5ff62b6c0f5f21f8b59c9dc116d23b81.ppt
- Количество слайдов: 13
 The Structure of the Atom Chapter 18. 1 Copyright © 2011 Interactive. Science. Lessons. com™
The Structure of the Atom Chapter 18. 1 Copyright © 2011 Interactive. Science. Lessons. com™
 Structure of the Atom • Definition: An atom is the smallest piece of matter that still retains the properties of the element. • Gold is made of gold atoms • Carbon is made of carbon atoms • Lead is made of lead atoms Copyright © 2011 Interactive. Science. Lessons. com™
Structure of the Atom • Definition: An atom is the smallest piece of matter that still retains the properties of the element. • Gold is made of gold atoms • Carbon is made of carbon atoms • Lead is made of lead atoms Copyright © 2011 Interactive. Science. Lessons. com™
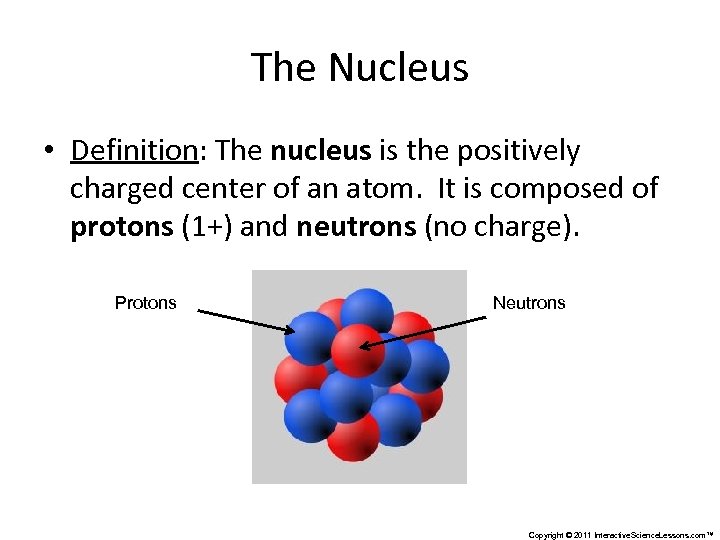 The Nucleus • Definition: The nucleus is the positively charged center of an atom. It is composed of protons (1+) and neutrons (no charge). Protons Neutrons Copyright © 2011 Interactive. Science. Lessons. com™
The Nucleus • Definition: The nucleus is the positively charged center of an atom. It is composed of protons (1+) and neutrons (no charge). Protons Neutrons Copyright © 2011 Interactive. Science. Lessons. com™
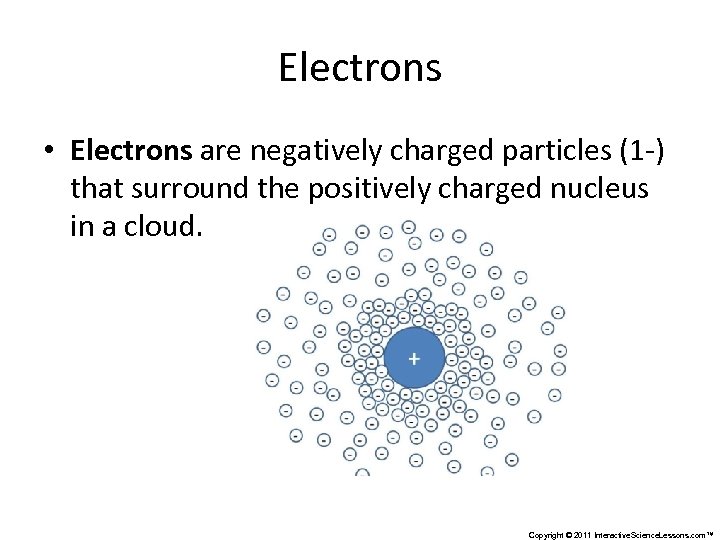 Electrons • Electrons are negatively charged particles (1 -) that surround the positively charged nucleus in a cloud. Copyright © 2011 Interactive. Science. Lessons. com™
Electrons • Electrons are negatively charged particles (1 -) that surround the positively charged nucleus in a cloud. Copyright © 2011 Interactive. Science. Lessons. com™
 The Concept of the Atom • 400 B. C. Democritus proposed the idea that all substances were made of atoms. • He called them atomos, meaning “uncuttable” • The great philosopher of the time, Aristotle, disagreed and so few accepted the idea of the atom. Copyright © 2011 Interactive. Science. Lessons. com™
The Concept of the Atom • 400 B. C. Democritus proposed the idea that all substances were made of atoms. • He called them atomos, meaning “uncuttable” • The great philosopher of the time, Aristotle, disagreed and so few accepted the idea of the atom. Copyright © 2011 Interactive. Science. Lessons. com™
