ae7eaa361686e7201e27a83adf93183b.ppt
- Количество слайдов: 50
 Therapies targeting the immune system: v Stimulation v Modulation v Suppression
Therapies targeting the immune system: v Stimulation v Modulation v Suppression
 IMMUNOMODULATORS modify the immune system either on a positive or at a negative way 1) Bacterial immunomodulators: Freund adjuvants (CFA)-mycobacterium tuberculosis human: BCG (mycobacterium) – melanoma, carcinoma Stimulation of Mf, T, B, Nk cells, IL-1 production muramyl dipeptide and derivatives, less toxic Staphylococcus aureus – superantigen – polyclonal stimuli Escherichia coli heatlabile enterotoxin (LT), CT - adjuvant effect 2) Cytokines: rekombinant proteins (IL-1. IL-2, Epo) cytokine antagonists: inhibitors of signaling soluble receptors: TNF, IL-1, IL-4 3) Antibodies: antibodies specific for cytokines, or cytokine receptors, or recognizing molecules on cell surface, receptors, co-stimulators etc.
IMMUNOMODULATORS modify the immune system either on a positive or at a negative way 1) Bacterial immunomodulators: Freund adjuvants (CFA)-mycobacterium tuberculosis human: BCG (mycobacterium) – melanoma, carcinoma Stimulation of Mf, T, B, Nk cells, IL-1 production muramyl dipeptide and derivatives, less toxic Staphylococcus aureus – superantigen – polyclonal stimuli Escherichia coli heatlabile enterotoxin (LT), CT - adjuvant effect 2) Cytokines: rekombinant proteins (IL-1. IL-2, Epo) cytokine antagonists: inhibitors of signaling soluble receptors: TNF, IL-1, IL-4 3) Antibodies: antibodies specific for cytokines, or cytokine receptors, or recognizing molecules on cell surface, receptors, co-stimulators etc.
 Applications: Autoimmune and allergic diseases Causal treatment: v Peptides – e. g. : DNA-mimotope peptides, epitópe peptides corresponding to the autoantigens in SLE v. Tolerance induction v. Inhibition of pathogenic antibody production (autoreactive, or Ig. E) v. Regulation of cytokine network v. Regulation od signaling v. Therapies influencing apoptosis
Applications: Autoimmune and allergic diseases Causal treatment: v Peptides – e. g. : DNA-mimotope peptides, epitópe peptides corresponding to the autoantigens in SLE v. Tolerance induction v. Inhibition of pathogenic antibody production (autoreactive, or Ig. E) v. Regulation of cytokine network v. Regulation od signaling v. Therapies influencing apoptosis
 1. Bacterial immunomodulators
1. Bacterial immunomodulators
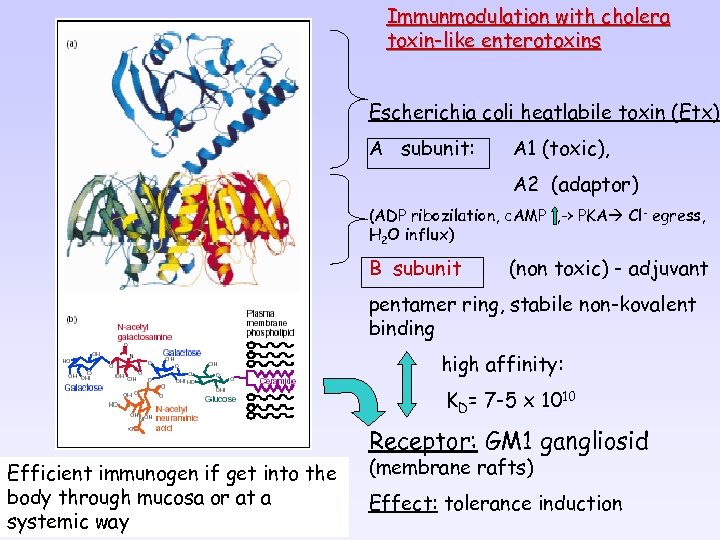 Immunmodulation with cholera toxin-like enterotoxins Escherichia coli heatlabile toxin (Etx) A subunit: A 1 (toxic), A 2 (adaptor) (ADP ribozilation, c. AMP , -> PKA Cl- egress, H 2 O influx) B subunit (non toxic) - adjuvant pentamer ring, stabile non-kovalent binding high affinity: KD= 7 -5 x 1010 Receptor: GM 1 gangliosid Efficient immunogen if get into the body through mucosa or at a systemic way (membrane rafts) Effect: tolerance induction
Immunmodulation with cholera toxin-like enterotoxins Escherichia coli heatlabile toxin (Etx) A subunit: A 1 (toxic), A 2 (adaptor) (ADP ribozilation, c. AMP , -> PKA Cl- egress, H 2 O influx) B subunit (non toxic) - adjuvant pentamer ring, stabile non-kovalent binding high affinity: KD= 7 -5 x 1010 Receptor: GM 1 gangliosid Efficient immunogen if get into the body through mucosa or at a systemic way (membrane rafts) Effect: tolerance induction
 When and how cholera-like enterotoxin is used for tolerance induction? Disease antigen prep immunization SRBC-Ctx. B p. o. BCG-Ctx. B p. o. EAE (rat) MBP-Ctx. B p. o. Diabetes insulin ins-XCtx. B p. o. Arthritis none ETXB s. c. Diabetes none CTx. B i. v. , i. p (Experimental Autoimmune Encephalomyelitis - mice model of multiple sclerosis) (NOD mice) (mucosal vaccination) Human: CTB – non-toxic, good adjuvant, vaccination against cholera oral vaccine: inactivated vibrio cholera +CTB Ig. A, memory Inducing tolerance in HSP uveitis: mucosal immunization tolerance (CTB-HSP peptide conjugates) small phase I/II trial in patients with Behcet’s disease (BD) was undertaken with very encouraging results
