73a608f328ad0cb147a2bfbb6d5e94ea.ppt
- Количество слайдов: 79
 The Role of Molecular Diagnostics in Infectious Disease SOMC Overview and Future Directions SOMC Grand Rounds January 8, 2016 Timothy R. Cassity, Ph. D. Microbiologist
The Role of Molecular Diagnostics in Infectious Disease SOMC Overview and Future Directions SOMC Grand Rounds January 8, 2016 Timothy R. Cassity, Ph. D. Microbiologist
 DISCLAIMER The opinions expressed in this presentation are those of the presenter. The material presented here does not necessarily reflect the views of Southern Ohio Medical Center.
DISCLAIMER The opinions expressed in this presentation are those of the presenter. The material presented here does not necessarily reflect the views of Southern Ohio Medical Center.
 DISCLAIMER The presenter has no financial interest in (i. e. is not being paid by) any diagnostic testing company or pharmaceutical company or product that may be mentioned in this presentation.
DISCLAIMER The presenter has no financial interest in (i. e. is not being paid by) any diagnostic testing company or pharmaceutical company or product that may be mentioned in this presentation.
 WARNING! Due to the nature of the topic, some slides may seem like an “infomercial. ” I apologize in advance!
WARNING! Due to the nature of the topic, some slides may seem like an “infomercial. ” I apologize in advance!
 Objectives Understand the advantages of molecular methods over traditional diagnostic microbiology tests.
Objectives Understand the advantages of molecular methods over traditional diagnostic microbiology tests.
 Objectives Identify situations where molecular methods are not beneficial.
Objectives Identify situations where molecular methods are not beneficial.
 Objectives Understand problems and limitations that can occur with molecular methods of detecting etiologic agents.
Objectives Understand problems and limitations that can occur with molecular methods of detecting etiologic agents.
 Acknowledgement The level of molecular testing offered at SOMC would not be possible without the support of Bridget Scott (Lab Administrative Direct) and Dr. Vincent Randaisi (Lab Medical Director) and the SOMC Administration!
Acknowledgement The level of molecular testing offered at SOMC would not be possible without the support of Bridget Scott (Lab Administrative Direct) and Dr. Vincent Randaisi (Lab Medical Director) and the SOMC Administration!
 Acknowledgement I would also like to express my appreciation to the physicians who have supported and championed molecular technology, and Dr. David Byers and Dr. Elie Saab in particular.
Acknowledgement I would also like to express my appreciation to the physicians who have supported and championed molecular technology, and Dr. David Byers and Dr. Elie Saab in particular.
 Acknowledgement I would like to acknowledge the invaluable guidance of Dr. Darren Adams in the design and implementation of the targets used by our OB/GYN providers.
Acknowledgement I would like to acknowledge the invaluable guidance of Dr. Darren Adams in the design and implementation of the targets used by our OB/GYN providers.

 Definitions What is “molecular diagnostic testing” in infectious diseases? Detection of etiologic agents of infectious disease by detecting a unique nucleic acid base sequence for specific organisms. The “goal” of molecular testing is no different from classical methods.
Definitions What is “molecular diagnostic testing” in infectious diseases? Detection of etiologic agents of infectious disease by detecting a unique nucleic acid base sequence for specific organisms. The “goal” of molecular testing is no different from classical methods.
 Definitions What is “molecular diagnostic testing” in infectious diseases? Every living organism (and some that aren’t living) are defined by the nucleotide base sequences on their DNA or RNA. For our purposes, each unique DNA or RNA sequence is loosely defined as a “target. ”
Definitions What is “molecular diagnostic testing” in infectious diseases? Every living organism (and some that aren’t living) are defined by the nucleotide base sequences on their DNA or RNA. For our purposes, each unique DNA or RNA sequence is loosely defined as a “target. ”
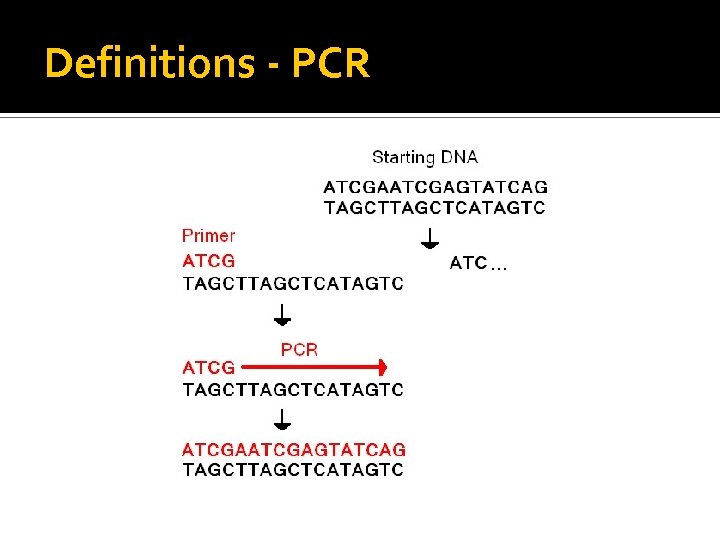
 Definitions - PCR What is PCR? Polymerase Chain Reaction It is the in vitro synthesis of strands of target DNA base sequences directed by homologous primers. There are many other methods of “amplifying” DNA, but I will use the term PCR to include all of those.
Definitions - PCR What is PCR? Polymerase Chain Reaction It is the in vitro synthesis of strands of target DNA base sequences directed by homologous primers. There are many other methods of “amplifying” DNA, but I will use the term PCR to include all of those.
 Definitions - PCR
Definitions - PCR
 Definitions - PCR What is PCR? There are many other methods of “amplifying” DNA, but I will use the term PCR to include all of those. At SOMC we also use: ▪ ▪ ▪ Strand Displacement Amplification Loop-Mediated isothermal DNA amplification (LAMP) Gold Nanoparticle-based Methods (Verigene) Rotor. Gene Q 5 channel in house developed PCR Alere™ i isothermal nucleic acid amplification
Definitions - PCR What is PCR? There are many other methods of “amplifying” DNA, but I will use the term PCR to include all of those. At SOMC we also use: ▪ ▪ ▪ Strand Displacement Amplification Loop-Mediated isothermal DNA amplification (LAMP) Gold Nanoparticle-based Methods (Verigene) Rotor. Gene Q 5 channel in house developed PCR Alere™ i isothermal nucleic acid amplification
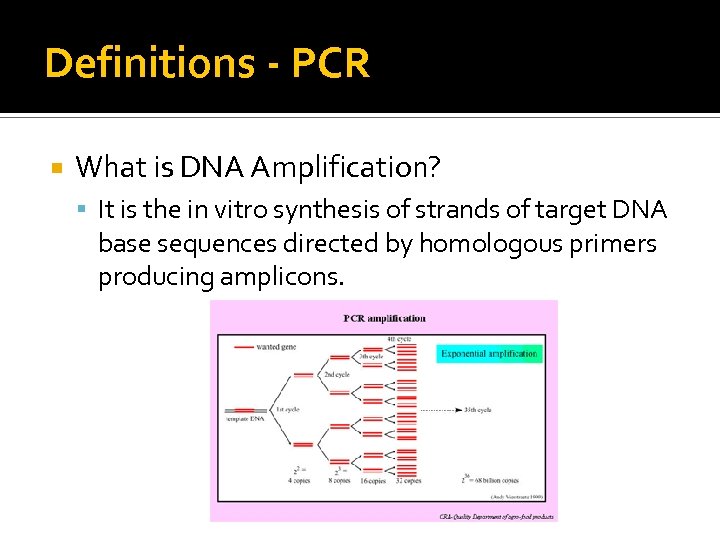 Definitions - PCR What is DNA Amplification? It is the in vitro synthesis of strands of target DNA base sequences directed by homologous primers producing amplicons.
