cf1b21b3b41f123c5ea6dcb2ff07c9b0.ppt
- Количество слайдов: 29
 The Society Perspective: How the Proposed Registries Will Work CRT 2012 Washington, DC February 2012 David R. Holmes, MD Mayo Clinic Rochester, MN
The Society Perspective: How the Proposed Registries Will Work CRT 2012 Washington, DC February 2012 David R. Holmes, MD Mayo Clinic Rochester, MN
 David R. Holmes, MD I have no real or apparent conflicts of interest to report.
David R. Holmes, MD I have no real or apparent conflicts of interest to report.

 Registries what we are actually doing and doing something about it (if “it” can be better!)
Registries what we are actually doing and doing something about it (if “it” can be better!)
 Registries Crucial Issues • Relevant data captured • Scope as broad as possible (all patients versus subsets) • Definitions harmonized • Accurate complete data entry (GI, GO) • Data audited • Avoid shoe strings • Data analytics responsive • Oversight
Registries Crucial Issues • Relevant data captured • Scope as broad as possible (all patients versus subsets) • Definitions harmonized • Accurate complete data entry (GI, GO) • Data audited • Avoid shoe strings • Data analytics responsive • Oversight
 Societal Registries What could they offer? • • • Harmonized objective audited data Sophisticated analytics without COI Blend clinical and administrative data Capture all procedures – numerator + denominator Capture medically treated patients Track device iterations and changes in patient selection • Serve as FDA, CMS mandated studies
Societal Registries What could they offer? • • • Harmonized objective audited data Sophisticated analytics without COI Blend clinical and administrative data Capture all procedures – numerator + denominator Capture medically treated patients Track device iterations and changes in patient selection • Serve as FDA, CMS mandated studies
 STS-ACC National TCV Registry The Vision • National Registry of ALL TAVR • Linkage of STS and ACC NCDR databases • STS Adult Cardiac Surgery Database • Present in 95% of the >1, 100 U. S. Heart Surgery Centers • >4 Million records • ACCF- NCDR • Present in 80% of ~1, 500 U. S. Interventional Catheterization Labs • >7 Million records • Develop TAVR new module harmonized with both databases • Linkage with CMS administrative database • Linkage with SSDMF • Linkage with ACC Pinnacle Outpatient Registry for all aortic stenosis management • Creation of a “research engine” for future studies
STS-ACC National TCV Registry The Vision • National Registry of ALL TAVR • Linkage of STS and ACC NCDR databases • STS Adult Cardiac Surgery Database • Present in 95% of the >1, 100 U. S. Heart Surgery Centers • >4 Million records • ACCF- NCDR • Present in 80% of ~1, 500 U. S. Interventional Catheterization Labs • >7 Million records • Develop TAVR new module harmonized with both databases • Linkage with CMS administrative database • Linkage with SSDMF • Linkage with ACC Pinnacle Outpatient Registry for all aortic stenosis management • Creation of a “research engine” for future studies
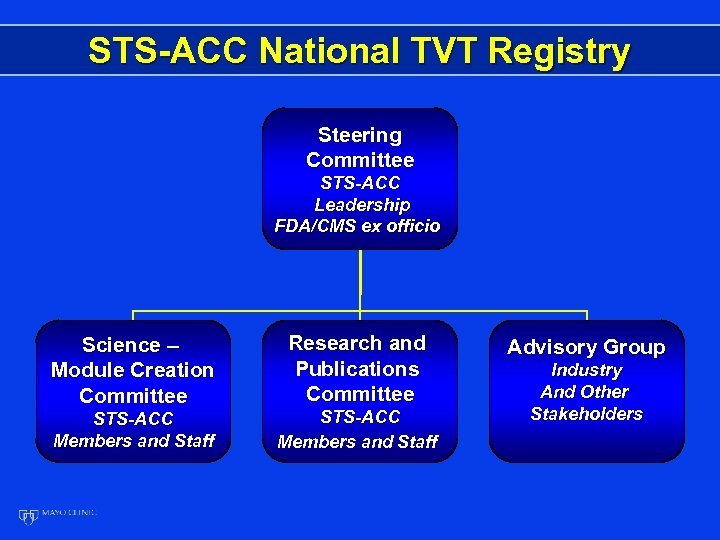 STS-ACC National TVT Registry Steering Committee STS-ACC Leadership FDA/CMS ex officio Science – Module Creation Committee STS-ACC Members and Staff Research and Publications Committee STS-ACC Members and Staff Advisory Group Industry And Other Stakeholders
