SPbU_Shinjo.pptx
- Количество слайдов: 21
 The way to prepare gold clusters -Every atom makes a difference Naoaki Shinjo Department of Chemistry, School of Science The University of Tokyo
The way to prepare gold clusters -Every atom makes a difference Naoaki Shinjo Department of Chemistry, School of Science The University of Tokyo
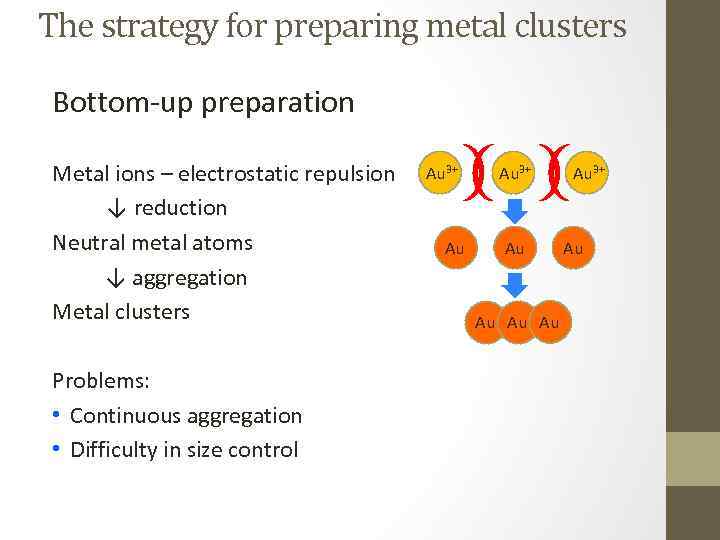 The strategy for preparing metal clusters Bottom-up preparation Metal ions – electrostatic repulsion ↓ reduction Neutral metal atoms ↓ aggregation Metal clusters Problems: • Continuous aggregation • Difficulty in size control Au 3+ Au Au Au 3+ Au
The strategy for preparing metal clusters Bottom-up preparation Metal ions – electrostatic repulsion ↓ reduction Neutral metal atoms ↓ aggregation Metal clusters Problems: • Continuous aggregation • Difficulty in size control Au 3+ Au Au Au 3+ Au
![“Magic numbers” for bare gold clusters n=9 (8 e-) Au: [Xe] 4 f 14 “Magic numbers” for bare gold clusters n=9 (8 e-) Au: [Xe] 4 f 14](https://present5.com/presentation/-101369386_437489824/image-3.jpg) “Magic numbers” for bare gold clusters n=9 (8 e-) Au: [Xe] 4 f 14 5 d 10 6 s 1 n = 21 (20 e-) n = 35 (34 e-) n = 58 (57 e-) Katakuse, I. et al. Int. J. Mass Spec. Ion Proc. 1985, 67, 229. “Superatom” – Stability is explained by electronic shell structure.
“Magic numbers” for bare gold clusters n=9 (8 e-) Au: [Xe] 4 f 14 5 d 10 6 s 1 n = 21 (20 e-) n = 35 (34 e-) n = 58 (57 e-) Katakuse, I. et al. Int. J. Mass Spec. Ion Proc. 1985, 67, 229. “Superatom” – Stability is explained by electronic shell structure.