Definitions - PCR What is DNA Amplification? It is the in vitro synthesis of strands of target DNA base sequences directed by homologous primers producing amplicons.
 Definitions - PCR What is DNA Amplification? We do the same thing with a culture, but with a culture we “amplify” by growing individual bacterial cells to a colony that we can easily detect visually. DNA amplification is a lot faster than growth! Note: Product of an artistic tech with too much time on her hands
Definitions - PCR What is DNA Amplification? We do the same thing with a culture, but with a culture we “amplify” by growing individual bacterial cells to a colony that we can easily detect visually. DNA amplification is a lot faster than growth! Note: Product of an artistic tech with too much time on her hands
 What is multiplexing? Multiplexing is the detection of multiple gene targets at the same time or on the same run. ▪ Respiratory Virus detects 17 targets simultaneously ▪ Blood culture detected 12 -15 targets simultaneously ▪ HSV 1 & 2, Trichomonas vaginalis, Atopobium vaginae, Gardnerella vaginalis, Candida albicans and Candida glabrata are multiplexed.
What is multiplexing? Multiplexing is the detection of multiple gene targets at the same time or on the same run. ▪ Respiratory Virus detects 17 targets simultaneously ▪ Blood culture detected 12 -15 targets simultaneously ▪ HSV 1 & 2, Trichomonas vaginalis, Atopobium vaginae, Gardnerella vaginalis, Candida albicans and Candida glabrata are multiplexed.
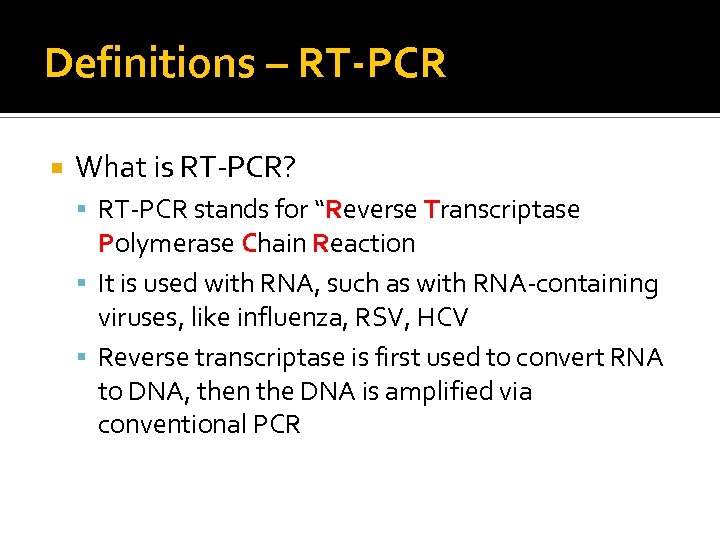 Definitions – RT-PCR What is RT-PCR? RT-PCR stands for “Reverse Transcriptase Polymerase Chain Reaction It is used with RNA, such as with RNA-containing viruses, like influenza, RSV, HCV Reverse transcriptase is first used to convert RNA to DNA, then the DNA is amplified via conventional PCR
Definitions – RT-PCR What is RT-PCR? RT-PCR stands for “Reverse Transcriptase Polymerase Chain Reaction It is used with RNA, such as with RNA-containing viruses, like influenza, RSV, HCV Reverse transcriptase is first used to convert RNA to DNA, then the DNA is amplified via conventional PCR
 Definitions – “Real-Time” PCR What is “Real Time” PCR or molecular detection? In “classical” PCR, amplification took place in a thermocycler and detection in a separate instrument. Real time combines amplification and detection into one process. This is faster and less hands on than classical PCR
Definitions – “Real-Time” PCR What is “Real Time” PCR or molecular detection? In “classical” PCR, amplification took place in a thermocycler and detection in a separate instrument. Real time combines amplification and detection into one process. This is faster and less hands on than classical PCR
 Definitions - q. PCR What is q. PCR or q. RT-PCR? The “q” refers to “quantitative” When we run a real time PCR, we generate a cycling time. i. e. the number of cycles of amplification it takes before the target is detected. The number of cycles it takes to detect a target is dependent on the number of targets present in the original sample.
Definitions - q. PCR What is q. PCR or q. RT-PCR? The “q” refers to “quantitative” When we run a real time PCR, we generate a cycling time. i. e. the number of cycles of amplification it takes before the target is detected. The number of cycles it takes to detect a target is dependent on the number of targets present in the original sample.
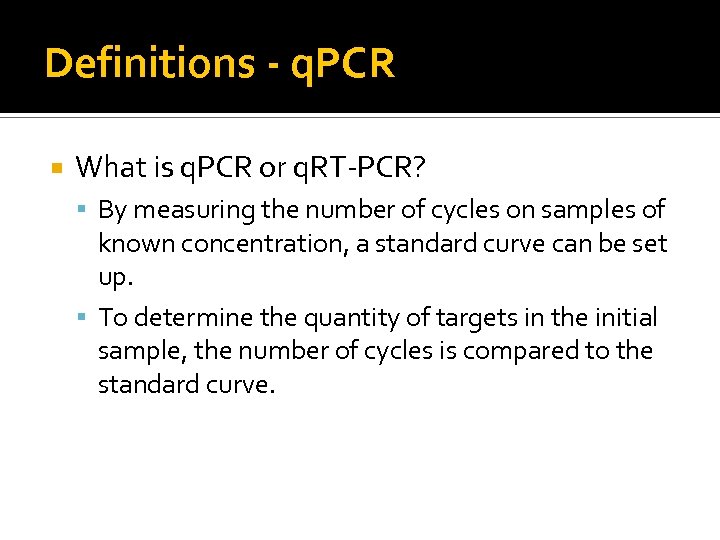 Definitions - q. PCR What is q. PCR or q. RT-PCR? By measuring the number of cycles on samples of known concentration, a standard curve can be set up. To determine the quantity of targets in the initial sample, the number of cycles is compared to the standard curve.
Definitions - q. PCR What is q. PCR or q. RT-PCR? By measuring the number of cycles on samples of known concentration, a standard curve can be set up. To determine the quantity of targets in the initial sample, the number of cycles is compared to the standard curve.
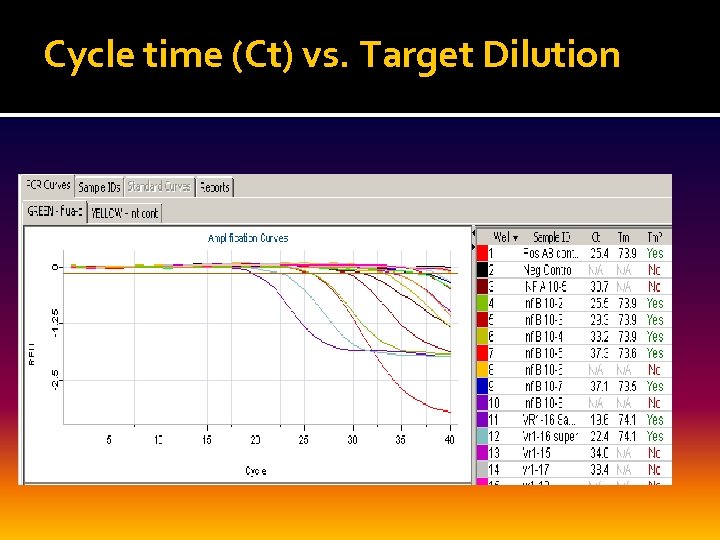 Cycle time (Ct) vs. Target Dilution
Cycle time (Ct) vs. Target Dilution
 How does it work?
How does it work?
 How does it work? Magic? Nay, Nay!
How does it work? Magic? Nay, Nay!
