abd006e960ab2390b5119425af5a607f.ppt
- Количество слайдов: 16
 The Surface Structure of Liquid Metals and Alloys * Current associates are underlined Non-Harvard Collaborators: Ben Ocko, Elaine Di. Masi, Olaf Magnussen Physics Dept. Brookhaven National Laboratory Moshe Deutsch: Bar Ilan University, Israel Binhua Lin, Mati Meron, Tim Graber, Jeff Gerbhardt, Advanced Photon Source Harvard Students/Postdocs Alexei Grigoriev, Patrick Huber, E. H. Kawamoto, Holger Tostmann, Mike Regan, Oleg Shpyrko. Support: DE-FG 02 -88 -ER 45379/NSF- DMR-0124936
The Surface Structure of Liquid Metals and Alloys * Current associates are underlined Non-Harvard Collaborators: Ben Ocko, Elaine Di. Masi, Olaf Magnussen Physics Dept. Brookhaven National Laboratory Moshe Deutsch: Bar Ilan University, Israel Binhua Lin, Mati Meron, Tim Graber, Jeff Gerbhardt, Advanced Photon Source Harvard Students/Postdocs Alexei Grigoriev, Patrick Huber, E. H. Kawamoto, Holger Tostmann, Mike Regan, Oleg Shpyrko. Support: DE-FG 02 -88 -ER 45379/NSF- DMR-0124936
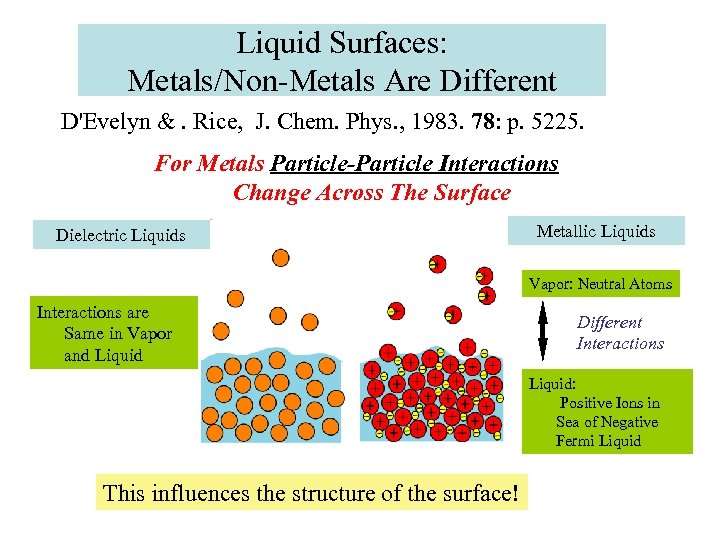 Liquid Surfaces: Metals/Non-Metals Are Different D'Evelyn &. Rice, J. Chem. Phys. , 1983. 78: p. 5225. For Metals Particle-Particle Interactions Change Across The Surface Dielectric Liquids Metallic Liquids Vapor: Neutral Atoms Interactions are Same in Vapor and Liquid Different Interactions Liquid: Positive Ions in Sea of Negative Fermi Liquid This influences the structure of the surface!
Liquid Surfaces: Metals/Non-Metals Are Different D'Evelyn &. Rice, J. Chem. Phys. , 1983. 78: p. 5225. For Metals Particle-Particle Interactions Change Across The Surface Dielectric Liquids Metallic Liquids Vapor: Neutral Atoms Interactions are Same in Vapor and Liquid Different Interactions Liquid: Positive Ions in Sea of Negative Fermi Liquid This influences the structure of the surface!
