edea510c5309a87308fcf0c1bacb9e7e.ppt
- Количество слайдов: 20
 THE TRIPLE A STUDY (ASTHMA, ALLERGIES & ADVICE) A SINGLE BLINDED RANDOMISED TRIAL Carol Bobb, Thomas Ritz, Gill Rowlands & Chris Griffiths
THE TRIPLE A STUDY (ASTHMA, ALLERGIES & ADVICE) A SINGLE BLINDED RANDOMISED TRIAL Carol Bobb, Thomas Ritz, Gill Rowlands & Chris Griffiths
 Aims q q To test the hypothesis that allergen avoidance advice with skin prick testing given at a primary care asthma review improves asthma control Replicating earlier findings of a pilot study that had shown lung function improvements with allergen avoidance advice (Bobb & Ritz, 2003, Respiratory Medicine)
Aims q q To test the hypothesis that allergen avoidance advice with skin prick testing given at a primary care asthma review improves asthma control Replicating earlier findings of a pilot study that had shown lung function improvements with allergen avoidance advice (Bobb & Ritz, 2003, Respiratory Medicine)
 Study Design q q Randomised, single-blinded, repeated measurement design Subjects were randomly assigned into two groups: 1 - Control (n=107) Spirometry and reversibility testing; standard asthma care, stepwise medication advice, inhaler technique training 2 -Treatment (n=107) The same programme as control; plus structured trigger & allergy assessment, skin prick testing and avoidance advice
Study Design q q Randomised, single-blinded, repeated measurement design Subjects were randomly assigned into two groups: 1 - Control (n=107) Spirometry and reversibility testing; standard asthma care, stepwise medication advice, inhaler technique training 2 -Treatment (n=107) The same programme as control; plus structured trigger & allergy assessment, skin prick testing and avoidance advice
 Location & Criteria 6 general practices in South London Selection criteria 18 -65 years q peak flow variation 15% to 20% q Not had skin testing in the last 10 years q Use of asthma inhalers during the past year Exclusions q. Pregnant patients q. Moderate to severe COPD or restriction q
Location & Criteria 6 general practices in South London Selection criteria 18 -65 years q peak flow variation 15% to 20% q Not had skin testing in the last 10 years q Use of asthma inhalers during the past year Exclusions q. Pregnant patients q. Moderate to severe COPD or restriction q
 Procedure q q Patients were invited twice, for a 45 min intervention (or regular care) session, and approximately 3 months later for a follow-up session Primary care nurses scheduled patients during regular office hours
Procedure q q Patients were invited twice, for a 45 min intervention (or regular care) session, and approximately 3 months later for a follow-up session Primary care nurses scheduled patients during regular office hours
 Outcome Measures Objective assessments by blinded assessors: Lung function tests: Forced expiratory volume in the first second (FEV 1), peak expiratory flow (PEF) 4 Questionnaires which subjects self completed: q Asthma control : 6 -item (Juniper 1999), q Asthma symptoms: 10 -item (Steen et al. , 1994), q q Perceived control of asthma: 11 -item (Katz et al. , 2002), Asthma self-efficacy: 20 -item (Wigal et al. , 1993).
Outcome Measures Objective assessments by blinded assessors: Lung function tests: Forced expiratory volume in the first second (FEV 1), peak expiratory flow (PEF) 4 Questionnaires which subjects self completed: q Asthma control : 6 -item (Juniper 1999), q Asthma symptoms: 10 -item (Steen et al. , 1994), q q Perceived control of asthma: 11 -item (Katz et al. , 2002), Asthma self-efficacy: 20 -item (Wigal et al. , 1993).
 Study power q q q 65 participants per group to detect a difference of 0. 5 in asthma control (ACQ) with 99% power at a significance level of 5% 38 per group needed to detect a clinically relevant 4 point difference in symptom control 45 per group for a 10% change in lung function (FEV 1).
Study power q q q 65 participants per group to detect a difference of 0. 5 in asthma control (ACQ) with 99% power at a significance level of 5% 38 per group needed to detect a clinically relevant 4 point difference in symptom control 45 per group for a 10% change in lung function (FEV 1).