When and how cholera-like enterotoxin is used for tolerance induction? Disease antigen prep immunization SRBC-Ctx. B p. o. BCG-Ctx. B p. o. EAE (rat) MBP-Ctx. B p. o. Diabetes insulin ins-XCtx. B p. o. Arthritis none ETXB s. c. Diabetes none CTx. B i. v. , i. p (Experimental Autoimmune Encephalomyelitis - mice model of multiple sclerosis) (NOD mice) (mucosal vaccination) Human: CTB – non-toxic, good adjuvant, vaccination against cholera oral vaccine: inactivated vibrio cholera +CTB Ig. A, memory Inducing tolerance in HSP uveitis: mucosal immunization tolerance (CTB-HSP peptide conjugates) small phase I/II trial in patients with Behcet’s disease (BD) was undertaken with very encouraging results
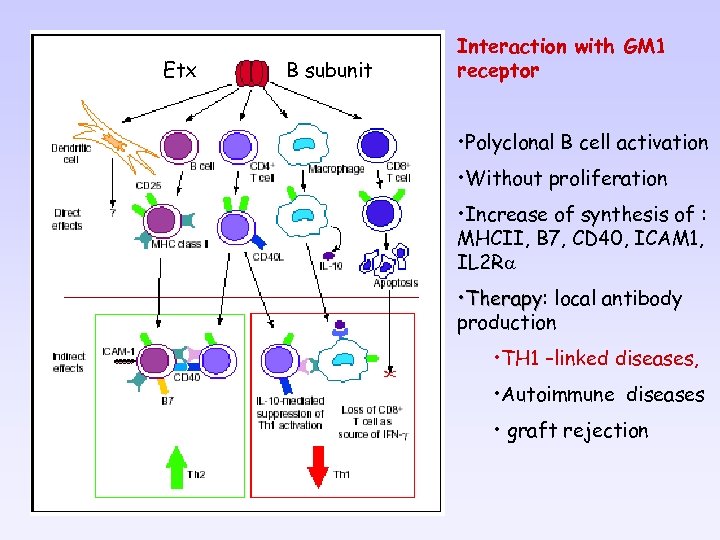 Etx B subunit Interaction with GM 1 receptor • Polyclonal B cell activation • Without proliferation • Increase of synthesis of : MHCII, B 7, CD 40, ICAM 1, IL 2 Ra • Therapy: local antibody Therapy production • TH 1 –linked diseases, • Autoimmune diseases • graft rejection
Etx B subunit Interaction with GM 1 receptor • Polyclonal B cell activation • Without proliferation • Increase of synthesis of : MHCII, B 7, CD 40, ICAM 1, IL 2 Ra • Therapy: local antibody Therapy production • TH 1 –linked diseases, • Autoimmune diseases • graft rejection
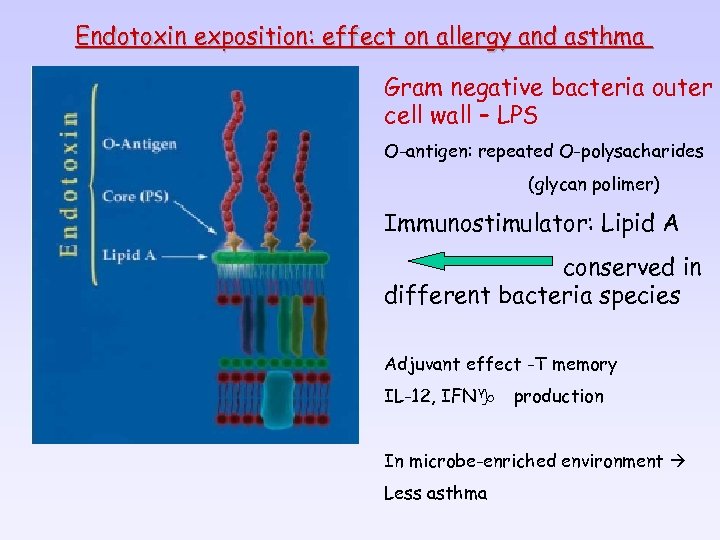 Endotoxin exposition: effect on allergy and asthma Gram negative bacteria outer cell wall – LPS O-antigen: repeated O-polysacharides (glycan polimer) Immunostimulator: Lipid A conserved in different bacteria species Adjuvant effect -T memory IL-12, IFNg production In microbe-enriched environment Less asthma
Endotoxin exposition: effect on allergy and asthma Gram negative bacteria outer cell wall – LPS O-antigen: repeated O-polysacharides (glycan polimer) Immunostimulator: Lipid A conserved in different bacteria species Adjuvant effect -T memory IL-12, IFNg production In microbe-enriched environment Less asthma
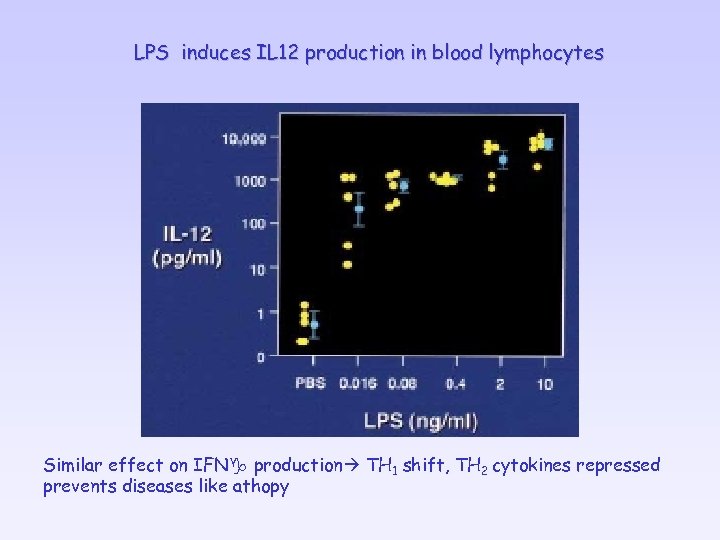 LPS induces IL 12 production in blood lymphocytes Similar effect on IFNg production TH 1 shift, TH 2 cytokines repressed prevents diseases like athopy
LPS induces IL 12 production in blood lymphocytes Similar effect on IFNg production TH 1 shift, TH 2 cytokines repressed prevents diseases like athopy
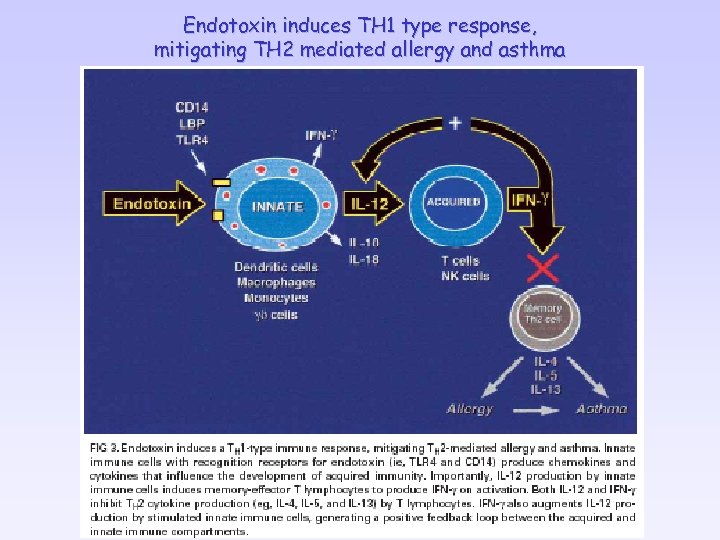 Endotoxin induces TH 1 type response, mitigating TH 2 mediated allergy and asthma
Endotoxin induces TH 1 type response, mitigating TH 2 mediated allergy and asthma

 Reverse correlation between exposure to microbe infection and the appearance of allergy and asthma ØHouse dust, animals, non-pasteurized milk lower number of children have allergy ØTowns >>> farms: ØAllergy ØFrequent infections in children communities (airways, intestinal infections „Hygiene hypothesis”
Reverse correlation between exposure to microbe infection and the appearance of allergy and asthma ØHouse dust, animals, non-pasteurized milk lower number of children have allergy ØTowns >>> farms: ØAllergy ØFrequent infections in children communities (airways, intestinal infections „Hygiene hypothesis”
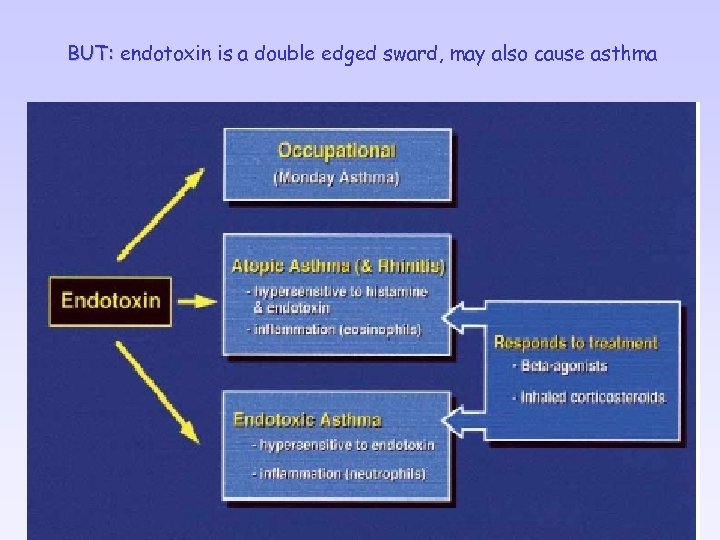 BUT: endotoxin is a double edged sward, may also cause asthma
BUT: endotoxin is a double edged sward, may also cause asthma
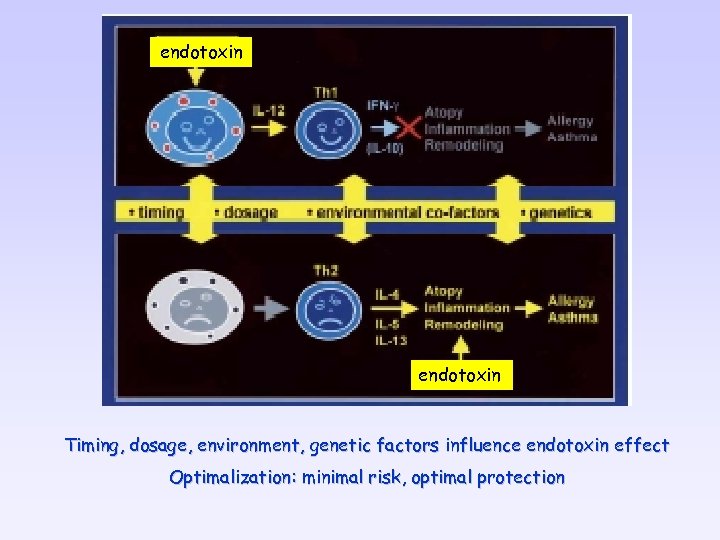 endotoxin Timing, dosage, environment, genetic factors influence endotoxin effect Optimalization: minimal risk, optimal protection
