6660aef93b04d96faa962e1bf5964331.ppt
- Количество слайдов: 36
 The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 Implementation in practice • Evaluation of a trial of two safety engineered iv cannulae • Setting: – Acute London hospital theatres dept – May/June 2007 The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Implementation in practice • Evaluation of a trial of two safety engineered iv cannulae • Setting: – Acute London hospital theatres dept – May/June 2007 The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 Background • MCEAG • Finance • Costs – it can be introduced at no extra cost – savings of about £ 5649 (rebate of £ 0. 20 per cannulae available) estimated The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Background • MCEAG • Finance • Costs – it can be introduced at no extra cost – savings of about £ 5649 (rebate of £ 0. 20 per cannulae available) estimated The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 Products evaluated • Ported, winged cannula (20, 22 & 24 G) • Non-ported, non winged cannula (14, 16, 18, 20, 22 & 24 G) • incorporate self-activating clip technology, preventing needlestick injury The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Products evaluated • Ported, winged cannula (20, 22 & 24 G) • Non-ported, non winged cannula (14, 16, 18, 20, 22 & 24 G) • incorporate self-activating clip technology, preventing needlestick injury The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
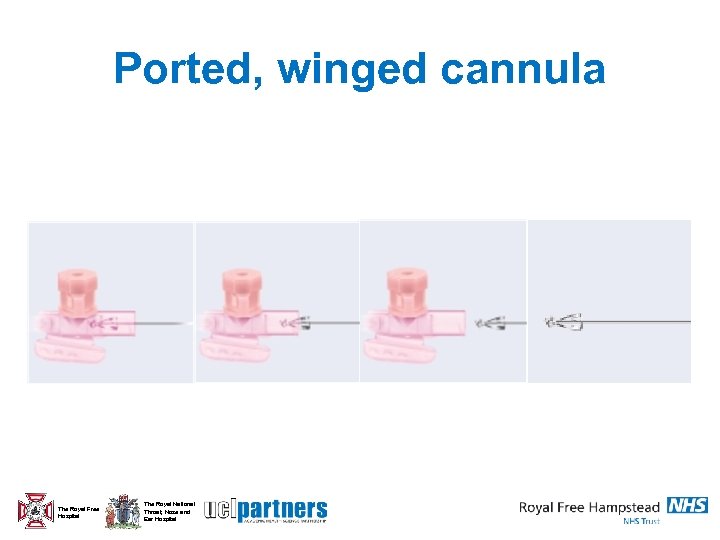 Ported, winged cannula The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Ported, winged cannula The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