 “What I'm going to accomplish goes far beyond magic. ” Victor (Frankenstein) to Jefferson and Rumplestiltskin Time – season 2, episode 5. - Once upon a
“What I'm going to accomplish goes far beyond magic. ” Victor (Frankenstein) to Jefferson and Rumplestiltskin Time – season 2, episode 5. - Once upon a
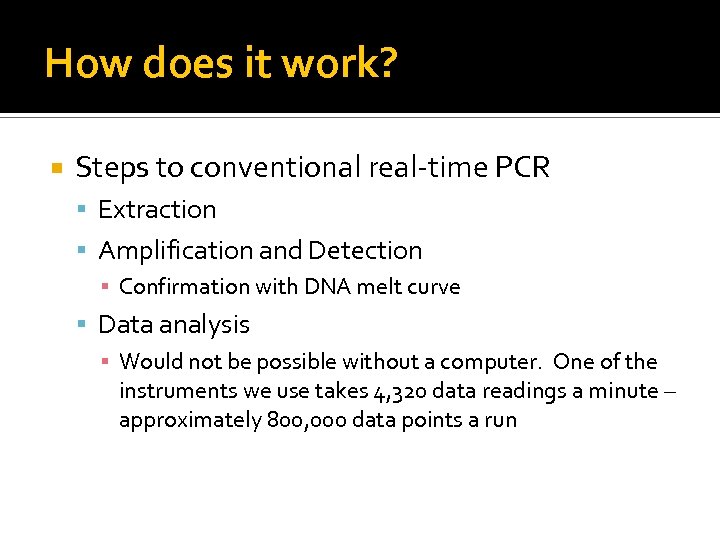 How does it work? Steps to conventional real-time PCR Extraction Amplification and Detection ▪ Confirmation with DNA melt curve Data analysis ▪ Would not be possible without a computer. One of the instruments we use takes 4, 320 data readings a minute – approximately 800, 000 data points a run
How does it work? Steps to conventional real-time PCR Extraction Amplification and Detection ▪ Confirmation with DNA melt curve Data analysis ▪ Would not be possible without a computer. One of the instruments we use takes 4, 320 data readings a minute – approximately 800, 000 data points a run
 How does it work? Nucleic Acid Extraction Lyses cells and releases nucleic acids Proteases are incorporated to inactivate nucleases and other enzymes Result is purified RNA and DNA
How does it work? Nucleic Acid Extraction Lyses cells and releases nucleic acids Proteases are incorporated to inactivate nucleases and other enzymes Result is purified RNA and DNA
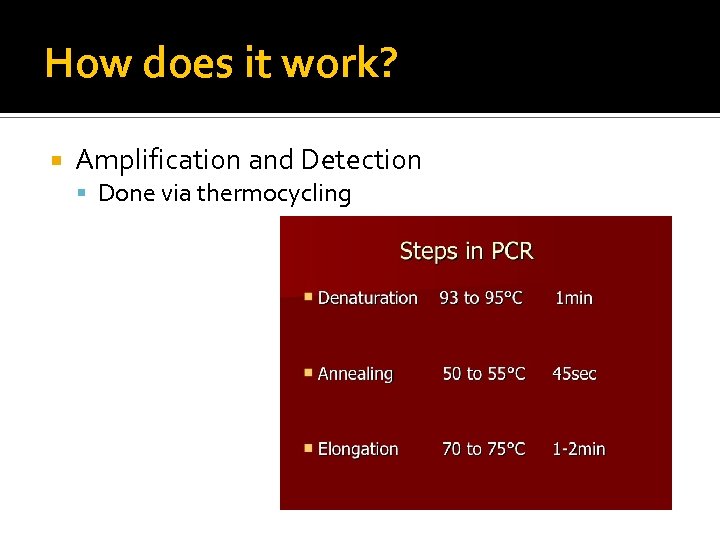 How does it work? Amplification and Detection Done via thermocycling
How does it work? Amplification and Detection Done via thermocycling
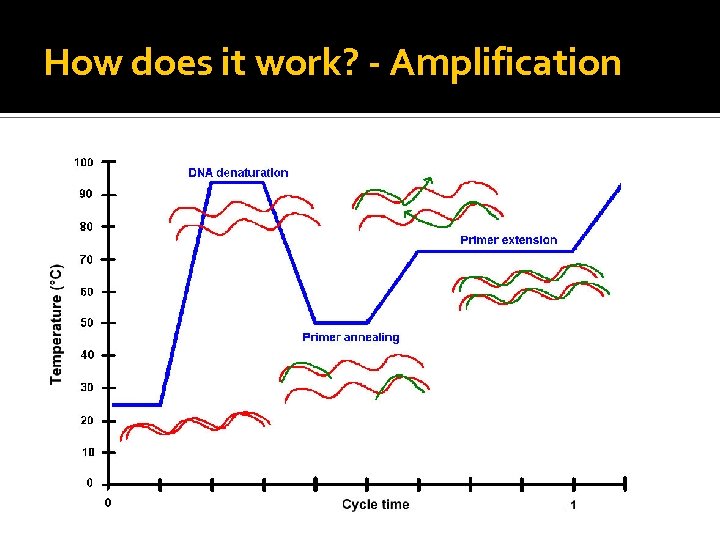 How does it work? - Amplification
How does it work? - Amplification
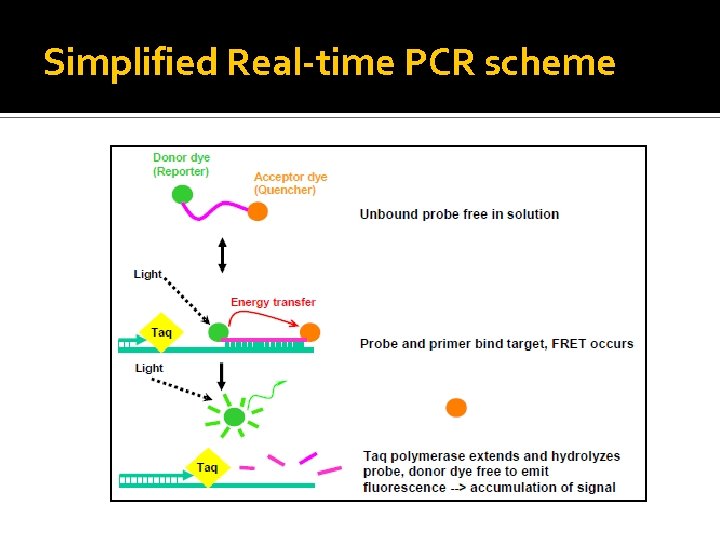 Simplified Real-time PCR scheme
Simplified Real-time PCR scheme
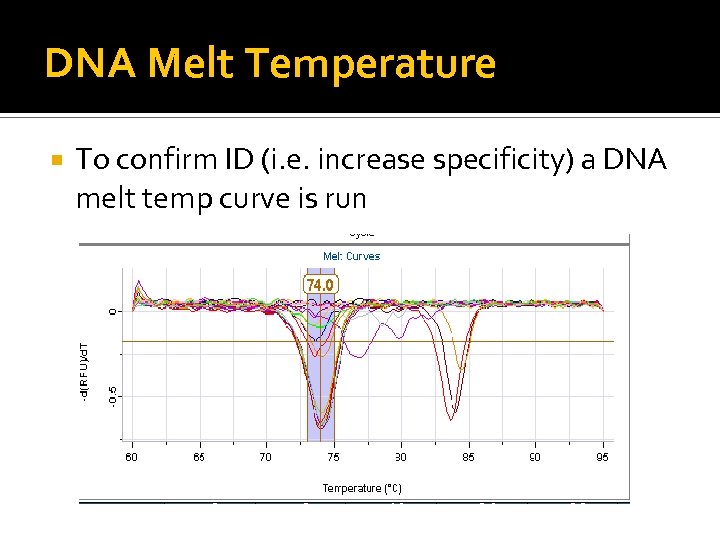 DNA Melt Temperature To confirm ID (i. e. increase specificity) a DNA melt temp curve is run
DNA Melt Temperature To confirm ID (i. e. increase specificity) a DNA melt temp curve is run
 Questions? ?