endotoxin Timing, dosage, environment, genetic factors influence endotoxin effect Optimalization: minimal risk, optimal protection
 2. Cytokines: • recombinant proteins (IL-1. IL-2, Epo) • cytokine antagonists: IL-1 RA signaling inhibitors • soluble receptors : TNF, IL-1, IL-4 • specific, high affinity binding, • natural occurence in body fluids (proteolitic cleavage or alternative splicing) • do not activate immune response • neutralise ligands • relative long life time • less immunogen
2. Cytokines: • recombinant proteins (IL-1. IL-2, Epo) • cytokine antagonists: IL-1 RA signaling inhibitors • soluble receptors : TNF, IL-1, IL-4 • specific, high affinity binding, • natural occurence in body fluids (proteolitic cleavage or alternative splicing) • do not activate immune response • neutralise ligands • relative long life time • less immunogen
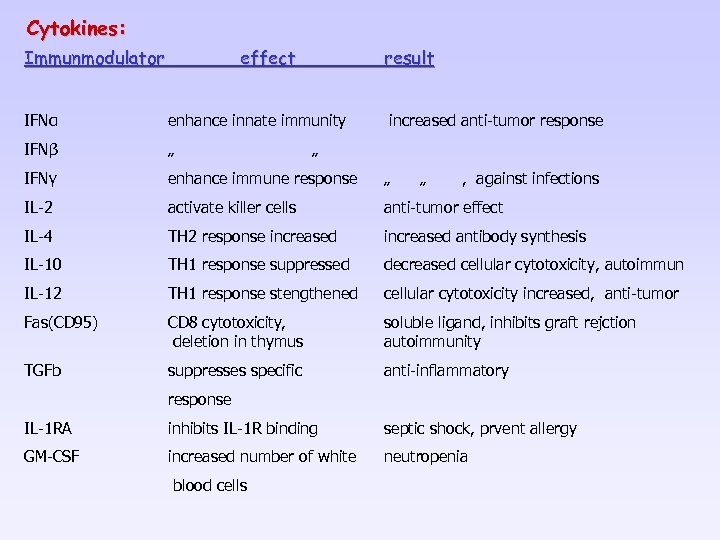 Cytokines: Immunmodulator effect result IFNα enhance innate immunity increased anti-tumor response IFNβ „ IFNγ enhance immune response „ IL-2 activate killer cells anti-tumor effect IL-4 TH 2 response increased antibody synthesis IL-10 TH 1 response suppressed decreased cellular cytotoxicity, autoimmun IL-12 TH 1 response stengthened cellular cytotoxicity increased, anti-tumor Fas(CD 95) CD 8 cytotoxicity, deletion in thymus soluble ligand, inhibits graft rejction autoimmunity TGFb suppresses specific anti-inflammatory „ „ , against infections response IL-1 RA inhibits IL-1 R binding septic shock, prvent allergy GM-CSF increased number of white neutropenia blood cells
Cytokines: Immunmodulator effect result IFNα enhance innate immunity increased anti-tumor response IFNβ „ IFNγ enhance immune response „ IL-2 activate killer cells anti-tumor effect IL-4 TH 2 response increased antibody synthesis IL-10 TH 1 response suppressed decreased cellular cytotoxicity, autoimmun IL-12 TH 1 response stengthened cellular cytotoxicity increased, anti-tumor Fas(CD 95) CD 8 cytotoxicity, deletion in thymus soluble ligand, inhibits graft rejction autoimmunity TGFb suppresses specific anti-inflammatory „ „ , against infections response IL-1 RA inhibits IL-1 R binding septic shock, prvent allergy GM-CSF increased number of white neutropenia blood cells


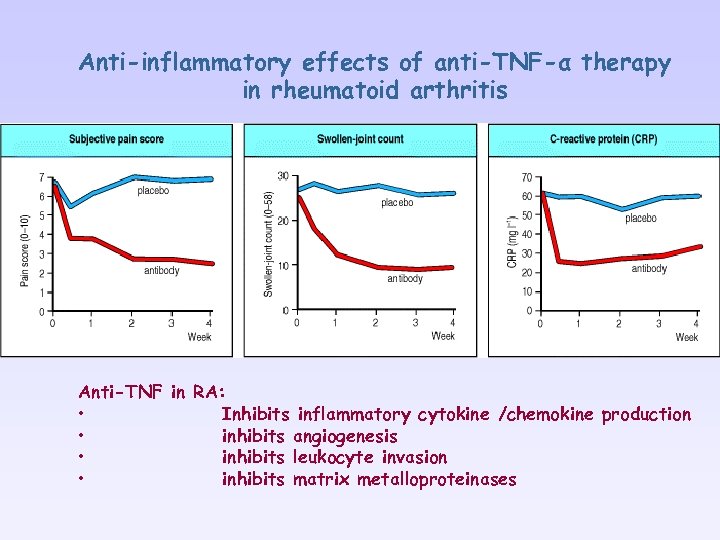 Anti-inflammatory effects of anti-TNF-α therapy in rheumatoid arthritis Anti-TNF in RA: • Inhibits inflammatory cytokine /chemokine production • inhibits angiogenesis • inhibits leukocyte invasion • inhibits matrix metalloproteinases
Anti-inflammatory effects of anti-TNF-α therapy in rheumatoid arthritis Anti-TNF in RA: • Inhibits inflammatory cytokine /chemokine production • inhibits angiogenesis • inhibits leukocyte invasion • inhibits matrix metalloproteinases
 3). Antibody mediated therapies: Ø elimination of the pathogen – hyperimmune sera (passive immunization) (rabies, hepatitis B, CMV, RSV, varicella/zoster) Ø prevention of infection : RSV (respiratory syncytial virus) Øtoxin neutralization e. g. snake toxin, tetanus Ø inhibition of blood coagulation Ø cell depletion: e. g. . anti-CD 20+ B cell depletion ØTargeting targeted therapies
3). Antibody mediated therapies: Ø elimination of the pathogen – hyperimmune sera (passive immunization) (rabies, hepatitis B, CMV, RSV, varicella/zoster) Ø prevention of infection : RSV (respiratory syncytial virus) Øtoxin neutralization e. g. snake toxin, tetanus Ø inhibition of blood coagulation Ø cell depletion: e. g. . anti-CD 20+ B cell depletion ØTargeting targeted therapies
 Targets of antibodies: ØCell surface receptors ØCytokines and their receptors: • Graft versus host disease (GVH) • Malignus tumor • Immunosuppression (a-MHCII, a-MHCI) • inflammation • Platelets aggregation Antibodies are applied for: üDiagnosis: detect malignant cells - in metastasis üPrognosis – based on detection of membrane markers üHyperimmune antibodies : intramuscular, intravenous application üAnti-inflammatory effect (IVIG): autoimmune dieases, allergy üSubstitution therapy : immunodefficieny, autoimmune diseases