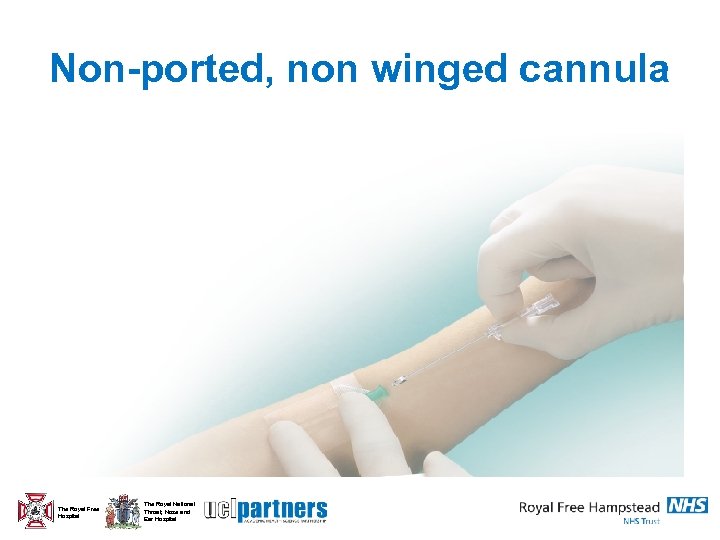 Non-ported, non winged cannula The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Non-ported, non winged cannula The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 Evaluation Plan • • Two week trial Evaluation form Covering letter Theatres • • Emergency Neurosurgery Liver Urology • Standard cannulae removed during trial The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Evaluation Plan • • Two week trial Evaluation form Covering letter Theatres • • Emergency Neurosurgery Liver Urology • Standard cannulae removed during trial The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 Coordinated by: • • Supplies Clinical Nurse Advisor Consultant in Occupational Medicine Consultant Anaesthetist Principal ODA, Theatres Theatre Manager Infection Control Lead Nurse Practice Development Lead Nurse The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Coordinated by: • • Supplies Clinical Nurse Advisor Consultant in Occupational Medicine Consultant Anaesthetist Principal ODA, Theatres Theatre Manager Infection Control Lead Nurse Practice Development Lead Nurse The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 Evaluation criteria • • Ease of use/requirement for training Dexterity Sharpness Flashback visualisation Patient experience Reliable operation of safety mechanism Sharps injury risk reduction (perception) The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Evaluation criteria • • Ease of use/requirement for training Dexterity Sharpness Flashback visualisation Patient experience Reliable operation of safety mechanism Sharps injury risk reduction (perception) The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
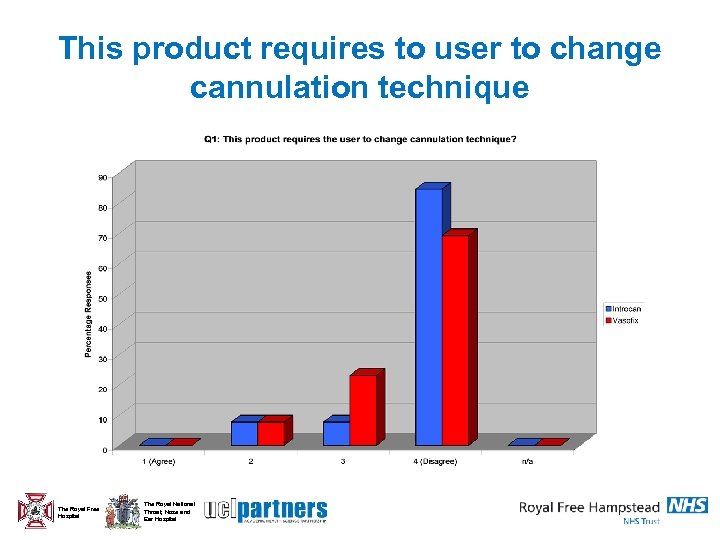 This product requires to user to change cannulation technique The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