Questions? ?
 Advantages of Molecular Tests
Advantages of Molecular Tests
 Advantages of Molecular Tests The Perfect Lab test would have 100% sensitivity (No false negatives) and 100% specificity (No false Positives)
Advantages of Molecular Tests The Perfect Lab test would have 100% sensitivity (No false negatives) and 100% specificity (No false Positives)
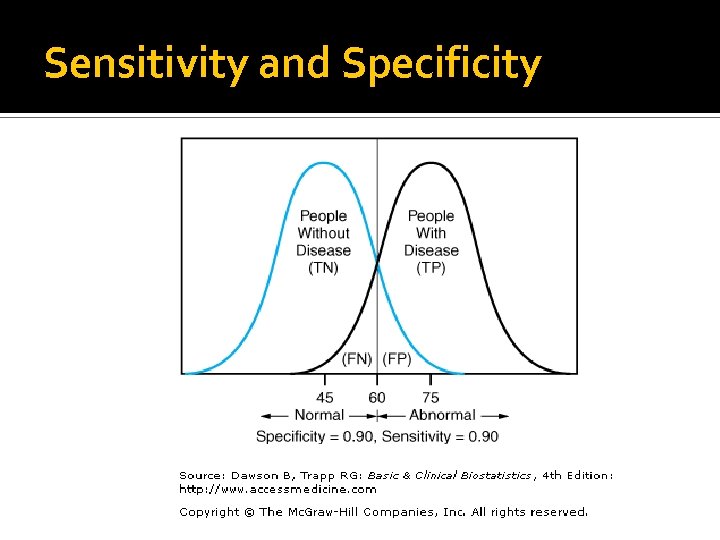 Sensitivity and Specificity
Sensitivity and Specificity
 Advantages of Molecular Tests Much more sensitive (i. e. few false negative tests) than most conventional diagnostic methods used with infectious agents. Example – only method acceptable for the detection of HSV in CSF Analogy – if PCR could be used to detect sugar, you could drop 2 sugar cubes into Lake Michigan and detect it in Lake Michigan water.
Advantages of Molecular Tests Much more sensitive (i. e. few false negative tests) than most conventional diagnostic methods used with infectious agents. Example – only method acceptable for the detection of HSV in CSF Analogy – if PCR could be used to detect sugar, you could drop 2 sugar cubes into Lake Michigan and detect it in Lake Michigan water.
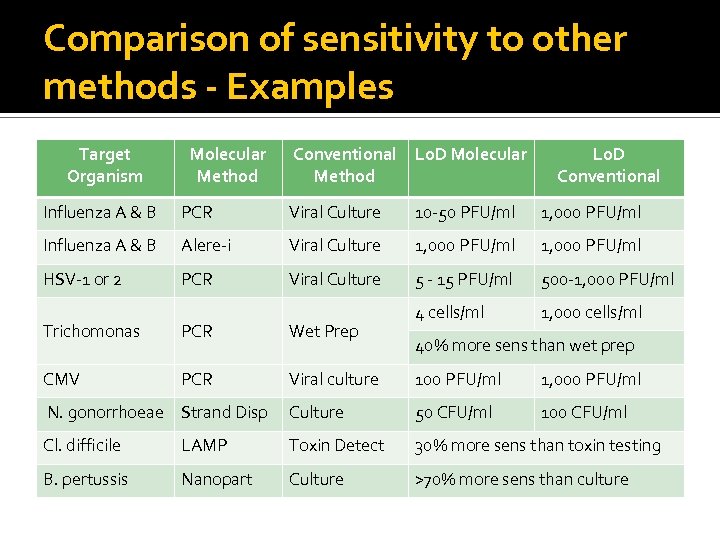 Comparison of sensitivity to other methods - Examples Target Organism Molecular Method Conventional Method Lo. D Molecular Lo. D Conventional Influenza A & B PCR Viral Culture 10 -50 PFU/ml 1, 000 PFU/ml Influenza A & B Alere-i Viral Culture 1, 000 PFU/ml HSV-1 or 2 PCR Viral Culture 5 - 15 PFU/ml 500 -1, 000 PFU/ml 4 cells/ml 1, 000 cells/ml Trichomonas PCR Wet Prep CMV PCR Viral culture 100 PFU/ml 1, 000 PFU/ml N. gonorrhoeae Strand Disp Culture 50 CFU/ml 100 CFU/ml Cl. difficile LAMP Toxin Detect 30% more sens than toxin testing B. pertussis Nanopart Culture >70% more sens than culture 40% more sens than wet prep
Comparison of sensitivity to other methods - Examples Target Organism Molecular Method Conventional Method Lo. D Molecular Lo. D Conventional Influenza A & B PCR Viral Culture 10 -50 PFU/ml 1, 000 PFU/ml Influenza A & B Alere-i Viral Culture 1, 000 PFU/ml HSV-1 or 2 PCR Viral Culture 5 - 15 PFU/ml 500 -1, 000 PFU/ml 4 cells/ml 1, 000 cells/ml Trichomonas PCR Wet Prep CMV PCR Viral culture 100 PFU/ml 1, 000 PFU/ml N. gonorrhoeae Strand Disp Culture 50 CFU/ml 100 CFU/ml Cl. difficile LAMP Toxin Detect 30% more sens than toxin testing B. pertussis Nanopart Culture >70% more sens than culture 40% more sens than wet prep
 Advantages of Molecular Tests If performed properly, specificity is also excellent! Few false positive results, but some are still possible
Advantages of Molecular Tests If performed properly, specificity is also excellent! Few false positive results, but some are still possible
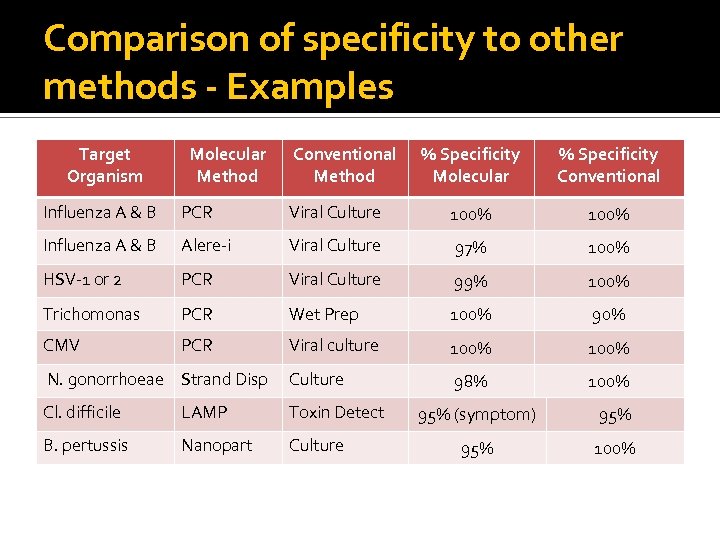 Comparison of specificity to other methods - Examples Target Organism Molecular Method Conventional Method % Specificity Molecular % Specificity Conventional Influenza A & B PCR Viral Culture 100% Influenza A & B Alere-i Viral Culture 97% 100% HSV-1 or 2 PCR Viral Culture 99% 100% Trichomonas PCR Wet Prep 100% 90% CMV PCR Viral culture 100% N. gonorrhoeae Strand Disp Culture 98% 100% Cl. difficile LAMP Toxin Detect B. pertussis Nanopart Culture 95% (symptom) 95% 100%
Comparison of specificity to other methods - Examples Target Organism Molecular Method Conventional Method % Specificity Molecular % Specificity Conventional Influenza A & B PCR Viral Culture 100% Influenza A & B Alere-i Viral Culture 97% 100% HSV-1 or 2 PCR Viral Culture 99% 100% Trichomonas PCR Wet Prep 100% 90% CMV PCR Viral culture 100% N. gonorrhoeae Strand Disp Culture 98% 100% Cl. difficile LAMP Toxin Detect B. pertussis Nanopart Culture 95% (symptom) 95% 100%
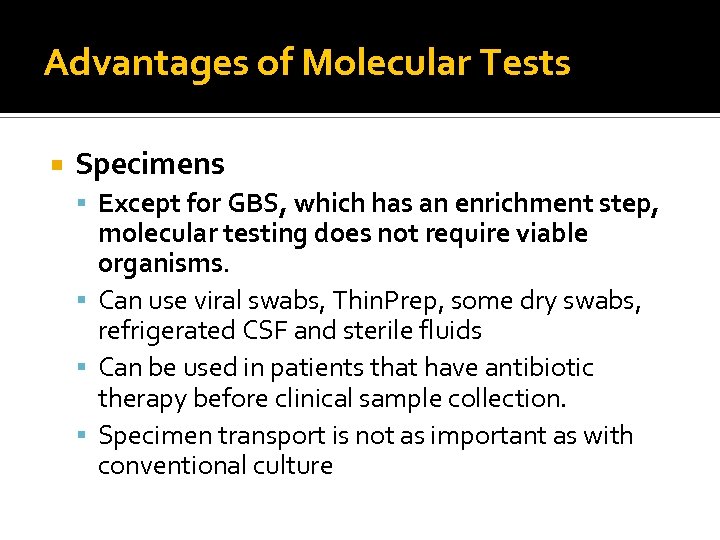 Advantages of Molecular Tests Specimens Except for GBS, which has an enrichment step, molecular testing does not require viable organisms. Can use viral swabs, Thin. Prep, some dry swabs, refrigerated CSF and sterile fluids Can be used in patients that have antibiotic therapy before clinical sample collection. Specimen transport is not as important as with conventional culture
