12aea5be1623b039fdbd01c321e3a581.ppt
- Количество слайдов: 27

The Value of Applying Quality by Design - Not Just Monoclonals, But Across Products and Systems Tony Mire-Sluis, Executive Director, Global Product Quality and Quality Sciences 1
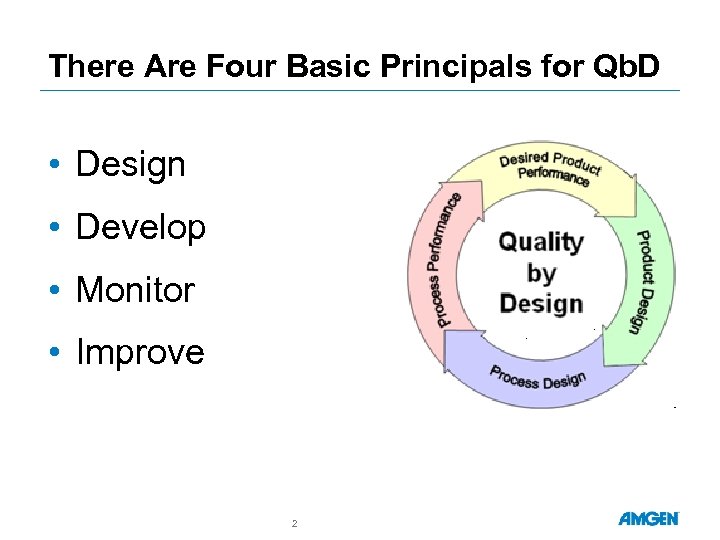
There Are Four Basic Principals for Qb. D • Design • Develop • Monitor • Improve 2
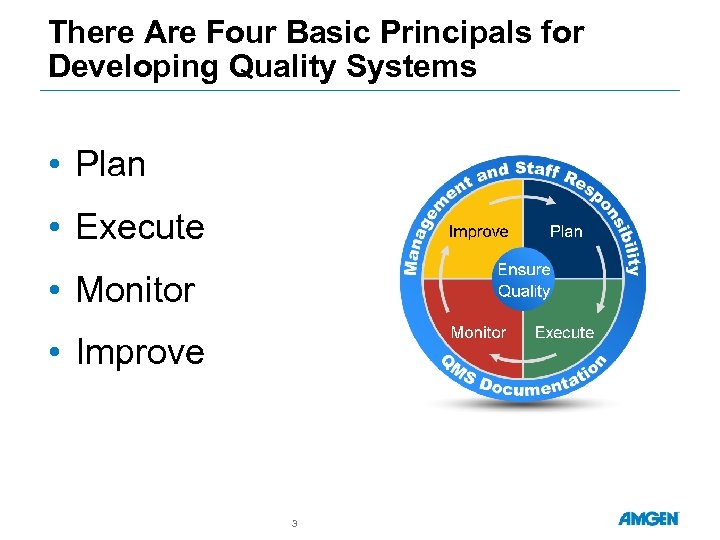
There Are Four Basic Principals for Developing Quality Systems • Plan • Execute • Monitor • Improve 3
The Principles of Qb. D Are Not Unique to a Product or Process! 4
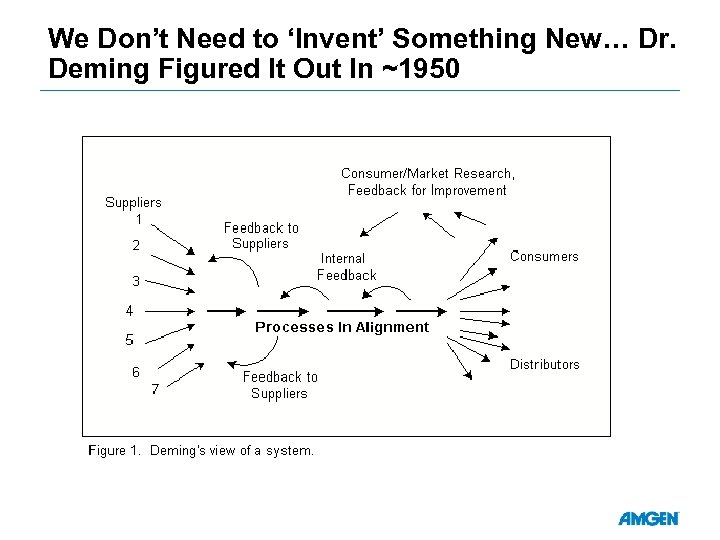
We Don’t Need to ‘Invent’ Something New… Dr. Deming Figured It Out In ~1950

The Principles of Qb. D are not ‘a Regulatory Strategy’ but Provide Business and Quality Benefits • Qb. D principles can provide: • Improved success rates for process and product development through increased understanding • Reducing risks • To patients, process failures, method invalids, NCs etc. • • • Improving product quality More robust and consistent processes Less manufacturing failures Reduced complaints Continuous improvement 6
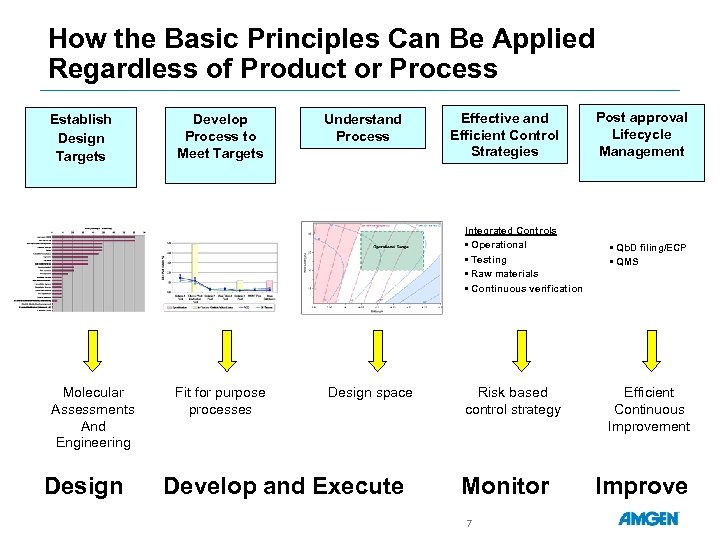
How the Basic Principles Can Be Applied Regardless of Product or Process Establish Design Targets Develop Process to Meet Targets Understand Process Effective and Efficient Control Strategies Integrated Controls • Operational • Testing • Raw materials • Continuous verification Molecular Assessments And Engineering Design Fit for purpose processes Design space Develop and Execute Risk based control strategy Monitor 7 Post approval Lifecycle Management • Qb. D filing/ECP • QMS Efficient Continuous Improvement Improve
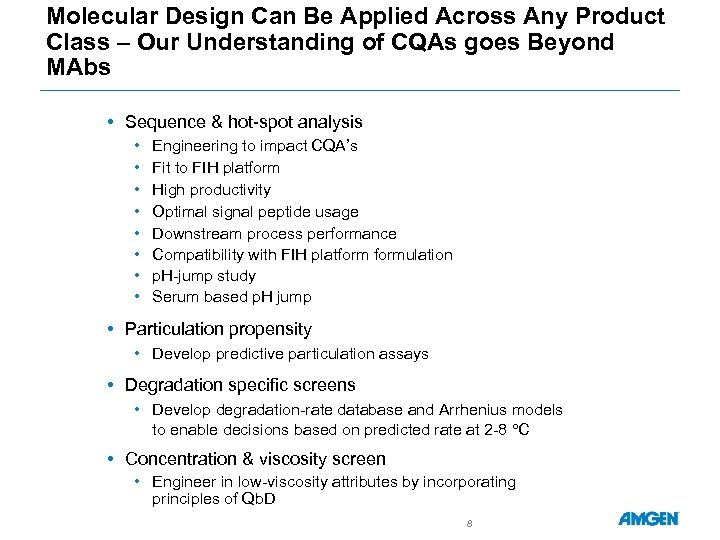