This product requires to user to change cannulation technique The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
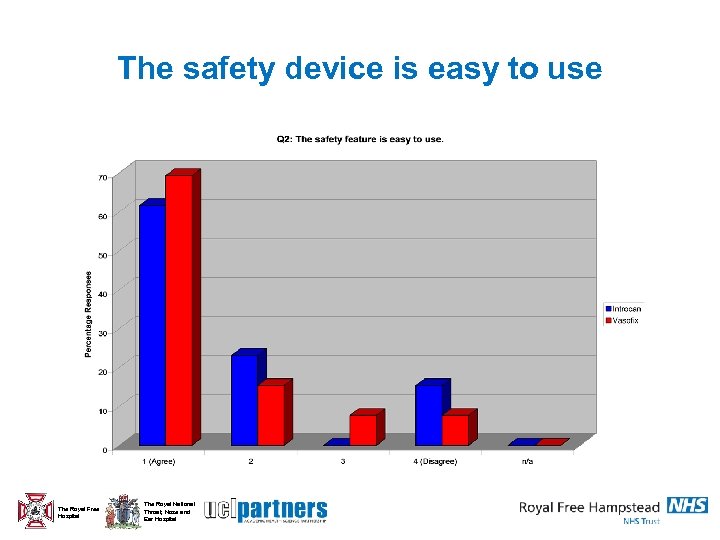 The safety device is easy to use The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
The safety device is easy to use The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
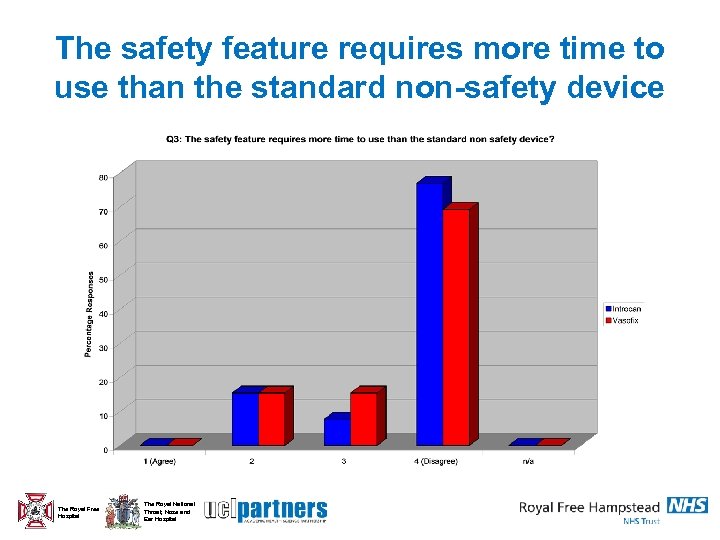 The safety feature requires more time to use than the standard non-safety device The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
The safety feature requires more time to use than the standard non-safety device The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
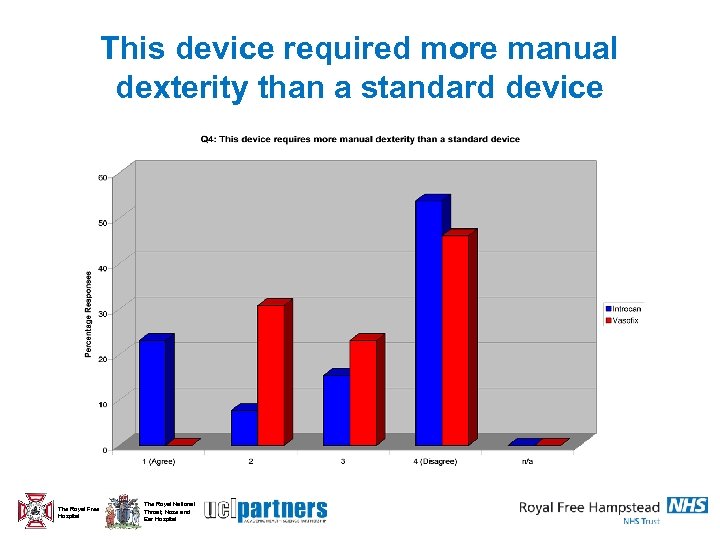 This device required more manual dexterity than a standard device The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
This device required more manual dexterity than a standard device The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
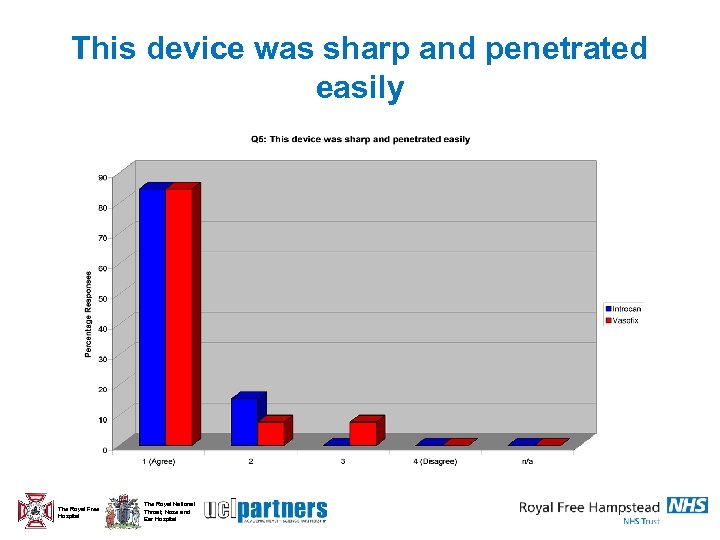 This device was sharp and penetrated easily The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
This device was sharp and penetrated easily The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
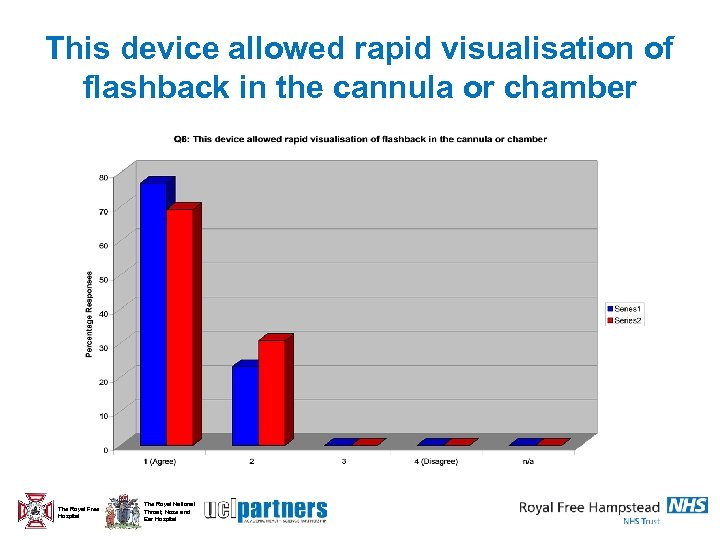 This device allowed rapid visualisation of flashback in the cannula or chamber The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
This device allowed rapid visualisation of flashback in the cannula or chamber The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
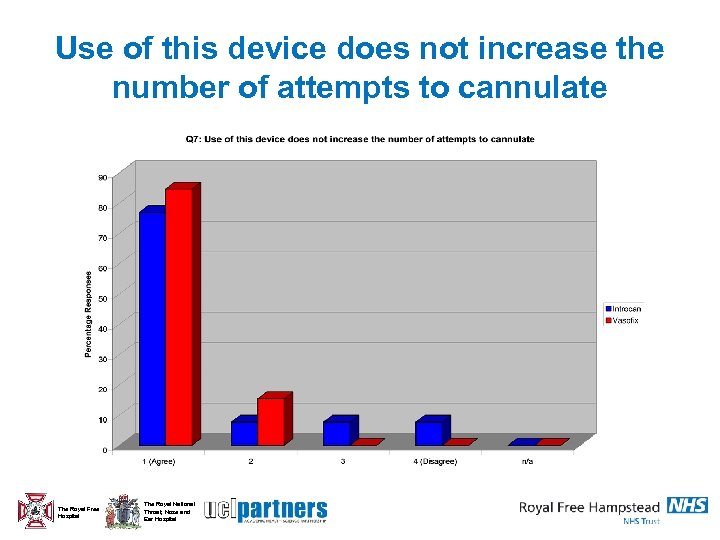 Use of this device does not increase the number of attempts to cannulate The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Use of this device does not increase the number of attempts to cannulate The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