Advantages of Molecular Tests Specimens Except for GBS, which has an enrichment step, molecular testing does not require viable organisms. Can use viral swabs, Thin. Prep, some dry swabs, refrigerated CSF and sterile fluids Can be used in patients that have antibiotic therapy before clinical sample collection. Specimen transport is not as important as with conventional culture
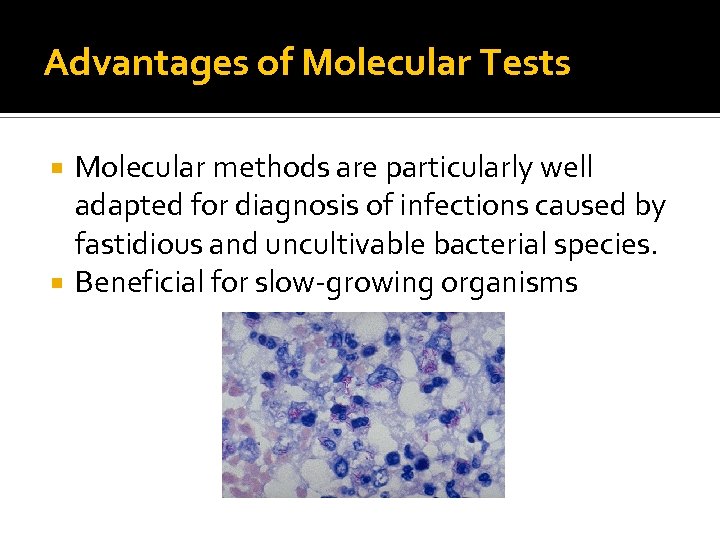 Advantages of Molecular Tests Molecular methods are particularly well adapted for diagnosis of infections caused by fastidious and uncultivable bacterial species. Beneficial for slow-growing organisms
Advantages of Molecular Tests Molecular methods are particularly well adapted for diagnosis of infections caused by fastidious and uncultivable bacterial species. Beneficial for slow-growing organisms
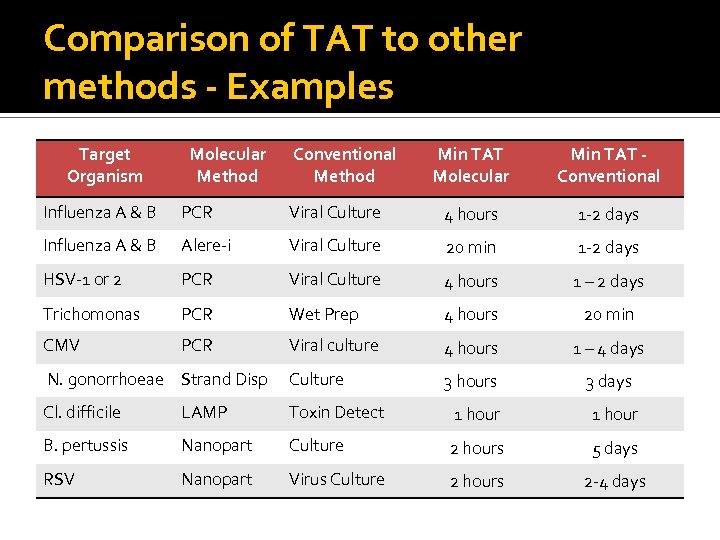 Comparison of TAT to other methods - Examples Target Organism Molecular Method Conventional Method Min TAT Molecular Min TAT Conventional Influenza A & B PCR Viral Culture 4 hours 1 -2 days Influenza A & B Alere-i Viral Culture 20 min 1 -2 days HSV-1 or 2 PCR Viral Culture 4 hours 1 – 2 days Trichomonas PCR Wet Prep 4 hours 20 min CMV PCR Viral culture 4 hours 1 – 4 days N. gonorrhoeae Strand Disp Culture 3 hours 3 days Cl. difficile LAMP Toxin Detect 1 hour B. pertussis Nanopart Culture 2 hours 5 days RSV Nanopart Virus Culture 2 hours 2 -4 days
Comparison of TAT to other methods - Examples Target Organism Molecular Method Conventional Method Min TAT Molecular Min TAT Conventional Influenza A & B PCR Viral Culture 4 hours 1 -2 days Influenza A & B Alere-i Viral Culture 20 min 1 -2 days HSV-1 or 2 PCR Viral Culture 4 hours 1 – 2 days Trichomonas PCR Wet Prep 4 hours 20 min CMV PCR Viral culture 4 hours 1 – 4 days N. gonorrhoeae Strand Disp Culture 3 hours 3 days Cl. difficile LAMP Toxin Detect 1 hour B. pertussis Nanopart Culture 2 hours 5 days RSV Nanopart Virus Culture 2 hours 2 -4 days
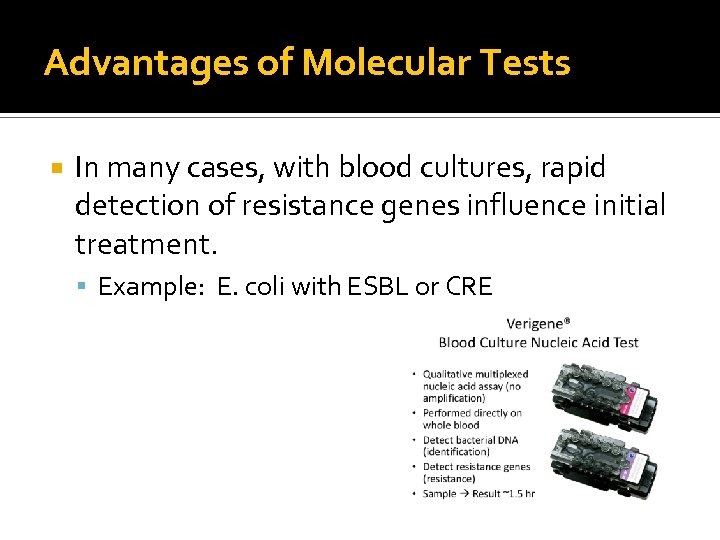 Advantages of Molecular Tests In many cases, with blood cultures, rapid detection of resistance genes influence initial treatment. Example: E. coli with ESBL or CRE
Advantages of Molecular Tests In many cases, with blood cultures, rapid detection of resistance genes influence initial treatment. Example: E. coli with ESBL or CRE
 Advantages of Molecular Tests Rapid results may permit discontinuance or de-escalation of antibiotics earlier than conventional culture. Can be an important part of antibiotic stewardship
Advantages of Molecular Tests Rapid results may permit discontinuance or de-escalation of antibiotics earlier than conventional culture. Can be an important part of antibiotic stewardship
 Advantages of Molecular Tests Can decrease length of stay Several studies have shown positive blood cultures identified by molecular detection average a 1. 2 day shorter length of stay
Advantages of Molecular Tests Can decrease length of stay Several studies have shown positive blood cultures identified by molecular detection average a 1. 2 day shorter length of stay
 Problems and Limitations with PCR
Problems and Limitations with PCR
 Potential Problems with PCR Too sensitive in some cases – can yield falsepositive results Example: C. difficile molecular detection Tests should be performed only on symptomatic patients PCR results must be correlated with clinical presentation and patient history
Potential Problems with PCR Too sensitive in some cases – can yield falsepositive results Example: C. difficile molecular detection Tests should be performed only on symptomatic patients PCR results must be correlated with clinical presentation and patient history
 Potential Problems with PCR The biggest potential problem with PCRbased tests is amplicon contamination. Samples cannot be handled in the same area they are amplified Instruments and the environment must be “disinfected” after every use!
