439c8859421ca3de2b867982e4ca4c94.ppt
- Количество слайдов: 27
 The Vaporization Enthalpies and Vapor Pressures of Two Insecticide Components, Muscalure and Empenthrin By Correlation Gas Chromatography Jessica Spencer and James Chickos Department of Chemistry and Biochemistry University of Missouri-St. Louis MO 63121 E-mail: jsc@umsl. edu A portion of the Science Complex at UMSL
The Vaporization Enthalpies and Vapor Pressures of Two Insecticide Components, Muscalure and Empenthrin By Correlation Gas Chromatography Jessica Spencer and James Chickos Department of Chemistry and Biochemistry University of Missouri-St. Louis MO 63121 E-mail: jsc@umsl. edu A portion of the Science Complex at UMSL
 Outline of the Presentation Properties of the targets Introduction to the fundamentals of correlation gas chromatography Demonstration of the method as applied to: Muscalure Application to evaluate the vaporization enthalpy and vapor pressure of empenthrin Comparison of the results on empenthrin with those obtained by another gas chromatographic retention time method that has been criticized recently 1 Ruzicka, K. ; Koutek, B. ; Fulem, M. ; Hoskovec, M. Indirect Determination of Vapor Pressures by Capillary Gas-Liquid Chromatography: Analysis of the Reference Vapor –Pressure Data and Their Treatment. J. Chem. Eng. Data 2011, 57, 1349 -68. 1
Outline of the Presentation Properties of the targets Introduction to the fundamentals of correlation gas chromatography Demonstration of the method as applied to: Muscalure Application to evaluate the vaporization enthalpy and vapor pressure of empenthrin Comparison of the results on empenthrin with those obtained by another gas chromatographic retention time method that has been criticized recently 1 Ruzicka, K. ; Koutek, B. ; Fulem, M. ; Hoskovec, M. Indirect Determination of Vapor Pressures by Capillary Gas-Liquid Chromatography: Analysis of the Reference Vapor –Pressure Data and Their Treatment. J. Chem. Eng. Data 2011, 57, 1349 -68. 1
 Information on the Compounds Investigated Muscalure: Z 9 -Tricosene, is a sex pheromone produce by female house flies (Musca domestica). Muscalure in combination with other fecal odors provides maximum attraction for male flies. It is used as a pesticide is in combination with fly paper or other traps. Z 9 -Tricosene also serves as a communication pheromone in the waggle dance of bees. The synthetic sample also contains a small amount of E 9 -Tricosene. Empenthrin: (E)-(RS)-1 -ethynyl-2 -methylpent-2 -enyl (1 RS) -cis-trans-2, 2 -dimethyl-3 -(2 -methylprop-1 -enyl)cyclopropane-carboxylate is a synthetic pyrethrin used as a pesticide. It has a broad spectrum of activity on various flying insects but relatively low mammalian toxicity. It consists of a racemic mixture of up to 4 possible diasteriomers. At least three of the diasteriomers were detected in the commercial product.