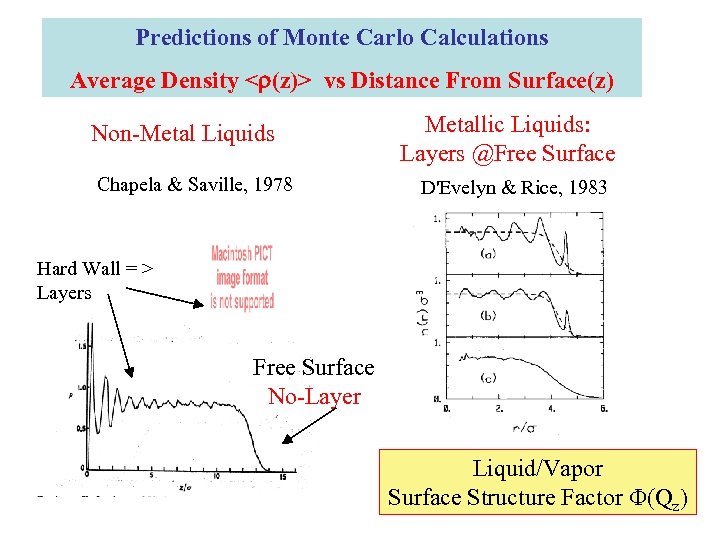 Predictions of Monte Carlo Calculations Average Density
Predictions of Monte Carlo Calculations Average Density
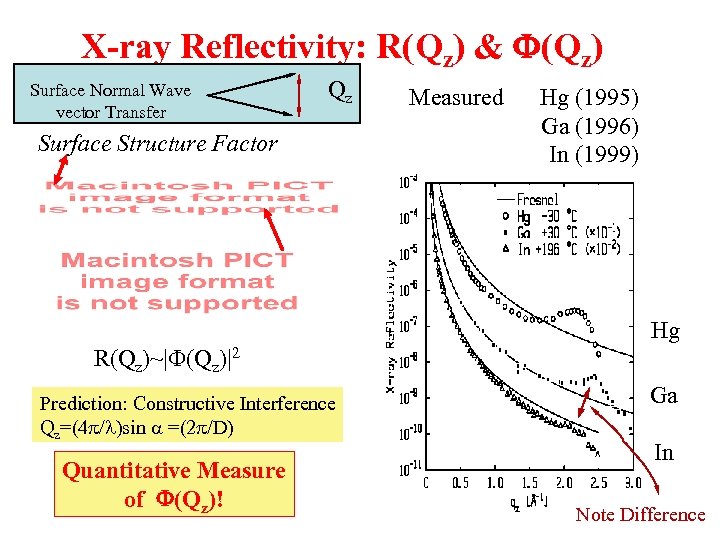 X-ray Reflectivity: R(Qz) & F(Qz) Surface Normal Wave vector Transfer Qz Surface Structure Factor Measured Hg (1995) Ga (1996) In (1999) Hg R(Qz)~|F(Qz)|2 Prediction: Constructive Interference Qz=(4 p/l)sin a =(2 p/D) Quantitative Measure of F(Qz)! Ga In Note Difference
X-ray Reflectivity: R(Qz) & F(Qz) Surface Normal Wave vector Transfer Qz Surface Structure Factor Measured Hg (1995) Ga (1996) In (1999) Hg R(Qz)~|F(Qz)|2 Prediction: Constructive Interference Qz=(4 p/l)sin a =(2 p/D) Quantitative Measure of F(Qz)! Ga In Note Difference
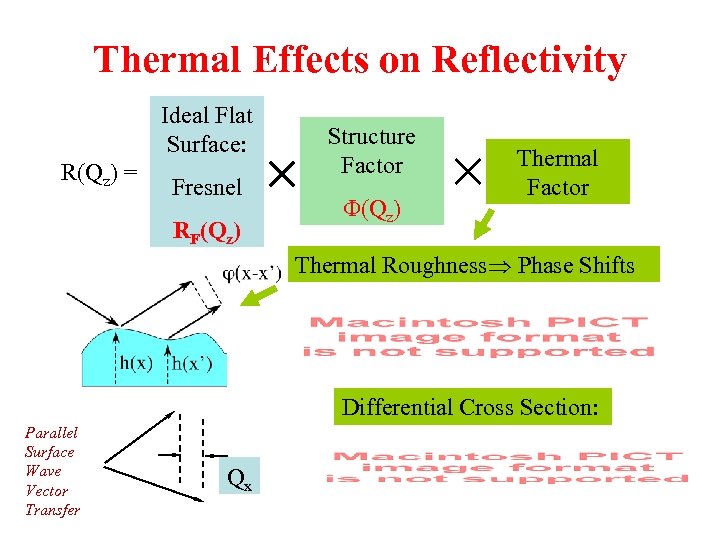 Thermal Effects on Reflectivity Ideal Flat Surface: R(Qz) = Fresnel RF(Qz) Structure Factor F(Qz) Thermal Factor Thermal Roughness Phase Shifts Differential Cross Section: Parallel Surface Wave Vector Transfer Qx