STS-ACC National TVT Registry Steering Committee STS-ACC Leadership FDA/CMS ex officio Science – Module Creation Committee STS-ACC Members and Staff Research and Publications Committee STS-ACC Members and Staff Advisory Group Industry And Other Stakeholders
 Post-market Surveillance: Goals • The numerator and denominator of patients receiving a device are both crucial. • Primary goals are the assessment of effectiveness and safety when applied in clinical practice for ALL patients who receive the device, not a subset. • Use existing infrastructure of national clinical data repositories to capture ALL patients undergoing device placement: ACC/NCDR and STS
Post-market Surveillance: Goals • The numerator and denominator of patients receiving a device are both crucial. • Primary goals are the assessment of effectiveness and safety when applied in clinical practice for ALL patients who receive the device, not a subset. • Use existing infrastructure of national clinical data repositories to capture ALL patients undergoing device placement: ACC/NCDR and STS
 STS-ACC TCV National Registry Steering Committee STS • Fred Edwards • Fred Grover • Michael Mack • Dave Shahian EX-OFFICIO FDA • Danica Marinac-Dabic CMS • Jyme Schafer INTERMACS • David Naftel ADVISORY GROUP • INDUSTRY • AATS • SCAI • Ralph Brindis ACC • Ralph Brindis • John Carroll • David Holmes • Murat Tuzcu NCDR Staff • John Rumsfeld • Kathleen Hewitt STS STAFF • Cynthia Shewan DATA MODULE CONSTRUCTION/ WAREHOUSING • NCDR DATA ANALYTICS • DCRI
STS-ACC TCV National Registry Steering Committee STS • Fred Edwards • Fred Grover • Michael Mack • Dave Shahian EX-OFFICIO FDA • Danica Marinac-Dabic CMS • Jyme Schafer INTERMACS • David Naftel ADVISORY GROUP • INDUSTRY • AATS • SCAI • Ralph Brindis ACC • Ralph Brindis • John Carroll • David Holmes • Murat Tuzcu NCDR Staff • John Rumsfeld • Kathleen Hewitt STS STAFF • Cynthia Shewan DATA MODULE CONSTRUCTION/ WAREHOUSING • NCDR DATA ANALYTICS • DCRI
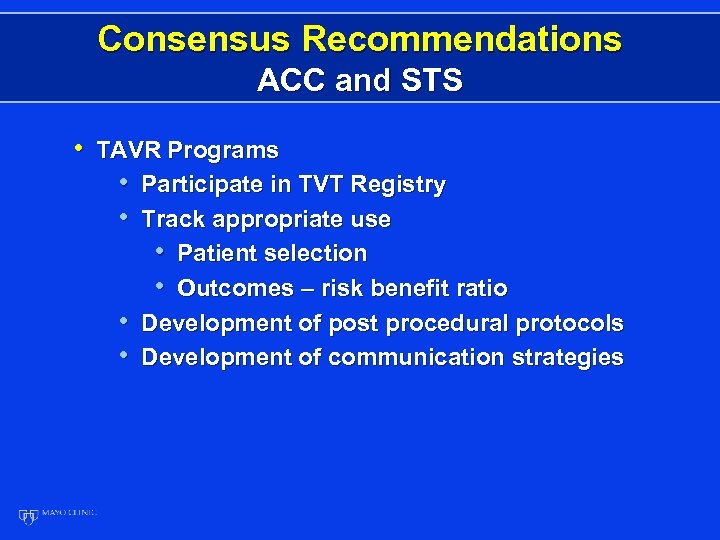 Consensus Recommendations ACC and STS • TAVR Programs • Participate in TVT Registry • Track appropriate use • Patient selection • Outcomes – risk benefit ratio • Development of post procedural protocols • Development of communication strategies
Consensus Recommendations ACC and STS • TAVR Programs • Participate in TVT Registry • Track appropriate use • Patient selection • Outcomes – risk benefit ratio • Development of post procedural protocols • Development of communication strategies
 STS-ACC National TVT Registry The Vision • Creation of a generic platform • Infrastructure for: • Pre market IDE device submission • Post market surveillance • Compliance with labeling- “indication creep” • Different devices • Device iterations • Develop comprehensive infrastructure for disease management • Comparative effectiveness analysis • Cost effectiveness research • Appropriateness of care analysis • Quality monitoring • Performance improvement opportunity • Observational and hypothesis-driven studies of “real world” practice