Molecular Design Can Be Applied Across Any Product Class – Our Understanding of CQAs goes Beyond MAbs • Sequence & hot-spot analysis • • Engineering to impact CQA’s Fit to FIH platform High productivity Optimal signal peptide usage Downstream process performance Compatibility with FIH platformulation p. H-jump study Serum based p. H jump • Particulation propensity • Develop predictive particulation assays • Degradation specific screens • Develop degradation-rate database and Arrhenius models to enable decisions based on predicted rate at 2 -8 C • Concentration & viscosity screen • Engineer in low-viscosity attributes by incorporating principles of Qb. D 8
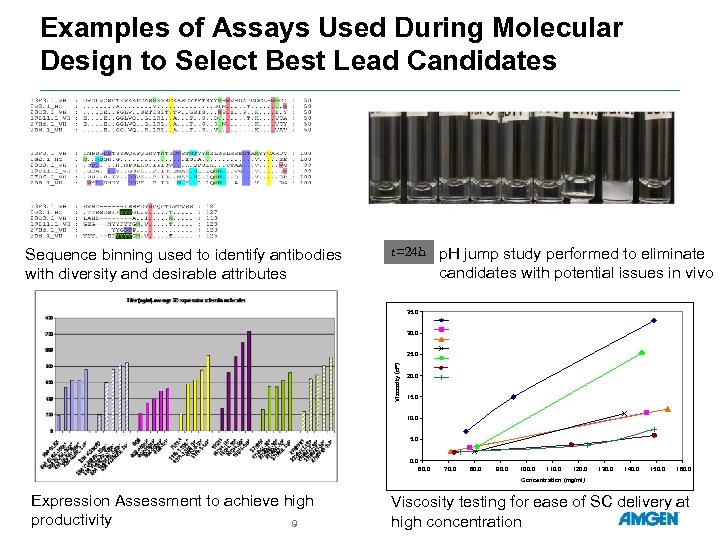
Examples of Assays Used During Molecular Design to Select Best Lead Candidates Sequence binning used to identify antibodies with diversity and desirable attributes t=24 h p. H jump study performed to eliminate candidates with potential issues in vivo 35. 0 30. 0 Viscosity (c. P) 25. 0 20. 0 15. 0 10. 0 5. 0 0. 0 60. 0 70. 0 80. 0 90. 0 100. 0 110. 0 120. 0 130. 0 140. 0 150. 0 160. 0 Concentration (mg/ml) Expression Assessment to achieve high productivity 9 Viscosity testing for ease of SC delivery at high concentration
Design Principles and their Benefits do Not Just Apply to Product or Process • Product • Manufacturing Process • Equipment • Facility • Utilities • Raw Materials • Containers • Transport 10
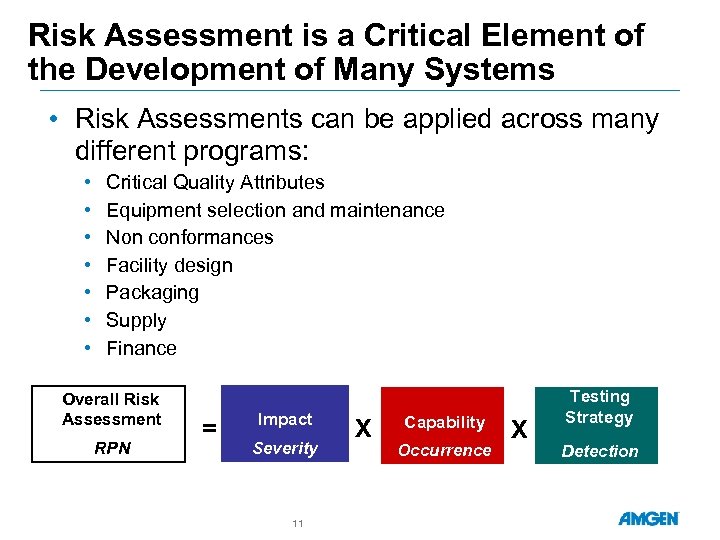
Risk Assessment is a Critical Element of the Development of Many Systems • Risk Assessments can be applied across many different programs: • • Critical Quality Attributes Equipment selection and maintenance Non conformances Facility design Packaging Supply Finance Overall Risk Assessment RPN = Impact Severity 11 X Capability Occurrence X Testing Strategy Detection
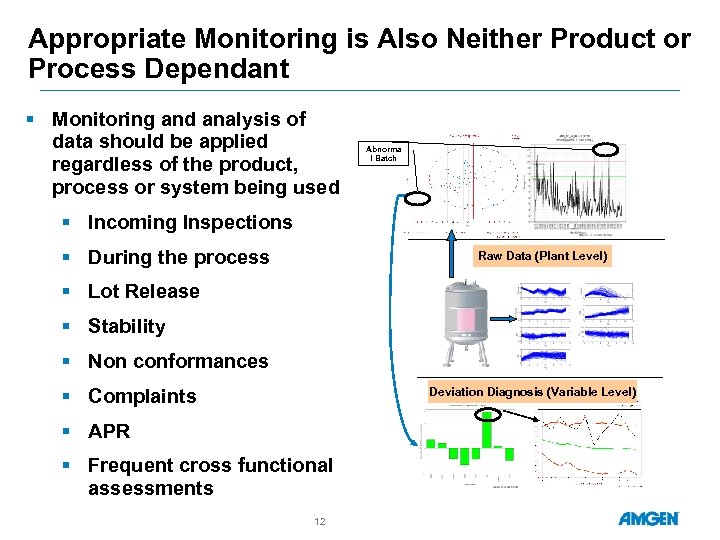