Thermal Effects on Reflectivity Ideal Flat Surface: R(Qz) = Fresnel RF(Qz) Structure Factor F(Qz) Thermal Factor Thermal Roughness Phase Shifts Differential Cross Section: Parallel Surface Wave Vector Transfer Qx
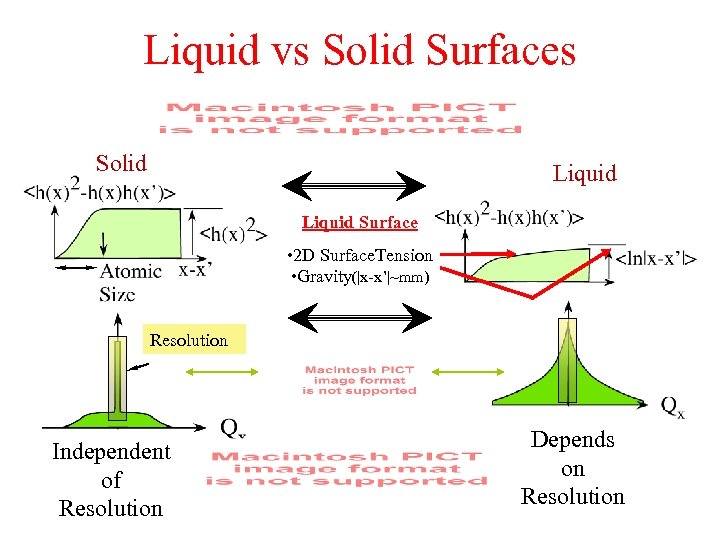 Liquid vs Solid Surfaces Solid Liquid Surface • 2 D Surface. Tension • Gravity(|x-x’|~mm) Resolution Independent of Resolution Depends on Resolution
Liquid vs Solid Surfaces Solid Liquid Surface • 2 D Surface. Tension • Gravity(|x-x’|~mm) Resolution Independent of Resolution Depends on Resolution
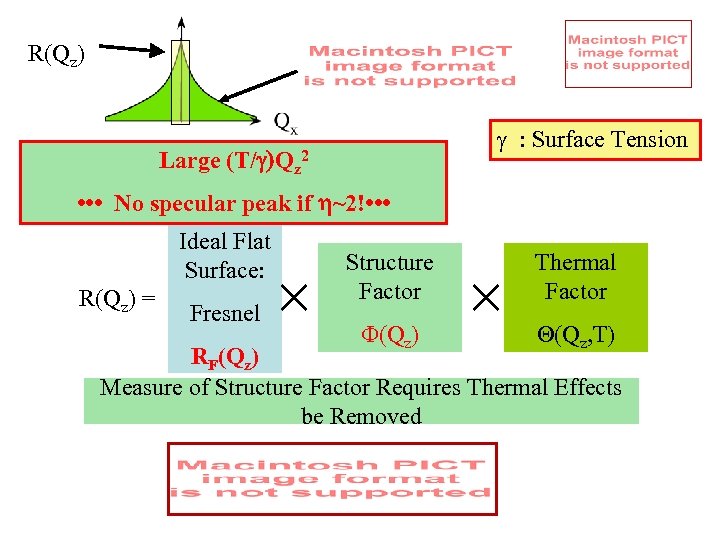 R(Qz) g : Surface Tension Large (T/ )Qz 2 • • • No specular peak if h~2! • • • Ideal Flat Surface: R(Qz) = Fresnel Structure Factor Thermal Factor F(Qz) Q(Qz, T) RF(Qz) Measure of Structure Factor Requires Thermal Effects be Removed
R(Qz) g : Surface Tension Large (T/ )Qz 2 • • • No specular peak if h~2! • • • Ideal Flat Surface: R(Qz) = Fresnel Structure Factor Thermal Factor F(Qz) Q(Qz, T) RF(Qz) Measure of Structure Factor Requires Thermal Effects be Removed