Targets of antibodies: ØCell surface receptors ØCytokines and their receptors: • Graft versus host disease (GVH) • Malignus tumor • Immunosuppression (a-MHCII, a-MHCI) • inflammation • Platelets aggregation Antibodies are applied for: üDiagnosis: detect malignant cells - in metastasis üPrognosis – based on detection of membrane markers üHyperimmune antibodies : intramuscular, intravenous application üAnti-inflammatory effect (IVIG): autoimmune dieases, allergy üSubstitution therapy : immunodefficieny, autoimmune diseases
 Polyclonal antibodies : Non-antigen specific immunosuppression Suppresssion of cellular immune responses: Ø anti-thymocyte serum, -globulin: inhibits T cell responses Ø anti-lymphocyte serum, anti-lymphocyte globulin Transplantation: inhibits graft rejection, GVH Problems: standardization, non-selective antigenicity –serum disease
Polyclonal antibodies : Non-antigen specific immunosuppression Suppresssion of cellular immune responses: Ø anti-thymocyte serum, -globulin: inhibits T cell responses Ø anti-lymphocyte serum, anti-lymphocyte globulin Transplantation: inhibits graft rejection, GVH Problems: standardization, non-selective antigenicity –serum disease
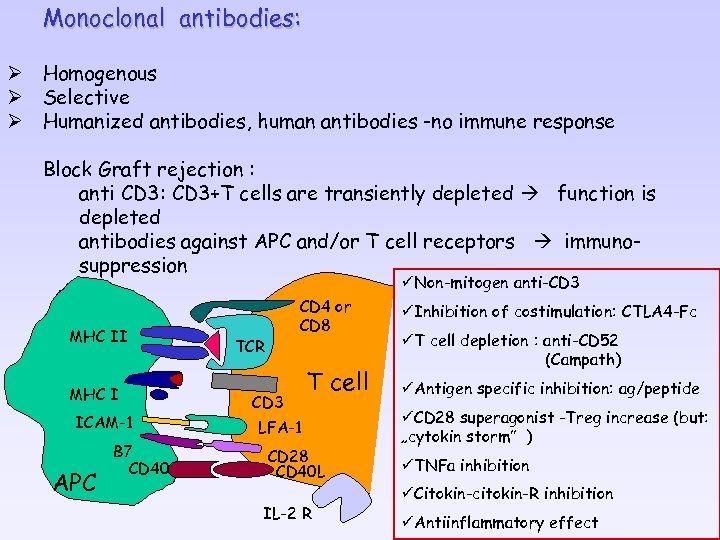 Monoclonal antibodies: Ø Homogenous Ø Selective Ø Humanized antibodies, human antibodies -no immune response Block Graft rejection : anti CD 3: CD 3+T cells are transiently depleted function is depleted antibodies against APC and/or T cell receptors immunosuppression üNon-mitogen anti-CD 3 MHC II MHC I ICAM-1 APC B 7 CD 40 CD 4 or CD 8 TCR CD 3 T cell LFA-1 CD 28 CD 40 L IL-2 R üInhibition of costimulation: CTLA 4 -Fc üT cell depletion : anti-CD 52 (Campath) üAntigen specific inhibition: ag/peptide üCD 28 superagonist -Treg increase (but: „cytokin storm” ) üTNFa inhibition üCitokin-citokin-R inhibition üAntiinflammatory effect
Monoclonal antibodies: Ø Homogenous Ø Selective Ø Humanized antibodies, human antibodies -no immune response Block Graft rejection : anti CD 3: CD 3+T cells are transiently depleted function is depleted antibodies against APC and/or T cell receptors immunosuppression üNon-mitogen anti-CD 3 MHC II MHC I ICAM-1 APC B 7 CD 40 CD 4 or CD 8 TCR CD 3 T cell LFA-1 CD 28 CD 40 L IL-2 R üInhibition of costimulation: CTLA 4 -Fc üT cell depletion : anti-CD 52 (Campath) üAntigen specific inhibition: ag/peptide üCD 28 superagonist -Treg increase (but: „cytokin storm” ) üTNFa inhibition üCitokin-citokin-R inhibition üAntiinflammatory effect
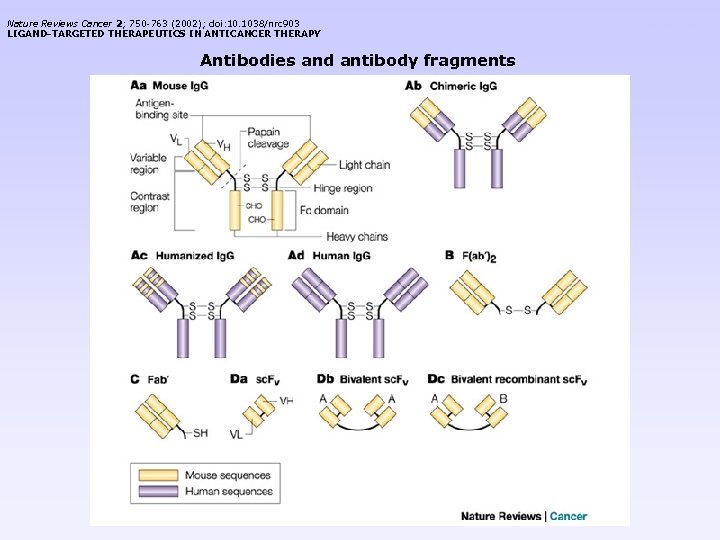 Nature Reviews Cancer 2; 750 -763 (2002); doi: 10. 1038/nrc 903 LIGAND-TARGETED THERAPEUTICS IN ANTICANCER THERAPY Antibodies and antibody fragments < previous next >
Nature Reviews Cancer 2; 750 -763 (2002); doi: 10. 1038/nrc 903 LIGAND-TARGETED THERAPEUTICS IN ANTICANCER THERAPY Antibodies and antibody fragments < previous next >


 Bio-similar, bio-better, me-better • Biosimilar antibodies are “generic” versions of “innovator” (or “originator”) antibodies with the same amino acid sequence, but produced from different clones and manufacturing processes. • Bio-better antibodies are antibodies that target the same validated epitope as a marketed antibody, but have been engineered to have improved properties, e. g. , optimized glycosylation profiles to enhance effector functions or an engineered Fc domain to increase the serum half-life • “Me better” antibodies with controlled and optimized glycosylation have been obtained in glyco-engineered CHO cells or yeast strains