Information on the Compounds Investigated Muscalure: Z 9 -Tricosene, is a sex pheromone produce by female house flies (Musca domestica). Muscalure in combination with other fecal odors provides maximum attraction for male flies. It is used as a pesticide is in combination with fly paper or other traps. Z 9 -Tricosene also serves as a communication pheromone in the waggle dance of bees. The synthetic sample also contains a small amount of E 9 -Tricosene. Empenthrin: (E)-(RS)-1 -ethynyl-2 -methylpent-2 -enyl (1 RS) -cis-trans-2, 2 -dimethyl-3 -(2 -methylprop-1 -enyl)cyclopropane-carboxylate is a synthetic pyrethrin used as a pesticide. It has a broad spectrum of activity on various flying insects but relatively low mammalian toxicity. It consists of a racemic mixture of up to 4 possible diasteriomers. At least three of the diasteriomers were detected in the commercial product.
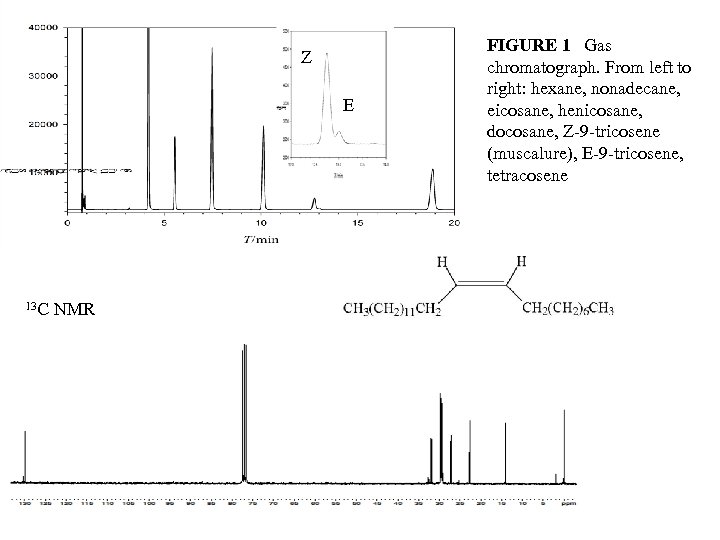 Z E 13 C NMR FIGURE 1 Gas chromatograph. From left to right: hexane, nonadecane, eicosane, henicosane, docosane, Z-9 -tricosene (muscalure), E-9 -tricosene, tetracosene
Z E 13 C NMR FIGURE 1 Gas chromatograph. From left to right: hexane, nonadecane, eicosane, henicosane, docosane, Z-9 -tricosene (muscalure), E-9 -tricosene, tetracosene
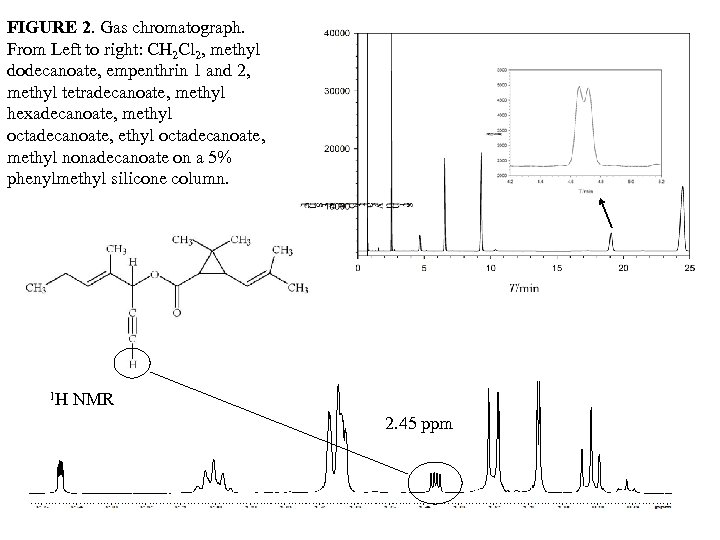 FIGURE 2. Gas chromatograph. From Left to right: CH 2 Cl 2, methyl dodecanoate, empenthrin 1 and 2, methyl tetradecanoate, methyl hexadecanoate, methyl octadecanoate, methyl nonadecanoate on a 5% phenylmethyl silicone column. 1 H NMR 2. 45 ppm
FIGURE 2. Gas chromatograph. From Left to right: CH 2 Cl 2, methyl dodecanoate, empenthrin 1 and 2, methyl tetradecanoate, methyl hexadecanoate, methyl octadecanoate, methyl nonadecanoate on a 5% phenylmethyl silicone column. 1 H NMR 2. 45 ppm
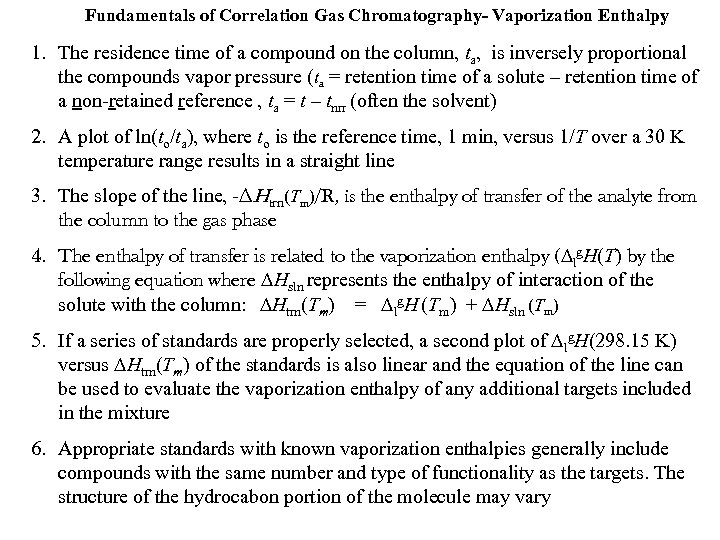 Fundamentals of Correlation Gas Chromatography- Vaporization Enthalpy 1. The residence time of a compound on the column, ta, is inversely proportional the compounds vapor pressure (ta = retention time of a solute – retention time of a non-retained reference , ta = t – tnrr (often the solvent) 2. A plot of ln(to/ta), where to is the reference time, 1 min, versus 1/T over a 30 K temperature range results in a straight line 3. The slope of the line, -∆Htrn(Tm)/R, is the enthalpy of transfer of the analyte from the column to the gas phase 4. The enthalpy of transfer is related to the vaporization enthalpy (∆lg. H(T) by the following equation where ∆Hsln represents the enthalpy of interaction of the solute with the column: ∆Htrn(Tm) = ∆lg. H (Tm) + ∆Hsln (Tm) 5. If a series of standards are properly selected, a second plot of ∆lg. H(298. 15 K) versus ∆Htrn(Tm) of the standards is also linear and the equation of the line can be used to evaluate the vaporization enthalpy of any additional targets included in the mixture 6. Appropriate standards with known vaporization enthalpies generally include compounds with the same number and type of functionality as the targets. The structure of the hydrocabon portion of the molecule may vary