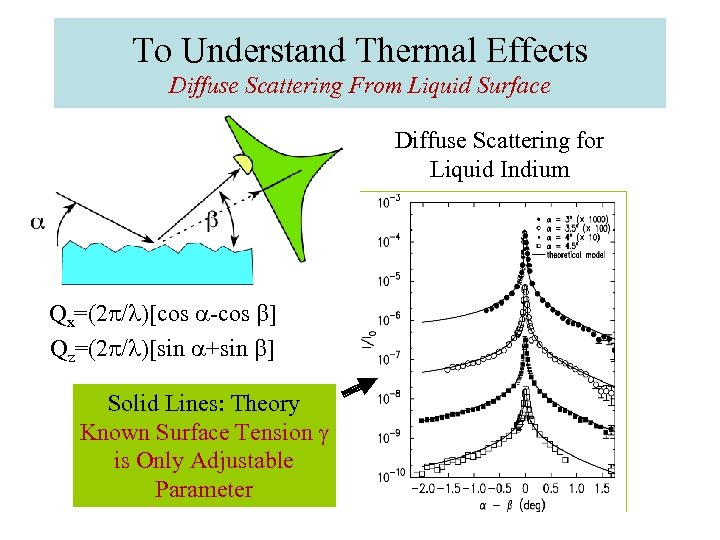 To Understand Thermal Effects Diffuse Scattering From Liquid Surface Diffuse Scattering for Liquid Indium Qx=(2 p/l)[cos a-cos b] Qz=(2 p/l)[sin a+sin b] Solid Lines: Theory Known Surface Tension g is Only Adjustable Parameter
To Understand Thermal Effects Diffuse Scattering From Liquid Surface Diffuse Scattering for Liquid Indium Qx=(2 p/l)[cos a-cos b] Qz=(2 p/l)[sin a+sin b] Solid Lines: Theory Known Surface Tension g is Only Adjustable Parameter
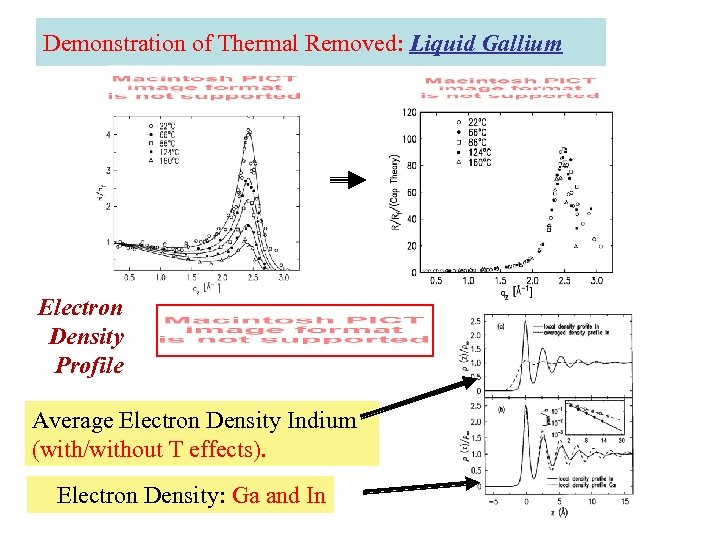 Demonstration of Thermal Removed: Liquid Gallium Electron Density Profile Average Electron Density Indium (with/without T effects). Electron Density: Ga and In
Demonstration of Thermal Removed: Liquid Gallium Electron Density Profile Average Electron Density Indium (with/without T effects). Electron Density: Ga and In
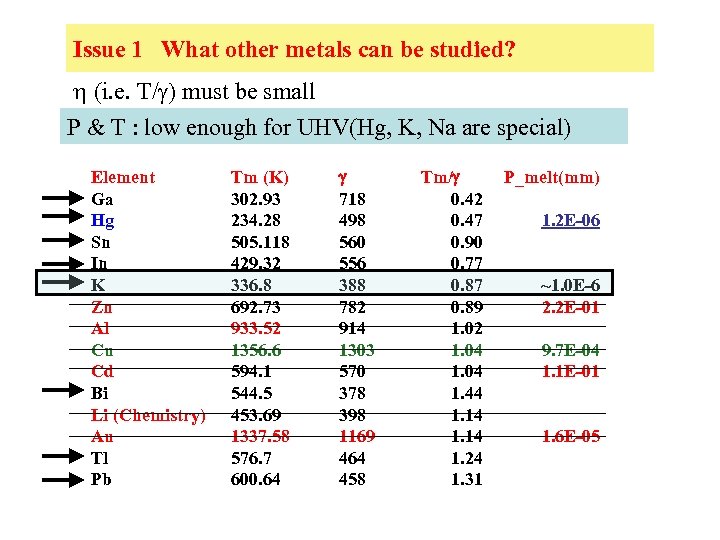 Issue 1 What other metals can be studied? h (i. e. T/g) must be small P & T : low enough for UHV(Hg, K, Na are special) Element Ga Hg Sn In K Zn Al Cu Cd Bi Li (Chemistry) Au Tl Pb Tm (K) 302. 93 234. 28 505. 