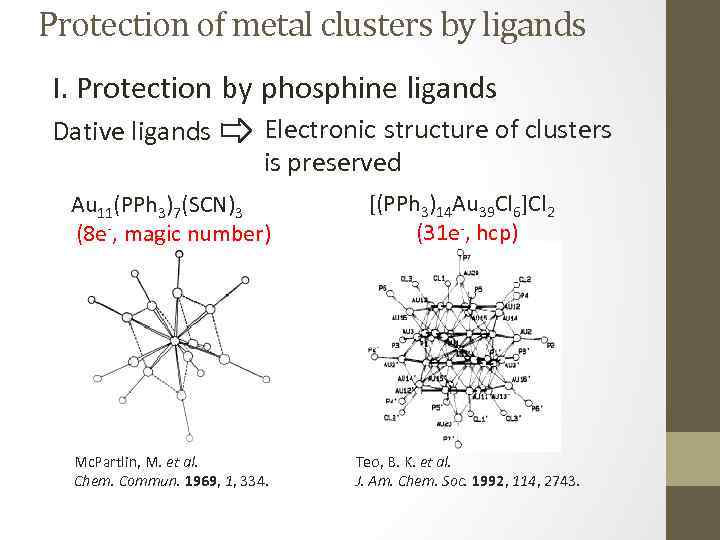 Protection of metal clusters by ligands I. Protection by phosphine ligands Dative ligands Electronic structure of clusters is preserved Au 11(PPh 3)7(SCN)3 (8 e-, magic number) Mc. Partlin, M. et al. Chem. Commun. 1969, 1, 334. [(PPh 3)14 Au 39 Cl 6]Cl 2 (31 e-, hcp) Teo, B. K. et al. J. Am. Chem. Soc. 1992, 114, 2743.
Protection of metal clusters by ligands I. Protection by phosphine ligands Dative ligands Electronic structure of clusters is preserved Au 11(PPh 3)7(SCN)3 (8 e-, magic number) Mc. Partlin, M. et al. Chem. Commun. 1969, 1, 334. [(PPh 3)14 Au 39 Cl 6]Cl 2 (31 e-, hcp) Teo, B. K. et al. J. Am. Chem. Soc. 1992, 114, 2743.
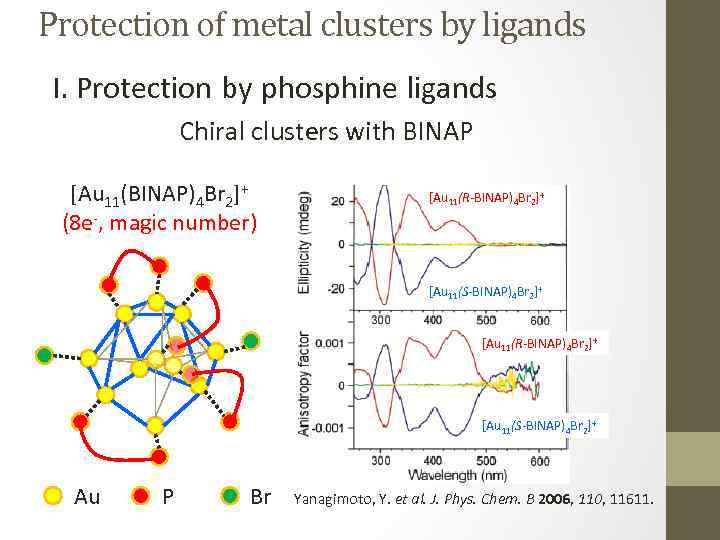 Protection of metal clusters by ligands I. Protection by phosphine ligands Chiral clusters with BINAP [Au 11(BINAP)4 Br 2]+ (8 e-, magic number) [Au 11(R-BINAP)4 Br 2]+ [Au 11(S-BINAP)4 Br 2]+ Au P Br Yanagimoto, Y. et al. J. Phys. Chem. B 2006, 110, 11611.
Protection of metal clusters by ligands I. Protection by phosphine ligands Chiral clusters with BINAP [Au 11(BINAP)4 Br 2]+ (8 e-, magic number) [Au 11(R-BINAP)4 Br 2]+ [Au 11(S-BINAP)4 Br 2]+ Au P Br Yanagimoto, Y. et al. J. Phys. Chem. B 2006, 110, 11611.