Fundamentals of Correlation Gas Chromatography- Vaporization Enthalpy 1. The residence time of a compound on the column, ta, is inversely proportional the compounds vapor pressure (ta = retention time of a solute – retention time of a non-retained reference , ta = t – tnrr (often the solvent) 2. A plot of ln(to/ta), where to is the reference time, 1 min, versus 1/T over a 30 K temperature range results in a straight line 3. The slope of the line, -∆Htrn(Tm)/R, is the enthalpy of transfer of the analyte from the column to the gas phase 4. The enthalpy of transfer is related to the vaporization enthalpy (∆lg. H(T) by the following equation where ∆Hsln represents the enthalpy of interaction of the solute with the column: ∆Htrn(Tm) = ∆lg. H (Tm) + ∆Hsln (Tm) 5. If a series of standards are properly selected, a second plot of ∆lg. H(298. 15 K) versus ∆Htrn(Tm) of the standards is also linear and the equation of the line can be used to evaluate the vaporization enthalpy of any additional targets included in the mixture 6. Appropriate standards with known vaporization enthalpies generally include compounds with the same number and type of functionality as the targets. The structure of the hydrocabon portion of the molecule may vary
 Fundamentals of Correlation Gas Chromatography - Vapor Pressure Use of appropriate standards with known vapor pressures also results in linear plots between ln(p/po) and ln(to/ta) The equation of the line plus values of ln(to/ta) of the targets results in their vapor pressures Performed over a range of temperatures can provide the vapor pressure temperature profile of the targets
Fundamentals of Correlation Gas Chromatography - Vapor Pressure Use of appropriate standards with known vapor pressures also results in linear plots between ln(p/po) and ln(to/ta) The equation of the line plus values of ln(to/ta) of the targets results in their vapor pressures Performed over a range of temperatures can provide the vapor pressure temperature profile of the targets
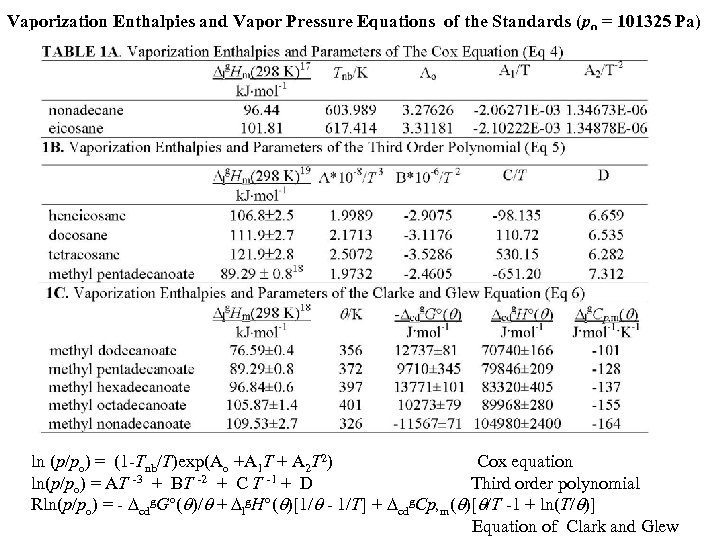 Vaporization Enthalpies and Vapor Pressure Equations of the Standards (po = 101325 Pa) ln (p/po) = (1 -Tnb/T)exp(Ao +A 1 T + A 2 T 2) Cox equation ln(p/po) = AT -3 + BT -2 + C T -1 + D Third order polynomial Rln(p/po) = - cdg. G°( )/ + lg. H°( )[1/ - 1/T] + cdg. Cp, m( )[ /T -1 + ln(T/ )] Equation of Clark and Glew
Vaporization Enthalpies and Vapor Pressure Equations of the Standards (po = 101325 Pa) ln (p/po) = (1 -Tnb/T)exp(Ao +A 1 T + A 2 T 2) Cox equation ln(p/po) = AT -3 + BT -2 + C T -1 + D Third order polynomial Rln(p/po) = - cdg. G°( )/ + lg. H°( )[1/ - 1/T] + cdg. Cp, m( )[ /T -1 + ln(T/ )] Equation of Clark and Glew
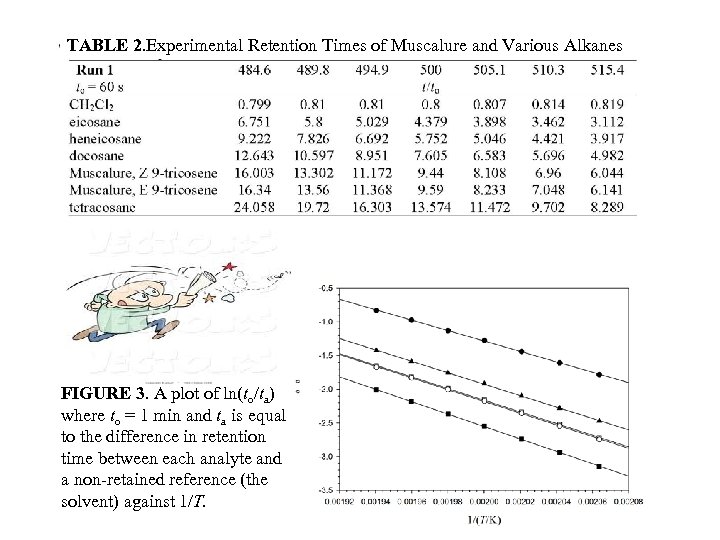 2 TABLE 2. Experimental Retention Times of Muscalure and Various Alkanes FIGURE 3. A plot of ln(to/ta) where to = 1 min and ta is equal to the difference in retention time between each analyte and a non-retained reference (the solvent) against 1/T.
