b63ab47b14044818f9b2ee951ce0f884.ppt
- Количество слайдов: 36
 The Search for Synergism A Data Analytic Approach L. Wouters, J. Van Dun, L. Bijnens May 2003 Three Country Corner Royal Statistical Society
The Search for Synergism A Data Analytic Approach L. Wouters, J. Van Dun, L. Bijnens May 2003 Three Country Corner Royal Statistical Society
 Overview § Combined action of drugs § Screening for synergism § Experimental Design § Fitting concentration response curves, estimation of IC 50 § Graphical analysis of combined action – isobolograms – fraction plots – combination index 2
Overview § Combined action of drugs § Screening for synergism § Experimental Design § Fitting concentration response curves, estimation of IC 50 § Graphical analysis of combined action – isobolograms – fraction plots – combination index 2
 Drug Combinations § Additive § Sub-additive: antagonism fight against one another § Super-additive: synergism work together 3
Drug Combinations § Additive § Sub-additive: antagonism fight against one another § Super-additive: synergism work together 3
 Drug Combinations: Antagonism - Synergism § Major therapeutic areas: – Oncology – Infectious disease § Ideal combination: – Synergistic for therapeutic activity – Antagonistic for toxicity 4
Drug Combinations: Antagonism - Synergism § Major therapeutic areas: – Oncology – Infectious disease § Ideal combination: – Synergistic for therapeutic activity – Antagonistic for toxicity 4
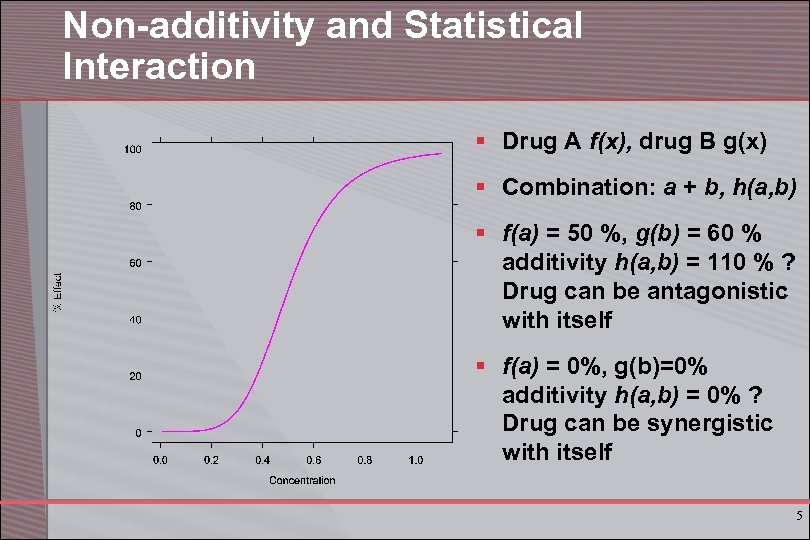 Non-additivity and Statistical Interaction § Drug A f(x), drug B g(x) § Combination: a + b, h(a, b) § f(a) = 50 %, g(b) = 60 % additivity h(a, b) = 110 % ? Drug can be antagonistic with itself § f(a) = 0%, g(b)=0% additivity h(a, b) = 0% ? Drug can be synergistic with itself 5