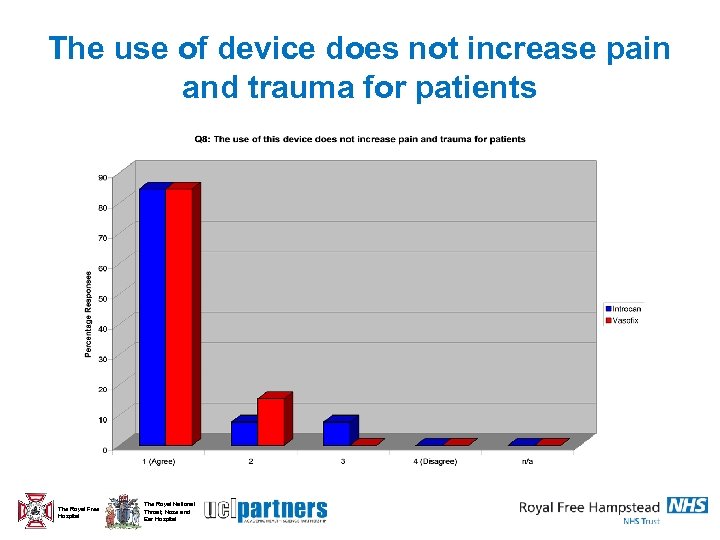 The use of device does not increase pain and trauma for patients The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
The use of device does not increase pain and trauma for patients The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
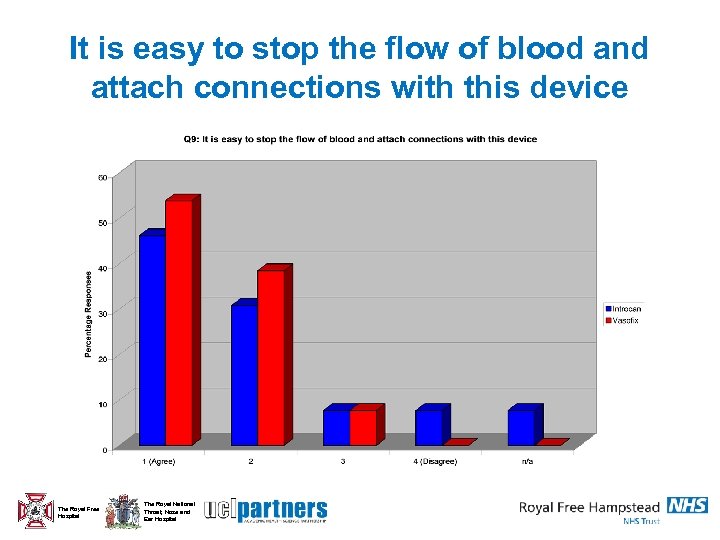 It is easy to stop the flow of blood and attach connections with this device The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
It is easy to stop the flow of blood and attach connections with this device The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
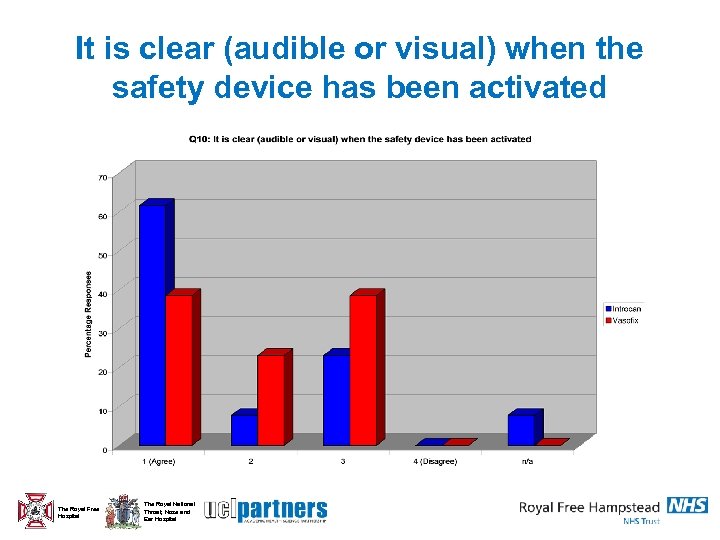 It is clear (audible or visual) when the safety device has been activated The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
It is clear (audible or visual) when the safety device has been activated The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
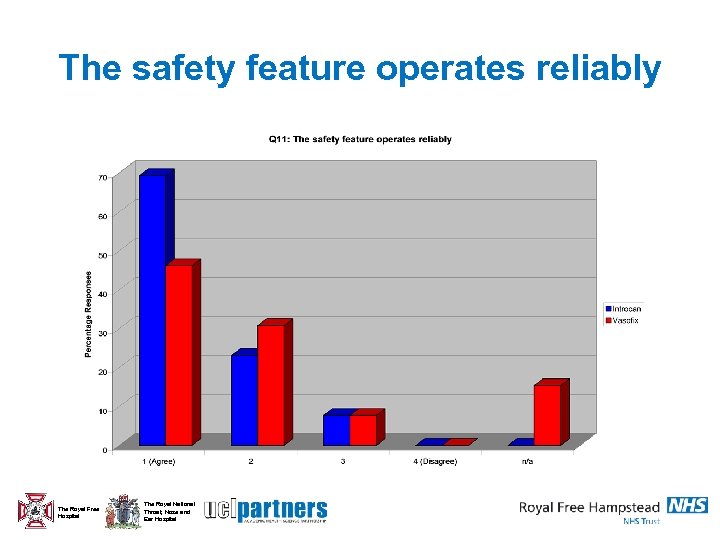 The safety feature operates reliably The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
The safety feature operates reliably The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
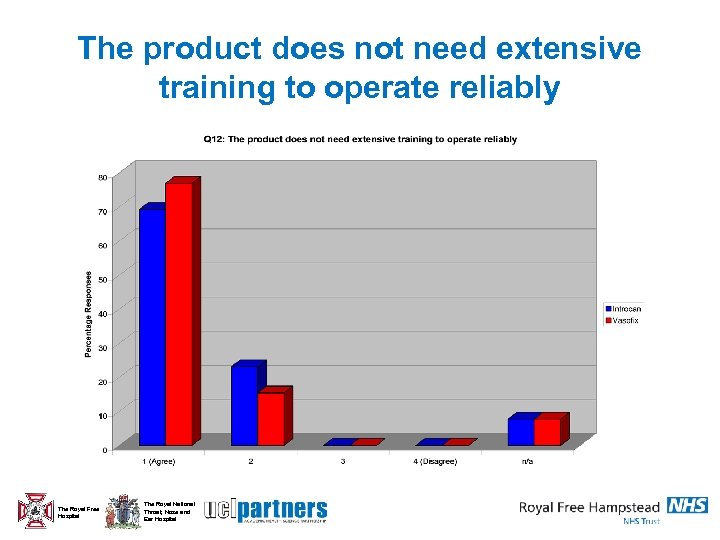 The product does not need extensive training to operate reliably The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
The product does not need extensive training to operate reliably The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
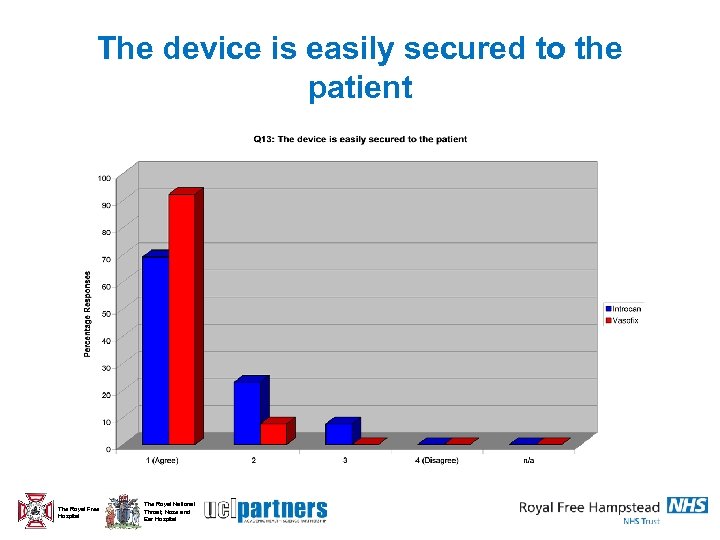 The device is easily secured to the patient The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
The device is easily secured to the patient The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
