fadfd648b8a685e5e1bdd32ec7b7e0c4.ppt
- Количество слайдов: 20

The Seprion Separation System Development of a feasible blood screening protocol for abnormal prion protein Stuart Wilson Microsens Biotechnologies
From oligomers to fibrils Amyloid fibrils (aggregates of thousands of molecules) Large, insoluble. Visible by staining. Found deposited in tissues eg. brain and spleen. Readily available and used extensively for spiking studies. Amyloid oligomers (>2 molecules) Small, soluble. Most likely form in blood. Invisible by staining.
Post-mortem tests and the use of protease The large aggregates of rogue prion protein in the brain are relatively resistant to protease allowing the normal prion protein (Pr. Pc) to be digested away prior to testing. But: • Problems with standardisation – lab to lab; sample to sample; tissue to tissue leading to false positives • Problems with automation • No guarantee that all rogue prion is protease resistant – Even post-mortem - atypical scrapie (nor 98) and BSE – Ante-mortem - is rogue prion in blood resistant to protease? – Most post-mortem tests cannot easily be applied to ante-mortem testing

Introduction to the Seprion Separation System

Technology background There is a wealth of scientific literature demonstrating that polyionic polymers can bind to rogue prion protein: histopathological stains, curing infected cell lines, delaying or preventing disease http: //www. priondata. org/ We have developed the use of polyionic polymers (Seprion) to specifically capture rogue prion protein and other amyloid type diseases and thus avoid the need for proteinase K
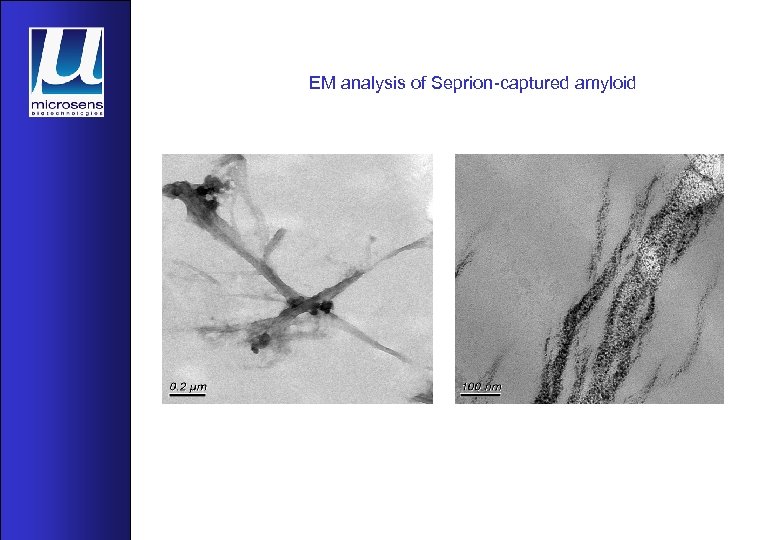
EM analysis of Seprion-captured amyloid
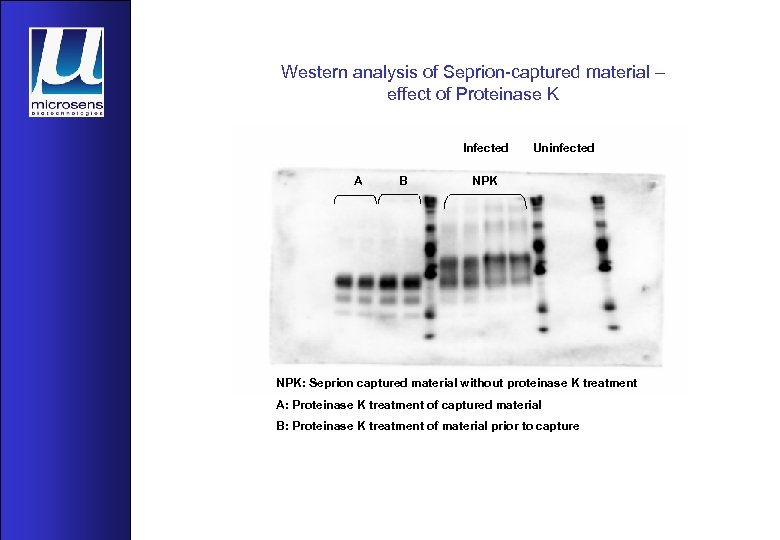
Western analysis of Seprion-captured material – effect of Proteinase K Infected A B Uninfected NPK: Seprion captured material without proteinase K treatment A: Proteinase K treatment of captured material B: Proteinase K treatment of material prior to capture
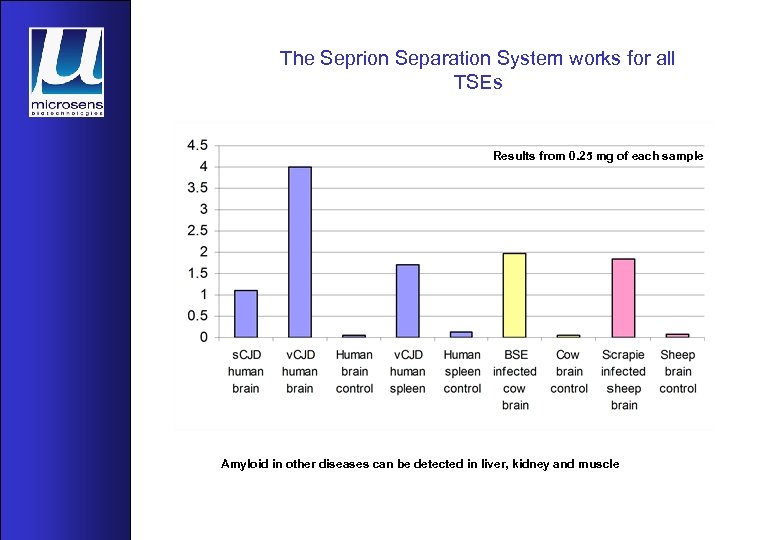
The Seprion Separation System works for all TSEs Results from 0. 25 mg of each sample Amyloid in other diseases can be detected in liver, kidney and muscle