Non-additivity and Statistical Interaction § Drug A f(x), drug B g(x) § Combination: a + b, h(a, b) § f(a) = 50 %, g(b) = 60 % additivity h(a, b) = 110 % ? Drug can be antagonistic with itself § f(a) = 0%, g(b)=0% additivity h(a, b) = 0% ? Drug can be synergistic with itself 5
 Problems with Synergism Antagonism § Synergism is controversial issue § Literature large but confusing § Different definitions § Different methods and experimental designs § Pharmacological - biostatistical approaches § Greco (1995) Pharmacol Rev 47: 331 -385 6
Problems with Synergism Antagonism § Synergism is controversial issue § Literature large but confusing § Different definitions § Different methods and experimental designs § Pharmacological - biostatistical approaches § Greco (1995) Pharmacol Rev 47: 331 -385 6
 Sarriselkä agreement (1992) 7
Sarriselkä agreement (1992) 7
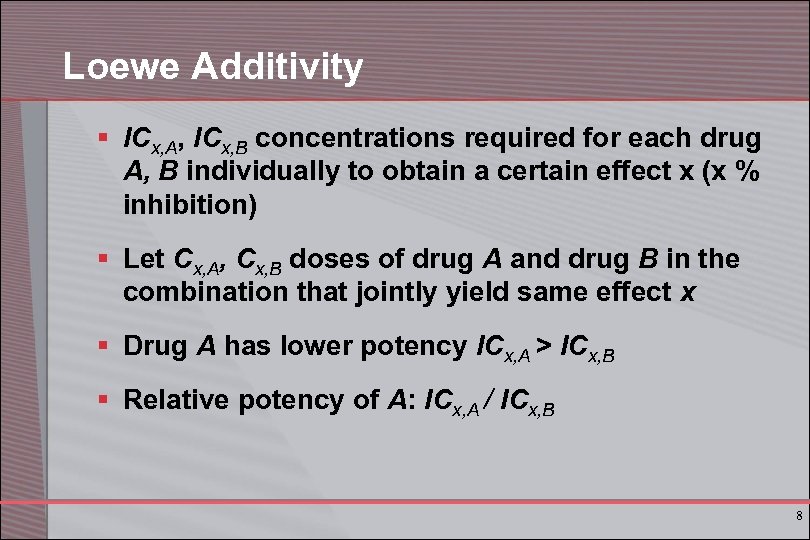 Loewe Additivity § ICx, A, ICx, B concentrations required for each drug A, B individually to obtain a certain effect x (x % inhibition) § Let Cx, A, Cx, B doses of drug A and drug B in the combination that jointly yield same effect x § Drug A has lower potency ICx, A > ICx, B § Relative potency of A: ICx, A / ICx, B 8
Loewe Additivity § ICx, A, ICx, B concentrations required for each drug A, B individually to obtain a certain effect x (x % inhibition) § Let Cx, A, Cx, B doses of drug A and drug B in the combination that jointly yield same effect x § Drug A has lower potency ICx, A > ICx, B § Relative potency of A: ICx, A / ICx, B 8
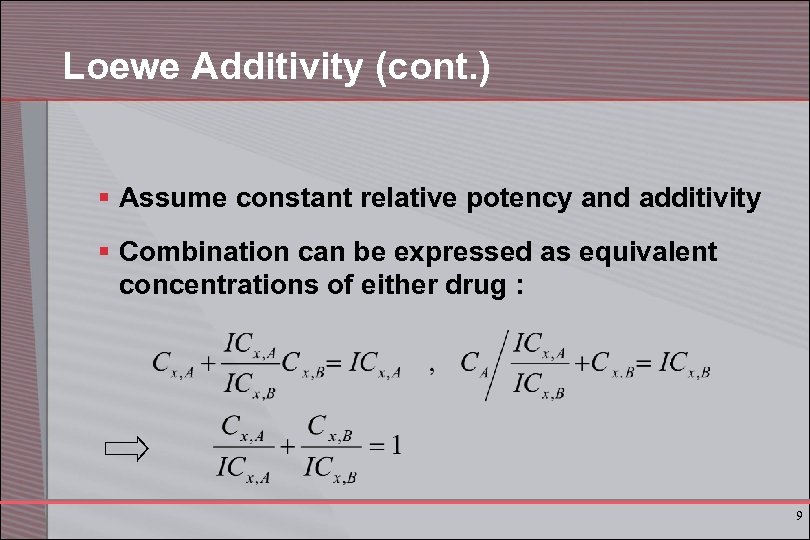 Loewe Additivity (cont. ) § Assume constant relative potency and additivity § Combination can be expressed as equivalent concentrations of either drug : 9