 John Dalton • In the early 1800’s, John Dalton proved that Democritus was correct about the existence of atoms. • Today, however, we know that atoms can be broken down into smaller subatomic units. Copyright © 2011 Interactive. Science. Lessons. com™
John Dalton • In the early 1800’s, John Dalton proved that Democritus was correct about the existence of atoms. • Today, however, we know that atoms can be broken down into smaller subatomic units. Copyright © 2011 Interactive. Science. Lessons. com™
 Models Of The Atom John Dalton (1803) Ø J. J. Thomson (1897) Ø Ernest Rutherford (1906) Ø Niels Bohr (1913) Ø Electron Cloud (Current) The Future Ø Copyright © 2011 Interactive. Science. Lessons. com™
Models Of The Atom John Dalton (1803) Ø J. J. Thomson (1897) Ø Ernest Rutherford (1906) Ø Niels Bohr (1913) Ø Electron Cloud (Current) The Future Ø Copyright © 2011 Interactive. Science. Lessons. com™
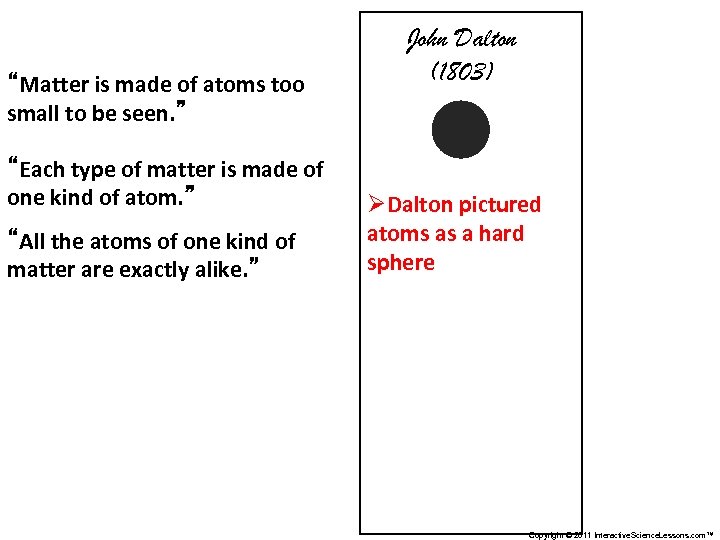 “Matter is made of atoms too small to be seen. ” “Each type of matter is made of one kind of atom. ” “All the atoms of one kind of matter are exactly alike. ” John Dalton (1803) ØDalton pictured atoms as a hard sphere Copyright © 2011 Interactive. Science. Lessons. com™
“Matter is made of atoms too small to be seen. ” “Each type of matter is made of one kind of atom. ” “All the atoms of one kind of matter are exactly alike. ” John Dalton (1803) ØDalton pictured atoms as a hard sphere Copyright © 2011 Interactive. Science. Lessons. com™
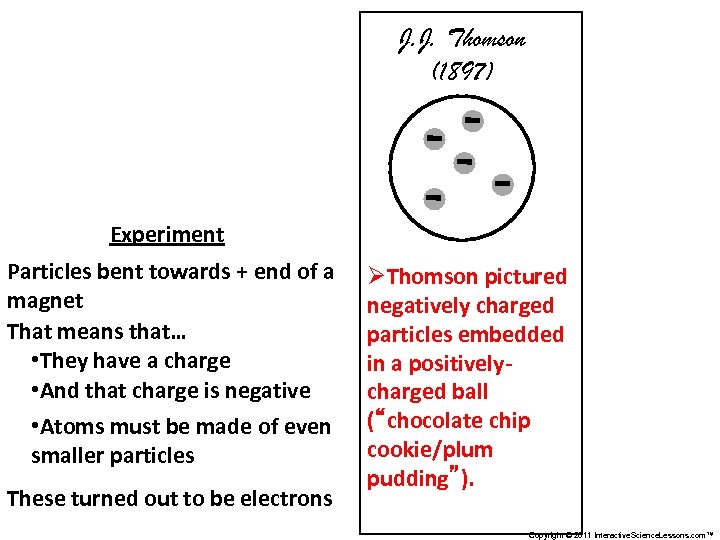 J. J. Thomson (1897) Experiment Particles bent towards + end of a magnet That means that… • They have a charge • And that charge is negative • Atoms must be made of even smaller particles These turned out to be electrons ØThomson pictured negatively charged particles embedded in a positivelycharged ball (“chocolate chip cookie/plum pudding”). Copyright © 2011 Interactive. Science. Lessons. com™
J. J. Thomson (1897) Experiment Particles bent towards + end of a magnet That means that… • They have a charge • And that charge is negative • Atoms must be made of even smaller particles These turned out to be electrons ØThomson pictured negatively charged particles embedded in a positivelycharged ball (“chocolate chip cookie/plum pudding”). Copyright © 2011 Interactive. Science. Lessons. com™