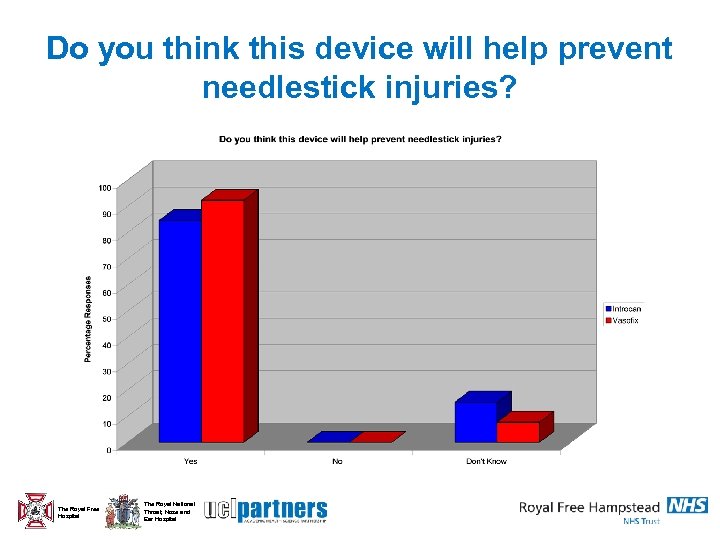 Do you think this device will help prevent needlestick injuries? The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Do you think this device will help prevent needlestick injuries? The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
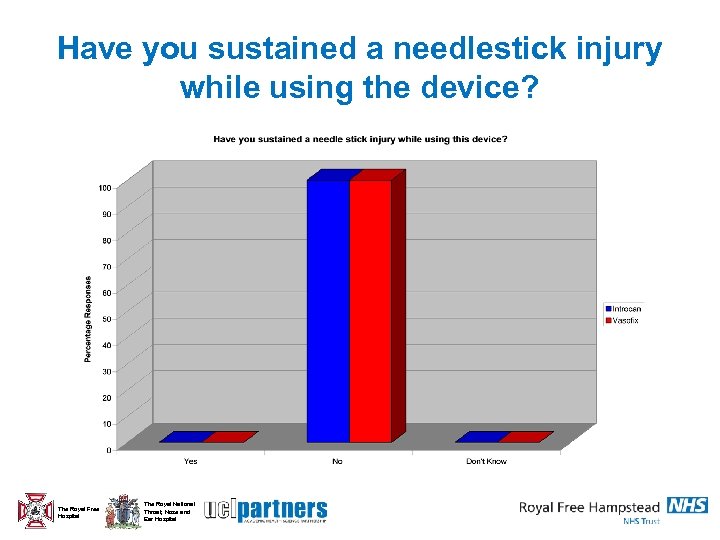 Have you sustained a needlestick injury while using the device? The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Have you sustained a needlestick injury while using the device? The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
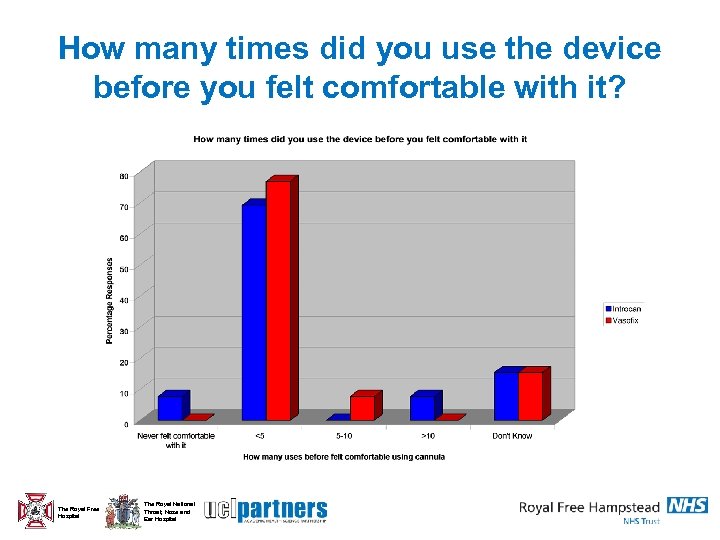 How many times did you use the device before you felt comfortable with it? The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
How many times did you use the device before you felt comfortable with it? The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 Conclusions • the two devices were well received and acceptable to users on all parameters The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Conclusions • the two devices were well received and acceptable to users on all parameters The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 Recommendations • Ported, winged cannula (20, 22, & 24 G) to be implemented only in the Theatres • Non-ported, non winged cannula (14, 16, 18, 20, 22, & 24 G) to be implemented throughout the Trust The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Recommendations • Ported, winged cannula (20, 22, & 24 G) to be implemented only in the Theatres • Non-ported, non winged cannula (14, 16, 18, 20, 22, & 24 G) to be implemented throughout the Trust The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 Implementation • August 2007 • Briefing letter for ward and departments • Training sessions e. g. for new junior doctors The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Implementation • August 2007 • Briefing letter for ward and departments • Training sessions e. g. for new junior doctors The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 October 2007 6 week post implementation • Majority of wards and departments had slowly started to use the smaller gauges of the non-ported, non-winged safety cannula • Large amount of old stock, especially the larger gauge sizes (18, 16 & 14 G) • These sizes are infrequently used on the wards, so it will take considerable time for stock to be moved and replaced by the non-ported, non-winged safety cannula The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
October 2007 6 week post implementation • Majority of wards and departments had slowly started to use the smaller gauges of the non-ported, non-winged safety cannula • Large amount of old stock, especially the larger gauge sizes (18, 16 & 14 G) • These sizes are infrequently used on the wards, so it will take considerable time for stock to be moved and replaced by the non-ported, non-winged safety cannula The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 October 2007, surgical ward • Ward had a large amount of stock of old ported product– sizes 18 & 20 G. • When questioned they said that they “had been using this cannula for a while”. The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
October 2007, surgical ward • Ward had a large amount of stock of old ported product– sizes 18 & 20 G. • When questioned they said that they “had been using this cannula for a while”. The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 October 2007, paediatric wards • These wards had stock of a 24 G IV cannulae and which were non-safety. • They did not appear to have the nonported, non-winged cannula yet. • Codes needed changing The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
October 2007, paediatric wards • These wards had stock of a 24 G IV cannulae and which were non-safety. • They did not appear to have the nonported, non-winged cannula yet. • Codes needed changing The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 October 2007, ITU • Had stock of old IV cannulae and nonsafety cannulae • They had 2 boxes of the non-ported, nonwinged safety cannulae only. The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
October 2007, ITU • Had stock of old IV cannulae and nonsafety cannulae • They had 2 boxes of the non-ported, nonwinged safety cannulae only. The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 October 2007, neonatal unit • Would like to continue evaluating the nonported, non-winged safety cannula. • This is being coordinated by Matron • They are currently successfully using the samples and Matron has said that the SHOs are now evaluating them. • Evaluation is slow as they do not use many IV cannulae. The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
October 2007, neonatal unit • Would like to continue evaluating the nonported, non-winged safety cannula. • This is being coordinated by Matron • They are currently successfully using the samples and Matron has said that the SHOs are now evaluating them. • Evaluation is slow as they do not use many IV cannulae. The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 October 2007, main theatres • Principal ODA said that the non-ported, nonwinged