STS-ACC National TVT Registry The Vision • Creation of a generic platform • Infrastructure for: • Pre market IDE device submission • Post market surveillance • Compliance with labeling- “indication creep” • Different devices • Device iterations • Develop comprehensive infrastructure for disease management • Comparative effectiveness analysis • Cost effectiveness research • Appropriateness of care analysis • Quality monitoring • Performance improvement opportunity • Observational and hypothesis-driven studies of “real world” practice
 Global Interest in Registries • Development of International Registries • Implemented in the US, EU, and interest in emerging markets • IT & Provision of Healthcare • Benefits of registries • Quality improvement deliverables
Global Interest in Registries • Development of International Registries • Implemented in the US, EU, and interest in emerging markets • IT & Provision of Healthcare • Benefits of registries • Quality improvement deliverables


 Consensus Recommendations ACC and STS • Focus TAVR Programs • Specialized Heart Centers • Multidisciplinary teams • Expert surgeons and interventionalists and • imagers • Expertise with high risk and structural heart disease patients • Ancillary personnel • Optimal facilities Protocol development • Patient selection • Procedural details • Complication management
Consensus Recommendations ACC and STS • Focus TAVR Programs • Specialized Heart Centers • Multidisciplinary teams • Expert surgeons and interventionalists and • imagers • Expertise with high risk and structural heart disease patients • Ancillary personnel • Optimal facilities Protocol development • Patient selection • Procedural details • Complication management
 Post Approval Studies Objectives Yearly follow-up of patients in premarket study through 5 -years post implant • Describe the 5 -year durability and QOL outcomes • The measure for durability will be the degree • of aortic insufficiency as measured via echocardiogram Quality of life will be measured by three instruments – • the Kansas City Cardiomyopathy Questionnaire (KCCQ), • SF-12 and • Euro. Qol (EQ)-5 D Utilities
Post Approval Studies Objectives Yearly follow-up of patients in premarket study through 5 -years post implant • Describe the 5 -year durability and QOL outcomes • The measure for durability will be the degree • of aortic insufficiency as measured via echocardiogram Quality of life will be measured by three instruments – • the Kansas City Cardiomyopathy Questionnaire (KCCQ), • SF-12 and • Euro. Qol (EQ)-5 D Utilities
 Post Approval Studies Objectives Follow-up of newly enrolled patients through 5 -years post implant • Evaluate the neurological and vascular outcomes at 30 days and annually through 5 -years post implant • “Assess the learning curve among surgical teams placing the device at 50 geographically disbursed sites with high, moderate and low volumes of potential patient participation” • Evaluate the composite safety and effectiveness endpoints at 30 days and annually through 5 -years post implant
Post Approval Studies Objectives Follow-up of newly enrolled patients through 5 -years post implant • Evaluate the neurological and vascular outcomes at 30 days and annually through 5 -years post implant • “Assess the learning curve among surgical teams placing the device at 50 geographically disbursed sites with high, moderate and low volumes of potential patient participation” • Evaluate the composite safety and effectiveness endpoints at 30 days and annually through 5 -years post implant
