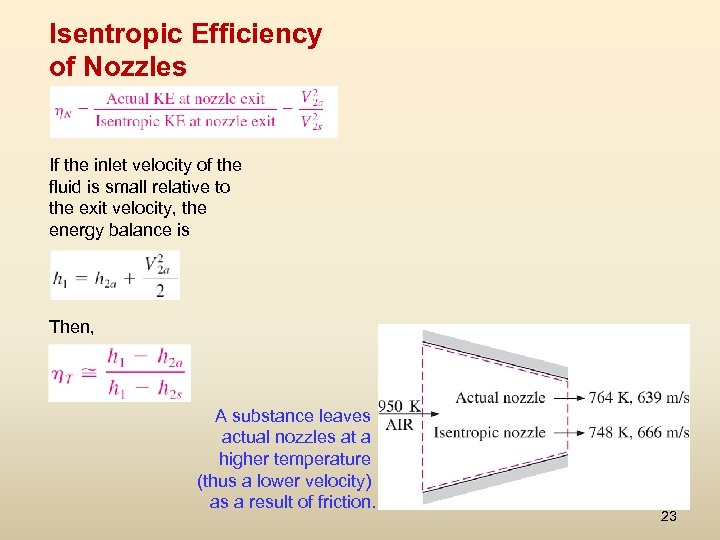 Isentropic Efficiency of Nozzles If the inlet velocity of the fluid is small relative to the exit velocity, the energy balance is Then, A substance leaves actual nozzles at a higher temperature (thus a lower velocity) as a result of friction. 23
Isentropic Efficiency of Nozzles If the inlet velocity of the fluid is small relative to the exit velocity, the energy balance is Then, A substance leaves actual nozzles at a higher temperature (thus a lower velocity) as a result of friction. 23
 ENTROPY BALANCE Entropy Change of a System, ∆Ssystem 24
ENTROPY BALANCE Entropy Change of a System, ∆Ssystem 24
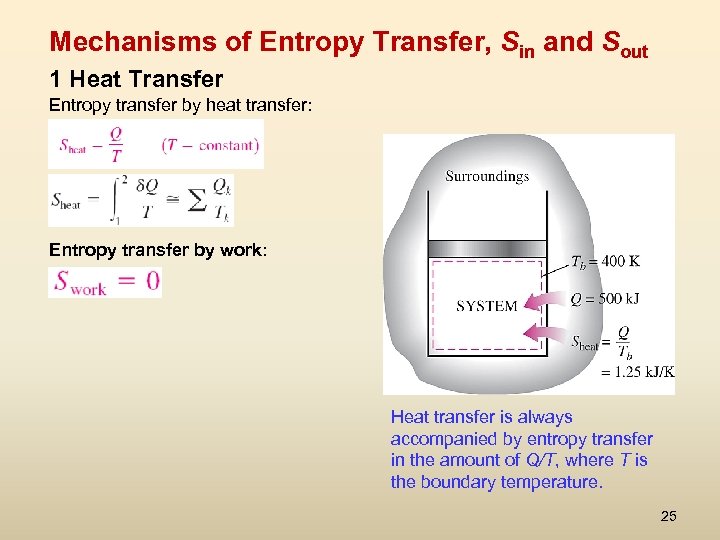 Mechanisms of Entropy Transfer, Sin and Sout 1 Heat Transfer Entropy transfer by heat transfer: Entropy transfer by work: Heat transfer is always accompanied by entropy transfer in the amount of Q/T, where T is the boundary temperature. 25
Mechanisms of Entropy Transfer, Sin and Sout 1 Heat Transfer Entropy transfer by heat transfer: Entropy transfer by work: Heat transfer is always accompanied by entropy transfer in the amount of Q/T, where T is the boundary temperature. 25
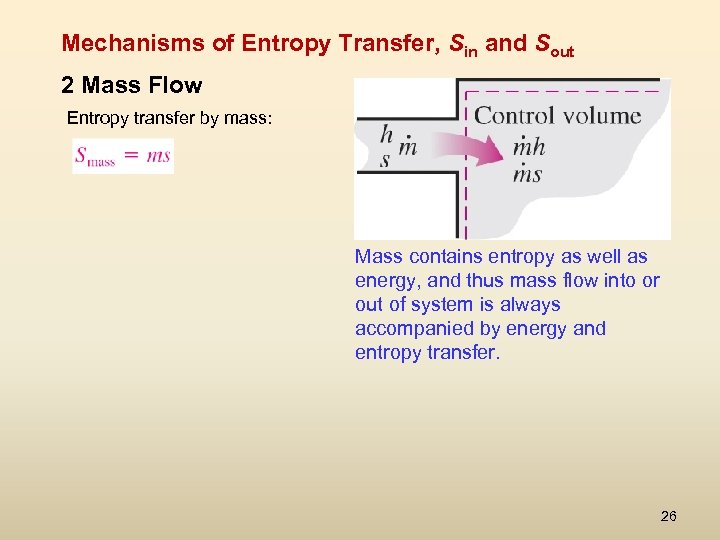 Mechanisms of Entropy Transfer, Sin and Sout 2 Mass Flow Entropy transfer by mass: Mass contains entropy as well as energy, and thus mass flow into or out of system is always accompanied by energy and entropy transfer. 26
Mechanisms of Entropy Transfer, Sin and Sout 2 Mass Flow Entropy transfer by mass: Mass contains entropy as well as energy, and thus mass flow into or out of system is always accompanied by energy and entropy transfer. 26