2 TABLE 2. Experimental Retention Times of Muscalure and Various Alkanes FIGURE 3. A plot of ln(to/ta) where to = 1 min and ta is equal to the difference in retention time between each analyte and a non-retained reference (the solvent) against 1/T.
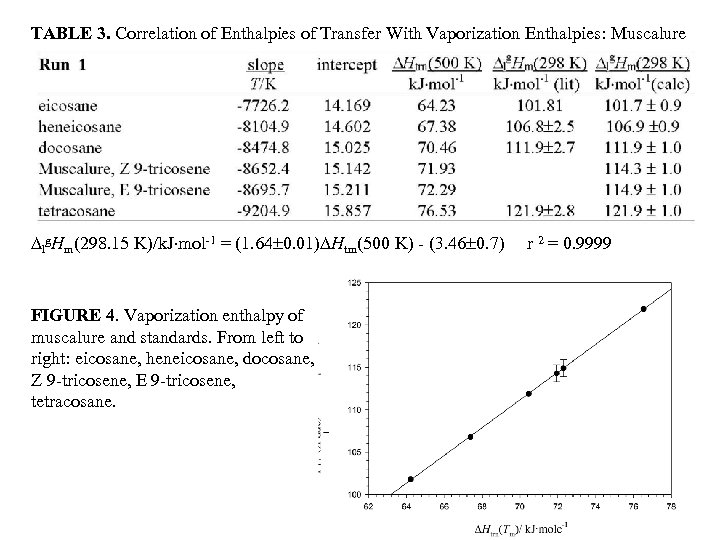 TABLE 3. Correlation of Enthalpies of Transfer With Vaporization Enthalpies: Muscalure lg. Hm(298. 15 K)/k. J mol-1 = (1. 64 0. 01) Htrn(500 K) - (3. 46 0. 7) FIGURE 4. Vaporization enthalpy of muscalure and standards. From left to right: eicosane, heneicosane, docosane, Z 9 -tricosene, E 9 -tricosene, tetracosane. r 2 = 0. 9999
TABLE 3. Correlation of Enthalpies of Transfer With Vaporization Enthalpies: Muscalure lg. Hm(298. 15 K)/k. J mol-1 = (1. 64 0. 01) Htrn(500 K) - (3. 46 0. 7) FIGURE 4. Vaporization enthalpy of muscalure and standards. From left to right: eicosane, heneicosane, docosane, Z 9 -tricosene, E 9 -tricosene, tetracosane. r 2 = 0. 9999
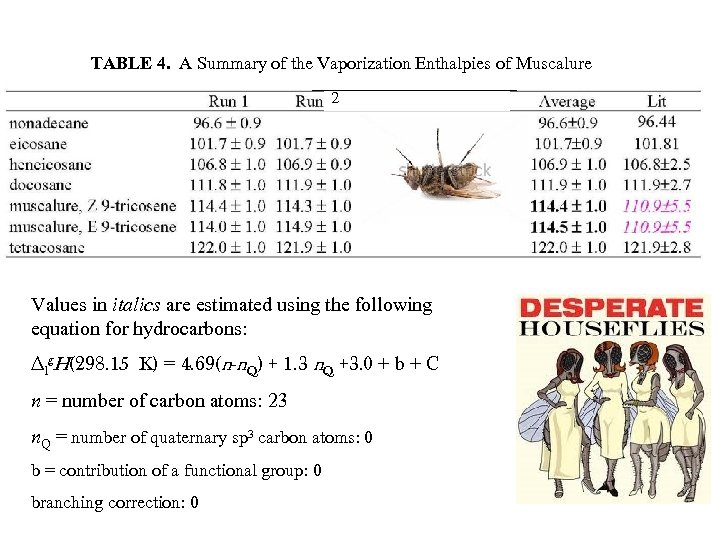 TABLE 4. A Summary of the Vaporization Enthalpies of Muscalure 2 Values in italics are estimated using the following equation for hydrocarbons: ∆lg. H(298. 15 K) = 4. 69(n-n. Q) + 1. 3 n. Q +3. 0 + b + C n = number of carbon atoms: 23 n. Q = number of quaternary sp 3 carbon atoms: 0 b = contribution of a functional group: 0 branching correction: 0
TABLE 4. A Summary of the Vaporization Enthalpies of Muscalure 2 Values in italics are estimated using the following equation for hydrocarbons: ∆lg. H(298. 15 K) = 4. 69(n-n. Q) + 1. 3 n. Q +3. 0 + b + C n = number of carbon atoms: 23 n. Q = number of quaternary sp 3 carbon atoms: 0 b = contribution of a functional group: 0 branching correction: 0
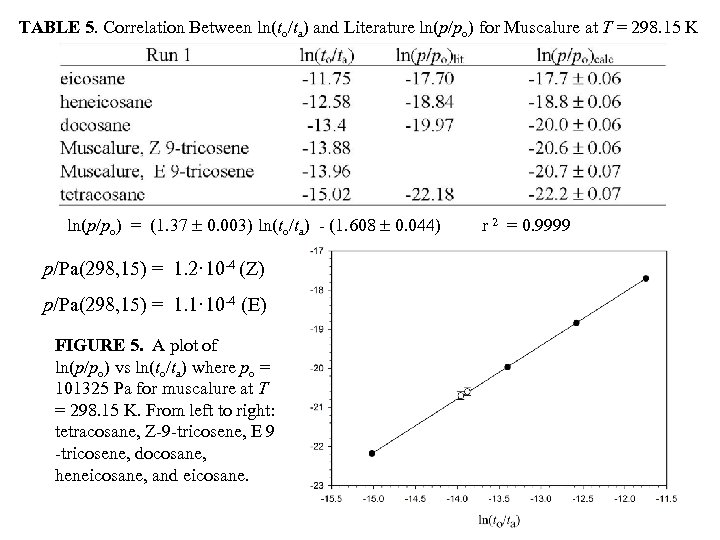 TABLE 5. Correlation Between ln(to/ta) and Literature ln(p/po) for Muscalure at T = 298. 15 K ln(p/po) = (1. 37 0. 003) ln(to/ta) - (1. 608 0. 044) p/Pa(298, 15) = 1. 2· 10 -4 (Z) p/Pa(298, 15) = 1. 1· 10 -4 (E) FIGURE 5. A plot of ln(p/po) vs ln(to/ta) where po = 101325 Pa for muscalure at T = 298. 15 K. From left to right: tetracosane, Z-9 -tricosene, E 9 -tricosene, docosane, heneicosane, and eicosane. r 2 = 0. 9999
TABLE 5. Correlation Between ln(to/ta) and Literature ln(p/po) for Muscalure at T = 298. 15 K ln(p/po) = (1. 37 0. 003) ln(to/ta) - (1. 608 0. 044) p/Pa(298, 15) = 1. 2· 10 -4 (Z) p/Pa(298, 15) = 1. 1· 10 -4 (E) FIGURE 5. A plot of ln(p/po) vs ln(to/ta) where po = 101325 Pa for muscalure at T = 298. 15 K. From left to right: tetracosane, Z-9 -tricosene, E 9 -tricosene, docosane, heneicosane, and eicosane. r 2 = 0. 9999
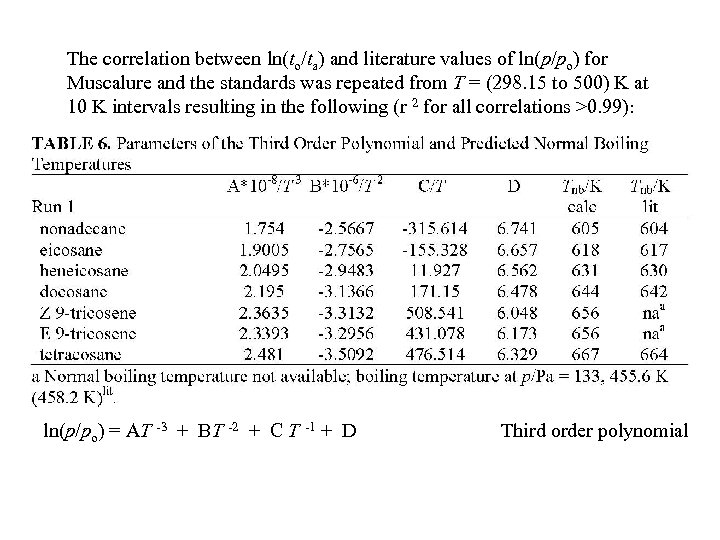 The correlation between ln(to/ta) and literature values of ln(p/po) for Muscalure and the standards was repeated from T = (298. 15 to 500) K at 10 K intervals resulting in the following (r 2 for all correlations >0. 99): ln(p/po) = AT -3 + BT -2 + C T -1 + D Third order polynomial