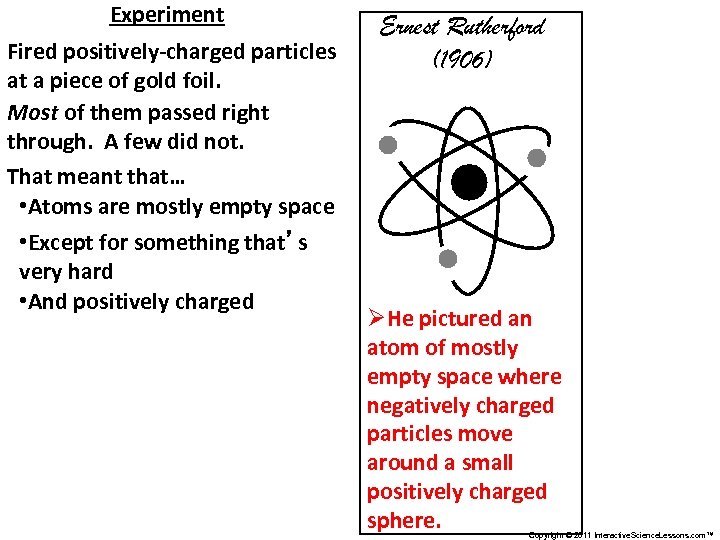 Experiment Fired positively-charged particles at a piece of gold foil. Most of them passed right through. A few did not. That meant that… • Atoms are mostly empty space • Except for something that’s very hard • And positively charged Ernest Rutherford (1906) ØHe pictured an atom of mostly empty space where negatively charged particles move around a small positively charged sphere. Copyright © 2011 Interactive. Science. Lessons. com™
Experiment Fired positively-charged particles at a piece of gold foil. Most of them passed right through. A few did not. That meant that… • Atoms are mostly empty space • Except for something that’s very hard • And positively charged Ernest Rutherford (1906) ØHe pictured an atom of mostly empty space where negatively charged particles move around a small positively charged sphere. Copyright © 2011 Interactive. Science. Lessons. com™
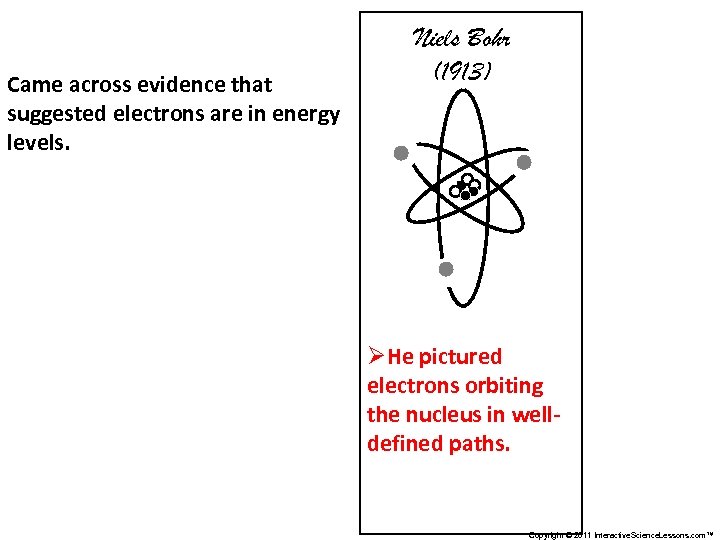 Came across evidence that suggested electrons are in energy levels. Niels Bohr (1913) ØHe pictured electrons orbiting the nucleus in welldefined paths. Copyright © 2011 Interactive. Science. Lessons. com™
Came across evidence that suggested electrons are in energy levels. Niels Bohr (1913) ØHe pictured electrons orbiting the nucleus in welldefined paths. Copyright © 2011 Interactive. Science. Lessons. com™
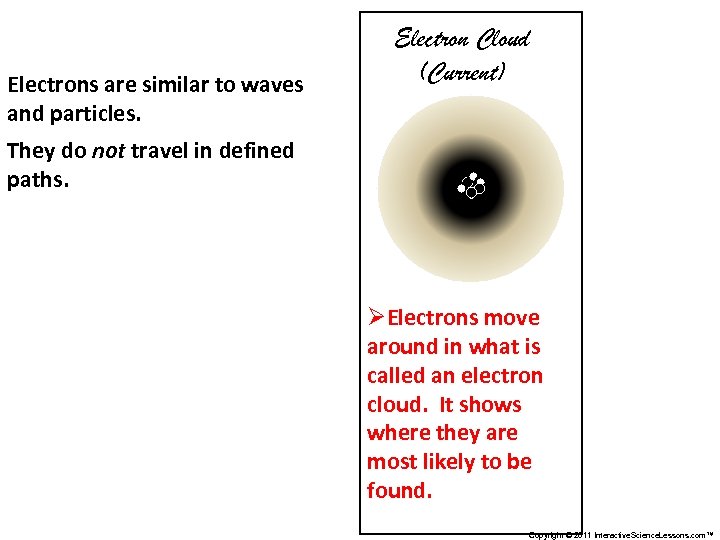 Electrons are similar to waves and particles. Electron Cloud (Current) They do not travel in defined paths. ØElectrons move around in what is called an electron cloud. It shows where they are most likely to be found. Copyright © 2011 Interactive. Science. Lessons. com™
Electrons are similar to waves and particles. Electron Cloud (Current) They do not travel in defined paths. ØElectrons move around in what is called an electron cloud. It shows where they are most likely to be found. Copyright © 2011 Interactive. Science. Lessons. com™
 The Future Copyright © 2011 Interactive. Science. Lessons. com™
The Future Copyright © 2011 Interactive. Science. Lessons. com™