Bio-similar, bio-better, me-better • Biosimilar antibodies are “generic” versions of “innovator” (or “originator”) antibodies with the same amino acid sequence, but produced from different clones and manufacturing processes. • Bio-better antibodies are antibodies that target the same validated epitope as a marketed antibody, but have been engineered to have improved properties, e. g. , optimized glycosylation profiles to enhance effector functions or an engineered Fc domain to increase the serum half-life • “Me better” antibodies with controlled and optimized glycosylation have been obtained in glyco-engineered CHO cells or yeast strains
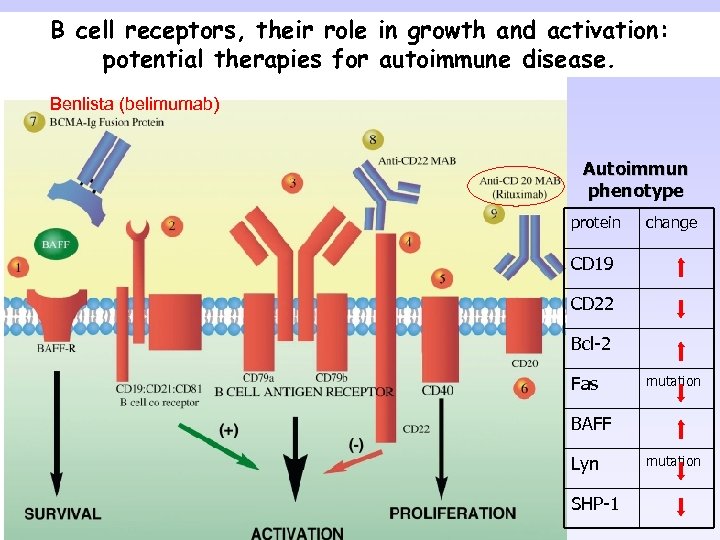 B cell receptors, their role in growth and activation: potential therapies for autoimmune disease. Benlista (belimumab) Autoimmun phenotype protein change CD 19 CD 22 Bcl-2 Fas mutation BAFF Lyn SHP-1 mutation
B cell receptors, their role in growth and activation: potential therapies for autoimmune disease. Benlista (belimumab) Autoimmun phenotype protein change CD 19 CD 22 Bcl-2 Fas mutation BAFF Lyn SHP-1 mutation
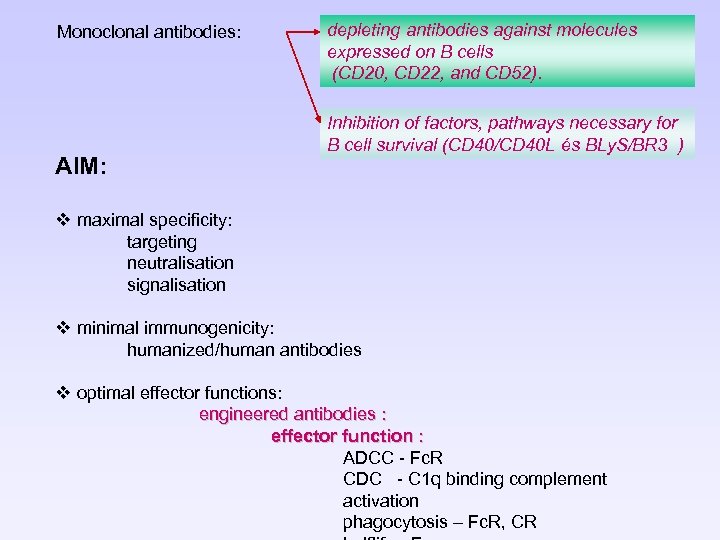 Monoclonal antibodies: AIM: depleting antibodies against molecules expressed on B cells (CD 20, CD 22, and CD 52). Inhibition of factors, pathways necessary for B cell survival (CD 40/CD 40 L és BLy. S/BR 3 ) v maximal specificity: targeting neutralisation signalisation v minimal immunogenicity: humanized/human antibodies v optimal effector functions: engineered antibodies : effector function : ADCC - Fc. R CDC - C 1 q binding complement activation phagocytosis – Fc. R, CR
Monoclonal antibodies: AIM: depleting antibodies against molecules expressed on B cells (CD 20, CD 22, and CD 52). Inhibition of factors, pathways necessary for B cell survival (CD 40/CD 40 L és BLy. S/BR 3 ) v maximal specificity: targeting neutralisation signalisation v minimal immunogenicity: humanized/human antibodies v optimal effector functions: engineered antibodies : effector function : ADCC - Fc. R CDC - C 1 q binding complement activation phagocytosis – Fc. R, CR
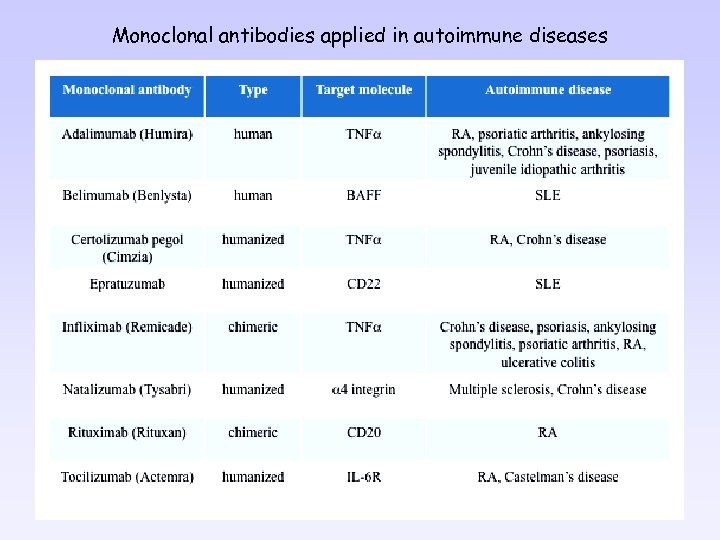 Monoclonal antibodies applied in autoimmune diseases
Monoclonal antibodies applied in autoimmune diseases
 Antibody therapies: substitution of antibodies Plasmapheresis: ~50 % removed (Ig. G 20 %, Ig. M ~50 %) Elimination of immunecomplexes autoimmune diseases: self-specific Ig. G: Goodpasture’s syndrom: lung, kidney, (antibodies against glomerulal basal membrane) myasthenia gravis (anti-acetilcholinreceptor) Antibody overproduction: Waldenström macroglobulinemia –Ig. M cold agglutinin haemolitic anemia -Ig. M
Antibody therapies: substitution of antibodies Plasmapheresis: ~50 % removed (Ig. G 20 %, Ig. M ~50 %) Elimination of immunecomplexes autoimmune diseases: self-specific Ig. G: Goodpasture’s syndrom: lung, kidney, (antibodies against glomerulal basal membrane) myasthenia gravis (anti-acetilcholinreceptor) Antibody overproduction: Waldenström macroglobulinemia –Ig. M cold agglutinin haemolitic anemia -Ig. M
 Intravenous Ig therapy, (IVIG) Immunmodulatory, anti-inflammatory effect IVIG therapy - examples • Neuroimmunological diseases : diseases with demyelination - inhibiting complement effect MS (? ) • Primairy immunodefficiencies: Ig < 400 mg/dl • Idiopathic trombocytopenia purpura: low platelet number - IVIG inhibits phagocytosis • CLL: against bacterial infections • infectious diseases, toxic shock (100 000/year) –sepsis • Kawasaki disease: chronic vasculitis - IVIG – neutralization effect ,
Intravenous Ig therapy, (IVIG) Immunmodulatory, anti-inflammatory effect IVIG therapy - examples • Neuroimmunological diseases : diseases with demyelination - inhibiting complement effect MS (? ) • Primairy immunodefficiencies: Ig < 400 mg/dl • Idiopathic trombocytopenia purpura: low platelet number - IVIG inhibits phagocytosis • CLL: against bacterial infections • infectious diseases, toxic shock (100 000/year) –sepsis • Kawasaki disease: chronic vasculitis - IVIG – neutralization effect ,