 Protection of metal clusters by ligands II. Protection by thiolate ligands – high affinity between Au and S (A) “Brust-Schiffrin Method” (B) Ligand exchange of phosphine-protected clusters (C) Thiolation of polymer-stabilized clusters
Protection of metal clusters by ligands II. Protection by thiolate ligands – high affinity between Au and S (A) “Brust-Schiffrin Method” (B) Ligand exchange of phosphine-protected clusters (C) Thiolation of polymer-stabilized clusters
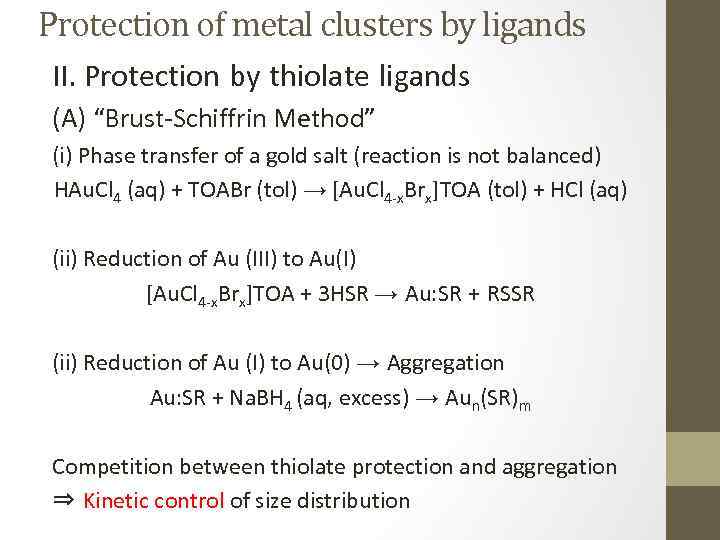 Protection of metal clusters by ligands II. Protection by thiolate ligands (A) “Brust-Schiffrin Method” (i) Phase transfer of a gold salt (reaction is not balanced) HAu. Cl 4 (aq) + TOABr (tol) → [Au. Cl 4 -x. Brx]TOA (tol) + HCl (aq) (ii) Reduction of Au (III) to Au(I) [Au. Cl 4 -x. Brx]TOA + 3 HSR → Au: SR + RSSR (ii) Reduction of Au (I) to Au(0) → Aggregation Au: SR + Na. BH 4 (aq, excess) → Aun(SR)m Competition between thiolate protection and aggregation ⇒ Kinetic control of size distribution
Protection of metal clusters by ligands II. Protection by thiolate ligands (A) “Brust-Schiffrin Method” (i) Phase transfer of a gold salt (reaction is not balanced) HAu. Cl 4 (aq) + TOABr (tol) → [Au. Cl 4 -x. Brx]TOA (tol) + HCl (aq) (ii) Reduction of Au (III) to Au(I) [Au. Cl 4 -x. Brx]TOA + 3 HSR → Au: SR + RSSR (ii) Reduction of Au (I) to Au(0) → Aggregation Au: SR + Na. BH 4 (aq, excess) → Aun(SR)m Competition between thiolate protection and aggregation ⇒ Kinetic control of size distribution
 Protection of metal clusters by ligands II. Protection by thiolate ligands (B) Ligand exchange of phosphine-stabilized clusters e. g. [Au 11(PPh 3)8 X 2]+, [Au 11(BINAP)4 X 2]+ (C) Thiolation of polymer-stabilized clusters e. g. Au: PVP Dependent on the stability of parent clusters ⇒ Thermodynamic control of size distribution