118 429. 32 336. 8 692. 73 933. 52 1356. 6 594. 1 544. 5 453. 69 1337. 58 576. 7 600. 64 718 498 560 556 388 782 914 1303 570 378 398 1169 464 458 Tm/ 0. 42 0. 47 0. 90 0. 77 0. 89 1. 02 1. 04 1. 44 1. 14 1. 24 1. 31 P_melt(mm) 1. 2 E-06 ~1. 0 E-6 2. 2 E-01 9. 7 E-04 1. 1 E-01 1. 6 E-05
Issue 1 What other metals can be studied? h (i. e. T/g) must be small P & T : low enough for UHV(Hg, K, Na are special) Element Ga Hg Sn In K Zn Al Cu Cd Bi Li (Chemistry) Au Tl Pb Tm (K) 302. 93 234. 28 505. 118 429. 32 336. 8 692. 73 933. 52 1356. 6 594. 1 544. 5 453. 69 1337. 58 576. 7 600. 64 718 498 560 556 388 782 914 1303 570 378 398 1169 464 458 Tm/ 0. 42 0. 47 0. 90 0. 77 0. 89 1. 02 1. 04 1. 44 1. 14 1. 24 1. 31 P_melt(mm) 1. 2 E-06 ~1. 0 E-6 2. 2 E-01 9. 7 E-04 1. 1 E-01 1. 6 E-05
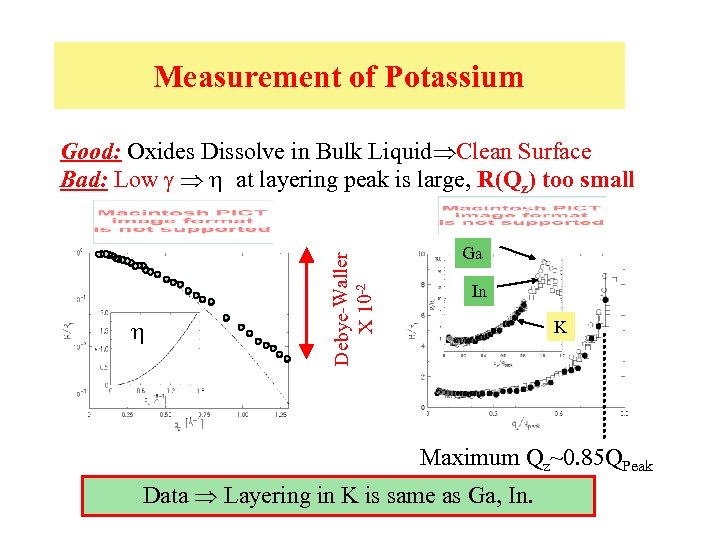 Measurement of Potassium h Debye-Waller X 10 -2 Good: Oxides Dissolve in Bulk Liquid Clean Surface Bad: Low g h at layering peak is large, R(Qz) too small Ga In K Maximum Qz~0. 85 QPeak Data Layering in K is same as Ga, In.
