 THE WATER
THE WATER
 The water is a chemical substance with the formula H 2 O. The water is very important for life, because it is vital. The animals or humans drink water because the body needs 75% water to do exercise for example: walk. Water covers 70% of the Earth. Like two thirds parts of the earth.
The water is a chemical substance with the formula H 2 O. The water is very important for life, because it is vital. The animals or humans drink water because the body needs 75% water to do exercise for example: walk. Water covers 70% of the Earth. Like two thirds parts of the earth.
 The bonds which hold the hydrogen and oxygen together are called covalent bonds - they are very strong. These are the electrons that are not involved in the covalent bonds. The pairs of electrons are left alone. In our picture they are represented by the double dots. These lone pairs are very negative containing two negative electrons each - and want to stay away from each other as much as possible. These repulsive forces act to push the hydrogens closer together
The bonds which hold the hydrogen and oxygen together are called covalent bonds - they are very strong. These are the electrons that are not involved in the covalent bonds. The pairs of electrons are left alone. In our picture they are represented by the double dots. These lone pairs are very negative containing two negative electrons each - and want to stay away from each other as much as possible. These repulsive forces act to push the hydrogens closer together
 Water is the only natural substance found in all three common states of matter liquid, solid, gas/vapor. Water is the only common substance less dense in solid form than in liquid form. Water can dissolve more substances than any other liquid.
Water is the only natural substance found in all three common states of matter liquid, solid, gas/vapor. Water is the only common substance less dense in solid form than in liquid form. Water can dissolve more substances than any other liquid.

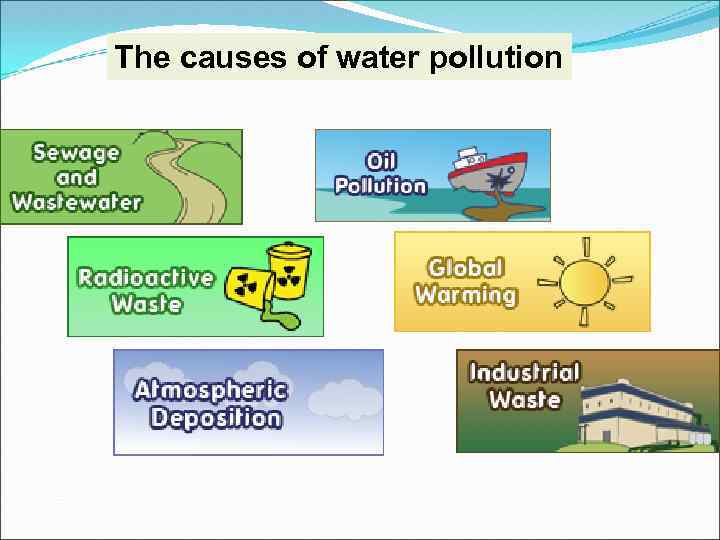 The causes of water pollution
The causes of water pollution
 Water Hardness Hard water is due to metal ions (minerals) that are dissolved in the ground water. These minerals include Ca 2+, Mg 2+, Fe 3+, SO 42 -, and HCO 3 The concentration of the Ca 2+ ions is greater than the concentration of any other metal ion in our water Water hardness is usually expressed in ppm Ca. CO 3
Water Hardness Hard water is due to metal ions (minerals) that are dissolved in the ground water. These minerals include Ca 2+, Mg 2+, Fe 3+, SO 42 -, and HCO 3 The concentration of the Ca 2+ ions is greater than the concentration of any other metal ion in our water Water hardness is usually expressed in ppm Ca. CO 3