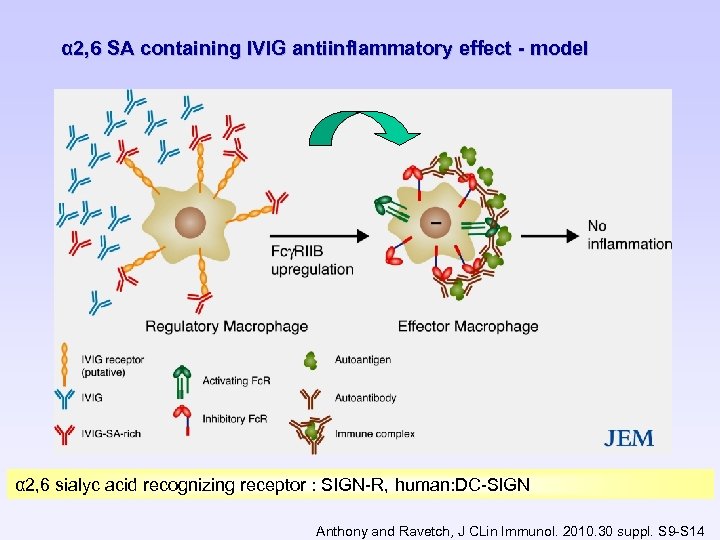 α 2, 6 SA containing IVIG antiinflammatory effect - model α 2, 6 sialyc acid recognizing receptor : SIGN-R, human: DC-SIGN Anthony and Ravetch, J CLin Immunol. 2010. 30 suppl. S 9 -S 14
α 2, 6 SA containing IVIG antiinflammatory effect - model α 2, 6 sialyc acid recognizing receptor : SIGN-R, human: DC-SIGN Anthony and Ravetch, J CLin Immunol. 2010. 30 suppl. S 9 -S 14

 IMMUNSUPPRESSION Block unwanted immunoresponse: - Allergy - Autoimmune diseases - transplantation: rejection, GVH Antigen specific immunsuppression – aim: to induce specific tolerance a, Antigen-specific (pl. oral tolerance) b, Non-antigen specific • corticosteroids • CY-A, FK 506, Rapamycin, • irradiation • Cytostatic agents
IMMUNSUPPRESSION Block unwanted immunoresponse: - Allergy - Autoimmune diseases - transplantation: rejection, GVH Antigen specific immunsuppression – aim: to induce specific tolerance a, Antigen-specific (pl. oral tolerance) b, Non-antigen specific • corticosteroids • CY-A, FK 506, Rapamycin, • irradiation • Cytostatic agents
 Antigen non-specific immunosuppression: Corticosteroids Inhibit inflammation Mechanisms: they act via hormon receptors Naturally occuring 21 C atoms steroid hormon: Corticosteroid product of cholesterin metabolism 1948: hydrocortison (Reumatoid arthritis)
Antigen non-specific immunosuppression: Corticosteroids Inhibit inflammation Mechanisms: they act via hormon receptors Naturally occuring 21 C atoms steroid hormon: Corticosteroid product of cholesterin metabolism 1948: hydrocortison (Reumatoid arthritis)
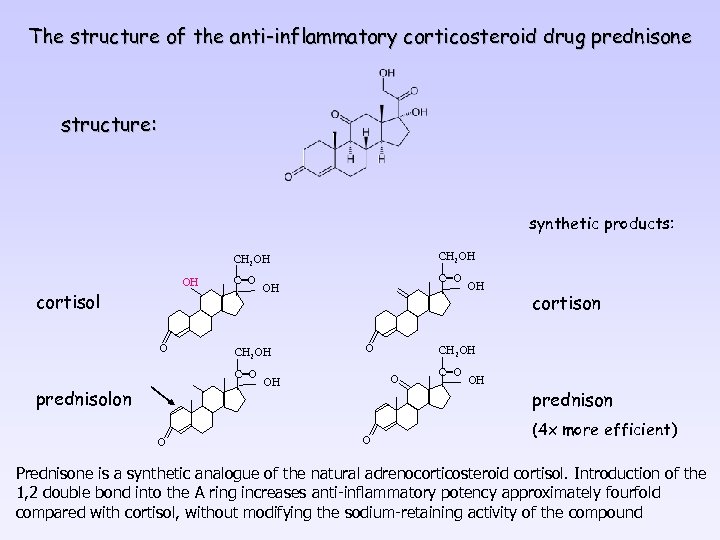 The structure of the anti-inflammatory corticosteroid drug prednisone structure: synthetic products: CH 2 OH OH cortisol O CH 2 OH C=O prednisolon O OH O O cortison CH 2 OH OH C=O OH prednison (4 x more efficient) Prednisone is a synthetic analogue of the natural adrenocorticosteroid cortisol. Introduction of the 1, 2 double bond into the A ring increases anti-inflammatory potency approximately fourfold compared with cortisol, without modifying the sodium-retaining activity of the compound
The structure of the anti-inflammatory corticosteroid drug prednisone structure: synthetic products: CH 2 OH OH cortisol O CH 2 OH C=O prednisolon O OH O O cortison CH 2 OH OH C=O OH prednison (4 x more efficient) Prednisone is a synthetic analogue of the natural adrenocorticosteroid cortisol. Introduction of the 1, 2 double bond into the A ring increases anti-inflammatory potency approximately fourfold compared with cortisol, without modifying the sodium-retaining activity of the compound
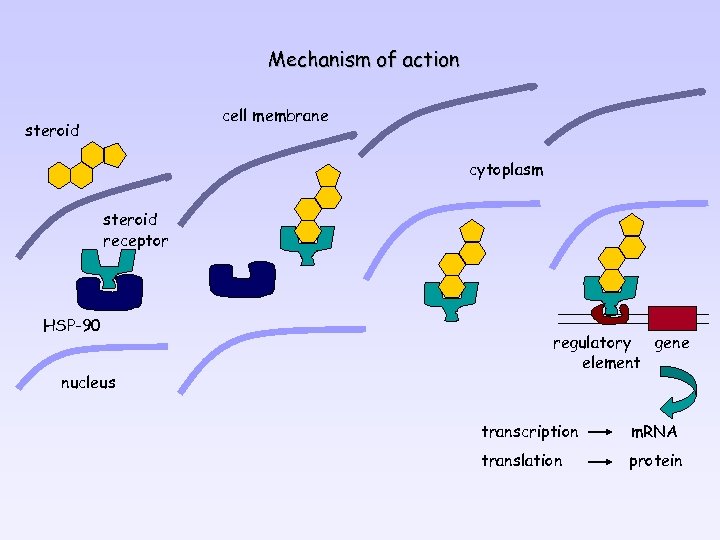 Mechanism of action cell membrane steroid cytoplasm steroid receptor HSP-90 nucleus regulatory gene element transcription m. RNA translation protein
Mechanism of action cell membrane steroid cytoplasm steroid receptor HSP-90 nucleus regulatory gene element transcription m. RNA translation protein
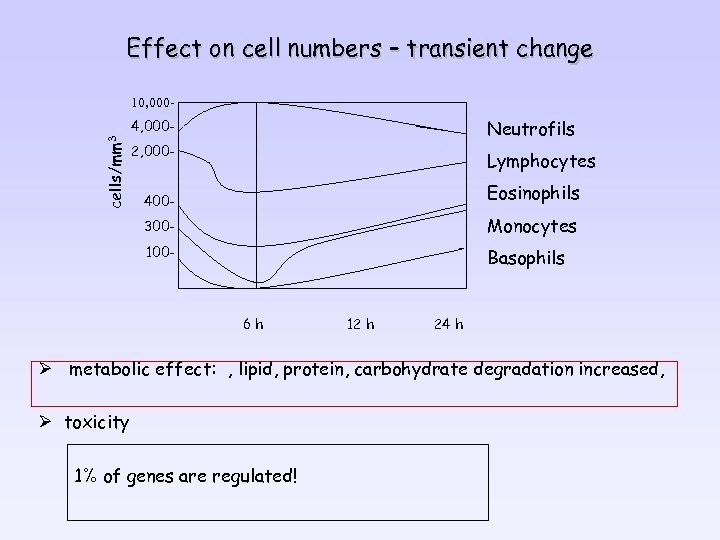 Effect on cell numbers – transient change 10, 000 - cells/mm 3 4, 000 - Neutrofils 2, 000 - Lymphocytes 400 - Eosinophils 300 - Monocytes 100 - Basophils 6 h 12 h 24 h Ø metabolic effect: , lipid, protein, carbohydrate degradation increased, Ø toxicity 1% of genes are regulated!
Effect on cell numbers – transient change 10, 000 - cells/mm 3 4, 000 - Neutrofils 2, 000 - Lymphocytes 400 - Eosinophils 300 - Monocytes 100 - Basophils 6 h 12 h 24 h Ø metabolic effect: , lipid, protein, carbohydrate degradation increased, Ø toxicity 1% of genes are regulated!