Measurement of Potassium h Debye-Waller X 10 -2 Good: Oxides Dissolve in Bulk Liquid Clean Surface Bad: Low g h at layering peak is large, R(Qz) too small Ga In K Maximum Qz~0. 85 QPeak Data Layering in K is same as Ga, In.
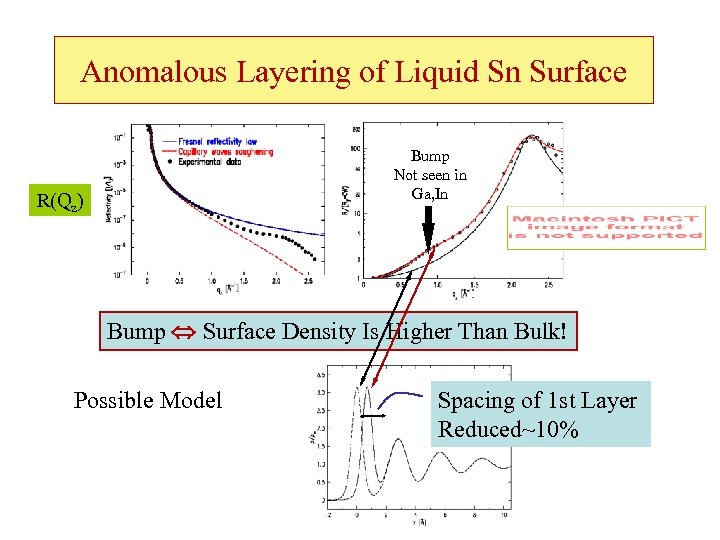 Anomalous Layering of Liquid Sn Surface Bump Not seen in Ga, In R(Qz) Bump Surface Density Is Higher Than Bulk! Possible Model Spacing of 1 st Layer Reduced~10%
Anomalous Layering of Liquid Sn Surface Bump Not seen in Ga, In R(Qz) Bump Surface Density Is Higher Than Bulk! Possible Model Spacing of 1 st Layer Reduced~10%
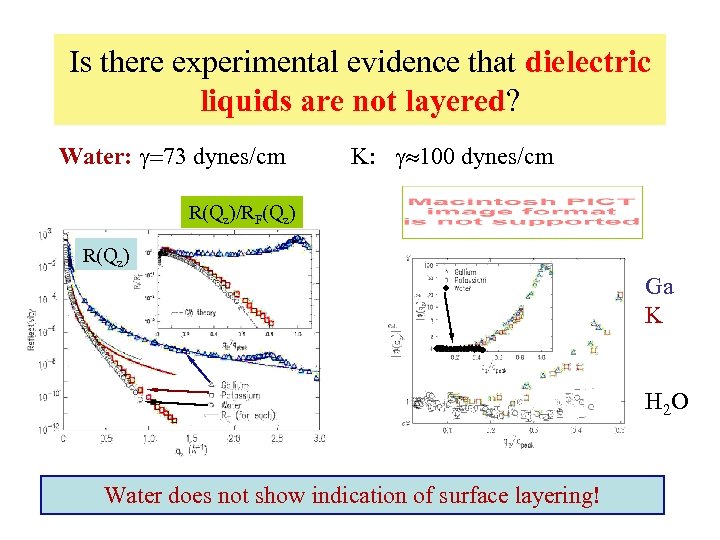 Is there experimental evidence that dielectric liquids are not layered? Water: g=73 dynes/cm K: g 100 dynes/cm R(Qz)/RF(Qz) R(Qz) Ga K H 2 O Water does not show indication of surface layering!
Is there experimental evidence that dielectric liquids are not layered? Water: g=73 dynes/cm K: g 100 dynes/cm R(Qz)/RF(Qz) R(Qz) Ga K H 2 O Water does not show indication of surface layering!