Appropriate Monitoring is Also Neither Product or Process Dependant § Monitoring and analysis of data should be applied regardless of the product, process or system being used Abnorma l Batch § Incoming Inspections § During the process Raw Data (Plant Level) § Lot Release § Stability § Non conformances § Complaints Deviation Diagnosis (Variable Level) § APR § Frequent cross functional assessments 12

Applying Qb. D Principles to Raw Materials – A Case Study of Vials 13

Raw Materials are an Essential part of both Product and Process • Raw materials and their controls are vital at all stages of manufacture • In fact, they can make up the product itself beyond just the protein – water, buffering components, tonicity agents, polysorbate, primary packaging etc. and Qb. D principles apply to RMs at all stages of manufacture • Application of the principles is carried out on a risk based approach depending where they are in the manufacturing process (downstream or upstream) or how much they impact the quality of the product (e. g. media components are downstream away from the final product but can have a tremendous impact on protein CQAs during fermentation) 14
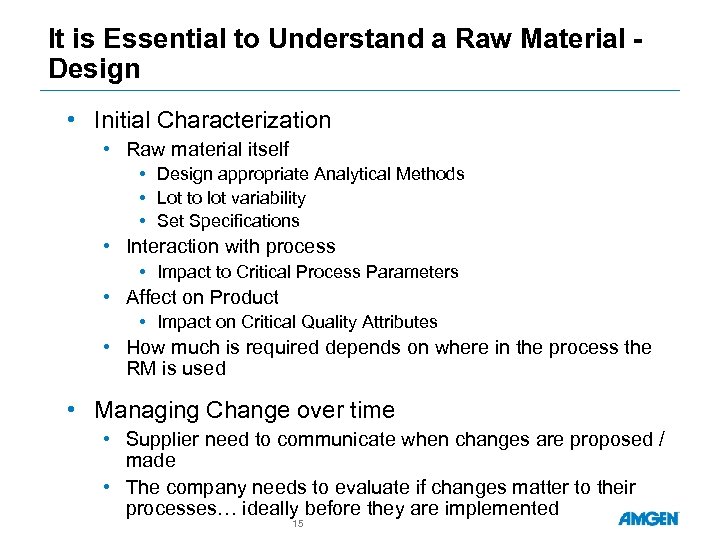
It is Essential to Understand a Raw Material Design • Initial Characterization • Raw material itself • Design appropriate Analytical Methods • Lot to lot variability • Set Specifications • Interaction with process • Impact to Critical Process Parameters • Affect on Product • Impact on Critical Quality Attributes • How much is required depends on where in the process the RM is used • Managing Change over time • Supplier need to communicate when changes are proposed / made • The company needs to evaluate if changes matter to their processes… ideally before they are implemented 15

What do you Need to Characterize in a Raw Material? – What is There • Relying on the manufacturer’s Co. A is often not be enough • One needs to understand all the components of the raw material as the manufacturer may not even measure the parameters you require control of: • Product Heterogeneity • Additives • Preservatives • Degradation products • Contaminants 16