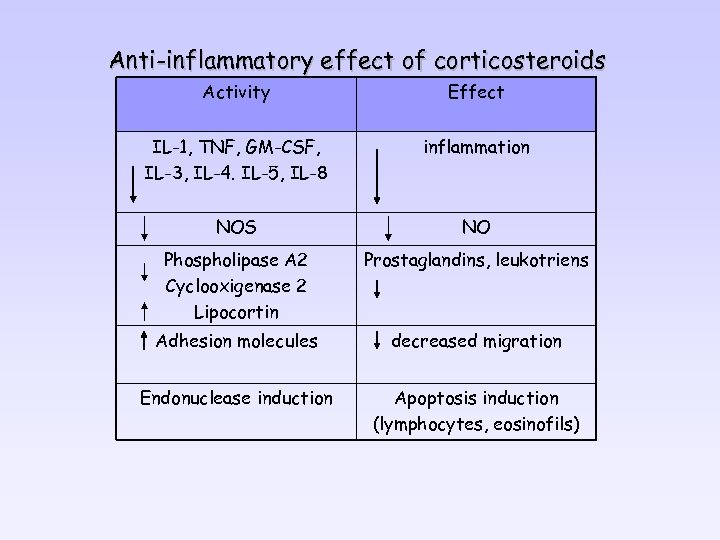 Anti-inflammatory effect of corticosteroids Activity Effect IL-1, TNF, GM-CSF, IL-3, IL-4. IL-5, IL-8 inflammation NOS NO Phospholipase A 2 Cyclooxigenase 2 Lipocortin Prostaglandins, leukotriens Adhesion molecules decreased migration Endonuclease induction Apoptosis induction (lymphocytes, eosinofils)
Anti-inflammatory effect of corticosteroids Activity Effect IL-1, TNF, GM-CSF, IL-3, IL-4. IL-5, IL-8 inflammation NOS NO Phospholipase A 2 Cyclooxigenase 2 Lipocortin Prostaglandins, leukotriens Adhesion molecules decreased migration Endonuclease induction Apoptosis induction (lymphocytes, eosinofils)
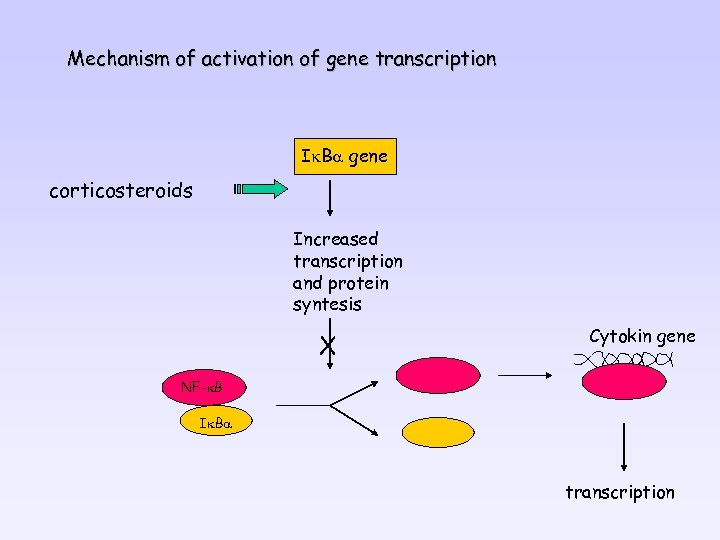 Mechanism of activation of gene transcription Ik. Ba gene corticosteroids Increased transcription and protein syntesis X Cytokin gene NF-k. B Ik. Ba transcription
Mechanism of activation of gene transcription Ik. Ba gene corticosteroids Increased transcription and protein syntesis X Cytokin gene NF-k. B Ik. Ba transcription
 Non-steroid anti-inflammatory agents 400 BC aszpirin (Salix alba)- Hippokrates synthetic production : 19. century today USA – 15 x 106 kg / year mechanism: cyclooxigenase inhibition prostaglandin production inhibited • active site : serine acetilation (irreversible) • arachidonic acid binding inhibition (reversible)
Non-steroid anti-inflammatory agents 400 BC aszpirin (Salix alba)- Hippokrates synthetic production : 19. century today USA – 15 x 106 kg / year mechanism: cyclooxigenase inhibition prostaglandin production inhibited • active site : serine acetilation (irreversible) • arachidonic acid binding inhibition (reversible)
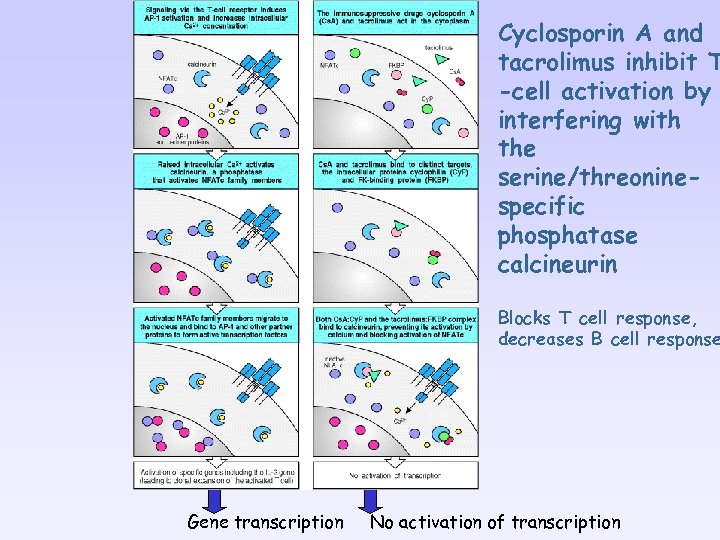 Cyclosporin A and tacrolimus inhibit T -cell activation by interfering with the serine/threoninespecific phosphatase calcineurin Blocks T cell response, decreases B cell response Gene transcription No activation of transcription
Cyclosporin A and tacrolimus inhibit T -cell activation by interfering with the serine/threoninespecific phosphatase calcineurin Blocks T cell response, decreases B cell response Gene transcription No activation of transcription
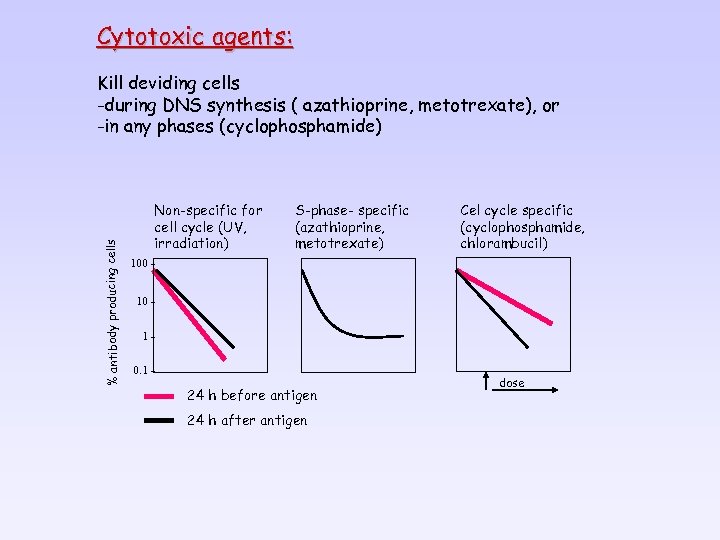 Cytotoxic agents: % antibody producing cells Kill deviding cells -during DNS synthesis ( azathioprine, metotrexate), or -in any phases (cyclophosphamide) Non-specific for cell cycle (UV, irradiation) S-phase- specific (azathioprine, metotrexate) Cel cycle specific (cyclophosphamide, chlorambucil) 100 10 10. 1 - 24 h before antigen 24 h after antigen dose
Cytotoxic agents: % antibody producing cells Kill deviding cells -during DNS synthesis ( azathioprine, metotrexate), or -in any phases (cyclophosphamide) Non-specific for cell cycle (UV, irradiation) S-phase- specific (azathioprine, metotrexate) Cel cycle specific (cyclophosphamide, chlorambucil) 100 10 10. 1 - 24 h before antigen 24 h after antigen dose
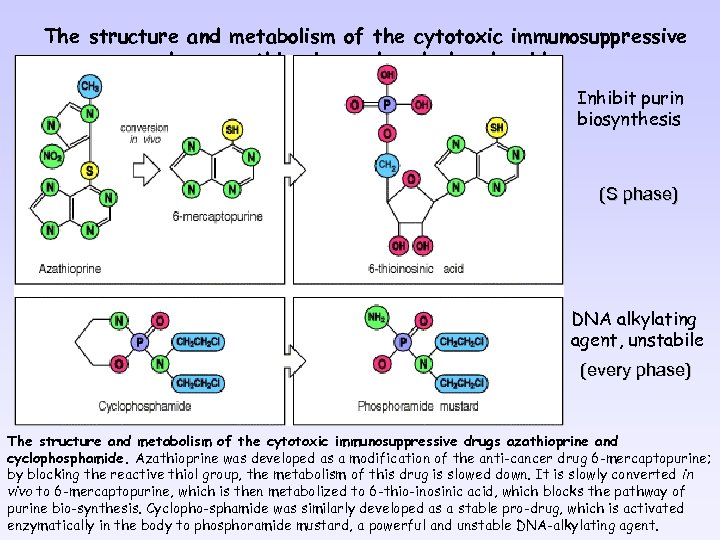 The structure and metabolism of the cytotoxic immunosuppressive drugs azathioprine and cyclophosphamide Inhibit purin biosynthesis (S phase) DNA alkylating agent, unstabile (every phase) The structure and metabolism of the cytotoxic immunosuppressive drugs azathioprine and cyclophosphamide. Azathioprine was developed as a modification of the anti-cancer drug 6 -mercaptopurine; by blocking the reactive thiol group, the metabolism of this drug is slowed down. It is slowly converted in vivo to 6 -mercaptopurine, which is then metabolized to 6 -thio-inosinic acid, which blocks the pathway of purine bio-synthesis. Cyclopho-sphamide was similarly developed as a stable pro-drug, which is activated enzymatically in the body to phosphoramide mustard, a powerful and unstable DNA-alkylating agent.