Loewe Additivity (cont. ) § Assume constant relative potency and additivity § Combination can be expressed as equivalent concentrations of either drug : 9
 Methods Based on Loewe Additivity § Isobologram § Interaction index of Berenbaum (1977) § Bivariate spline fitting method of Sühnel (1990) § Hypothesis testing approach of Laska (1994) § Response surface methodology of Greco (1990), Machado (1994) 10
Methods Based on Loewe Additivity § Isobologram § Interaction index of Berenbaum (1977) § Bivariate spline fitting method of Sühnel (1990) § Hypothesis testing approach of Laska (1994) § Response surface methodology of Greco (1990), Machado (1994) 10
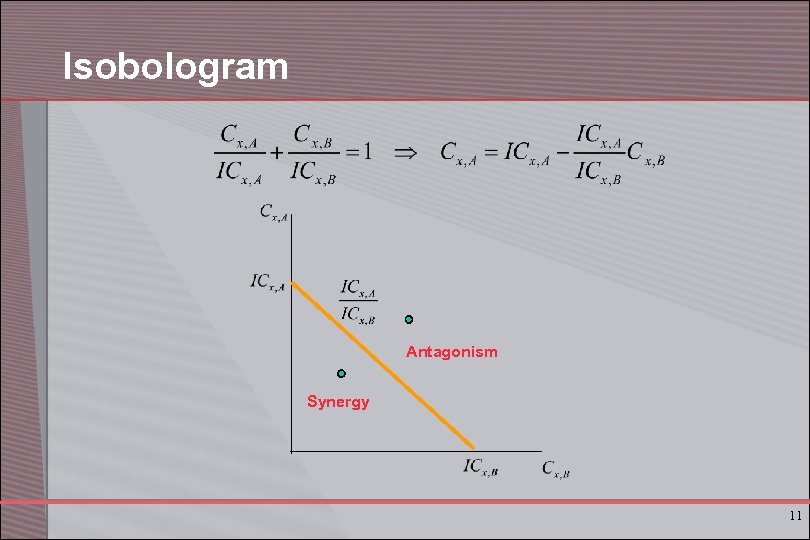 Isobologram Antagonism Synergy 11
Isobologram Antagonism Synergy 11
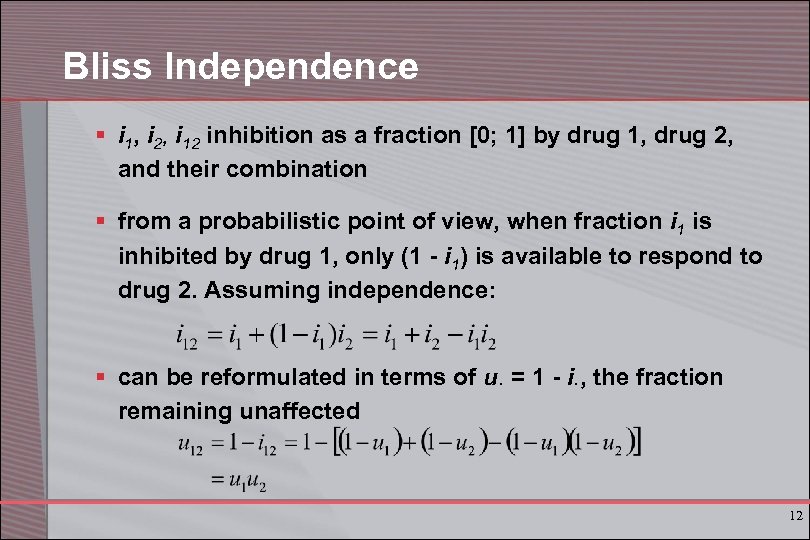 Bliss Independence § i 1, i 2, i 12 inhibition as a fraction [0; 1] by drug 1, drug 2, and their combination § from a probabilistic point of view, when fraction i 1 is inhibited by drug 1, only (1 - i 1) is available to respond to drug 2. Assuming independence: § can be reformulated in terms of u. = 1 - i. , the fraction remaining unaffected 12
Bliss Independence § i 1, i 2, i 12 inhibition as a fraction [0; 1] by drug 1, drug 2, and their combination § from a probabilistic point of view, when fraction i 1 is inhibited by drug 1, only (1 - i 1) is available to respond to drug 2. Assuming independence: § can be reformulated in terms of u. = 1 - i. , the fraction remaining unaffected 12
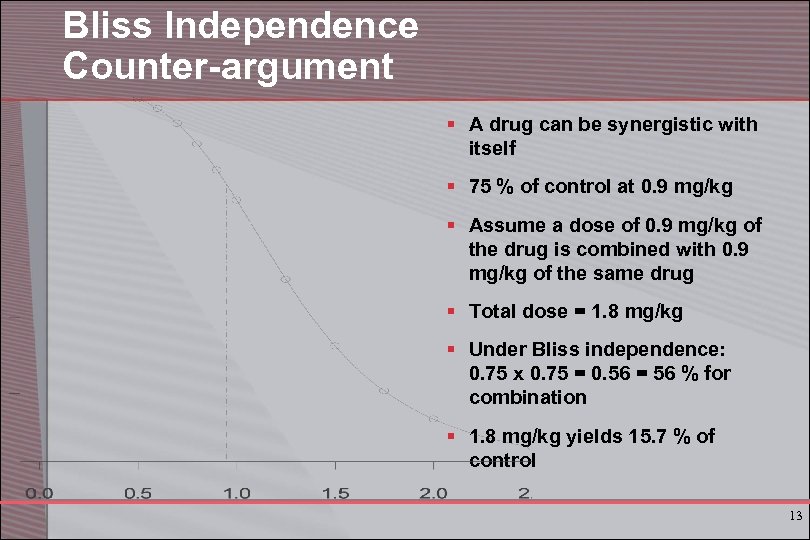 Bliss Independence Counter-argument § A drug can be synergistic with itself § 75 % of control at 0. 9 mg/kg § Assume a dose of 0. 9 mg/kg of the drug is combined with 0. 9 mg/kg of the same drug § Total dose = 1. 8 mg/kg § Under Bliss independence: 0. 75 x 0. 75 = 0. 56 = 56 % for combination § 1. 8 mg/kg yields 15. 7 % of control 13