 Device Approval What happens next? The 64 Million Dollar Question
Device Approval What happens next? The 64 Million Dollar Question
 Post Approval Studies History • Typically FDA mandated, industry supported • Little harmonization of definitions or data specifications • Specific patient populations – numerator known but no denominator • • • Do not track device iterations Conflict of interest Typically no blend of clinical and administrative data
Post Approval Studies History • Typically FDA mandated, industry supported • Little harmonization of definitions or data specifications • Specific patient populations – numerator known but no denominator • • • Do not track device iterations Conflict of interest Typically no blend of clinical and administrative data
 Post Approval Studies • Vary in design and scope • Assessment of device performance and • • risk benefit ratio through PLC Study specific endpoints of concern – stroke Identify unexpected serious risks – e. g. device failure May focus on population used for approval or expand to other populations Track real world introduction including selection creep, device iteration
Post Approval Studies • Vary in design and scope • Assessment of device performance and • • risk benefit ratio through PLC Study specific endpoints of concern – stroke Identify unexpected serious risks – e. g. device failure May focus on population used for approval or expand to other populations Track real world introduction including selection creep, device iteration
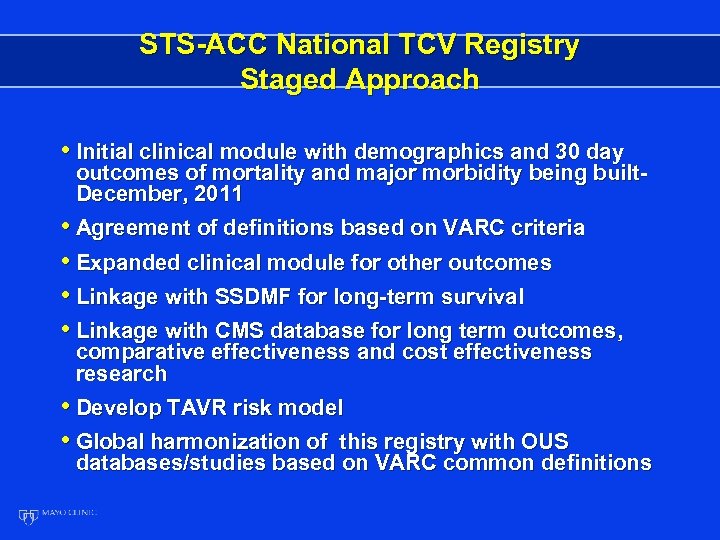 STS-ACC National TCV Registry Staged Approach • Initial clinical module with demographics and 30 day outcomes of mortality and major morbidity being built. December, 2011 • Agreement of definitions based on VARC criteria • Expanded clinical module for other outcomes • Linkage with SSDMF for long-term survival • Linkage with CMS database for long term outcomes, comparative effectiveness and cost effectiveness research • Develop TAVR risk model • Global harmonization of this registry with OUS databases/studies based on VARC common definitions
STS-ACC National TCV Registry Staged Approach • Initial clinical module with demographics and 30 day outcomes of mortality and major morbidity being built. December, 2011 • Agreement of definitions based on VARC criteria • Expanded clinical module for other outcomes • Linkage with SSDMF for long-term survival • Linkage with CMS database for long term outcomes, comparative effectiveness and cost effectiveness research • Develop TAVR risk model • Global harmonization of this registry with OUS databases/studies based on VARC common definitions
 Post-market Surveillance: What is Involved • Infrastructure, well-designed data forms to allow seamless collection of data for: • New iterations of the device(s) • New adjunctive strategies (embolic protection, etc. ) • Changes in approach (transapical, subclavian, transfemoral) • Changes in patient selection criteria and outcome over time
Post-market Surveillance: What is Involved • Infrastructure, well-designed data forms to allow seamless collection of data for: • New iterations of the device(s) • New adjunctive strategies (embolic protection, etc. ) • Changes in approach (transapical, subclavian, transfemoral) • Changes in patient selection criteria and outcome over time