What do you Need to Characterize in a Raw Material? – What Might Appear • There must be a thorough understanding of the degradation pathway of the raw material – relying on an expiration date from the manufacturer may not be enough • The impact of degradants on the product or process is needed • There needs to be an understanding of the use of raw materials over time and the impact to the expiration date of the product • The impact of handling the raw material over time must be assessed (aliquoting, light, temperature, oxygen etc. ) 17

Supplier Management is Necessary for Ensuring Raw Material Quality Expectations must be clear • We need to understand the manufacturing processes for raw materials so we know how they could impact product quality, allow us to set appropriate specifications and help us during investigations – Design and Monitoring • Notification of change - Monitoring • Thorough investigation of defects – Continuous Improvement Supplier site visits are key • Audit of Quality system - Plan • Technical visits to understand processes, process capability and identify indirect product contact materials - Design • Technical visits to evaluate changes and ensure that they are managed properly - Monitor 18
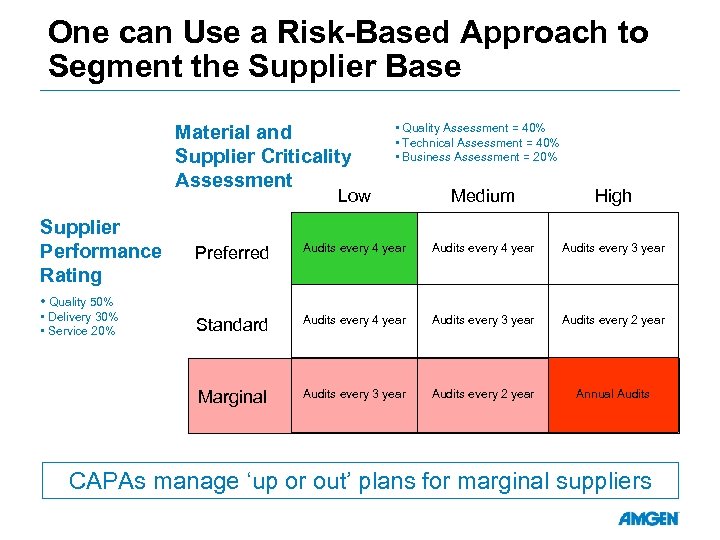
One can Use a Risk-Based Approach to Segment the Supplier Base Material and Supplier Criticality Assessment • Quality Assessment = 40% • Technical Assessment = 40% • Business Assessment = 20% Low High Preferred Audits every 4 year Audits every 3 year Standard Audits every 4 year Audits every 3 year Audits every 2 year Marginal Supplier Performance Rating Medium Audits every 3 year Audits every 2 year Annual Audits • Quality 50% • Delivery 30% • Service 20% CAPAs manage ‘up or out’ plans for marginal suppliers