Protection of metal clusters by ligands II. Protection by thiolate ligands (B) Ligand exchange of phosphine-stabilized clusters e. g. [Au 11(PPh 3)8 X 2]+, [Au 11(BINAP)4 X 2]+ (C) Thiolation of polymer-stabilized clusters e. g. Au: PVP Dependent on the stability of parent clusters ⇒ Thermodynamic control of size distribution
 Fractionation of gold clusters Atomic monodispersity is difficult to achieve ↓ Fractionation (A) Polyacrylamide gel electrophoresis (PAGE) (B) Gel permeation chromatography (GPC) (C) Size-selective etching
Fractionation of gold clusters Atomic monodispersity is difficult to achieve ↓ Fractionation (A) Polyacrylamide gel electrophoresis (PAGE) (B) Gel permeation chromatography (GPC) (C) Size-selective etching
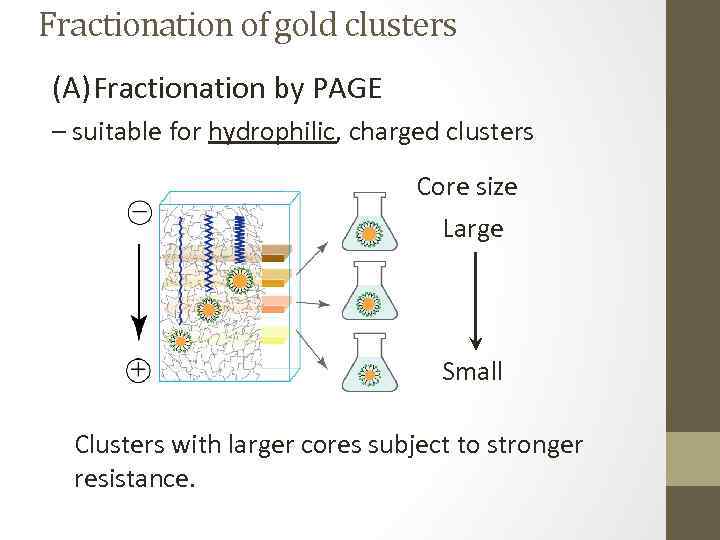 Fractionation of gold clusters (A) Fractionation by PAGE – suitable for hydrophilic, charged clusters Core size Large Small Clusters with larger cores subject to stronger resistance.
Fractionation of gold clusters (A) Fractionation by PAGE – suitable for hydrophilic, charged clusters Core size Large Small Clusters with larger cores subject to stronger resistance.
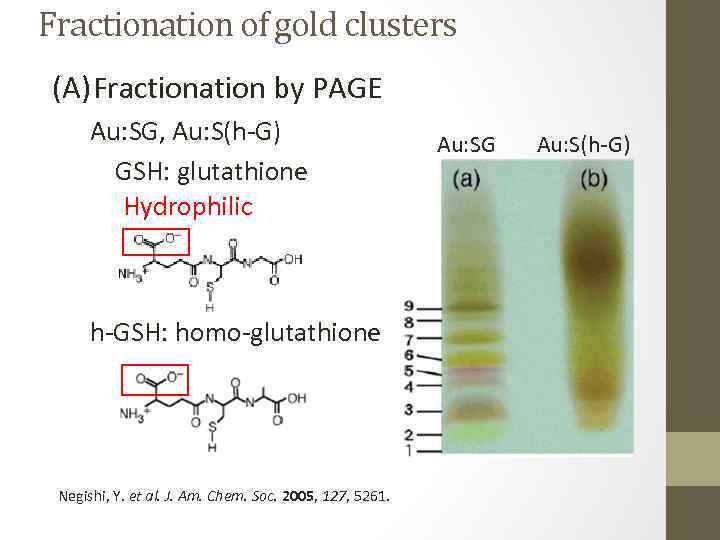 Fractionation of gold clusters (A) Fractionation by PAGE Au: SG, Au: S(h-G) GSH: glutathione Hydrophilic h-GSH: homo-glutathione Negishi, Y. et al. J. Am. Chem. Soc. 2005, 127, 5261. Au: SG Au: S(h-G)
Fractionation of gold clusters (A) Fractionation by PAGE Au: SG, Au: S(h-G) GSH: glutathione Hydrophilic h-GSH: homo-glutathione Negishi, Y. et al. J. Am. Chem. Soc. 2005, 127, 5261. Au: SG Au: S(h-G)
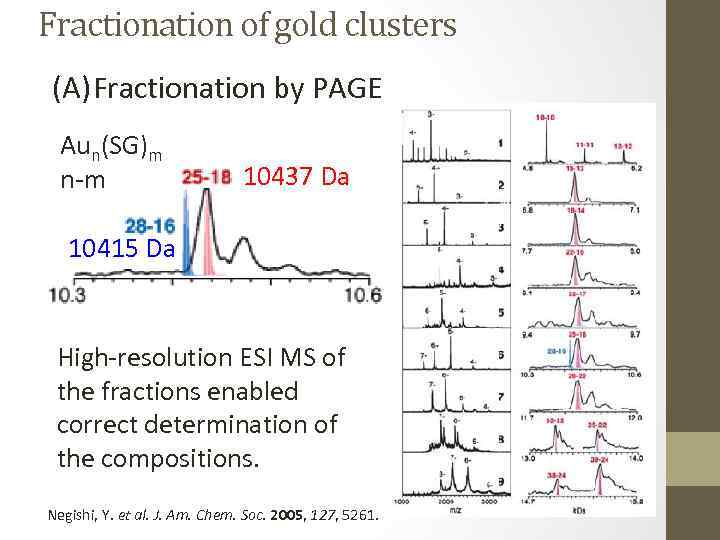 Fractionation of gold clusters (A) Fractionation by PAGE Aun(SG)m n-m 10437 Da 10415 Da High-resolution ESI MS of the fractions enabled correct determination of the compositions. Negishi, Y. et al. J. Am. Chem. Soc. 2005, 127, 5261.