The correlation between ln(to/ta) and literature values of ln(p/po) for Muscalure and the standards was repeated from T = (298. 15 to 500) K at 10 K intervals resulting in the following (r 2 for all correlations >0. 99): ln(p/po) = AT -3 + BT -2 + C T -1 + D Third order polynomial
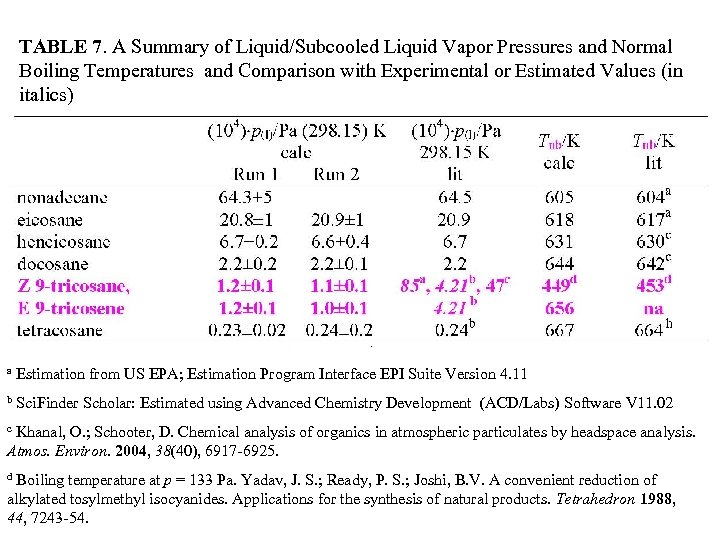 TABLE 7. A Summary of Liquid/Subcooled Liquid Vapor Pressures and Normal Boiling Temperatures and Comparison with Experimental or Estimated Values (in italics) a Estimation from US EPA; Estimation Program Interface EPI Suite Version 4. 11 b Sci. Finder Scholar: Estimated using Advanced Chemistry Development (ACD/Labs) Software V 11. 02 Khanal, O. ; Schooter, D. Chemical analysis of organics in atmospheric particulates by headspace analysis. Atmos. Environ. 2004, 38(40), 6917 -6925. c Boiling temperature at p = 133 Pa. Yadav, J. S. ; Ready, P. S. ; Joshi, B. V. A convenient reduction of alkylated tosylmethyl isocyanides. Applications for the synthesis of natural products. Tetrahedron 1988, 44, 7243 -54. d
TABLE 7. A Summary of Liquid/Subcooled Liquid Vapor Pressures and Normal Boiling Temperatures and Comparison with Experimental or Estimated Values (in italics) a Estimation from US EPA; Estimation Program Interface EPI Suite Version 4. 11 b Sci. Finder Scholar: Estimated using Advanced Chemistry Development (ACD/Labs) Software V 11. 02 Khanal, O. ; Schooter, D. Chemical analysis of organics in atmospheric particulates by headspace analysis. Atmos. Environ. 2004, 38(40), 6917 -6925. c Boiling temperature at p = 133 Pa. Yadav, J. S. ; Ready, P. S. ; Joshi, B. V. A convenient reduction of alkylated tosylmethyl isocyanides. Applications for the synthesis of natural products. Tetrahedron 1988, 44, 7243 -54. d
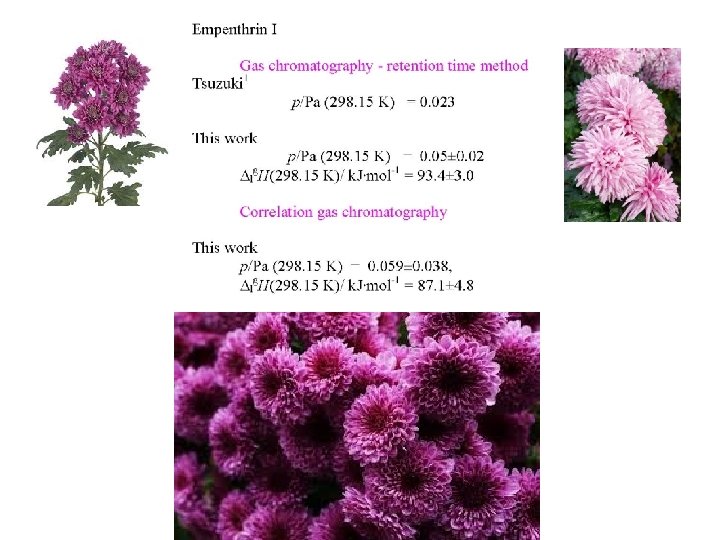
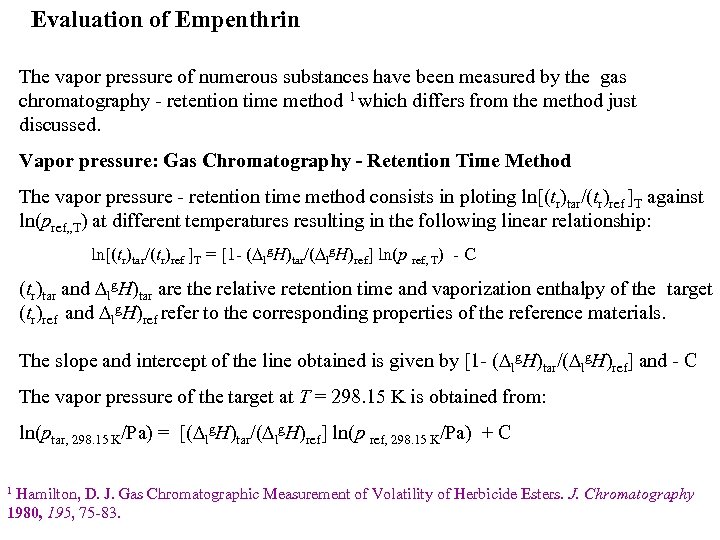 Evaluation of Empenthrin The vapor pressure of numerous substances have been measured by the gas chromatography - retention time method 1 which differs from the method just discussed. Vapor pressure: Gas Chromatography - Retention Time Method The vapor pressure - retention time method consists in ploting ln[(tr)tar/(tr)ref ]T against ln(pref, , T) at different temperatures resulting in the following linear relationship: ln[(tr)tar/(tr)ref ]T = [1 - ( lg. H)tar/( lg. H)ref] ln(p ref, T) - C (tr)tar and lg. H)tar are the relative retention time and vaporization enthalpy of the target (tr)ref and lg. H)ref refer to the corresponding properties of the reference materials. The slope and intercept of the line obtained is given by [1 - ( lg. H)tar/( lg. H)ref] and - C The vapor pressure of the target at T = 298. 15 K is obtained from: ln(ptar, 298. 15 K/Pa) = [( lg. H)tar/( lg. H)ref] ln(p ref, 298. 15 K/Pa) + C Hamilton, D. J. Gas Chromatographic Measurement of Volatility of Herbicide Esters. J. Chromatography 1980, 195, 75 -83. 1