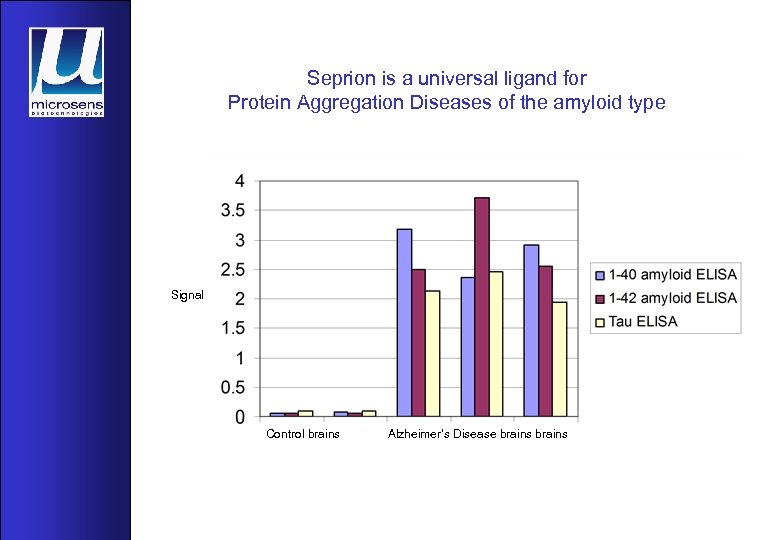
Seprion is a universal ligand for Protein Aggregation Diseases of the amyloid type Signal Control brains Alzheimer’s Disease brains
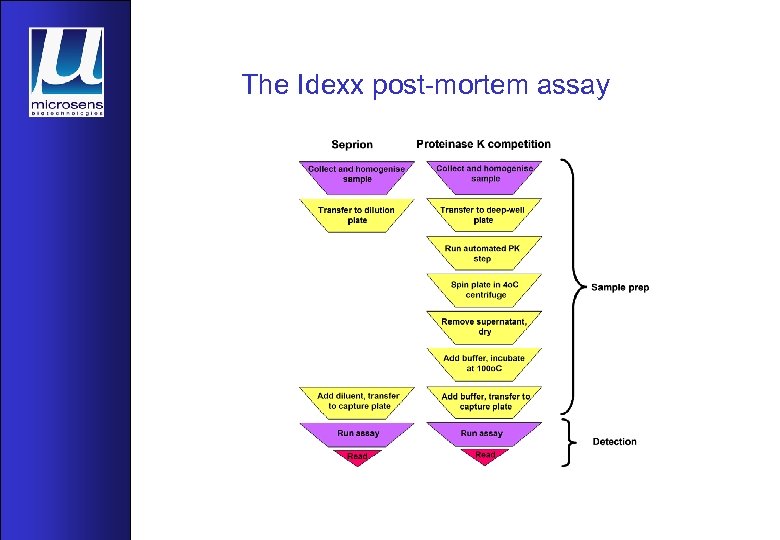
The Idexx post-mortem assay

TSE post-mortem commercial summary • The Idexx BSE and CWD commercial assay has 100% specificity and 100% sensitivity compared to existing EU approved tests on brain and lymph nodes • USDA approval for BSE and CWD • EU approval for BSE and scrapie • 100% sensitivity and specificity compared to IHC on ante -mortem scrapie RAMALT testing

The Seprion Separation System applied to blood screening for TSEs

Keeping blood safe • • • The size of the problem is still unknown Blood related transmission has occurred Reduce risk by exclusion – Donor exclusion. Incomplete. – Leucodepletion. Animal models demonstrate that 55% of infectivity remains (Rohwer, 2004) – Filtration. Pall Corp, PRDT. Animal organ spiking models. Endogenous infection may be species specific. Prion in plasma may be complexed with other proteins/lipid rafts. Quality assurance? Epidemiology? • Abrogate risk through blood testing