Fractionation of gold clusters (A) Fractionation by PAGE Aun(SG)m n-m 10437 Da 10415 Da High-resolution ESI MS of the fractions enabled correct determination of the compositions. Negishi, Y. et al. J. Am. Chem. Soc. 2005, 127, 5261.
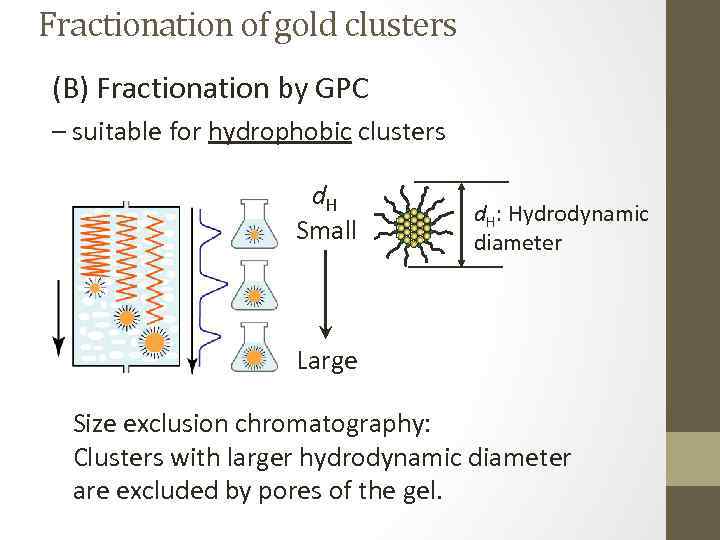 Fractionation of gold clusters (B) Fractionation by GPC – suitable for hydrophobic clusters d. H Small d. H: Hydrodynamic diameter Large Size exclusion chromatography: Clusters with larger hydrodynamic diameter are excluded by pores of the gel.
Fractionation of gold clusters (B) Fractionation by GPC – suitable for hydrophobic clusters d. H Small d. H: Hydrodynamic diameter Large Size exclusion chromatography: Clusters with larger hydrodynamic diameter are excluded by pores of the gel.