Evaluation of Empenthrin The vapor pressure of numerous substances have been measured by the gas chromatography - retention time method 1 which differs from the method just discussed. Vapor pressure: Gas Chromatography - Retention Time Method The vapor pressure - retention time method consists in ploting ln[(tr)tar/(tr)ref ]T against ln(pref, , T) at different temperatures resulting in the following linear relationship: ln[(tr)tar/(tr)ref ]T = [1 - ( lg. H)tar/( lg. H)ref] ln(p ref, T) - C (tr)tar and lg. H)tar are the relative retention time and vaporization enthalpy of the target (tr)ref and lg. H)ref refer to the corresponding properties of the reference materials. The slope and intercept of the line obtained is given by [1 - ( lg. H)tar/( lg. H)ref] and - C The vapor pressure of the target at T = 298. 15 K is obtained from: ln(ptar, 298. 15 K/Pa) = [( lg. H)tar/( lg. H)ref] ln(p ref, 298. 15 K/Pa) + C Hamilton, D. J. Gas Chromatographic Measurement of Volatility of Herbicide Esters. J. Chromatography 1980, 195, 75 -83. 1
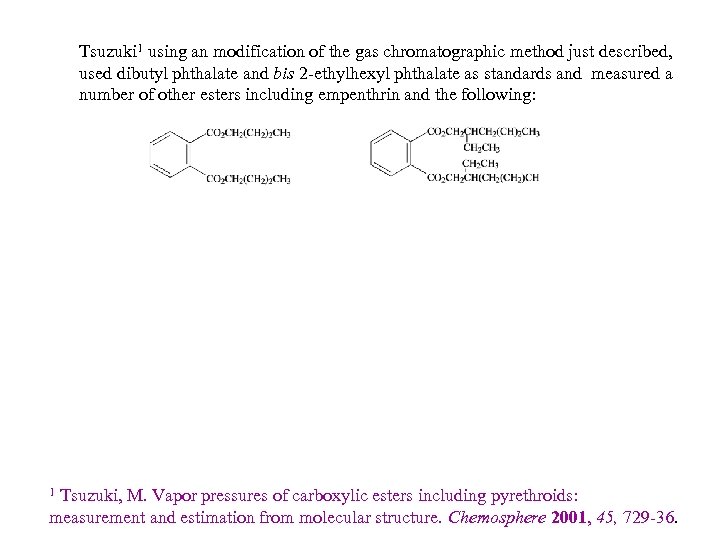 Tsuzuki 1 using an modification of the gas chromatographic method just described, used dibutyl phthalate and bis 2 -ethylhexyl phthalate as standards and measured a number of other esters including empenthrin and the following: It is not clear well phthalate diesters can serve as standards to these pyrethrinoids which in addition to being single esters have a variety of other functional groups. . Tsuzuki, M. Vapor pressures of carboxylic esters including pyrethroids: measurement and estimation from molecular structure. Chemosphere 2001, 45, 729 -36. 1
Tsuzuki 1 using an modification of the gas chromatographic method just described, used dibutyl phthalate and bis 2 -ethylhexyl phthalate as standards and measured a number of other esters including empenthrin and the following: It is not clear well phthalate diesters can serve as standards to these pyrethrinoids which in addition to being single esters have a variety of other functional groups. . Tsuzuki, M. Vapor pressures of carboxylic esters including pyrethroids: measurement and estimation from molecular structure. Chemosphere 2001, 45, 729 -36. 1
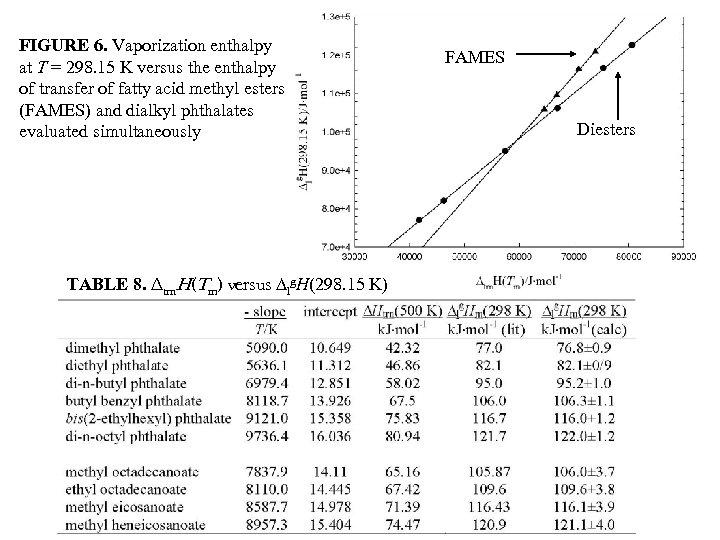 FIGURE 6. Vaporization enthalpy at T = 298. 15 K versus the enthalpy of transfer of fatty acid methyl esters (FAMES) and dialkyl phthalates evaluated simultaneously TABLE 8. ∆trn. H(Tm) versus ∆lg. H(298. 15 K) FAMES Diesters
FIGURE 6. Vaporization enthalpy at T = 298. 15 K versus the enthalpy of transfer of fatty acid methyl esters (FAMES) and dialkyl phthalates evaluated simultaneously TABLE 8. ∆trn. H(Tm) versus ∆lg. H(298. 15 K) FAMES Diesters
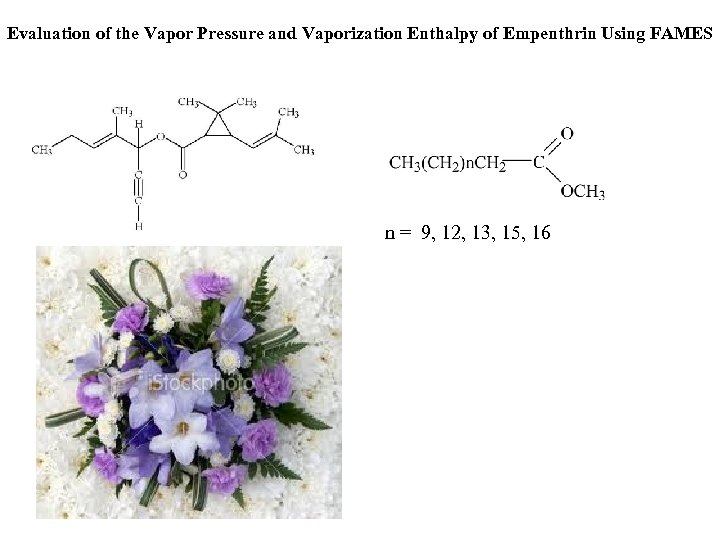 Evaluation of the Vapor Pressure and Vaporization Enthalpy of Empenthrin Using FAMES n = 9, 12, 13, 15, 16
Evaluation of the Vapor Pressure and Vaporization Enthalpy of Empenthrin Using FAMES n = 9, 12, 13, 15, 16
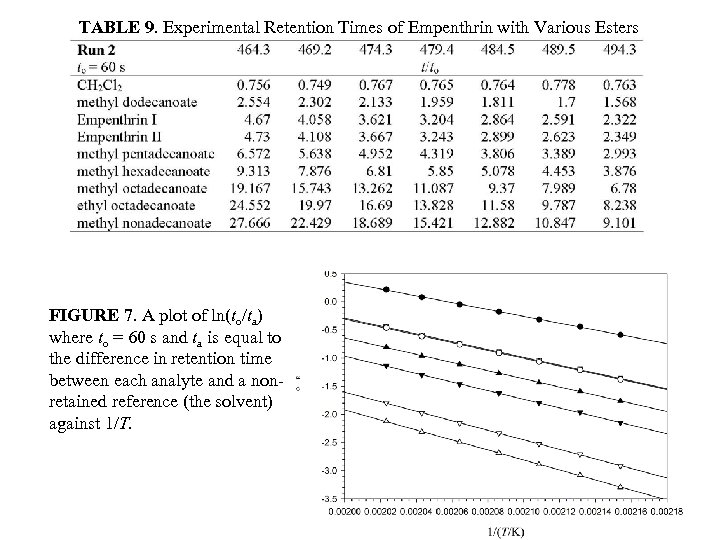 TABLE 9. Experimental Retention Times of Empenthrin with Various Esters FIGURE 7. A plot of ln(to/ta) where to = 60 s and ta is equal to the difference in retention time between each analyte and a nonretained reference (the solvent) against 1/T.