The structure and metabolism of the cytotoxic immunosuppressive drugs azathioprine and cyclophosphamide Inhibit purin biosynthesis (S phase) DNA alkylating agent, unstabile (every phase) The structure and metabolism of the cytotoxic immunosuppressive drugs azathioprine and cyclophosphamide. Azathioprine was developed as a modification of the anti-cancer drug 6 -mercaptopurine; by blocking the reactive thiol group, the metabolism of this drug is slowed down. It is slowly converted in vivo to 6 -mercaptopurine, which is then metabolized to 6 -thio-inosinic acid, which blocks the pathway of purine bio-synthesis. Cyclopho-sphamide was similarly developed as a stable pro-drug, which is activated enzymatically in the body to phosphoramide mustard, a powerful and unstable DNA-alkylating agent.

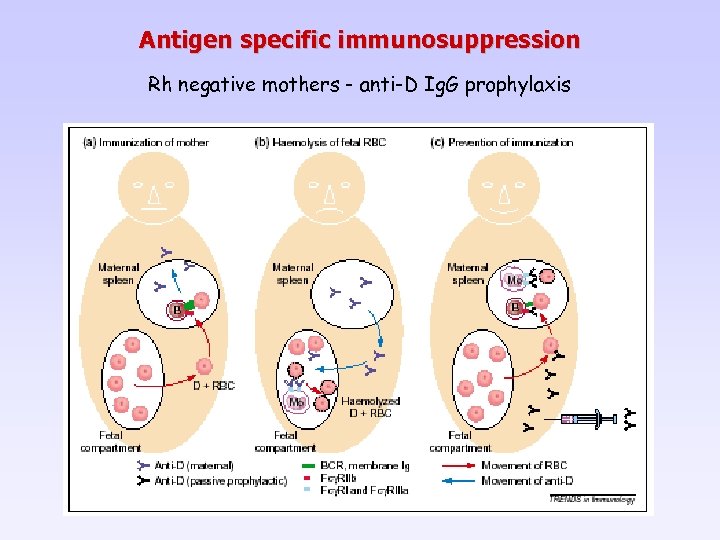 Antigen specific immunosuppression Rh negative mothers - anti-D Ig. G prophylaxis
Antigen specific immunosuppression Rh negative mothers - anti-D Ig. G prophylaxis
 INDUCTION ORAL TOLERANCE • Myelin basic protein (MBP) • Insulin • Collagen II-IV Local effect on mucosal immunsystem (Th 2 activation, TGF ß production enhanced)
INDUCTION ORAL TOLERANCE • Myelin basic protein (MBP) • Insulin • Collagen II-IV Local effect on mucosal immunsystem (Th 2 activation, TGF ß production enhanced)
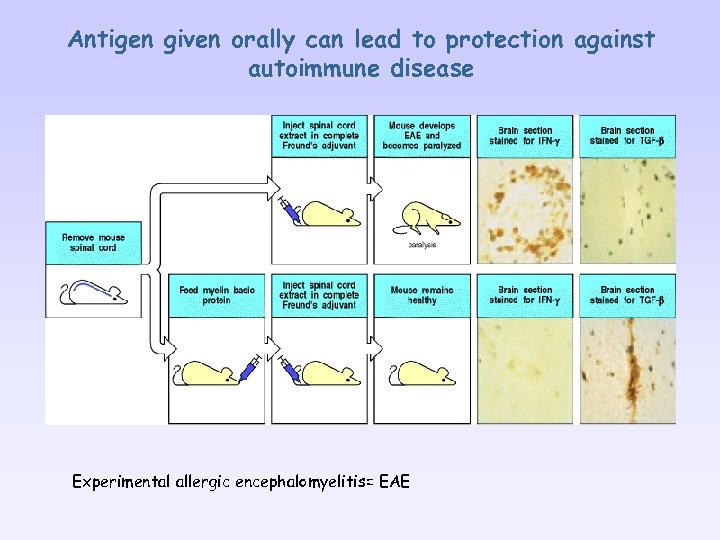 Antigen given orally can lead to protection against autoimmune disease Experimental allergic encephalomyelitis= EAE
Antigen given orally can lead to protection against autoimmune disease Experimental allergic encephalomyelitis= EAE
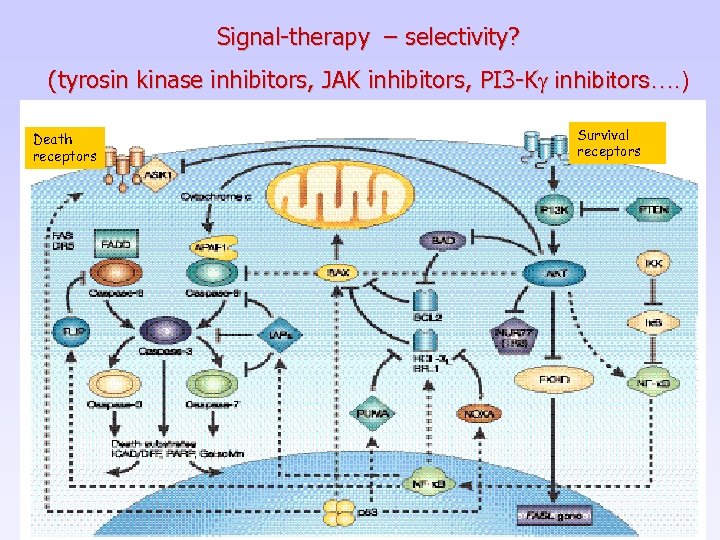 Signal-therapy – selectivity? (tyrosin kinase inhibitors, JAK inhibitors, PI 3 -Kg inhibitors…. ) Death receptors Survival receptors
Signal-therapy – selectivity? (tyrosin kinase inhibitors, JAK inhibitors, PI 3 -Kg inhibitors…. ) Death receptors Survival receptors