cannulae were being ordered and slowly used, however old stock was being ordered incorrectly. • Consultant Anaesthetist said that the anaesthetists are slowly getting used to the difference. • A lunch meeting with the anaesthetists planned for 30 th October to discuss the use of the nonported, non-winged cannulae The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
October 2007, main theatres • Principal ODA said that the non-ported, nonwinged cannulae were being ordered and slowly used, however old stock was being ordered incorrectly. • Consultant Anaesthetist said that the anaesthetists are slowly getting used to the difference. • A lunch meeting with the anaesthetists planned for 30 th October to discuss the use of the nonported, non-winged cannulae The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 October 2007, planned investigation and treatment unit and midwifery • Matron and Practice Development Nurse to co-ordinate a cannulation training programme for nurses and midwives The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
October 2007, planned investigation and treatment unit and midwifery • Matron and Practice Development Nurse to co-ordinate a cannulation training programme for nurses and midwives The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
 Implementation planning • • Groundwork, stakeholders Business case Trial/pilot Training Evaluate Feedback, information Monitor, evaluate, review The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital
Implementation planning • • Groundwork, stakeholders Business case Trial/pilot Training Evaluate Feedback, information Monitor, evaluate, review The Royal Free Hospital The Royal National Throat, Nose and Ear Hospital