 Intervention Tools n n Tayside Asthma Audit Cards. Asthma Trigger Inventory (Ritz, Steptoe, Bobb, Edwards & Harris, Psychosomatic Medicine, 68. 2006) Structured Allergy Questionnaire (Bobb & Ritz, Respiratory Medicine 97, 1180 -1187 2003) Skin prick testing (SPT)
Intervention Tools n n Tayside Asthma Audit Cards. Asthma Trigger Inventory (Ritz, Steptoe, Bobb, Edwards & Harris, Psychosomatic Medicine, 68. 2006) Structured Allergy Questionnaire (Bobb & Ritz, Respiratory Medicine 97, 1180 -1187 2003) Skin prick testing (SPT)
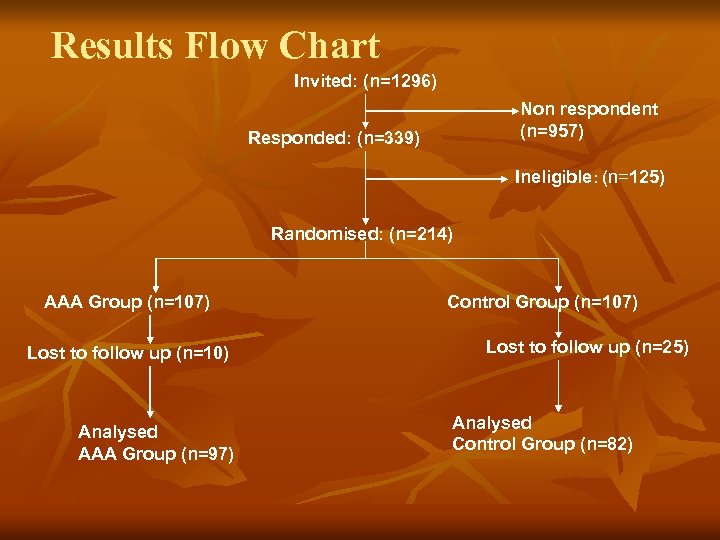 Results Flow Chart Invited: (n=1296) Non respondent (n=957) Responded: (n=339) Ineligible: (n=125) Randomised: (n=214) AAA Group (n=107) Lost to follow up (n=10) Analysed AAA Group (n=97) Control Group (n=107) Lost to follow up (n=25) Analysed Control Group (n=82)
Results Flow Chart Invited: (n=1296) Non respondent (n=957) Responded: (n=339) Ineligible: (n=125) Randomised: (n=214) AAA Group (n=107) Lost to follow up (n=10) Analysed AAA Group (n=97) Control Group (n=107) Lost to follow up (n=25) Analysed Control Group (n=82)
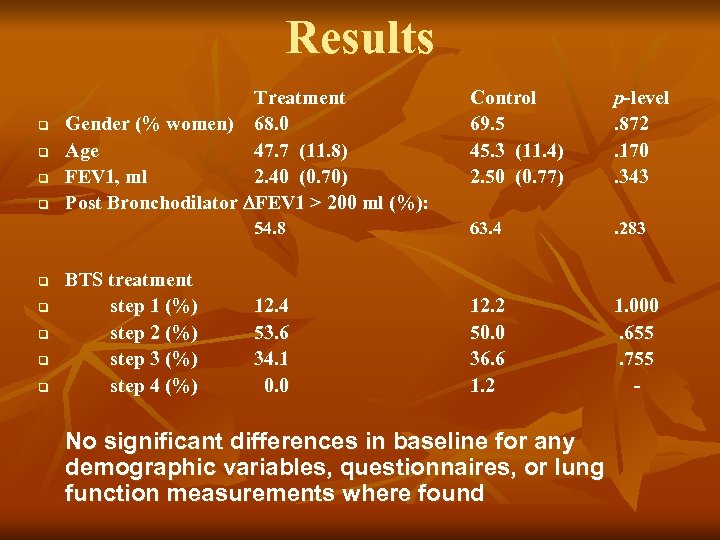 Results q q q q BTS treatment step 1 (%) step 2 (%) step 3 (%) step 4 (%) Control 69. 5 45. 3 (11. 4) 2. 50 (0. 77) p-level. 872. 170. 343 54. 8 q Treatment Gender (% women) 68. 0 Age 47. 7 (11. 8) FEV 1, ml 2. 40 (0. 70) Post Bronchodilator FEV 1 > 200 ml (%): 63. 4 . 283 12. 4 53. 6 34. 1 0. 0 12. 2 50. 0 36. 6 1. 2 1. 000. 655. 755 - No significant differences in baseline for any demographic variables, questionnaires, or lung function measurements where found
Results q q q q BTS treatment step 1 (%) step 2 (%) step 3 (%) step 4 (%) Control 69. 5 45. 3 (11. 4) 2. 50 (0. 77) p-level. 872. 170. 343 54. 8 q Treatment Gender (% women) 68. 0 Age 47. 7 (11. 8) FEV 1, ml 2. 40 (0. 70) Post Bronchodilator FEV 1 > 200 ml (%): 63. 4 . 283 12. 4 53. 6 34. 1 0. 0 12. 2 50. 0 36. 6 1. 2 1. 000. 655. 755 - No significant differences in baseline for any demographic variables, questionnaires, or lung function measurements where found
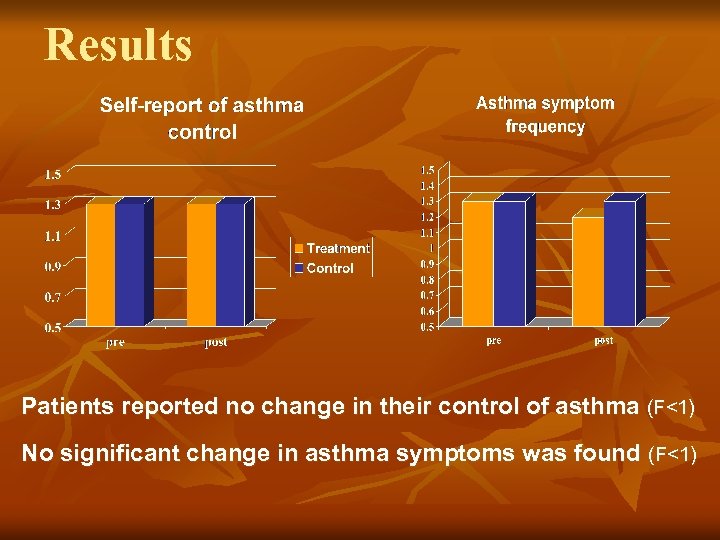 Results Patients reported no change in their control of asthma (F<1) No significant change in asthma symptoms was found (F<1)