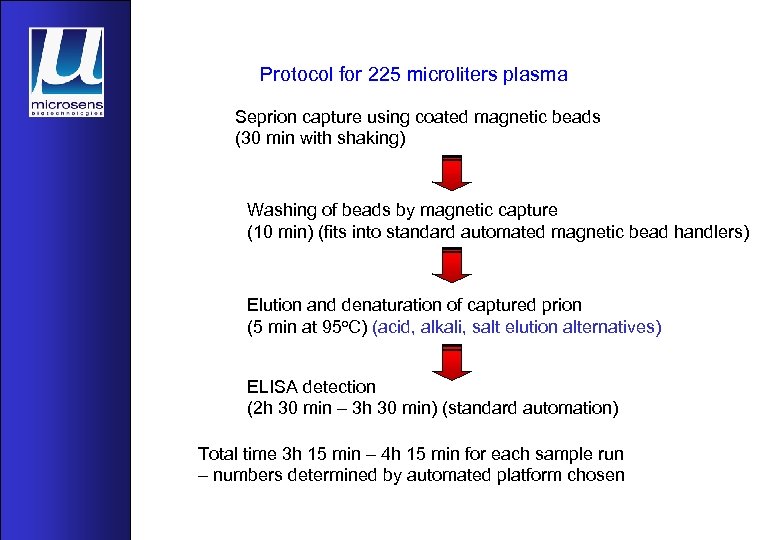
Protocol for 225 microliters plasma Seprion capture using coated magnetic beads (30 min with shaking) Washing of beads by magnetic capture (10 min) (fits into standard automated magnetic bead handlers) Elution and denaturation of captured prion (5 min at 95 o. C) (acid, alkali, salt elution alternatives) ELISA detection (2 h 30 min – 3 h 30 min) (standard automation) Total time 3 h 15 min – 4 h 15 min for each sample run – numbers determined by automated platform chosen
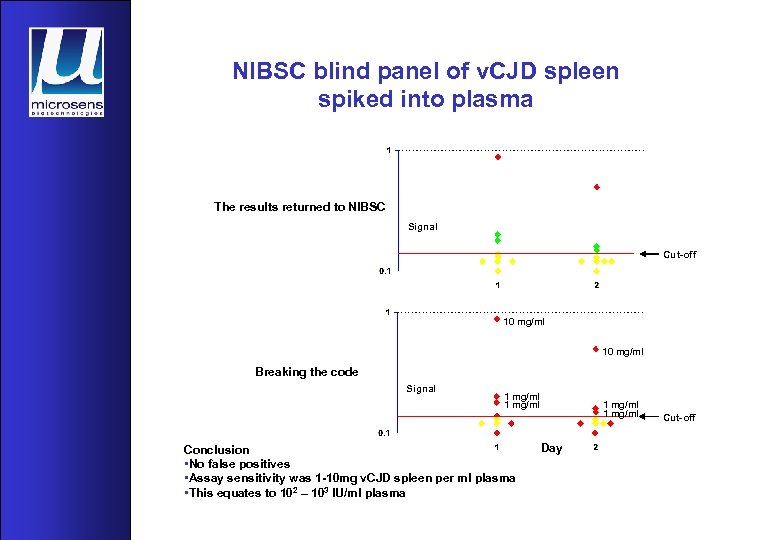
NIBSC blind panel of v. CJD spleen spiked into plasma The results returned to NIBSC Signal Cut-off 10 mg/ml Breaking the code Signal 1 mg/ml Conclusion • No false positives • Assay sensitivity was 1 -10 mg v. CJD spleen per ml plasma • This equates to 102 – 103 IU/ml plasma 1 mg/ml Day Cut-off
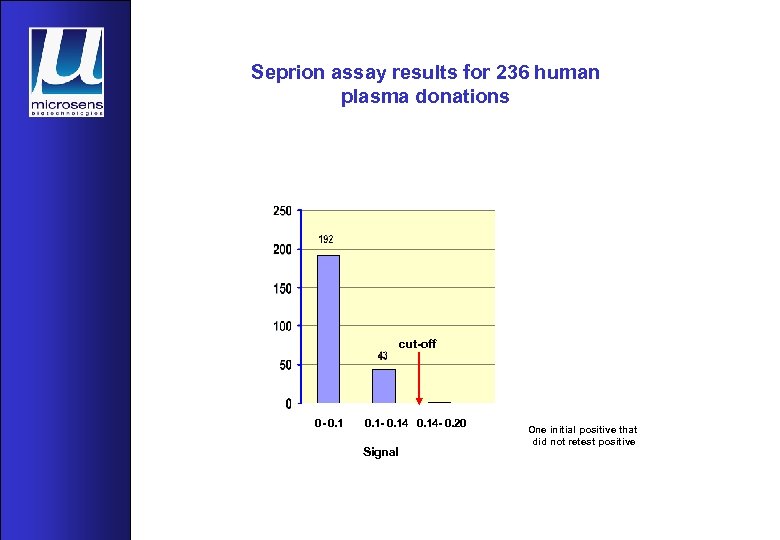
Seprion assay results for 236 human plasma donations cut-off 0 - 0. 1 - 0. 14 - 0. 20 Signal One initial positive that did not retest positive
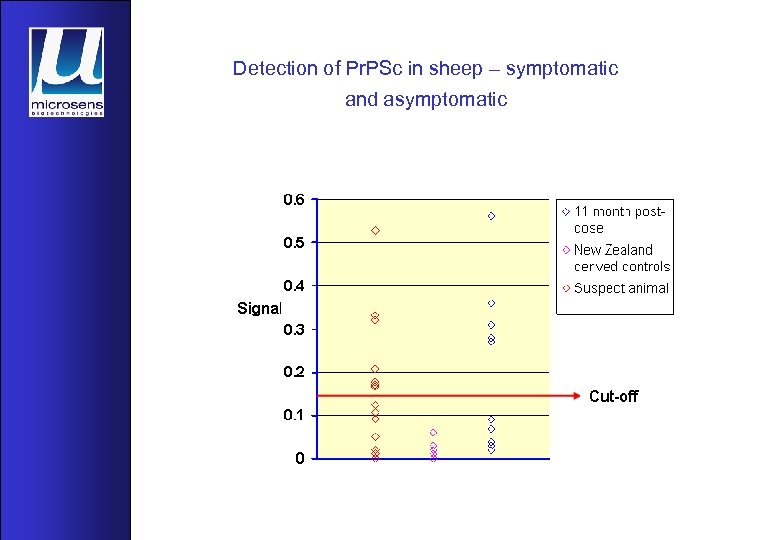