Bliss Independence Counter-argument § A drug can be synergistic with itself § 75 % of control at 0. 9 mg/kg § Assume a dose of 0. 9 mg/kg of the drug is combined with 0. 9 mg/kg of the same drug § Total dose = 1. 8 mg/kg § Under Bliss independence: 0. 75 x 0. 75 = 0. 56 = 56 % for combination § 1. 8 mg/kg yields 15. 7 % of control 13
 Screening for Synergism in Oncology § Screening experiment – as simple as possible with limited resources – carried out on a routine basis – analysis must be automated § Screening experiments on tumor cells grown in 96 -well microtiter plates 14
Screening for Synergism in Oncology § Screening experiment – as simple as possible with limited resources – carried out on a routine basis – analysis must be automated § Screening experiments on tumor cells grown in 96 -well microtiter plates 14
 Screening Experiment Requirements – Unbiased estimates of responses – Avoidance of confounding of random error and drug effects – Elimination of plate effects and plate location effects in 96 -well plates 15
Screening Experiment Requirements – Unbiased estimates of responses – Avoidance of confounding of random error and drug effects – Elimination of plate effects and plate location effects in 96 -well plates 15
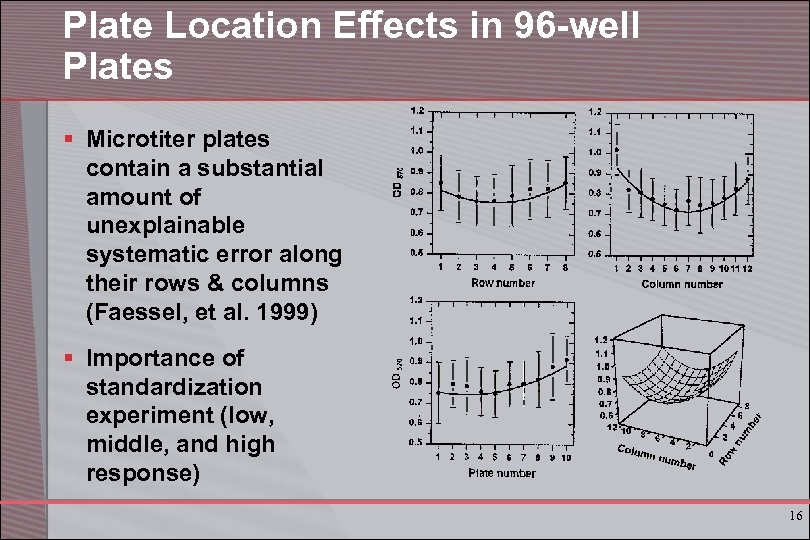 Plate Location Effects in 96 -well Plates § Microtiter plates contain a substantial amount of unexplainable systematic error along their rows & columns (Faessel, et al. 1999) § Importance of standardization experiment (low, middle, and high response) 16
Plate Location Effects in 96 -well Plates § Microtiter plates contain a substantial amount of unexplainable systematic error along their rows & columns (Faessel, et al. 1999) § Importance of standardization experiment (low, middle, and high response) 16