Vial FMEAs were conducted by a Global team - Design • Vials are an essential part of a product in which they are filled, critical to product quality and close to the patient • FMEAs were conducted at fill finish lines across internal and contractor manufacturing sites • Risks were identified, mitigation plans developed, and CAPAs documented completion • Identifying what were the important attributes for vials was essential to develop appropriate specifications: • Extractables/Leachables • Interactions with equipment • Handling and Storage • Possible glass defects 20
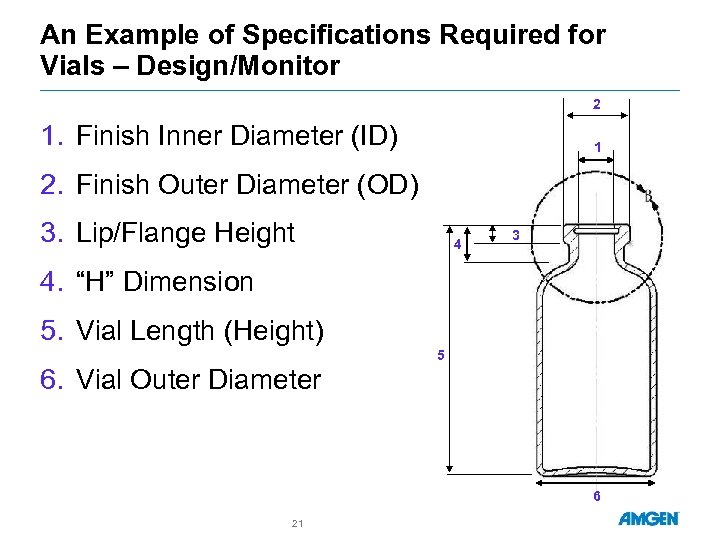
An Example of Specifications Required for Vials – Design/Monitor 2 1. Finish Inner Diameter (ID) 1 2. Finish Outer Diameter (OD) 3. Lip/Flange Height 4 3 4. “H” Dimension 5. Vial Length (Height) 5 6. Vial Outer Diameter 6 21
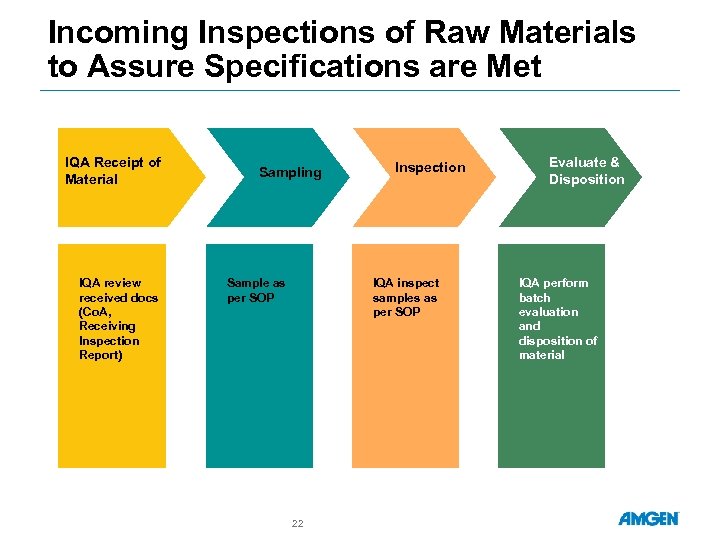
Incoming Inspections of Raw Materials to Assure Specifications are Met IQA Receipt of Material IQA review received docs (Co. A, Receiving Inspection Report) Sampling Sample as per SOP Inspection IQA inspect samples as per SOP 22 Evaluate & Disposition IQA perform batch evaluation and disposition of material
Inspection for Tubular Glass Vials Includes over 50 Criteria • Defect Classification: Critical Visual Examples: • Wrong component • Split on vial interior with nonremovable, marked deposit • Internal Airline – Elongated gaseous inclusion that appears as a vertical line. • Chipped (Broken Finish) – (if the seal is compromised) A finish that has actual piece of glass broken out of it. • Contamination – Foreign substance (particle, stain, dirt) deposited on the internal surface • Crack – Fracture that penetrates completely through the glass wall • Malformed – Finish is grossly distorted or deformed, if seal is compromised (lip malformed). 23