Detection of Pr. PSc in sheep – symptomatic and asymptomatic
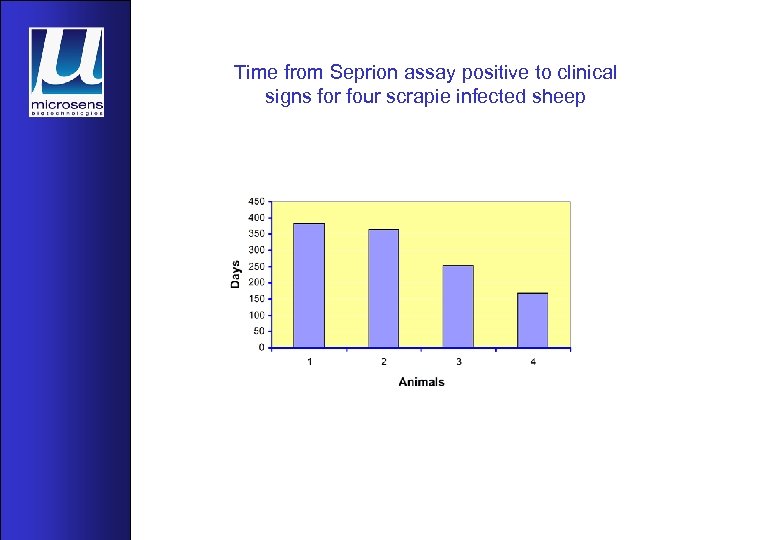
Time from Seprion assay positive to clinical signs for four scrapie infected sheep
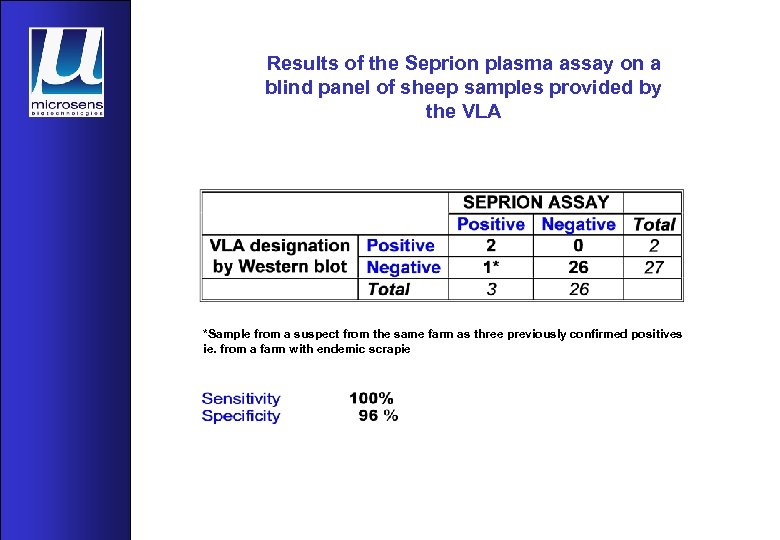
Results of the Seprion plasma assay on a blind panel of sheep samples provided by the VLA *Sample from a suspect from the same farm as three previously confirmed positives ie. from a farm with endemic scrapie

Summary • The Seprion Separation System has been extensively validated on TSE post-mortem brain samples and the plate based system has been approved by the USDA and EU. • A more flexible bead-based approach has been developed for use in therapeutic screening, for other tissues and for other protein aggregation diseases such as Alzheimer’s Disease. • This same protocol was used to investigate scrapie in sheep plasma. • Abnormal prion could be detected in symptomatic and asymptomatic pre-clinical animals. • Scrapie blind panel studies have been completed and a time course study is underway. • We are poised to receive the human plasma panel
fadfd648b8a685e5e1bdd32ec7b7e0c4.ppt