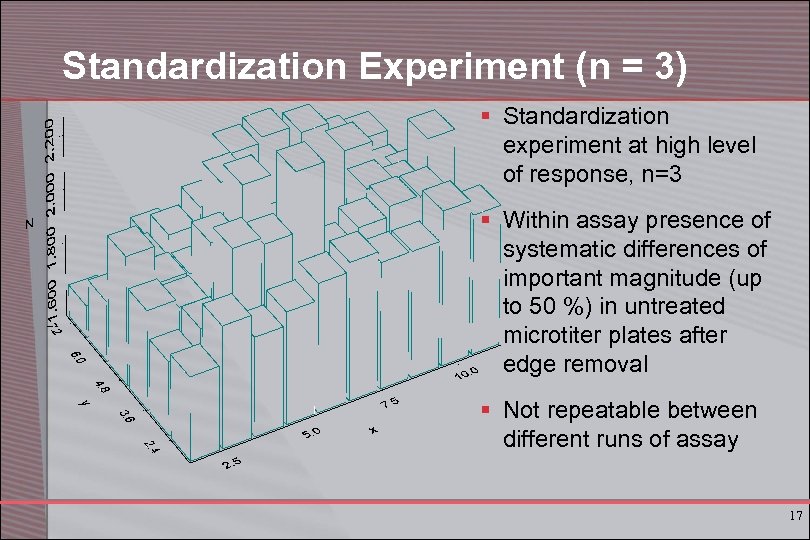 Standardization Experiment (n = 3) § Standardization experiment at high level of response, n=3 § Within assay presence of systematic differences of important magnitude (up to 50 %) in untreated microtiter plates after edge removal § Not repeatable between different runs of assay 17
Standardization Experiment (n = 3) § Standardization experiment at high level of response, n=3 § Within assay presence of systematic differences of important magnitude (up to 50 %) in untreated microtiter plates after edge removal § Not repeatable between different runs of assay 17
 How to Eliminate Bias & Confounding ? § Randomization assures: – Equal probability to attain a specific response for each well – Independence of results – Absence of confounding – Proper estimation of random error 18
How to Eliminate Bias & Confounding ? § Randomization assures: – Equal probability to attain a specific response for each well – Independence of results – Absence of confounding – Proper estimation of random error 18
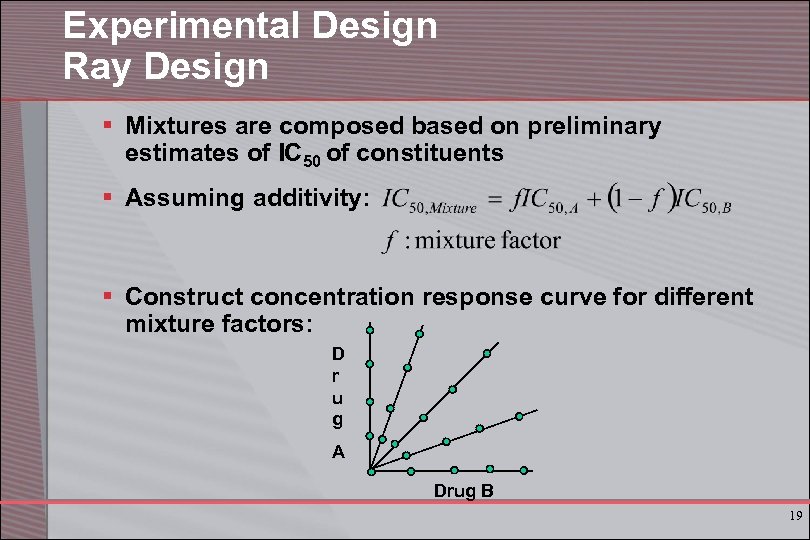 Experimental Design Ray Design § Mixtures are composed based on preliminary estimates of IC 50 of constituents § Assuming additivity: § Construct concentration response curve for different mixture factors: D r u g A Drug B 19
Experimental Design Ray Design § Mixtures are composed based on preliminary estimates of IC 50 of constituents § Assuming additivity: § Construct concentration response curve for different mixture factors: D r u g A Drug B 19
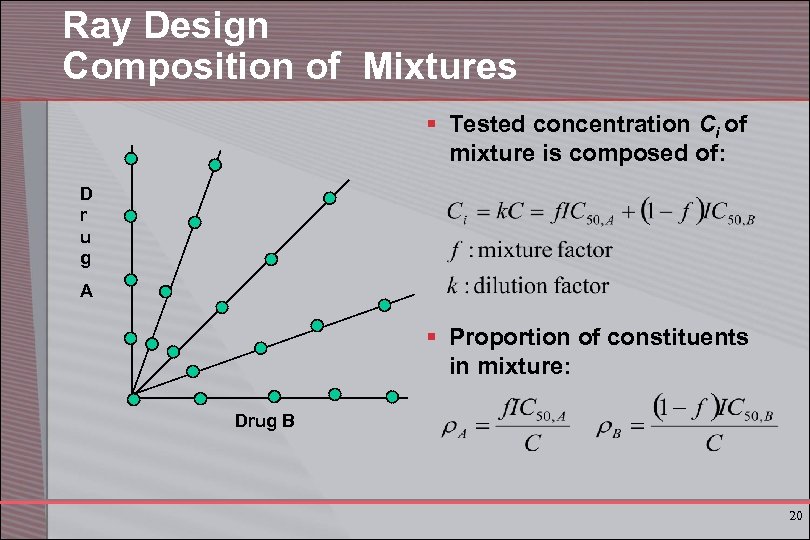 Ray Design Composition of Mixtures § Tested concentration Ci of mixture is composed of: D r u g A § Proportion of constituents in mixture: Drug B 20