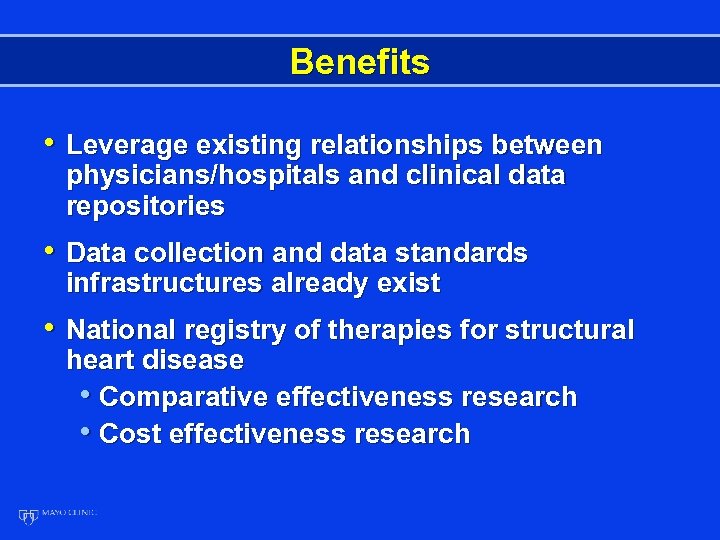 Benefits • Leverage existing relationships between physicians/hospitals and clinical data repositories • Data collection and data standards infrastructures already exist • National registry of therapies for structural heart disease • Comparative effectiveness research • Cost effectiveness research
Benefits • Leverage existing relationships between physicians/hospitals and clinical data repositories • Data collection and data standards infrastructures already exist • National registry of therapies for structural heart disease • Comparative effectiveness research • Cost effectiveness research
 Clinical Data Repositories • Patient safety • Device/therapeutic effectiveness • Specific devices/therapies • Comparative effectiveness • Quality improvement • Compliance
Clinical Data Repositories • Patient safety • Device/therapeutic effectiveness • Specific devices/therapies • Comparative effectiveness • Quality improvement • Compliance
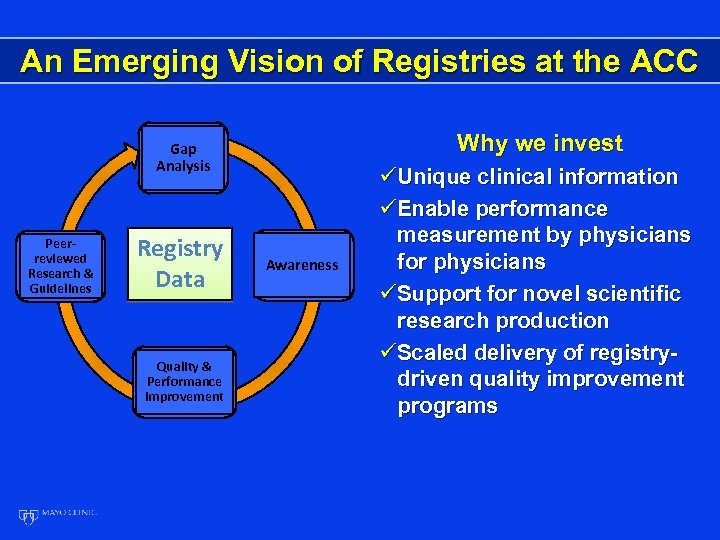 An Emerging Vision of Registries at the ACC Why we invest Gap Analysis Peerreviewed Research & Guidelines Registry Data Quality & Performance Improvement Awareness üUnique clinical information üEnable performance measurement by physicians for physicians üSupport for novel scientific research production üScaled delivery of registrydriven quality improvement programs
An Emerging Vision of Registries at the ACC Why we invest Gap Analysis Peerreviewed Research & Guidelines Registry Data Quality & Performance Improvement Awareness üUnique clinical information üEnable performance measurement by physicians for physicians üSupport for novel scientific research production üScaled delivery of registrydriven quality improvement programs
 The Mission of the ACC To advocate for quality cardiovascular care – through education, research promotion, development and application of standards and guidelines – and to influence health care policy.
The Mission of the ACC To advocate for quality cardiovascular care – through education, research promotion, development and application of standards and guidelines – and to influence health care policy.
 STS-ACC National TCV Registry The Vision • National Registry of ALL TAVR • Linkage of STS and ACC NCDR databases • STS Adult Cardiac Surgery Database • Present in 95% of the >1, 100 U. S. Heart Surgery Centers • >4 Million records • ACCF- NCDR • Present in 80% of ~1, 500 U. S. Interventional Catheterization Labs • >7 Million records • Develop TAVR new module harmonized with both databases • Linkage with CMS administrative database • Linkage with SSDMF • Linkage with ACC Pinnacle Outpatient Registry for all aortic stenosis management • Creation of a “research engine” for future studies
STS-ACC National TCV Registry The Vision • National Registry of ALL TAVR • Linkage of STS and ACC NCDR databases • STS Adult Cardiac Surgery Database • Present in 95% of the >1, 100 U. S. Heart Surgery Centers • >4 Million records • ACCF- NCDR • Present in 80% of ~1, 500 U. S. Interventional Catheterization Labs • >7 Million records • Develop TAVR new module harmonized with both databases • Linkage with CMS administrative database • Linkage with SSDMF • Linkage with ACC Pinnacle Outpatient Registry for all aortic stenosis management • Creation of a “research engine” for future studies