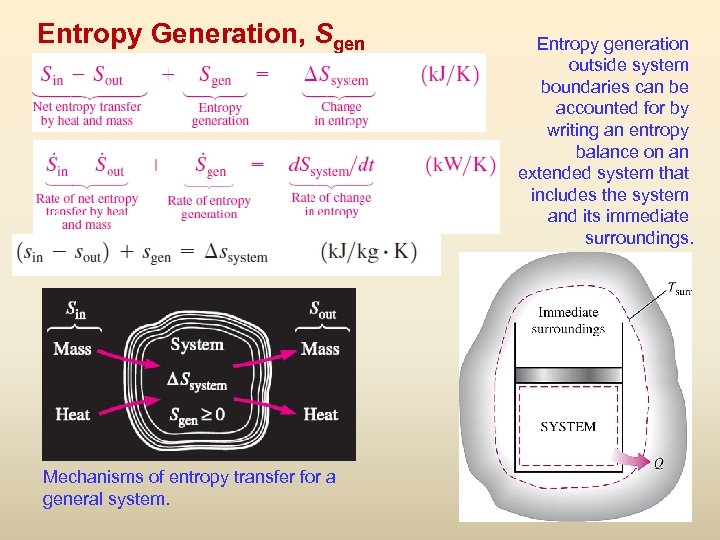 Entropy Generation, Sgen Entropy generation outside system boundaries can be accounted for by writing an entropy balance on an extended system that includes the system and its immediate surroundings. Mechanisms of entropy transfer for a general system. 27
Entropy Generation, Sgen Entropy generation outside system boundaries can be accounted for by writing an entropy balance on an extended system that includes the system and its immediate surroundings. Mechanisms of entropy transfer for a general system. 27
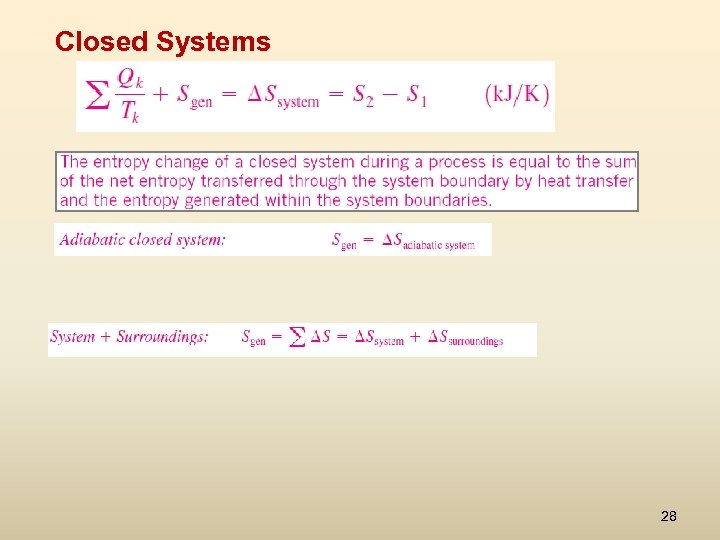 Closed Systems 28
Closed Systems 28
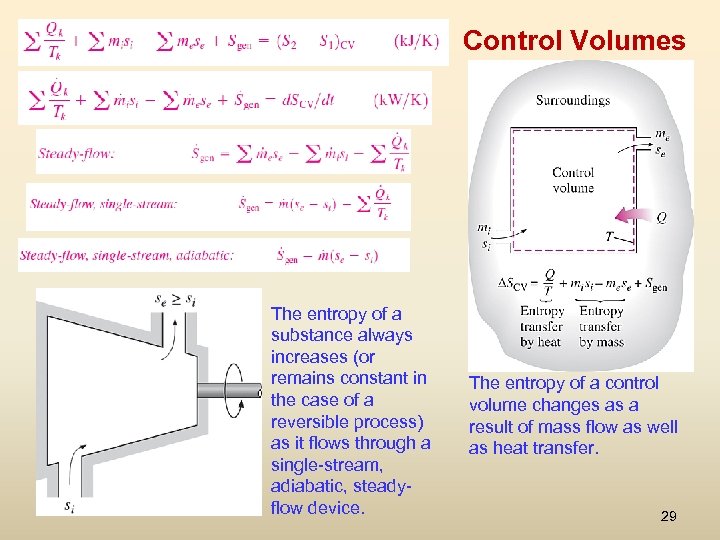 Control Volumes The entropy of a substance always increases (or remains constant in the case of a reversible process) as it flows through a single-stream, adiabatic, steadyflow device. The entropy of a control volume changes as a result of mass flow as well as heat transfer. 29
Control Volumes The entropy of a substance always increases (or remains constant in the case of a reversible process) as it flows through a single-stream, adiabatic, steadyflow device. The entropy of a control volume changes as a result of mass flow as well as heat transfer. 29
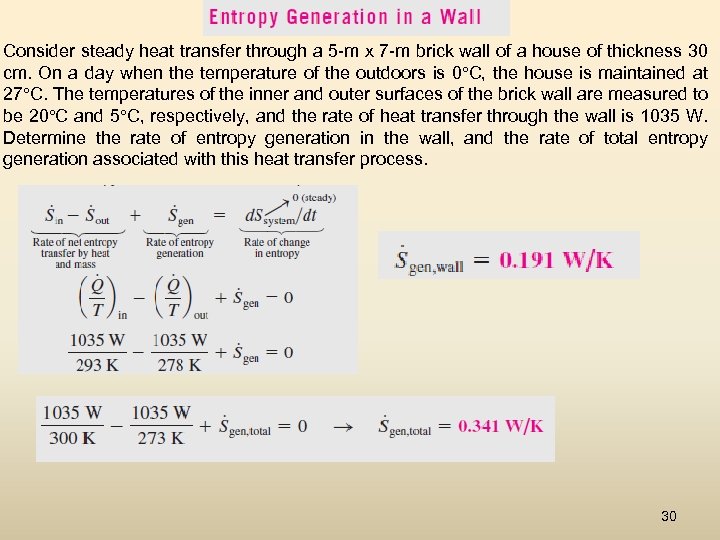 Consider steady heat transfer through a 5 -m x 7 -m brick wall of a house of thickness 30 cm. On a day when the temperature of the outdoors is 0°C, the house is maintained at 27°C. The temperatures of the inner and outer surfaces of the