1e61243b3c4dc41988bb13af51ba50e2.ppt
- Количество слайдов: 17
The Role of the Bacterioneuston in -Sea Gas Exchange Air Emma Harrison University of Newcastle-upon-Tyne, UK

Bacterioneuston? ?

Sea Surface Microlayer • Widespread, unique and dynamic habitat covering ~70% of the Earth’s surface • Microlayer ranges between 1 – 1000 μm • Definition highly debatable • Rich and diverse community of microorganisms which thrive on the interaction between the atmosphere and the water column
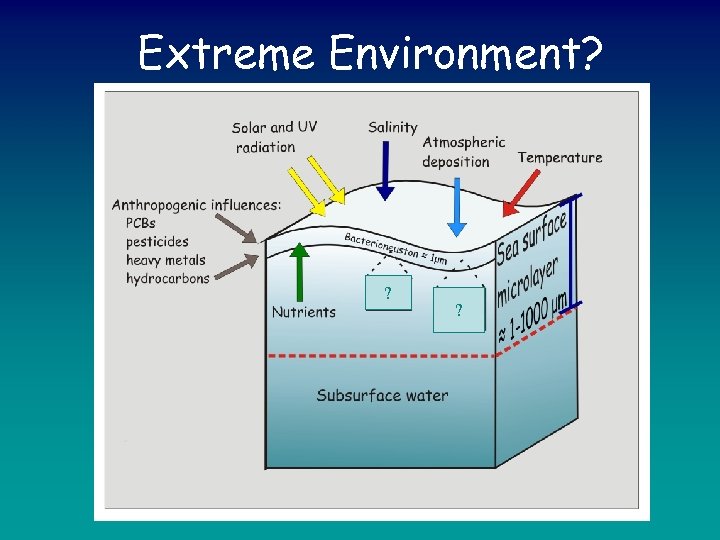
Extreme Environment? ? ?
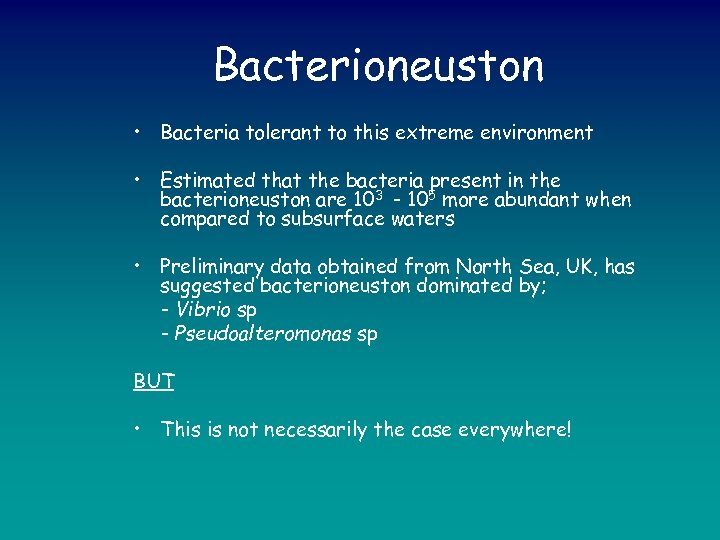
Bacterioneuston • Bacteria tolerant to this extreme environment • Estimated that the bacteria present in the bacterioneuston are 103 - 105 more abundant when compared to subsurface waters • Preliminary data obtained from North Sea, UK, has suggested bacterioneuston dominated by; - Vibrio sp - Pseudoalteromonas sp BUT • This is not necessarily the case everywhere!

Why is the bacterioneuston important?
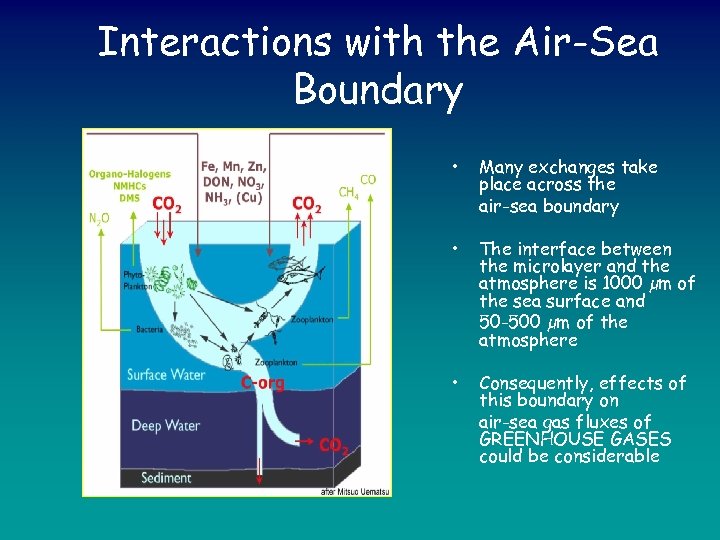
Interactions with the Air-Sea Boundary • Many exchanges take place across the air-sea boundary • The interface between the microlayer and the atmosphere is 1000 µm of the sea surface and 50 -500 µm of the atmosphere • Consequently, effects of this boundary on air-sea gas fluxes of GREENHOUSE GASES could be considerable