 Why Be Concerned About Hard Water? Originally, water hardness was defined as the measure of the capacity of the water to precipitate soap Hard water does cause soap scum, clogs pipes and clogs boilers as limescale
Why Be Concerned About Hard Water? Originally, water hardness was defined as the measure of the capacity of the water to precipitate soap Hard water does cause soap scum, clogs pipes and clogs boilers as limescale
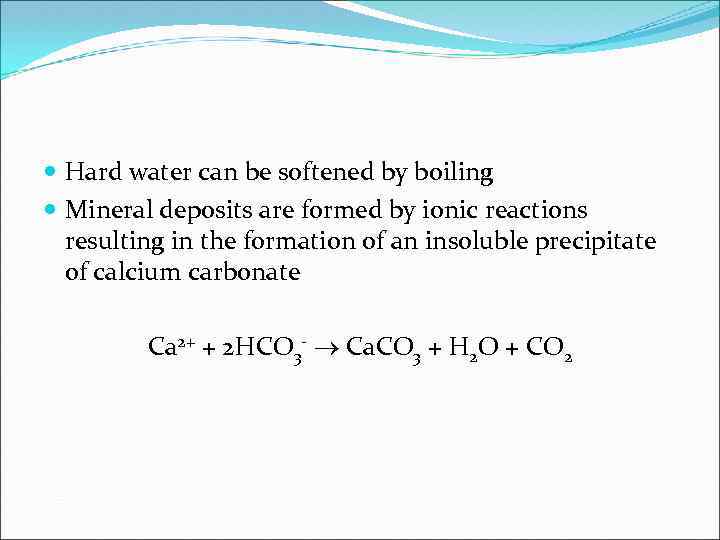 Hard water can be softened by boiling Mineral deposits are formed by ionic reactions resulting in the formation of an insoluble precipitate of calcium carbonate Ca 2+ + 2 HCO 3 - Ca. CO 3 + H 2 O + CO 2
Hard water can be softened by boiling Mineral deposits are formed by ionic reactions resulting in the formation of an insoluble precipitate of calcium carbonate Ca 2+ + 2 HCO 3 - Ca. CO 3 + H 2 O + CO 2
 Soaps are long chain fatty acids Soap scum is formed when the Ca 2+ ion binds with the soap. This causes an insoluble compound that precipitates to form the scum you see. Soap actually softens hard water by removing the Ca 2+ ions from the water When hard water is heated, Ca. CO 3 precipitates out, which then clogs pipes and industrial boilers. This leads to malfunction or damage and is expensive to remove
Soaps are long chain fatty acids Soap scum is formed when the Ca 2+ ion binds with the soap. This causes an insoluble compound that precipitates to form the scum you see. Soap actually softens hard water by removing the Ca 2+ ions from the water When hard water is heated, Ca. CO 3 precipitates out, which then clogs pipes and industrial boilers. This leads to malfunction or damage and is expensive to remove
 Temporary Hardness is due to the bicarbonate ion, HCO 3 -, being present in the water. This type of hardness can be removed by boiling the water to expel the CO 2, as indicated by the following equation Ca 2+ + 2 HCO 3 - Ca. CO 3 + H 2 O + CO 2 Bicarbonate hardness is classified as temporary hardness
Temporary Hardness is due to the bicarbonate ion, HCO 3 -, being present in the water. This type of hardness can be removed by boiling the water to expel the CO 2, as indicated by the following equation Ca 2+ + 2 HCO 3 - Ca. CO 3 + H 2 O + CO 2 Bicarbonate hardness is classified as temporary hardness
 Permanent hardness is due to the presence of the ions Ca 2+, Mg+2, Fe 3+ and SO 42 -. This type of hardness cannot be eliminated by boiling The water with this type of hardness is said to be permanently hard
Permanent hardness is due to the presence of the ions Ca 2+, Mg+2, Fe 3+ and SO 42 -. This type of hardness cannot be eliminated by boiling The water with this type of hardness is said to be permanently hard