TABLE 9. Experimental Retention Times of Empenthrin with Various Esters FIGURE 7. A plot of ln(to/ta) where to = 60 s and ta is equal to the difference in retention time between each analyte and a nonretained reference (the solvent) against 1/T.
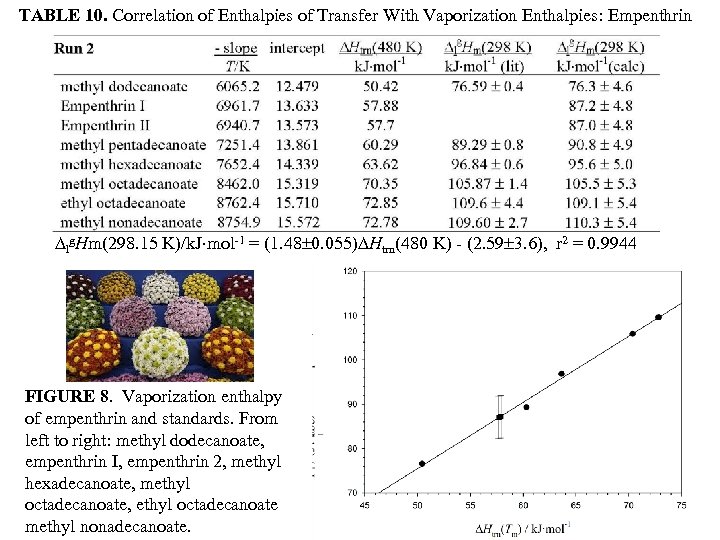 TABLE 10. Correlation of Enthalpies of Transfer With Vaporization Enthalpies: Empenthrin lg. Hm(298. 15 K)/k. J mol-1 = (1. 48 0. 055) Htrn(480 K) - (2. 59 3. 6), r 2 = 0. 9944 FIGURE 8. Vaporization enthalpy of empenthrin and standards. From left to right: methyl dodecanoate, empenthrin I, empenthrin 2, methyl hexadecanoate, methyl octadecanoate, ethyl octadecanoate methyl nonadecanoate.
TABLE 10. Correlation of Enthalpies of Transfer With Vaporization Enthalpies: Empenthrin lg. Hm(298. 15 K)/k. J mol-1 = (1. 48 0. 055) Htrn(480 K) - (2. 59 3. 6), r 2 = 0. 9944 FIGURE 8. Vaporization enthalpy of empenthrin and standards. From left to right: methyl dodecanoate, empenthrin I, empenthrin 2, methyl hexadecanoate, methyl octadecanoate, ethyl octadecanoate methyl nonadecanoate.
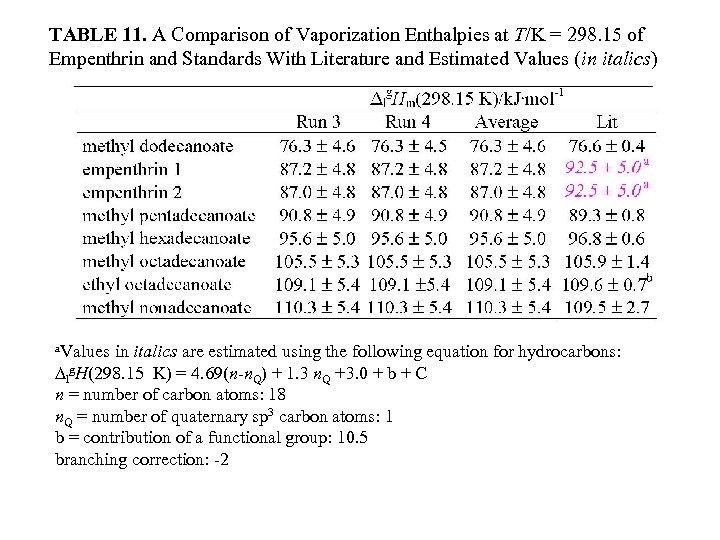 TABLE 11. A Comparison of Vaporization Enthalpies at T/K = 298. 15 of Empenthrin and Standards With Literature and Estimated Values (in italics) a. Values in italics are estimated using the following equation for hydrocarbons: ∆lg. H(298. 15 K) = 4. 69(n-n. Q) + 1. 3 n. Q +3. 0 + b + C n = number of carbon atoms: 18 n. Q = number of quaternary sp 3 carbon atoms: 1 b = contribution of a functional group: 10. 5 branching correction: -2
TABLE 11. A Comparison of Vaporization Enthalpies at T/K = 298. 15 of Empenthrin and Standards With Literature and Estimated Values (in italics) a. Values in italics are estimated using the following equation for hydrocarbons: ∆lg. H(298. 15 K) = 4. 69(n-n. Q) + 1. 3 n. Q +3. 0 + b + C n = number of carbon atoms: 18 n. Q = number of quaternary sp 3 carbon atoms: 1 b = contribution of a functional group: 10. 5 branching correction: -2
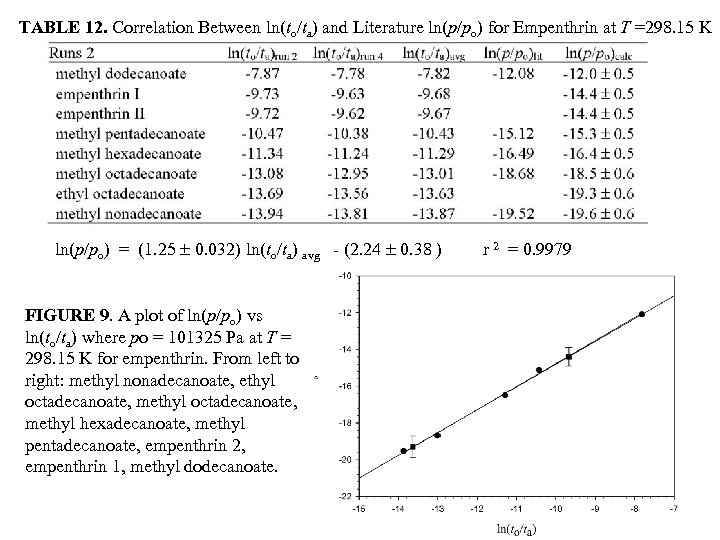 TABLE 12. Correlation Between ln(to/ta) and Literature ln(p/po) for Empenthrin at T =298. 15 K ln(p/po) = (1. 25 0. 032) ln(to/ta) avg - (2. 24 0. 38 ) FIGURE 9. A plot of ln(p/po) vs ln(to/ta) where po = 101325 Pa at T = 298. 15 K for empenthrin. From left to right: methyl nonadecanoate, ethyl octadecanoate, methyl hexadecanoate, methyl pentadecanoate, empenthrin 2, empenthrin 1, methyl dodecanoate. r 2 = 0. 9979
TABLE 12. Correlation Between ln(to/ta) and Literature ln(p/po) for Empenthrin at T =298. 15 K ln(p/po) = (1. 25 0. 032) ln(to/ta) avg - (2. 24 0. 38 ) FIGURE 9. A plot of ln(p/po) vs ln(to/ta) where po = 101325 Pa at T = 298. 15 K for empenthrin. From left to right: methyl nonadecanoate, ethyl octadecanoate, methyl hexadecanoate, methyl pentadecanoate, empenthrin 2, empenthrin 1, methyl dodecanoate. r 2 = 0. 9979