Ray Design Composition of Mixtures § Tested concentration Ci of mixture is composed of: D r u g A § Proportion of constituents in mixture: Drug B 20
 Advantages of strategy § Simplified analysis: – Consider mixture as new drug – Fit concentration response curve to different dilutions of mixture § Easy to carry out in laboratory § Limited number of samples 21
Advantages of strategy § Simplified analysis: – Consider mixture as new drug – Fit concentration response curve to different dilutions of mixture § Easy to carry out in laboratory § Limited number of samples 21
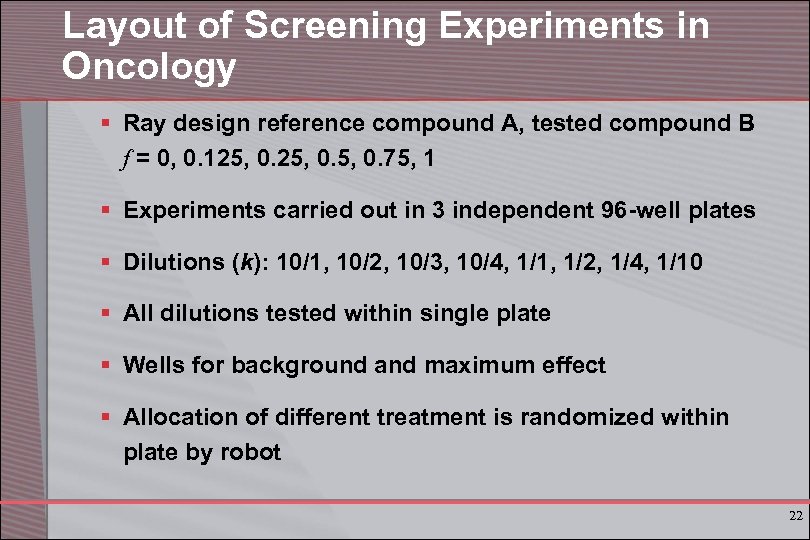 Layout of Screening Experiments in Oncology § Ray design reference compound A, tested compound B f = 0, 0. 125, 0. 75, 1 § Experiments carried out in 3 independent 96 -well plates § Dilutions (k): 10/1, 10/2, 10/3, 10/4, 1/1, 1/2, 1/4, 1/10 § All dilutions tested within single plate § Wells for background and maximum effect § Allocation of different treatment is randomized within plate by robot 22
Layout of Screening Experiments in Oncology § Ray design reference compound A, tested compound B f = 0, 0. 125, 0. 75, 1 § Experiments carried out in 3 independent 96 -well plates § Dilutions (k): 10/1, 10/2, 10/3, 10/4, 1/1, 1/2, 1/4, 1/10 § All dilutions tested within single plate § Wells for background and maximum effect § Allocation of different treatment is randomized within plate by robot 22
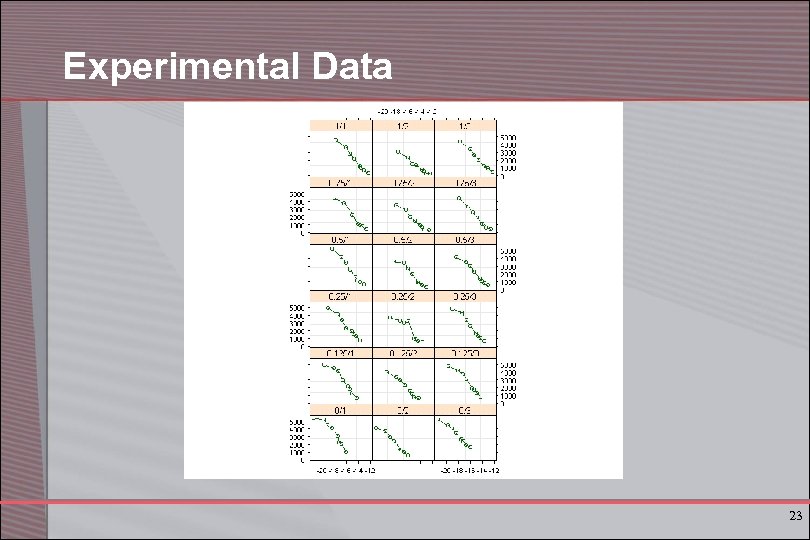 Experimental Data 23
Experimental Data 23
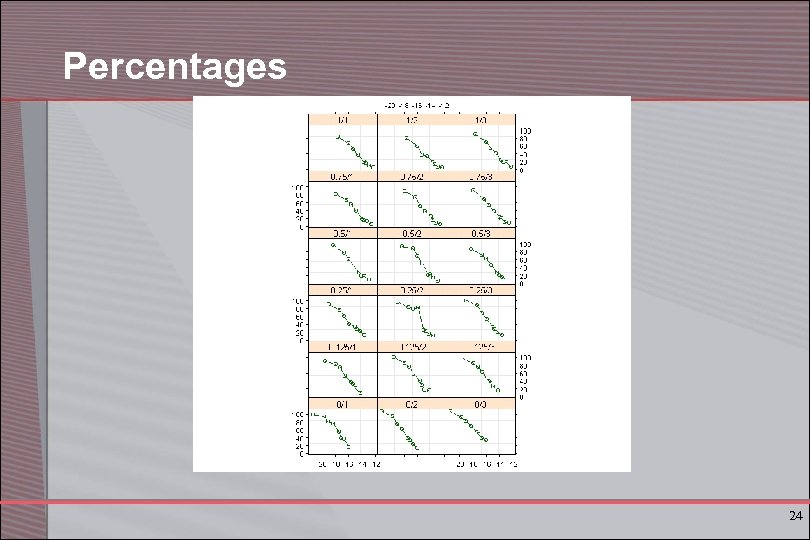 Percentages 24
Percentages 24
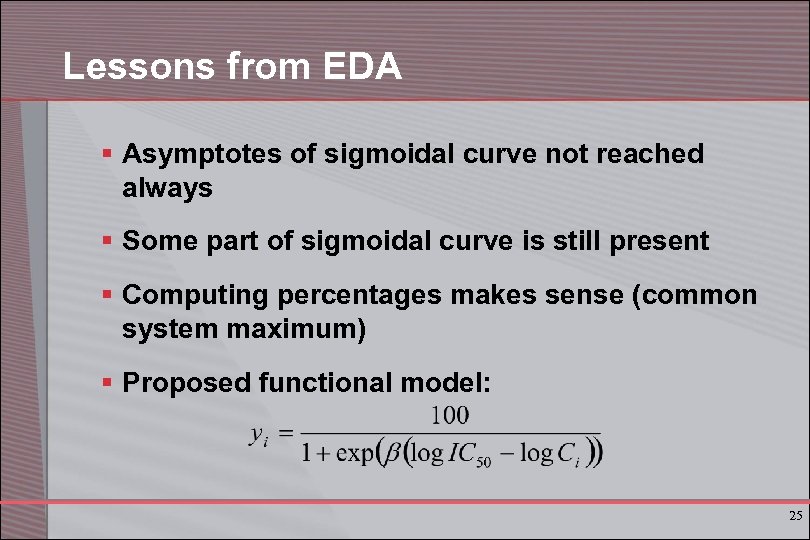 Lessons from EDA § Asymptotes of sigmoidal curve not reached always § Some part of sigmoidal curve is still present § Computing percentages makes sense (common system maximum) § Proposed functional model: 25