 Objectives To quantitatively determine Total, Permanent, and Calcium hardness in a sample of tap water To gain some basic analytical knowledge through analysis of water samples To become familiar with terminology such as ppm and to apply techniques learned from volumetric analysis to basic environmental analysis
Objectives To quantitatively determine Total, Permanent, and Calcium hardness in a sample of tap water To gain some basic analytical knowledge through analysis of water samples To become familiar with terminology such as ppm and to apply techniques learned from volumetric analysis to basic environmental analysis
 Complexometric Titration Water hardness is usually determined by titrating with a standard solution of ethylenediamminetetraacetic acid, EDTA is a complexing, or chelating agent used to capture the metal ions This causes the water to become softened, but the metal ions are not removed from the water EDTA simply binds the metal ions to it very tightly
Complexometric Titration Water hardness is usually determined by titrating with a standard solution of ethylenediamminetetraacetic acid, EDTA is a complexing, or chelating agent used to capture the metal ions This causes the water to become softened, but the metal ions are not removed from the water EDTA simply binds the metal ions to it very tightly
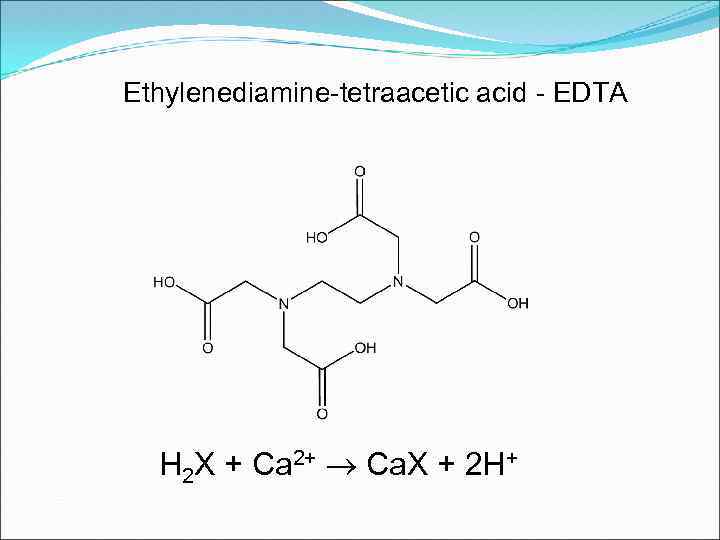 Ethylenediamine-tetraacetic acid - EDTA H 2 X + Ca 2+ Ca. X + 2 H+
Ethylenediamine-tetraacetic acid - EDTA H 2 X + Ca 2+ Ca. X + 2 H+
 EDTA is a versatile chelating agent A chelating agent is a substance whose molecules can form several bonds to a single metal ion Chelating agents are multi-dentate ligands. A ligand is a substance that binds with metal ions to form a complex ion Multidentate ligands are many clawed, holding onto the metal ion to form a very stable complex EDTA can form four or six bonds with a metal ion
EDTA is a versatile chelating agent A chelating agent is a substance whose molecules can form several bonds to a single metal ion Chelating agents are multi-dentate ligands. A ligand is a substance that binds with metal ions to form a complex ion Multidentate ligands are many clawed, holding onto the metal ion to form a very stable complex EDTA can form four or six bonds with a metal ion
 EDTA It is frequently used in soaps and detergents because it forms complexes with calcium and magnesium ions Certain enzymes are responsible for food spoilage. EDTA is used to remove metal ions from these enzymes Used to promote colour retention, and to improve flavour retention in foods
EDTA It is frequently used in soaps and detergents because it forms complexes with calcium and magnesium ions Certain enzymes are responsible for food spoilage. EDTA is used to remove metal ions from these enzymes Used to promote colour retention, and to improve flavour retention in foods