Potential Problems with PCR The biggest potential problem with PCRbased tests is amplicon contamination. Samples cannot be handled in the same area they are amplified Instruments and the environment must be “disinfected” after every use!
 Potential Problems with PCR
Potential Problems with PCR
 Potential Problems with PCR tests cannot accurately differentiate infection from commensalism or chronic carriage Not good for opportunistic pathogens and for clinical specimens collected at nonsterile sites.
Potential Problems with PCR tests cannot accurately differentiate infection from commensalism or chronic carriage Not good for opportunistic pathogens and for clinical specimens collected at nonsterile sites.
 Potential Problems with PCR Genetic Drift Genomes are not necessarily static If your target sequence changes in an organism you can no longer detect it! ▪ Example – Staphylococcus aureus and mec. A gene cassette ▪ Some organisms such as Streptococcus sp. do not show much genetic change. Others, like influenza viruses, show genetic change more often
Potential Problems with PCR Genetic Drift Genomes are not necessarily static If your target sequence changes in an organism you can no longer detect it! ▪ Example – Staphylococcus aureus and mec. A gene cassette ▪ Some organisms such as Streptococcus sp. do not show much genetic change. Others, like influenza viruses, show genetic change more often
 Limitations There are methods to detect some genes responsible for resistance, such as mec. A, some ESBLs, and some CREs. However, there are many more that we cannot test, or don’t even know about. Therefore, for organisms that can have variable susceptibility to antibiotics, culture and conventional susceptibility testing is still necessary.
Limitations There are methods to detect some genes responsible for resistance, such as mec. A, some ESBLs, and some CREs. However, there are many more that we cannot test, or don’t even know about. Therefore, for organisms that can have variable susceptibility to antibiotics, culture and conventional susceptibility testing is still necessary.
 Limitations – Expensive! Molecular-based diagnostics are much more expensive than convention culture or antigen testing! But for now, reimbursement is very good!
Limitations – Expensive! Molecular-based diagnostics are much more expensive than convention culture or antigen testing! But for now, reimbursement is very good!
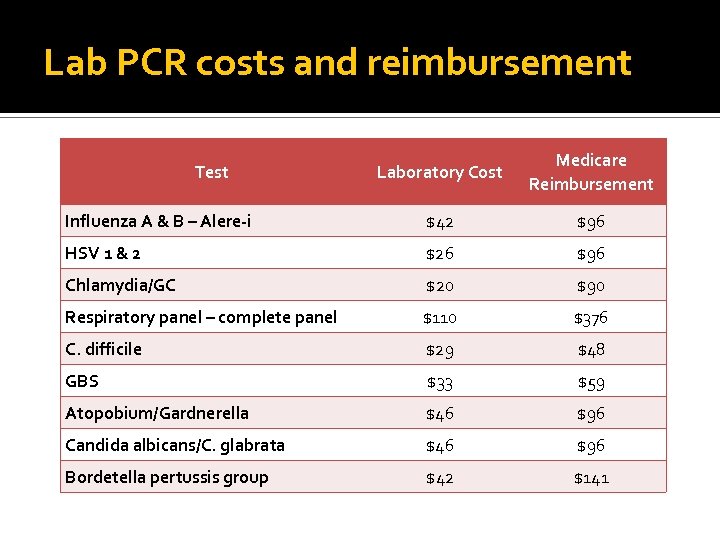 Lab PCR costs and reimbursement Laboratory Cost Medicare Reimbursement Influenza A & B – Alere-i $42 $96 HSV 1 & 2 $26 $96 Chlamydia/GC $20 $90 Respiratory panel – complete panel $110 $376 C. difficile $29 $48 GBS $33 $59 Atopobium/Gardnerella $46 $96 Candida albicans/C. glabrata $46 $96 Bordetella pertussis group $42 $141 Test
Lab PCR costs and reimbursement Laboratory Cost Medicare Reimbursement Influenza A & B – Alere-i $42 $96 HSV 1 & 2 $26 $96 Chlamydia/GC $20 $90 Respiratory panel – complete panel $110 $376 C. difficile $29 $48 GBS $33 $59 Atopobium/Gardnerella $46 $96 Candida albicans/C. glabrata $46 $96 Bordetella pertussis group $42 $141 Test
 Questions? ? ?
Questions? ? ?