Results Patients reported no change in their control of asthma (F<1) No significant change in asthma symptoms was found (F<1)
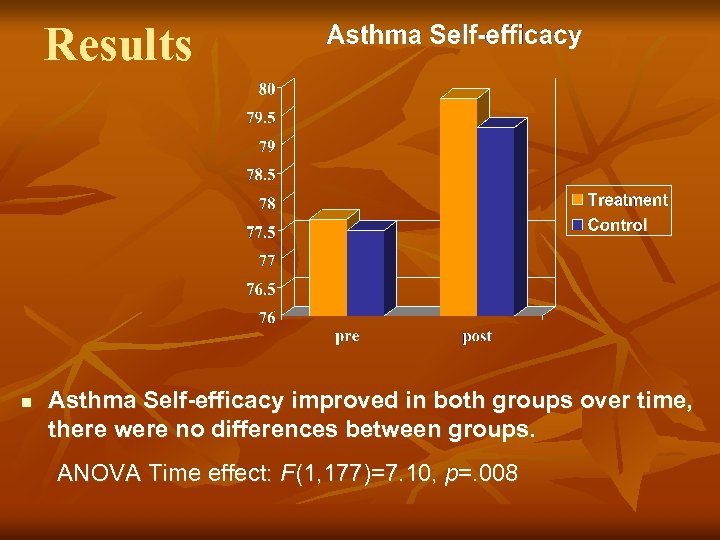 Results n Asthma Self-efficacy improved in both groups over time, there were no differences between groups. ANOVA Time effect: F(1, 177)=7. 10, p=. 008
Results n Asthma Self-efficacy improved in both groups over time, there were no differences between groups. ANOVA Time effect: F(1, 177)=7. 10, p=. 008
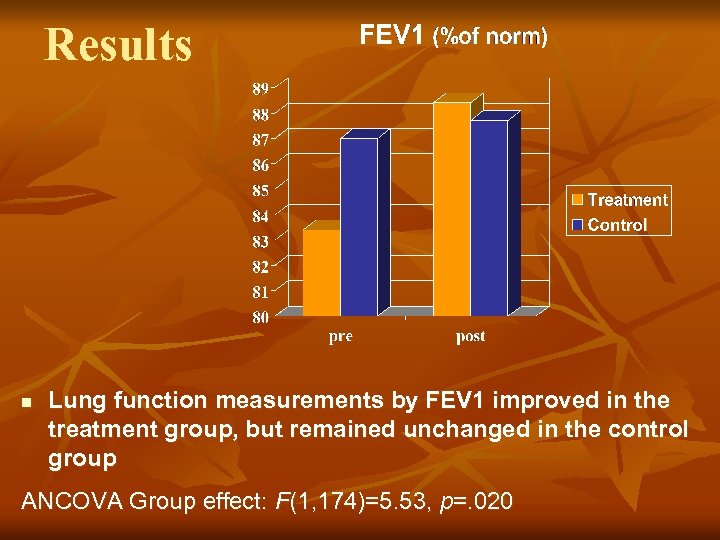 Results n Lung function measurements by FEV 1 improved in the treatment group, but remained unchanged in the control group ANCOVA Group effect: F(1, 174)=5. 53, p=. 020
Results n Lung function measurements by FEV 1 improved in the treatment group, but remained unchanged in the control group ANCOVA Group effect: F(1, 174)=5. 53, p=. 020
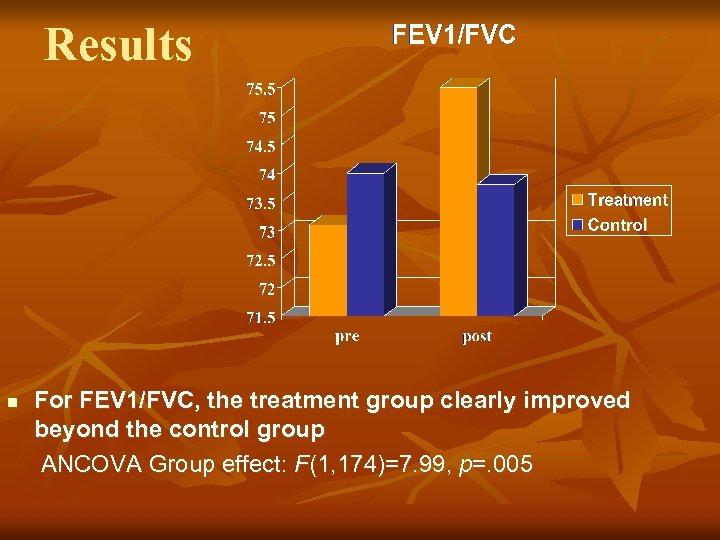 Results n For FEV 1/FVC, the treatment group clearly improved beyond the control group ANCOVA Group effect: F(1, 174)=7. 99, p=. 005
Results n For FEV 1/FVC, the treatment group clearly improved beyond the control group ANCOVA Group effect: F(1, 174)=7. 99, p=. 005
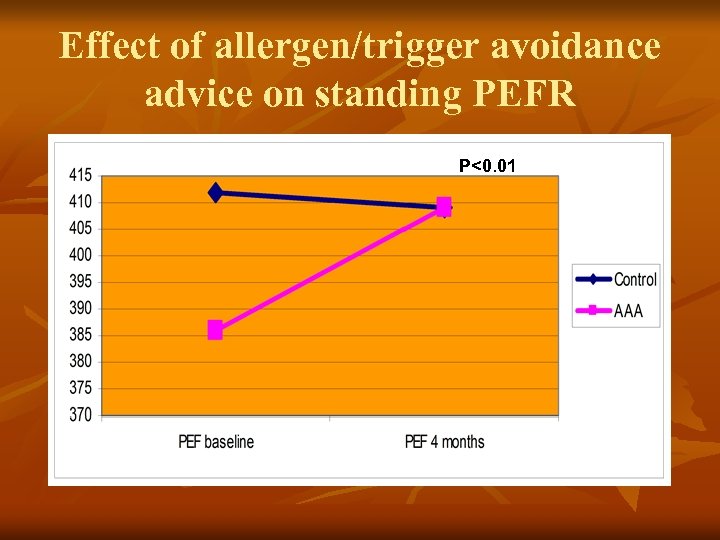 Effect of allergen/trigger avoidance advice on standing PEFR P<0. 01
Effect of allergen/trigger avoidance advice on standing PEFR P<0. 01
 Results Additional analysis: Intention to treat: Repeating the analysis with the initial sample of N=107 in each group, and baseline values carried forward: demonstrated the same significant results Medication change: Analysis with only those patients whose BTS step (medication level) was unchanged (n=80 treatment, n=70 control): same significant results
Results Additional analysis: Intention to treat: Repeating the analysis with the initial sample of N=107 in each group, and baseline values carried forward: demonstrated the same significant results Medication change: Analysis with only those patients whose BTS step (medication level) was unchanged (n=80 treatment, n=70 control): same significant results