 Titrations Use one tablet of indicator to develop a good colour Titrate water with EDTA until colour changes from red to blue
Titrations Use one tablet of indicator to develop a good colour Titrate water with EDTA until colour changes from red to blue
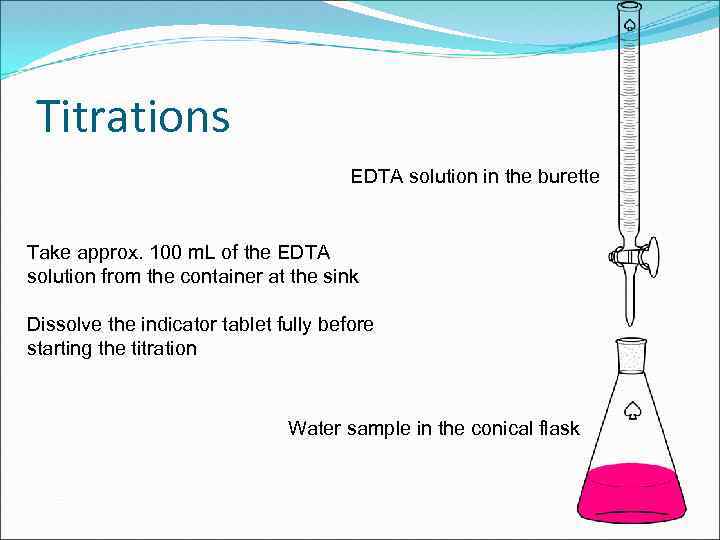 Titrations EDTA solution in the burette Take approx. 100 m. L of the EDTA solution from the container at the sink Dissolve the indicator tablet fully before starting the titration Water sample in the conical flask
Titrations EDTA solution in the burette Take approx. 100 m. L of the EDTA solution from the container at the sink Dissolve the indicator tablet fully before starting the titration Water sample in the conical flask
 Titrations Titrate for total hardness Titrate a boiled sample for permanent and hence temporary hardness Add murexide to a sample at p. H 12 to precipitate any Mg 2+ as Mg(OH)2. Then titrate to obtain Calcium and hence Magnesium hardness
Titrations Titrate for total hardness Titrate a boiled sample for permanent and hence temporary hardness Add murexide to a sample at p. H 12 to precipitate any Mg 2+ as Mg(OH)2. Then titrate to obtain Calcium and hence Magnesium hardness
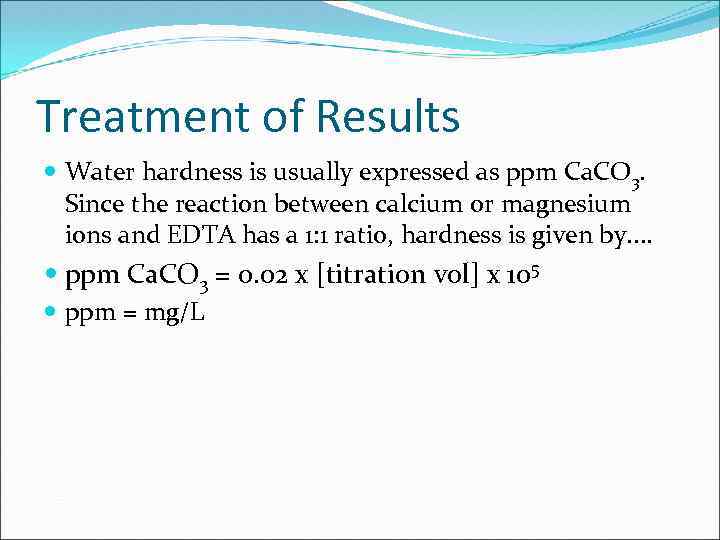 Treatment of Results Water hardness is usually expressed as ppm Ca. CO 3. Since the reaction between calcium or magnesium ions and EDTA has a 1: 1 ratio, hardness is given by. . ppm Ca. CO 3 = 0. 02 x [titration vol] x 105 ppm = mg/L
Treatment of Results Water hardness is usually expressed as ppm Ca. CO 3. Since the reaction between calcium or magnesium ions and EDTA has a 1: 1 ratio, hardness is given by. . ppm Ca. CO 3 = 0. 02 x [titration vol] x 105 ppm = mg/L
![ppm Ca. CO 3 = 0. 02 x [titration vol] x 105 M 1 ppm Ca. CO 3 = 0. 02 x [titration vol] x 105 M 1](https://present5.com/presentation/-58201108_300341003/image-22.jpg) ppm Ca. CO 3 = 0. 02 x [titration vol] x 105 M 1 V 1 = M 2 V 2 [n 1, n 2=1] M 1 x 10 -2 = 0. 02 x [volume] M 1 = 0. 02 x [volume] x 102 Mol mass Ca. CO 3 = 100 g mol-1 g. L-1 = 0. 02 x [volume] x 102 ppm = 0. 02 x [volume] x 105 Temporary hardness = total – permanent Magnesium hardness = total – calcium
ppm Ca. CO 3 = 0. 02 x [titration vol] x 105 M 1 V 1 = M 2 V 2 [n 1, n 2=1] M 1 x 10 -2 = 0. 02 x [volume] M 1 = 0. 02 x [volume] x 102 Mol mass Ca. CO 3 = 100 g mol-1 g. L-1 = 0. 02 x [volume] x 102 ppm = 0. 02 x [volume] x 105 Temporary hardness = total – permanent Magnesium hardness = total – calcium
 Report Experimental observations Balanced chemical equations All titration results Calculations Completed table
Report Experimental observations Balanced chemical equations All titration results Calculations Completed table