Lessons from EDA § Asymptotes of sigmoidal curve not reached always § Some part of sigmoidal curve is still present § Computing percentages makes sense (common system maximum) § Proposed functional model: 25
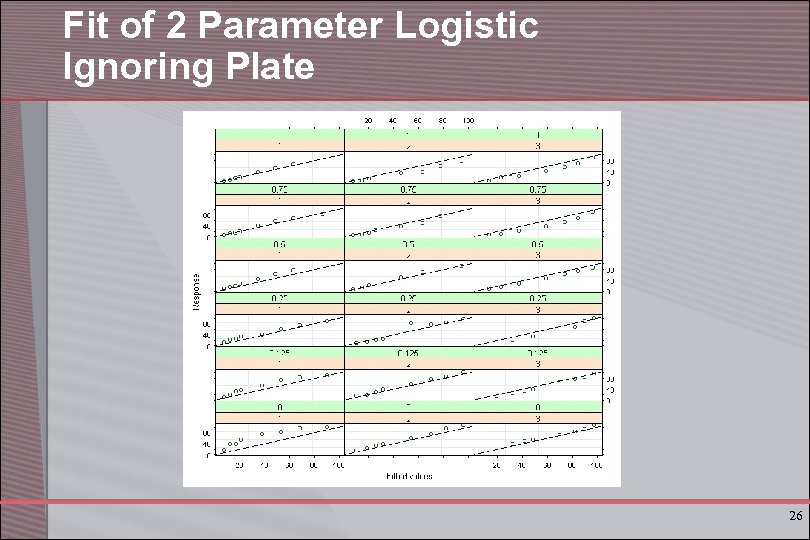 Fit of 2 Parameter Logistic Ignoring Plate 26
Fit of 2 Parameter Logistic Ignoring Plate 26
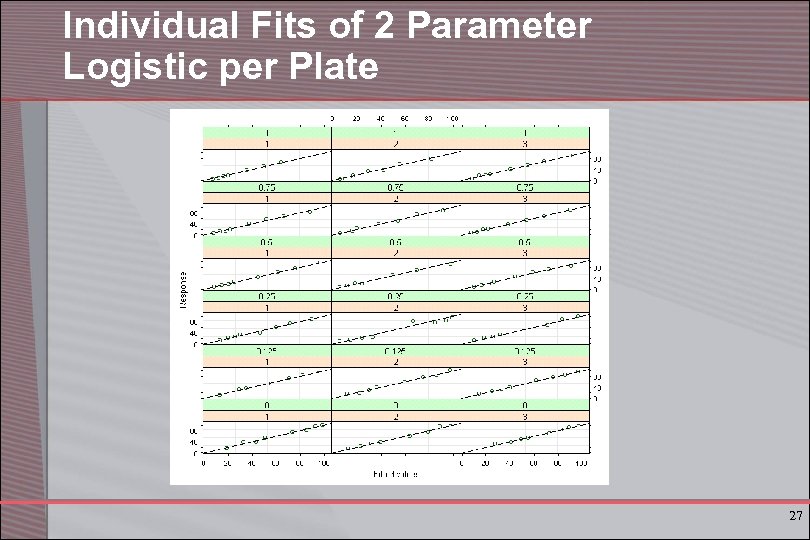 Individual Fits of 2 Parameter Logistic per Plate 27
Individual Fits of 2 Parameter Logistic per Plate 27
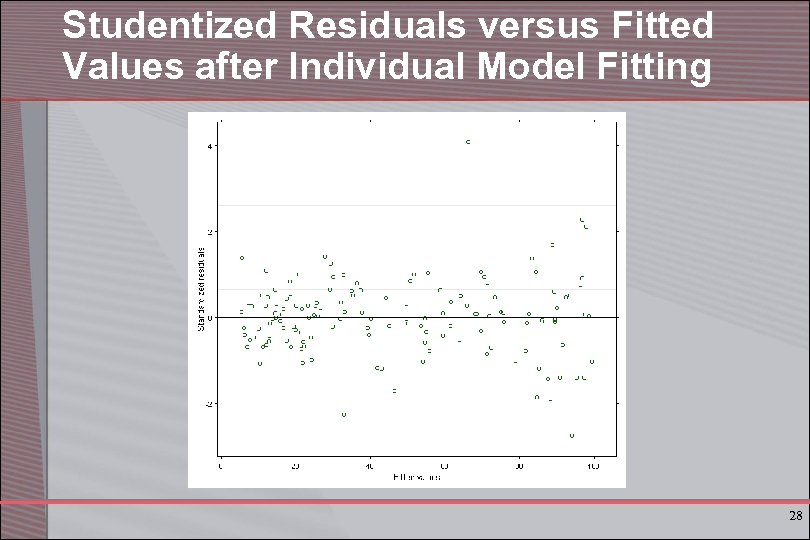 Studentized Residuals versus Fitted Values after Individual Model Fitting 28
Studentized Residuals versus Fitted Values after Individual Model Fitting 28
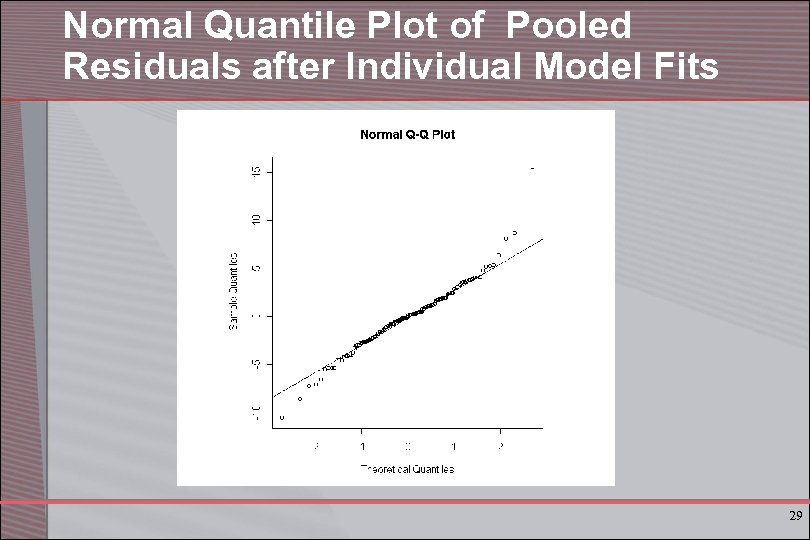 Normal Quantile Plot of Pooled Residuals after Individual Model Fits 29
Normal Quantile Plot of Pooled Residuals after Individual Model Fits 29
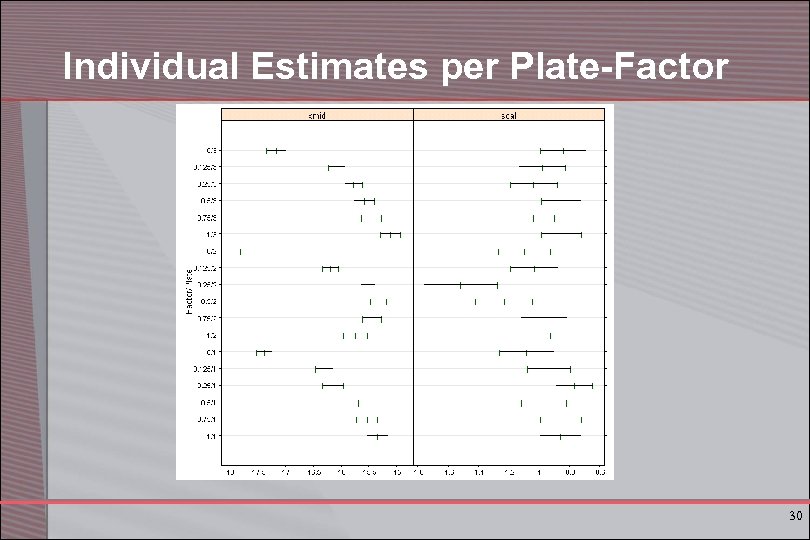 Individual Estimates per Plate-Factor 30
Individual Estimates per Plate-Factor 30
 Lessons from EDA for Functional Model Fitting § Sigmoidal shape as described by 2 -parameter logistic model § Importance of plate effect even after correcting for background, etc. by calculating percentages § How to obtain reliable estimate of IC 50 and standard errors ? 31
Lessons from EDA for Functional Model Fitting § Sigmoidal shape as described by 2 -parameter logistic model § Importance of plate effect even after correcting for background, etc. by calculating percentages § How to obtain reliable estimate of IC 50 and standard errors ? 31
 Nonlinear Mixed Effects § Nonlinear Mixed Effects Model (Pinheiro, Bates) allows to model individual response curves within plates and provides reliable estimate of standard error § Result = estimates and standard errors of model parameters as fixed effects 32