 Concluding remarks q. Our intervention improved lung function but not self-report measures of asthma control q Trials of allergen avoidance show conflicting results q Debate about content of asthma reviews in primary care q Our data suggests that skin prick testing and allergen avoidance can benefit patients in an asthma review
Concluding remarks q. Our intervention improved lung function but not self-report measures of asthma control q Trials of allergen avoidance show conflicting results q Debate about content of asthma reviews in primary care q Our data suggests that skin prick testing and allergen avoidance can benefit patients in an asthma review
 Acknowledgements q Asthma UK (for funding the study) q Practices participating with principal GP: Sydenham Green Group Practice-Dr A Platman Beckett House Group Practice-Dr E Mc. Ginn Crown Dale Group Practice-Dr P White Robinhood Group Practice-Dr R Seyan Grantham Road – Dr S Wickramsinge Landor Road Practice- Dr I Ferrier q Nurses: Carol Bobb BSc NP Asthma Dip Ross Walker RGN Asthma Dip Susan Rae RGN Asthma Dip Michele Withers RGN Asthma Dip Lyn Hunt RGN Lucia Gouveia RGN Tina Green RGN Medical Students: Ali Tasleem Barbara Hefferman Helen Cowie Dr Clara Kalu
Acknowledgements q Asthma UK (for funding the study) q Practices participating with principal GP: Sydenham Green Group Practice-Dr A Platman Beckett House Group Practice-Dr E Mc. Ginn Crown Dale Group Practice-Dr P White Robinhood Group Practice-Dr R Seyan Grantham Road – Dr S Wickramsinge Landor Road Practice- Dr I Ferrier q Nurses: Carol Bobb BSc NP Asthma Dip Ross Walker RGN Asthma Dip Susan Rae RGN Asthma Dip Michele Withers RGN Asthma Dip Lyn Hunt RGN Lucia Gouveia RGN Tina Green RGN Medical Students: Ali Tasleem Barbara Hefferman Helen Cowie Dr Clara Kalu
 References q Bobb C, Ritz T (2003). Do asthma patients in general practice profit from a structured allergen evaluation and skin prick testing? A pilot study. Respiratory Medicine 2003, 97, 1180 -1187 q Ritz T, Steptoe, Bobb C, Harris A. & Edwards M (in press). The Asthma Trigger Inventory: Development and evaluation of a questionnaire on perceived triggers of asthma. Psychosomatic Medicine, 68: 2006 q Brydon M (1993) Allergy Protocol