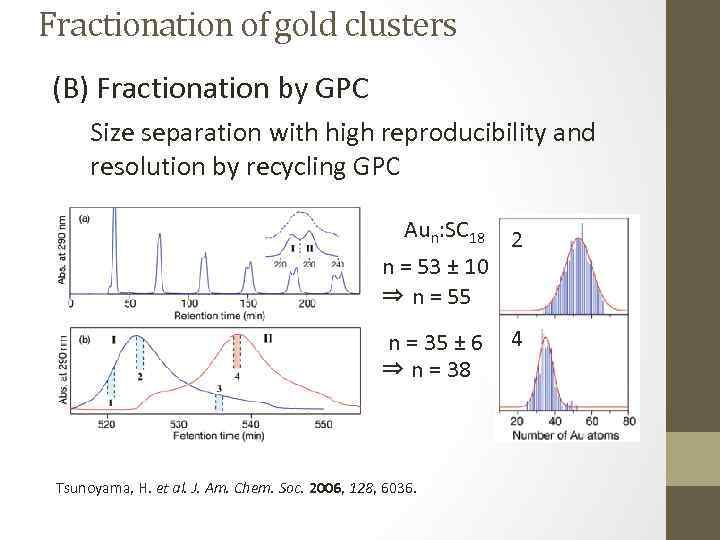 Fractionation of gold clusters (B) Fractionation by GPC Size separation with high reproducibility and resolution by recycling GPC Aun: SC 18 n = 53 ± 10 ⇒ n = 55 n = 35 ± 6 ⇒ n = 38 Tsunoyama, H. et al. J. Am. Chem. Soc. 2006, 128, 6036. 2 4
Fractionation of gold clusters (B) Fractionation by GPC Size separation with high reproducibility and resolution by recycling GPC Aun: SC 18 n = 53 ± 10 ⇒ n = 55 n = 35 ± 6 ⇒ n = 38 Tsunoyama, H. et al. J. Am. Chem. Soc. 2006, 128, 6036. 2 4
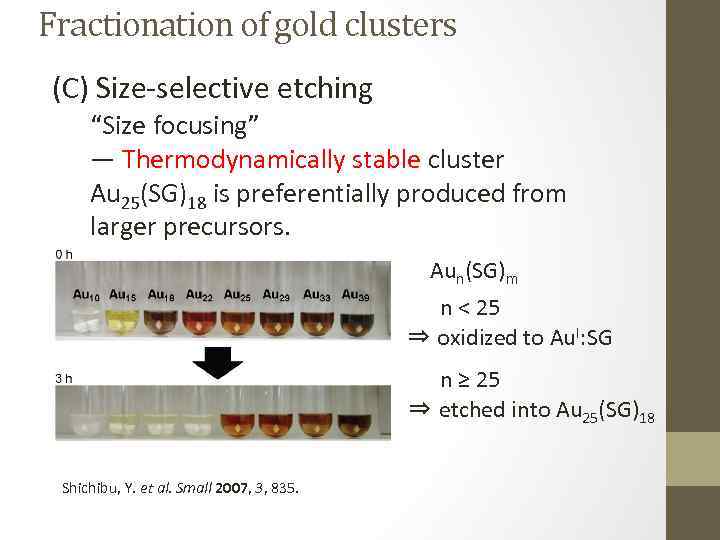 Fractionation of gold clusters (C) Size-selective etching “Size focusing” ― Thermodynamically stable cluster Au 25(SG)18 is preferentially produced from larger precursors. Aun(SG)m n < 25 ⇒ oxidized to Au. I: SG n ≥ 25 ⇒ etched into Au 25(SG)18 Shichibu, Y. et al. Small 2007, 3, 835.
Fractionation of gold clusters (C) Size-selective etching “Size focusing” ― Thermodynamically stable cluster Au 25(SG)18 is preferentially produced from larger precursors. Aun(SG)m n < 25 ⇒ oxidized to Au. I: SG n ≥ 25 ⇒ etched into Au 25(SG)18 Shichibu, Y. et al. Small 2007, 3, 835.