brick wall are measured to be 20°C and 5°C, respectively, and the rate of heat transfer through the wall is 1035 W. Determine the rate of entropy generation in the wall, and the rate of total entropy generation associated with this heat transfer process. 30
Consider steady heat transfer through a 5 -m x 7 -m brick wall of a house of thickness 30 cm. On a day when the temperature of the outdoors is 0°C, the house is maintained at 27°C. The temperatures of the inner and outer surfaces of the brick wall are measured to be 20°C and 5°C, respectively, and the rate of heat transfer through the wall is 1035 W. Determine the rate of entropy generation in the wall, and the rate of total entropy generation associated with this heat transfer process. 30
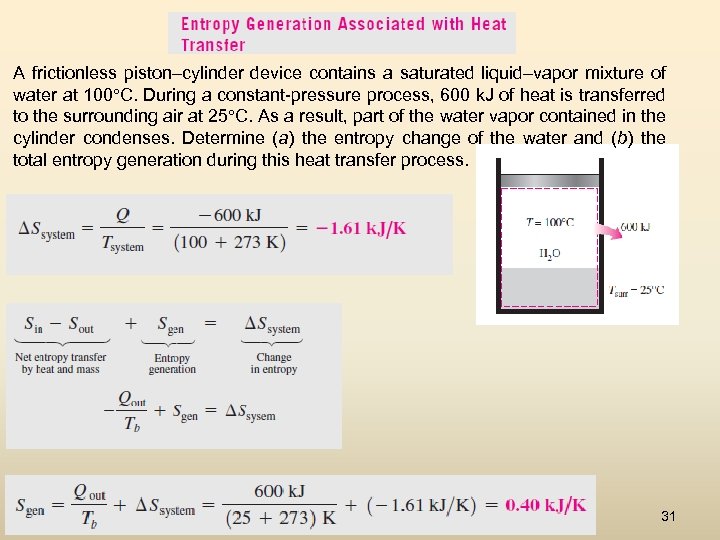 A frictionless piston–cylinder device contains a saturated liquid–vapor mixture of water at 100°C. During a constant-pressure process, 600 k. J of heat is transferred to the surrounding air at 25°C. As a result, part of the water vapor contained in the cylinder condenses. Determine (a) the entropy change of the water and (b) the total entropy generation during this heat transfer process. 31
A frictionless piston–cylinder device contains a saturated liquid–vapor mixture of water at 100°C. During a constant-pressure process, 600 k. J of heat is transferred to the surrounding air at 25°C. As a result, part of the water vapor contained in the cylinder condenses. Determine (a) the entropy change of the water and (b) the total entropy generation during this heat transfer process. 31