Practice Nursing 17 th August 1993 pg 21 q Evans S, Day S, Royston P. Minimisation program for allocating patients to treatment in clinical trials The London Hosp. Medical College q Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR (1999) Development and validation of a questionaire to measure asthma control. Eur Respir J 14: 902 -907 q q q Katz PP et al (1997) Perceived control of asthma: development & validation of a questionnaire. AMJ Respir Crit Care Med 155. 577 -82. Steen N et al (1994) Development of a symptom based outcome measure for asthma BMJ Vol 309 1065 -68 Oct 1994. Wigal JK, Stout C, Brandon M, Winder JA, Mc. Connaughy K, Creer TL, Kotses H. The Knowledge, Attitude and Self-Efficacy Asthma Questionnaire. Chest 1993 104: 1144 -8.
References q Bobb C, Ritz T (2003). Do asthma patients in general practice profit from a structured allergen evaluation and skin prick testing? A pilot study. Respiratory Medicine 2003, 97, 1180 -1187 q Ritz T, Steptoe, Bobb C, Harris A. & Edwards M (in press). The Asthma Trigger Inventory: Development and evaluation of a questionnaire on perceived triggers of asthma. Psychosomatic Medicine, 68: 2006 q Brydon M (1993) Allergy Protocol Practice Nursing 17 th August 1993 pg 21 q Evans S, Day S, Royston P. Minimisation program for allocating patients to treatment in clinical trials The London Hosp. Medical College q Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR (1999) Development and validation of a questionaire to measure asthma control. Eur Respir J 14: 902 -907 q q q Katz PP et al (1997) Perceived control of asthma: development & validation of a questionnaire. AMJ Respir Crit Care Med 155. 577 -82. Steen N et al (1994) Development of a symptom based outcome measure for asthma BMJ Vol 309 1065 -68 Oct 1994. Wigal JK, Stout C, Brandon M, Winder JA, Mc. Connaughy K, Creer TL, Kotses H. The Knowledge, Attitude and Self-Efficacy Asthma Questionnaire. Chest 1993 104: 1144 -8.
 q Thank you to the NAPC for inviting us to present our study today and thank you all for listening……………. .
q Thank you to the NAPC for inviting us to present our study today and thank you all for listening……………. .