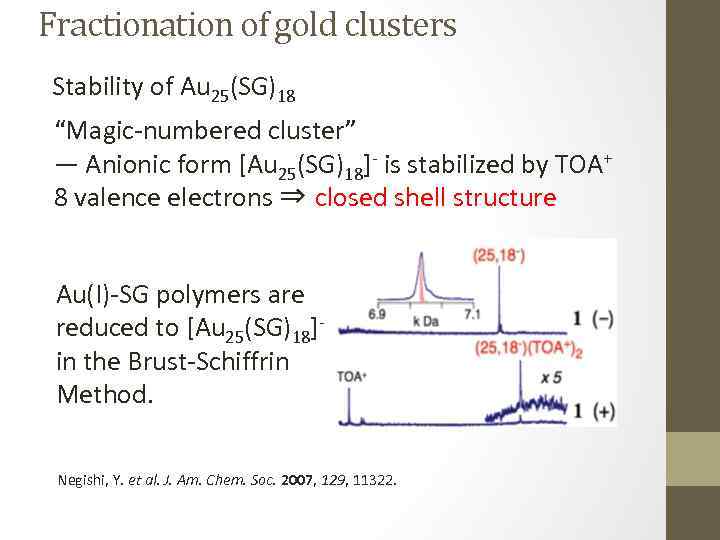 Fractionation of gold clusters Stability of Au 25(SG)18 “Magic-numbered cluster” ― Anionic form [Au 25(SG)18]- is stabilized by TOA+ 8 valence electrons ⇒ closed shell structure Au(I)-SG polymers are reduced to [Au 25(SG)18]in the Brust-Schiffrin Method. Negishi, Y. et al. J. Am. Chem. Soc. 2007, 129, 11322.
Fractionation of gold clusters Stability of Au 25(SG)18 “Magic-numbered cluster” ― Anionic form [Au 25(SG)18]- is stabilized by TOA+ 8 valence electrons ⇒ closed shell structure Au(I)-SG polymers are reduced to [Au 25(SG)18]in the Brust-Schiffrin Method. Negishi, Y. et al. J. Am. Chem. Soc. 2007, 129, 11322.
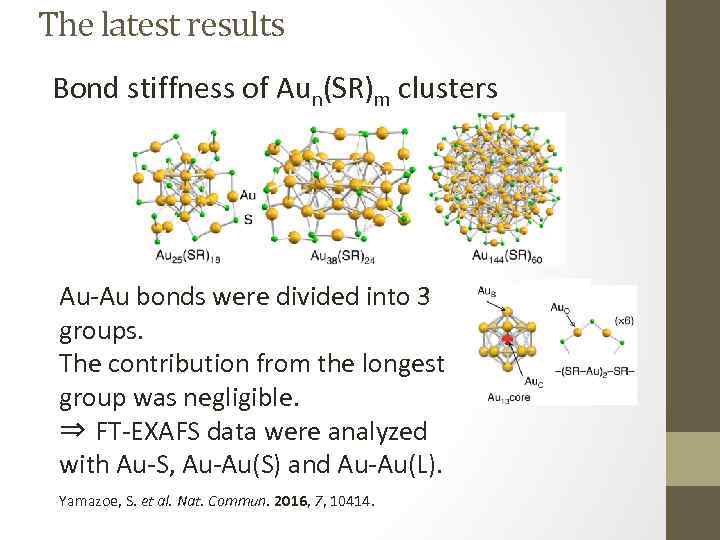 The latest results Bond stiffness of Aun(SR)m clusters Au-Au bonds were divided into 3 groups. The contribution from the longest group was negligible. ⇒ FT-EXAFS data were analyzed with Au-S, Au-Au(S) and Au-Au(L). Yamazoe, S. et al. Nat. Commun. 2016, 7, 10414.
The latest results Bond stiffness of Aun(SR)m clusters Au-Au bonds were divided into 3 groups. The contribution from the longest group was negligible. ⇒ FT-EXAFS data were analyzed with Au-S, Au-Au(S) and Au-Au(L). Yamazoe, S. et al. Nat. Commun. 2016, 7, 10414.