 Current SOMC Molecular Targets Currently we have molecular tests for 58 infectious disease targets! 15 Gram positive blood culture targets 14 Gram negative blood culture targets 18 Respiratory virus and pertussis targets 10 STD and OB/GYN targets 1 GI target (C. difficile), with 10 more under development
Current SOMC Molecular Targets Currently we have molecular tests for 58 infectious disease targets! 15 Gram positive blood culture targets 14 Gram negative blood culture targets 18 Respiratory virus and pertussis targets 10 STD and OB/GYN targets 1 GI target (C. difficile), with 10 more under development
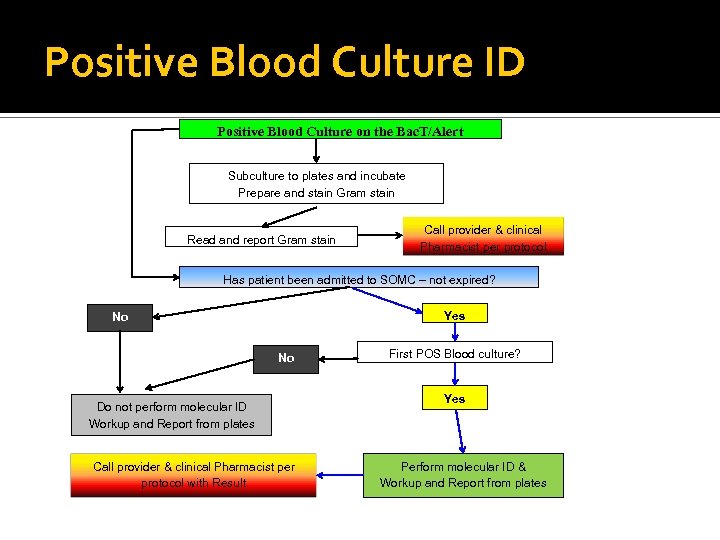 Positive Blood Culture ID Positive Blood Culture on the Bac. T/Alert Subculture to plates and incubate Prepare and stain Gram stain Read and report Gram stain Call provider & clinical Pharmacist per protocol Has patient been admitted to SOMC – not expired? Yes No No Do not perform molecular ID Workup and Report from plates Call provider & clinical Pharmacist per protocol with Result First POS Blood culture? Yes Perform molecular ID & Workup and Report from plates
Positive Blood Culture ID Positive Blood Culture on the Bac. T/Alert Subculture to plates and incubate Prepare and stain Gram stain Read and report Gram stain Call provider & clinical Pharmacist per protocol Has patient been admitted to SOMC – not expired? Yes No No Do not perform molecular ID Workup and Report from plates Call provider & clinical Pharmacist per protocol with Result First POS Blood culture? Yes Perform molecular ID & Workup and Report from plates
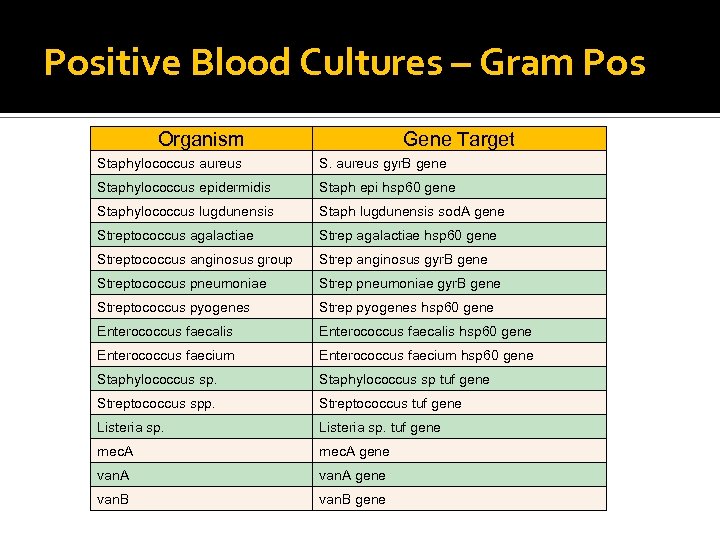 Positive Blood Cultures – Gram Pos Organism Gene Target Staphylococcus aureus S. aureus gyr. B gene Staphylococcus epidermidis Staph epi hsp 60 gene Staphylococcus lugdunensis Staph lugdunensis sod. A gene Streptococcus agalactiae Strep agalactiae hsp 60 gene Streptococcus anginosus group Strep anginosus gyr. B gene Streptococcus pneumoniae Strep pneumoniae gyr. B gene Streptococcus pyogenes Strep pyogenes hsp 60 gene Enterococcus faecalis hsp 60 gene Enterococcus faecium hsp 60 gene Staphylococcus sp tuf gene Streptococcus spp. Streptococcus tuf gene Listeria sp. tuf gene mec. A gene van. B gene
Positive Blood Cultures – Gram Pos Organism Gene Target Staphylococcus aureus S. aureus gyr. B gene Staphylococcus epidermidis Staph epi hsp 60 gene Staphylococcus lugdunensis Staph lugdunensis sod. A gene Streptococcus agalactiae Strep agalactiae hsp 60 gene Streptococcus anginosus group Strep anginosus gyr. B gene Streptococcus pneumoniae Strep pneumoniae gyr. B gene Streptococcus pyogenes Strep pyogenes hsp 60 gene Enterococcus faecalis hsp 60 gene Enterococcus faecium hsp 60 gene Staphylococcus sp tuf gene Streptococcus spp. Streptococcus tuf gene Listeria sp. tuf gene mec. A gene van. B gene
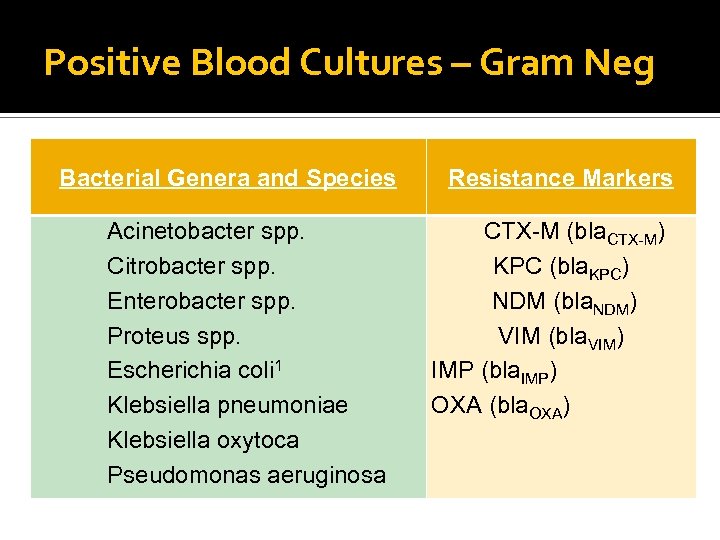 Positive Blood Cultures – Gram Neg Bacterial Genera and Species Acinetobacter spp. Citrobacter spp. Enterobacter spp. Proteus spp. Escherichia coli 1 Klebsiella pneumoniae Klebsiella oxytoca Pseudomonas aeruginosa Resistance Markers CTX-M (bla. CTX-M) KPC (bla. KPC) NDM (bla. NDM) VIM (bla. VIM) IMP (bla. IMP) OXA (bla. OXA)
Positive Blood Cultures – Gram Neg Bacterial Genera and Species Acinetobacter spp. Citrobacter spp. Enterobacter spp. Proteus spp. Escherichia coli 1 Klebsiella pneumoniae Klebsiella oxytoca Pseudomonas aeruginosa Resistance Markers CTX-M (bla. CTX-M) KPC (bla. KPC) NDM (bla. NDM) VIM (bla. VIM) IMP (bla. IMP) OXA (bla. OXA)
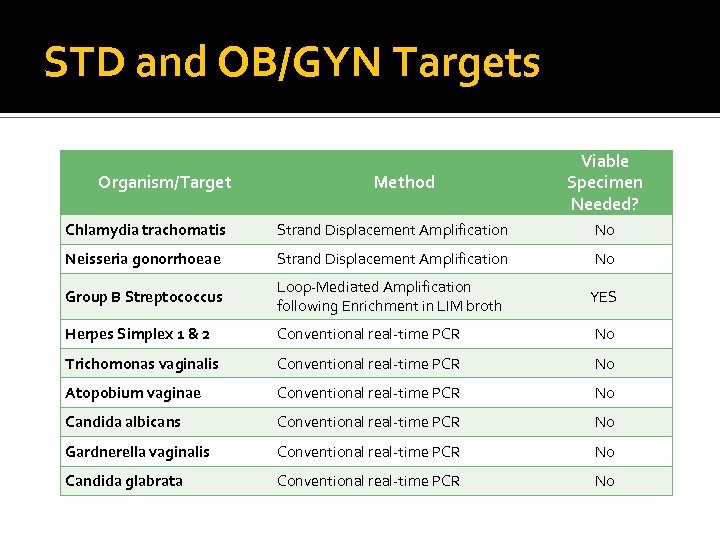 STD and OB/GYN Targets Organism/Target Method Viable Specimen Needed? Chlamydia trachomatis Strand Displacement Amplification No Neisseria gonorrhoeae Strand Displacement Amplification No Group B Streptococcus Loop-Mediated Amplification following Enrichment in LIM broth YES Herpes Simplex 1 & 2 Conventional real-time PCR No Trichomonas vaginalis Conventional real-time PCR No Atopobium vaginae Conventional real-time PCR No Candida albicans Conventional real-time PCR No Gardnerella vaginalis Conventional real-time PCR No Candida glabrata Conventional real-time PCR No
STD and OB/GYN Targets Organism/Target Method Viable Specimen Needed? Chlamydia trachomatis Strand Displacement Amplification No Neisseria gonorrhoeae Strand Displacement Amplification No Group B Streptococcus Loop-Mediated Amplification following Enrichment in LIM broth YES Herpes Simplex 1 & 2 Conventional real-time PCR No Trichomonas vaginalis Conventional real-time PCR No Atopobium vaginae Conventional real-time PCR No Candida albicans Conventional real-time PCR No Gardnerella vaginalis Conventional real-time PCR No Candida glabrata Conventional real-time PCR No
 Respiratory Tract Targets Typically Ordered by Groups, but can be ordered individually if desired. Groups include: Complete panel (12 targets) Influenza A & B (2 to 4 targets) RSV only (2 targets) Bordetella pertussis (3 targets) Can be customized per provider request
Respiratory Tract Targets Typically Ordered by Groups, but can be ordered individually if desired. Groups include: Complete panel (12 targets) Influenza A & B (2 to 4 targets) RSV only (2 targets) Bordetella pertussis (3 targets) Can be customized per provider request
 Respiratory Tract Targets Influenza A & B – Rapid Method – Alere-i Two targets – influenza A & influenza B Done on a dry swab or viral swab in UTM Requires approximately 20 min to perform Unlike rapid antigen tests, it does not require PCR or culture backup CLIA-waived, suitable for point of care
Respiratory Tract Targets Influenza A & B – Rapid Method – Alere-i Two targets – influenza A & influenza B Done on a dry swab or viral swab in UTM Requires approximately 20 min to perform Unlike rapid antigen tests, it does not require PCR or culture backup CLIA-waived, suitable for point of care