Microbial metabolism of climatically active trace gases such as CH 4, N 2 O, CO, DMS and methyl halides in the bacterioneuston may exert important controls on air-sea gas exchange Determining the diversity, abundance and activity of the major groups of microorganisms in the bacterioneuston and their involvement in trace gas cycling are a high priority in the UK SOLAS projects
Research Aims Specific objectives; 1. To determine the bacterial community structure of the bacterioneuston with specific reference to bacteria that metabolise trace gases 2. Investigate the role of the microbial populations on gas exchange rates in controlled laboratory gas exchange tank experiments 3. To measure rates of invasive (i. e. air to water) and evasive (i. e. water to air) air-sea exchange of selected atmospheric trace gases • Project in collaboration with Warwick University, UK

Objective One “Analysis of the bacterial community structure of the bacterioneuston” • Bacterioneuston sampled by sterile, polycarbonate filters • Removes the top layer of water from the interface through surface tension • Construct gene libraries representative of the microbial community by use of 16 S r. RNA sequence data • Application of PCR and DGGE allows the study of these complex communities • Overcomes; - small sample size - poor culturability of neuston bacteria

Acquiring Bacterioneuston Samples Sampling at Blyth Harbour, North East England
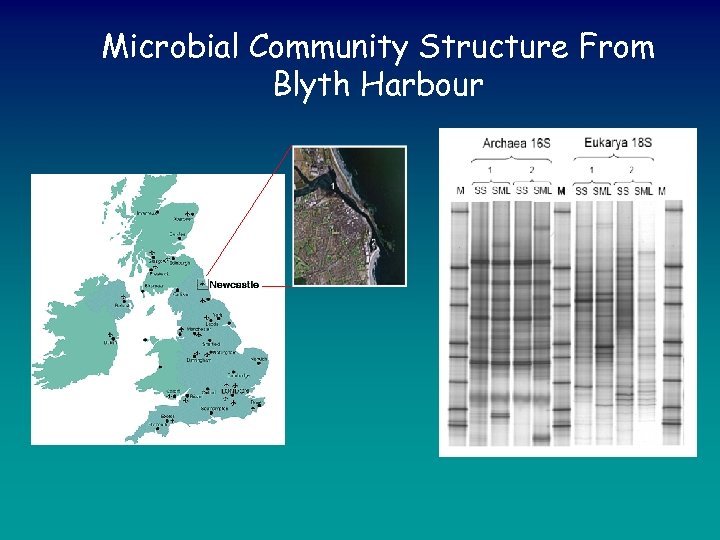
Microbial Community Structure From Blyth Harbour
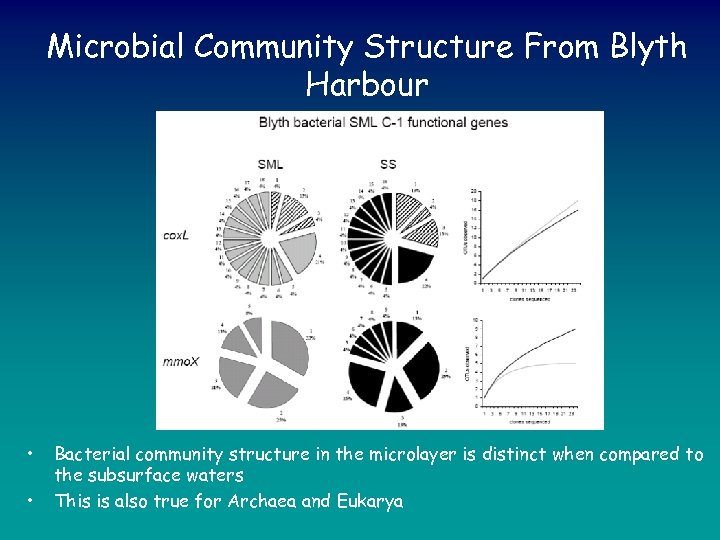
Microbial Community Structure From Blyth Harbour • • Bacterial community structure in the microlayer is distinct when compared to the subsurface waters This is also true for Archaea and Eukarya

Objective Two “Roles of the bacterioneuston investigated through gas exchange tank” • Purpose built gas exchange tank • Closed system • The microbial community structure will be correlated with changes in the gas exchange rates • Conditions altered and experiments will use local seawater (North Sea), river water (River Tyne), Milli-RO and artificial seawater prepared in Milli-RO

Objective Three “Measure the invasive and evasive rates of atmospheric trace gases” • Coupling of two gas chromatographs with gas tank to create a fully automated system to measure CH 4, CO, N 2 O and SF 6 concurrently • Tank headspace circulated through gas chromatographs • Gas fluxes quantified by estimating their transfer velocities, kw • Estimate kw by measuring evasion rates of inert volatile tracer, SF 6 with Schmidt number based scaling for each individual trace gas

Conclusion • Knowledge of the biology and population structure within the bacterioneuston is still in its infancy • Unclear what role these microorganisms play • Is clear the sea surface microlayer has the potential to impact the cycling of reactive trace gases and the exchange rate of these gases across the air-sea boundary • Using a combination of molecular ecology techniques and an understanding of gas exchange, the knowledge of this unique and dynamic environment will be greatly improved

Acknowledgements Many thanks to the following… • Supervisors; Rob Upstill-Goddard (University of Newcastle) Colin Murrell (University of Warwick) • Michael Cunliffe (University of Warwick) for his support and advice on molecular ecology and for microbial community structure work • Grant Forster for technical assistance • UK SOLAS Project • Natural Environment Research Council, UK
1e61243b3c4dc41988bb13af51ba50e2.ppt