Nonlinear Mixed Effects § Nonlinear Mixed Effects Model (Pinheiro, Bates) allows to model individual response curves within plates and provides reliable estimate of standard error § Result = estimates and standard errors of model parameters as fixed effects 32
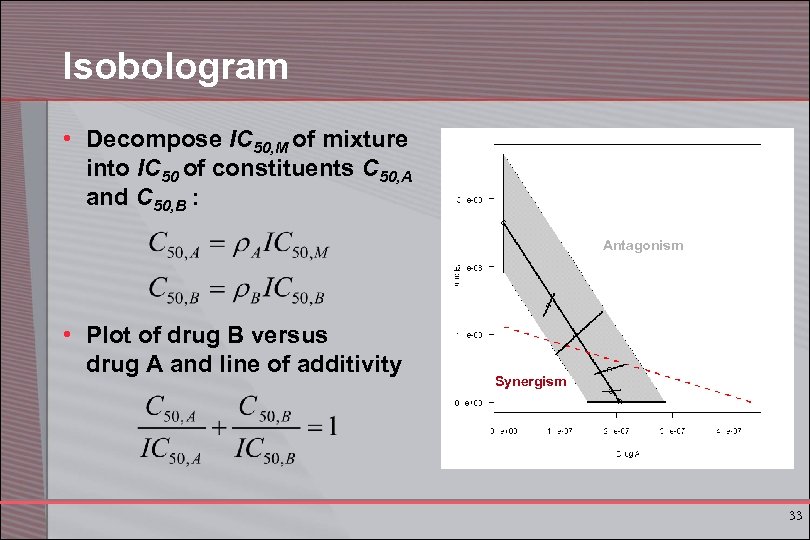 Isobologram • Decompose IC 50, M of mixture into IC 50 of constituents C 50, A and C 50, B : Antagonism • Plot of drug B versus drug A and line of additivity Synergism 33
Isobologram • Decompose IC 50, M of mixture into IC 50 of constituents C 50, A and C 50, B : Antagonism • Plot of drug B versus drug A and line of additivity Synergism 33
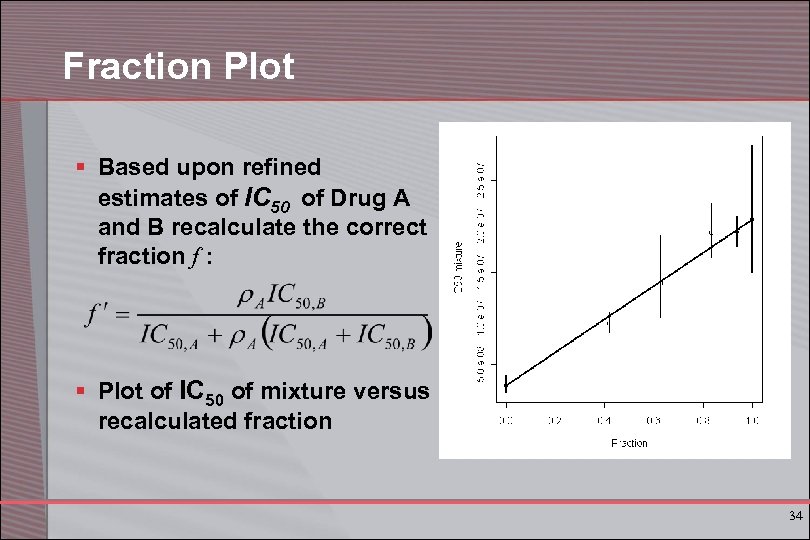 Fraction Plot § Based upon refined estimates of IC 50 of Drug A and B recalculate the correct fraction f : § Plot of IC 50 of mixture versus recalculated fraction 34
Fraction Plot § Based upon refined estimates of IC 50 of Drug A and B recalculate the correct fraction f : § Plot of IC 50 of mixture versus recalculated fraction 34
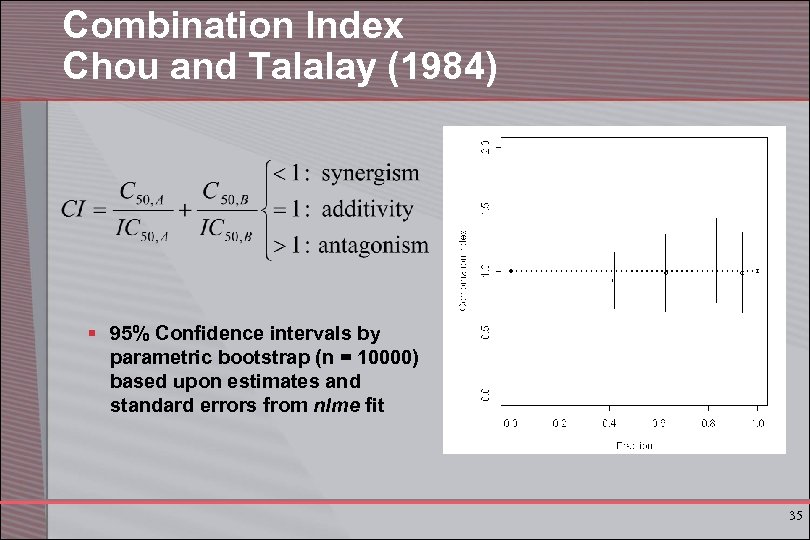 Combination Index Chou and Talalay (1984) § 95% Confidence intervals by parametric bootstrap (n = 10000) based upon estimates and standard errors from nlme fit 35
Combination Index Chou and Talalay (1984) § 95% Confidence intervals by parametric bootstrap (n = 10000) based upon estimates and standard errors from nlme fit 35
 Conclusions § Present graphical approach appealing to scientists § Still a lot to be done – T. O’Brien’s approach (TOB) – Incorporating design issues in TOB – Alternative distributions (e. g. gamma) – Optimal design 36
Conclusions § Present graphical approach appealing to scientists § Still a lot to be done – T. O’Brien’s approach (TOB) – Incorporating design issues in TOB – Alternative distributions (e. g. gamma) – Optimal design 36