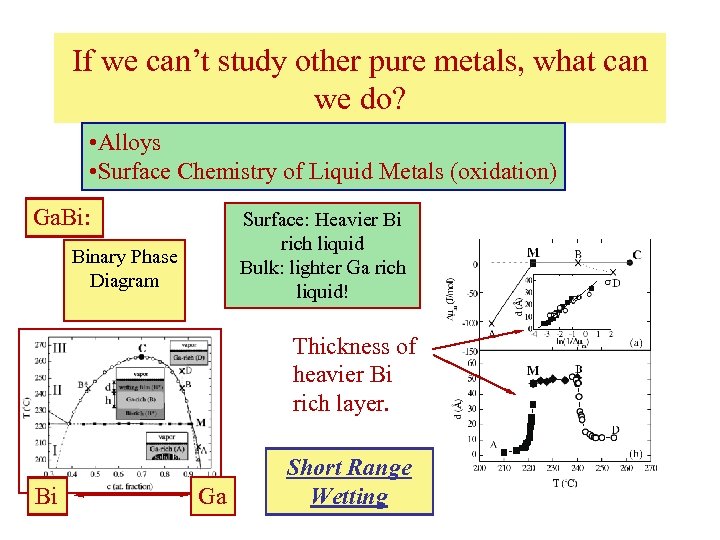 If we can’t study other pure metals, what can we do? • Alloys • Surface Chemistry of Liquid Metals (oxidation) Ga. Bi: Surface: Heavier Bi rich liquid Bulk: lighter Ga rich liquid! Binary Phase Diagram Thickness of heavier Bi rich layer. Bi Ga Short Range Wetting
If we can’t study other pure metals, what can we do? • Alloys • Surface Chemistry of Liquid Metals (oxidation) Ga. Bi: Surface: Heavier Bi rich liquid Bulk: lighter Ga rich liquid! Binary Phase Diagram Thickness of heavier Bi rich layer. Bi Ga Short Range Wetting
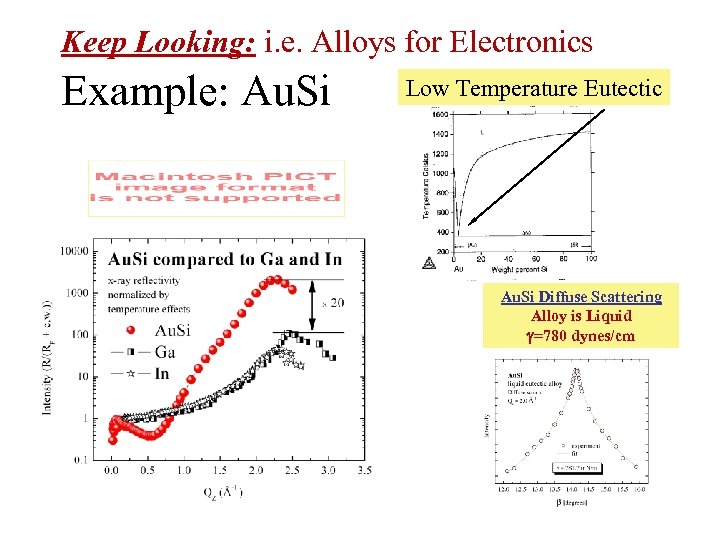 Keep Looking: i. e. Alloys for Electronics Example: Au. Si Low Temperature Eutectic Au. Si Diffuse Scattering Alloy is Liquid =780 dynes/cm
Keep Looking: i. e. Alloys for Electronics Example: Au. Si Low Temperature Eutectic Au. Si Diffuse Scattering Alloy is Liquid =780 dynes/cm
 Summary 1 - To Measure Liquid Surface Structure Factor |F(Qz)| Effects of Capillary Waves. 2 - |F(Qz)| of Free Surface of Liquid Metals Exhibits Atomic Layering! 3 - Water does Not! What about other non-metallic liquids. 4 - Surfaces of Liquid Alloys: Interesting Physics Sn: Anomalous Surface Ga. Bi: Short Range Wetting Au. Si: Enormously Intense Future
Summary 1 - To Measure Liquid Surface Structure Factor |F(Qz)| Effects of Capillary Waves. 2 - |F(Qz)| of Free Surface of Liquid Metals Exhibits Atomic Layering! 3 - Water does Not! What about other non-metallic liquids. 4 - Surfaces of Liquid Alloys: Interesting Physics Sn: Anomalous Surface Ga. Bi: Short Range Wetting Au. Si: Enormously Intense Future