 Respiratory Tract Targets Influenza A & B – Verigene Done on a variety of specimens – swab in UTM or other transport medium, BAL, Bronch Wash Four targets Gives H-type ID (ex. Influenza A H 1) Can be done in about 2. 5 hours >10 X more sensitive than the Alere-i
Respiratory Tract Targets Influenza A & B – Verigene Done on a variety of specimens – swab in UTM or other transport medium, BAL, Bronch Wash Four targets Gives H-type ID (ex. Influenza A H 1) Can be done in about 2. 5 hours >10 X more sensitive than the Alere-i
 Respiratory Tract Targets Influenza A & B – Conventional RT-PCR Done on a variety of specimens – swab in UTM or other transport medium, BAL, Bronch Wash Two targets – influenza A & influenza B Most sensitive method – Lo. D < 50 PFU Can be done in about 4. 5 hours More labor-intensive – we do not do this much anymore. Used only as backup or to resolve questionable results.
Respiratory Tract Targets Influenza A & B – Conventional RT-PCR Done on a variety of specimens – swab in UTM or other transport medium, BAL, Bronch Wash Two targets – influenza A & influenza B Most sensitive method – Lo. D < 50 PFU Can be done in about 4. 5 hours More labor-intensive – we do not do this much anymore. Used only as backup or to resolve questionable results.
 Respiratory Tract Targets RSV (only) – Done on the Verigene Done on a variety of specimens – swab in UTM or other transport medium, BAL, Bronch Wash Two targets (RSV A & RSV B) Can be done in about 2. 5 hours More sensitive than rapid antigen tests Can be done on adult patients as well as children
Respiratory Tract Targets RSV (only) – Done on the Verigene Done on a variety of specimens – swab in UTM or other transport medium, BAL, Bronch Wash Two targets (RSV A & RSV B) Can be done in about 2. 5 hours More sensitive than rapid antigen tests Can be done on adult patients as well as children
 Respiratory Tract Targets Common Respiratory Viruses Comprehensive Panel that includes: ▪ Influenza A & B, with influenza A H subtypes ▪ RSV A & RSV B ▪ Parainfluenza 1, 2, 3 & 4 ▪ Human metapneumovirus ▪ Adenovirus ▪ Rhinovirus (it’s free so we throw it in for good measure)
Respiratory Tract Targets Common Respiratory Viruses Comprehensive Panel that includes: ▪ Influenza A & B, with influenza A H subtypes ▪ RSV A & RSV B ▪ Parainfluenza 1, 2, 3 & 4 ▪ Human metapneumovirus ▪ Adenovirus ▪ Rhinovirus (it’s free so we throw it in for good measure)
 Respiratory Tract Targets Bordetella pertussis panel Include B. pertussis, B. holmesii, and B. parapertussis/B. bronchiseptica Should be used only with symptomatic patients Used to diagnose whooping cough type illnesses
Respiratory Tract Targets Bordetella pertussis panel Include B. pertussis, B. holmesii, and B. parapertussis/B. bronchiseptica Should be used only with symptomatic patients Used to diagnose whooping cough type illnesses
 Respiratory Tract Targets HSV and CMV These can also be ordered on respiratory tract specimens. More relevant in immunosuppressed patients than immunocompetent ones HSV-1 found in around 30 -40% of specimens, but most are not clinically significant These were routinely done with viral cultures
Respiratory Tract Targets HSV and CMV These can also be ordered on respiratory tract specimens. More relevant in immunosuppressed patients than immunocompetent ones HSV-1 found in around 30 -40% of specimens, but most are not clinically significant These were routinely done with viral cultures
 GI Targets Currently, only C. difficile is offered Very sensitive - can detect asymptomatic carriage – must be performed only on symptomatic patients 30% more sensitive than toxin testing – only 1 specimen needed to rule out C. difficile
GI Targets Currently, only C. difficile is offered Very sensitive - can detect asymptomatic carriage – must be performed only on symptomatic patients 30% more sensitive than toxin testing – only 1 specimen needed to rule out C. difficile
 GI Targets Molecular replacement for stool culture under development – Spring 2016 Stool culture TAT – 48 – 72 hours Molecular enteric pathogen TAT – 3 hours Expanded spectrum of etiologic agents Disadvantages Stool culture for susceptibility testing will be needed if Shigella sp. (and some Salmonella sp. ) are found.
GI Targets Molecular replacement for stool culture under development – Spring 2016 Stool culture TAT – 48 – 72 hours Molecular enteric pathogen TAT – 3 hours Expanded spectrum of etiologic agents Disadvantages Stool culture for susceptibility testing will be needed if Shigella sp. (and some Salmonella sp. ) are found.
 GI Targets will include: Bacteria Toxins Viruses Campylobacter Group Shiga Toxin 1 (stx 1) Norovirus Salmonella spp. Shiga Toxin 2 (stx 2) Rotavirus Shigella spp. Vibrio Group Yersinia enterocolitica
GI Targets will include: Bacteria Toxins Viruses Campylobacter Group Shiga Toxin 1 (stx 1) Norovirus Salmonella spp. Shiga Toxin 2 (stx 2) Rotavirus Shigella spp. Vibrio Group Yersinia enterocolitica
 Future Targets Infectious Agents GI pathogens – estimated Spring, 2016 Ureaplasma urealyticum HPV Mycoplasma genitalium (maybe)
Future Targets Infectious Agents GI pathogens – estimated Spring, 2016 Ureaplasma urealyticum HPV Mycoplasma genitalium (maybe)
 Future Targets Non-infectious targets BRAF Mutation Analysis EGFR (Epidermal Growth Factor Receptor) JAK 2 (Janus Kinase 2) KRAS mutation (Kirsten rat sarcoma viral oncogene homolog)
Future Targets Non-infectious targets BRAF Mutation Analysis EGFR (Epidermal Growth Factor Receptor) JAK 2 (Janus Kinase 2) KRAS mutation (Kirsten rat sarcoma viral oncogene homolog)
 Questions?
Questions?
 I would like to thank Grace Martin and the Scioto County Medical Society for this opportunity!
I would like to thank Grace Martin and the Scioto County Medical Society for this opportunity!