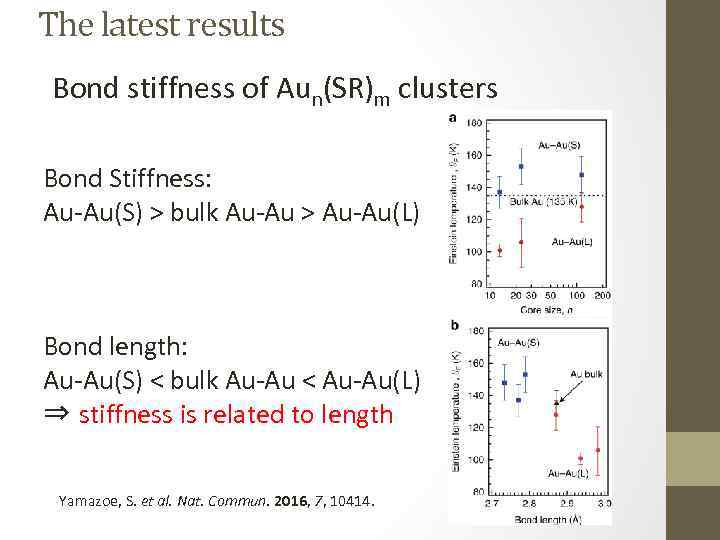 The latest results Bond stiffness of Aun(SR)m clusters Bond Stiffness: Au-Au(S) > bulk Au-Au > Au-Au(L) Bond length: Au-Au(S) < bulk Au-Au < Au-Au(L) ⇒ stiffness is related to length Yamazoe, S. et al. Nat. Commun. 2016, 7, 10414.
The latest results Bond stiffness of Aun(SR)m clusters Bond Stiffness: Au-Au(S) > bulk Au-Au > Au-Au(L) Bond length: Au-Au(S) < bulk Au-Au < Au-Au(L) ⇒ stiffness is related to length Yamazoe, S. et al. Nat. Commun. 2016, 7, 10414.
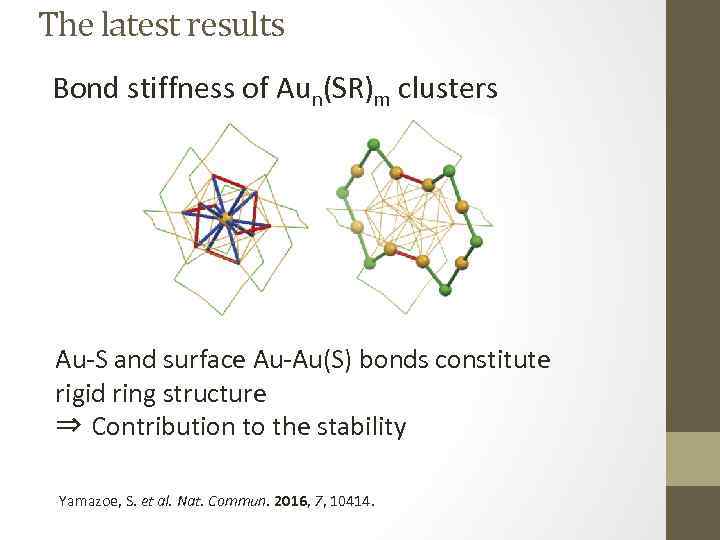 The latest results Bond stiffness of Aun(SR)m clusters Au-S and surface Au-Au(S) bonds constitute rigid ring structure ⇒ Contribution to the stability Yamazoe, S. et al. Nat. Commun. 2016, 7, 10414.
The latest results Bond stiffness of Aun(SR)m clusters Au-S and surface Au-Au(S) bonds constitute rigid ring structure ⇒ Contribution to the stability Yamazoe, S. et al. Nat. Commun. 2016, 7, 10414.
 Conclusion • Bottom-up preparation of gold clusters can be achieved by protection with ligands. • Monodisperse clusters are obtained by the fractionation (PAGE, GPC, and size-selective etching). • Magic-numbered clusters are thermodynamically stable and forms dominantly. The stability is explained by their electronic or geometric structures.
Conclusion • Bottom-up preparation of gold clusters can be achieved by protection with ligands. • Monodisperse clusters are obtained by the fractionation (PAGE, GPC, and size-selective etching). • Magic-numbered clusters are thermodynamically stable and forms dominantly. The stability is explained by their electronic or geometric structures.
 Thank you for listening!!
Thank you for listening!!