 Repeating this process at 10 K intervals from T = (298. 15 to 480) K resulted in the following vapor pressure – temperature profile; the data were fit to the following equation: ln(p/po) = AT -3 + BT -2 + C T -1 + D. All r 2 > 0. 99. TABLE 13. A Summary of Liquid/Subcooled Liquid Vapor Pressures and Normal Boiling Temperatures and Comparison with Experimental Values 1 Tsuzuki, M. Vapor pressures of carboxylic esters including pyrethroids: measurement and estimation from molecular structure. Chemosphere 2001, 45, 729 -36. 2 Sci. Finder Scholar; obtained from Syracuse Research Corporation of Syracuse, New York. 3 Sci. Finder Scholar, estimate.
Repeating this process at 10 K intervals from T = (298. 15 to 480) K resulted in the following vapor pressure – temperature profile; the data were fit to the following equation: ln(p/po) = AT -3 + BT -2 + C T -1 + D. All r 2 > 0. 99. TABLE 13. A Summary of Liquid/Subcooled Liquid Vapor Pressures and Normal Boiling Temperatures and Comparison with Experimental Values 1 Tsuzuki, M. Vapor pressures of carboxylic esters including pyrethroids: measurement and estimation from molecular structure. Chemosphere 2001, 45, 729 -36. 2 Sci. Finder Scholar; obtained from Syracuse Research Corporation of Syracuse, New York. 3 Sci. Finder Scholar, estimate.
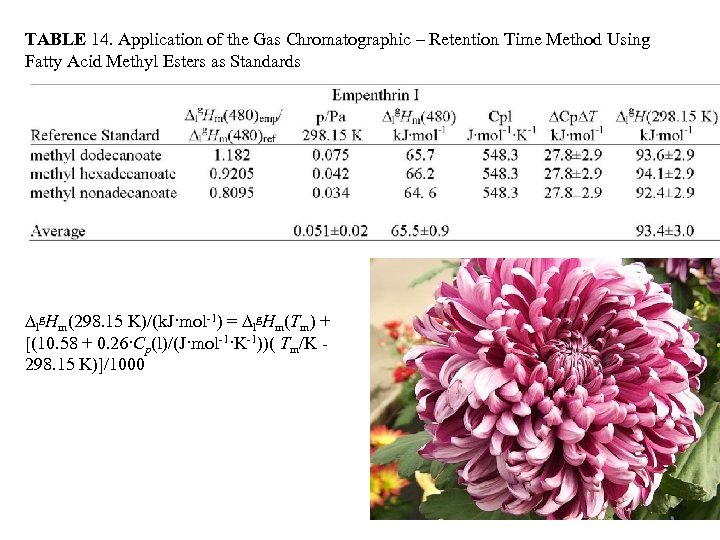 TABLE 14. Application of the Gas Chromatographic – Retention Time Method Using Fatty Acid Methyl Esters as Standards lg. Hm(298. 15 K)/(k. J·mol-1) = lg. Hm(Tm) + [(10. 58 + 0. 26·Cp(l)/(J·mol-1·K-1))( Tm/K 298. 15 K)]/1000
TABLE 14. Application of the Gas Chromatographic – Retention Time Method Using Fatty Acid Methyl Esters as Standards lg. Hm(298. 15 K)/(k. J·mol-1) = lg. Hm(Tm) + [(10. 58 + 0. 26·Cp(l)/(J·mol-1·K-1))( Tm/K 298. 15 K)]/1000
 Acknowledgements: Jessica Spencer and FKS Inc for financial support
Acknowledgements: Jessica Spencer and FKS Inc for financial support
 Vaporization Enthalpies and Vapor Pressures of Two Insecticide Components, Muscalure and Empenthrin, by Correlation Gas Chromatography. Spencer, J. ; Chickos, J. Chem. Eng. Data 2013, 59, 3513 -20. Ruzicka, K. ; Koutek, B. ; Fulem, M. ; Hoskovec, M. Indirect Determination of Vapor Pressures by Capillary Gas - Liquid Chromatography: Analysis of the Reference Vapor –Pressure Data and Their Treatment. J. Chem. Eng. Data 2011, 57, 1349 -68.
Vaporization Enthalpies and Vapor Pressures of Two Insecticide Components, Muscalure and Empenthrin, by Correlation Gas Chromatography. Spencer, J. ; Chickos, J. Chem. Eng. Data 2013, 59, 3513 -20. Ruzicka, K. ; Koutek, B. ; Fulem, M. ; Hoskovec, M. Indirect Determination of Vapor Pressures by Capillary Gas - Liquid Chromatography: Analysis of the Reference Vapor –Pressure Data and Their Treatment. J. Chem. Eng. Data 2011, 57, 1349 -68.