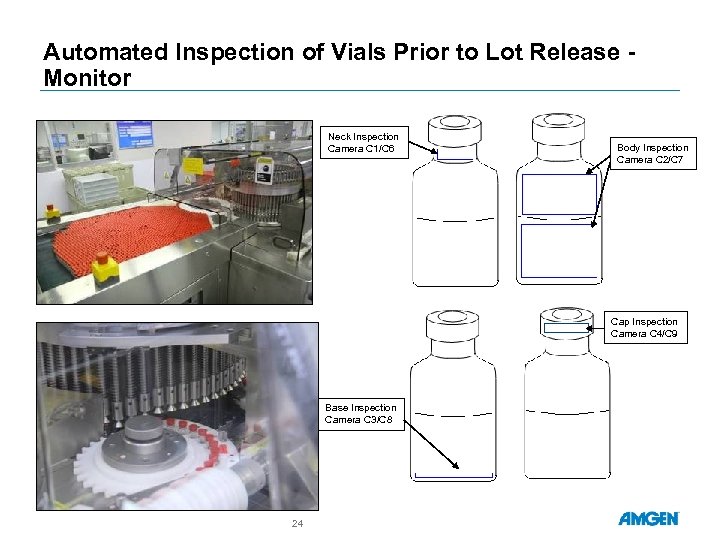
Automated Inspection of Vials Prior to Lot Release Monitor Neck Inspection Camera C 1/C 6 Body Inspection Camera C 2/C 7 Cap Inspection Camera C 4/C 9 Base Inspection Camera C 3/C 8 24
A Global Primary Container Team was established to Monitor Vial Performance • The Primary Container Team: • Comprises of all Amgen site, quality and technical groups • Tracks key glass metrics from the glass process monitoring, NCs, and Product Complaints • Identifies and implements Glass Handling best practices • Alignment to new equipment requirements • Identifies Quality/Process improvements and container standardization 25
Embedding the Quality System - Improve • Primary packaging issues are monitored globally and lessons learned applied • A governance body is covering vial platform projects and tasks • A global team is ensures Amgen wide scope and communication of tasks and work streams • Product Complaints Risk Assessment Primary Packaging Network Glass Handling Project Glass Process Monitoring Δ Control NC The team monitors Amgen production and CMC activities The creation of a global team allows for continuous monitoring and improvement 26

In Summary • The basic principals of Qb. D can (and should) be applied across product types as well as multiple processes and systems • We don’t have to label it Qb. D and treat it like something special, it just makes good business sense and we have been doing it to a certain extent anyway and it can be applied to all products and across multiple systems/processes • Using the principals of Qb. D in many cases does not require a great deal of cost – mostly good planning, increased understanding and more successful execution • A risk based approach can be used to help appropriately focus Qb. D efforts 27
12aea5be1623b039fdbd01c321e3a581.ppt