IUI_0 - копия.pptx
- Количество слайдов: 25
 The role of intrauterine infections in the developing of pathology in postvaccinal period
The role of intrauterine infections in the developing of pathology in postvaccinal period
 • About 2500 different infections are known in modern medical science. • Theoretically every infection in the period of pregnancy can influence badly on the embryo and the foetus. • If a pregnant woman have a light infection even without symptoms, it can lead to grave injures and even death of the foetus. • The range of the perinatal infections is very wide.
• About 2500 different infections are known in modern medical science. • Theoretically every infection in the period of pregnancy can influence badly on the embryo and the foetus. • If a pregnant woman have a light infection even without symptoms, it can lead to grave injures and even death of the foetus. • The range of the perinatal infections is very wide.
 "TORCH" • • A group of infections widespread in a population, which have similar clinical presentations and cause firm structural defects of different systems and organs of the foetus. Т — toxoplasmosis О — other (syphilis, chlamydia, enterovirus, hepatitis A, В, gonorrhoea, listeriosis, measles, parotiditis and papilloma virus infection) R — rubeola С — cytomegalia • Н — herpes
"TORCH" • • A group of infections widespread in a population, which have similar clinical presentations and cause firm structural defects of different systems and organs of the foetus. Т — toxoplasmosis О — other (syphilis, chlamydia, enterovirus, hepatitis A, В, gonorrhoea, listeriosis, measles, parotiditis and papilloma virus infection) R — rubeola С — cytomegalia • Н — herpes
 The rate of the Intrauterine Infections as the main reason of the perinatal mortality has grown up the last years by 3, 5 -4, 2 times. The reasons: • Deterioration of the women’s health level of the reproductive age before the pregnancy • The increase of extragenital diseases, severe anaemia, diseases of the urino-genital system by 2 -16 times • The decrease of the immuno-endocrine status
The rate of the Intrauterine Infections as the main reason of the perinatal mortality has grown up the last years by 3, 5 -4, 2 times. The reasons: • Deterioration of the women’s health level of the reproductive age before the pregnancy • The increase of extragenital diseases, severe anaemia, diseases of the urino-genital system by 2 -16 times • The decrease of the immuno-endocrine status
 According to some literary facts, in the autopsy material from the dead foetuses and newborns the revelation of the causative agents of the Interauterine Infections was detected in more than 60%. This fact points out considerably higher importance of the infectious pathology in the development of the perinatal mortality in comparison to the facts of the official statistic accountancy.
According to some literary facts, in the autopsy material from the dead foetuses and newborns the revelation of the causative agents of the Interauterine Infections was detected in more than 60%. This fact points out considerably higher importance of the infectious pathology in the development of the perinatal mortality in comparison to the facts of the official statistic accountancy.
 Factors of risk of the development of the intrauterine infection • Aggravation of the chronic infection that a pregnant woman have (chronic diseases of the breathing organs, digestion, caries, tonsillitis) • Urogenital infections (pyelonephritis, bacteriuria, colpitis, endocervicitis) • Disbacteriosis of the intestine and bacterial vaginosis • Complication of the pregnancy: anemia, gestosis, fetoplacental insufficiency, acute respiratory viral infection - in the second half of pregnancy • Acute respiratory viral infection in the childbirth, prenatal moving of amniotic fluid away, pathology of childbirth activity, using of obstetric supplies
Factors of risk of the development of the intrauterine infection • Aggravation of the chronic infection that a pregnant woman have (chronic diseases of the breathing organs, digestion, caries, tonsillitis) • Urogenital infections (pyelonephritis, bacteriuria, colpitis, endocervicitis) • Disbacteriosis of the intestine and bacterial vaginosis • Complication of the pregnancy: anemia, gestosis, fetoplacental insufficiency, acute respiratory viral infection - in the second half of pregnancy • Acute respiratory viral infection in the childbirth, prenatal moving of amniotic fluid away, pathology of childbirth activity, using of obstetric supplies
 The risk of infections • in the I trimester of pregnancy - 15% • in the II trimester - 45% • in the III trimester - 70%
The risk of infections • in the I trimester of pregnancy - 15% • in the II trimester - 45% • in the III trimester - 70%
 Analyzable materials • Medical documentation: • individual card of the pregnant woman and the puerpera • history of the childbirth • history of the development of the newborn • history of the development of the child • card of preventive vaccinations form 063/у • conclusion of an expert in forensic medicine or a pathoanatomist • conclusion of the commission of investigation of postvaccinal complications • Ready histological materials
Analyzable materials • Medical documentation: • individual card of the pregnant woman and the puerpera • history of the childbirth • history of the development of the newborn • history of the development of the child • card of preventive vaccinations form 063/у • conclusion of an expert in forensic medicine or a pathoanatomist • conclusion of the commission of investigation of postvaccinal complications • Ready histological materials
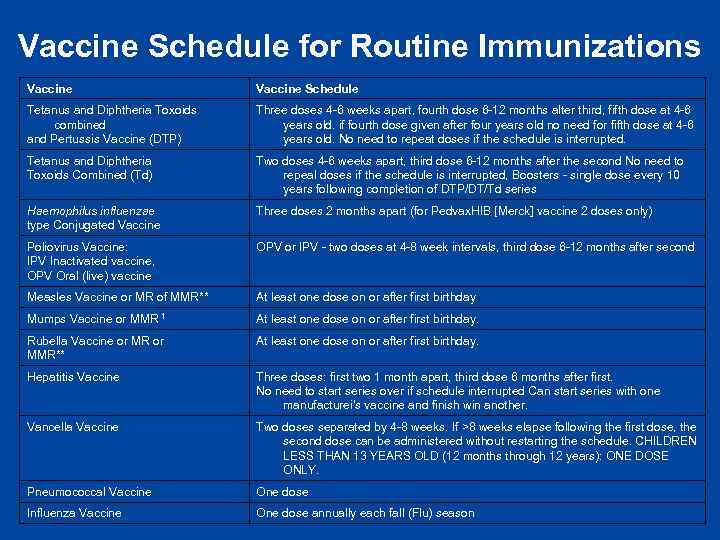 Vaccine Schedule for Routine Immunizations Vaccine Schedule Tetanus and Diphtheria Toxoids combined and Pertussis Vaccine (DTP) Three doses 4 -6 weeks apart, fourth dose 6 -12 months alter third, fifth dose at 4 -6 years old. if fourth dose given after four years old no need for fifth dose at 4 -6 years old. No need to repeat doses if the schedule is interrupted. Tetanus and Diphtheria Toxoids Combined (Td) Two doses 4 -6 weeks apart, third dose 6 -12 months after the second No need to repeal doses if the schedule is interrupted, Boosters - single dose every 10 years following completion of DTP/DT/Td series Haemophilus influenzae type Conjugated Vaccine Three doses 2 months apart (for Pedvax. HIB [Merck] vaccine 2 doses only) Poliovirus Vaccine: IPV Inactivated vaccine, OPV Oral (live) vaccine OPV or IPV - two doses at 4 -8 week intervals, third dose 6 -12 months after second Measles Vaccine or MR of MMR** At least one dose on or after first birthday Mumps Vaccine or MMR 1 At least one dose on or after first birthday. Rubella Vaccine or MR or MMR** At least one dose on or after first birthday. Hepatitis Vaccine Three doses: first two 1 month apart, third dose 6 months after first. No need to start series over if schedule interrupted Can start series with one manufacturei's vaccine and finish win another. Vancella Vaccine Two doses separated by 4 -8 weeks. If >8 weeks elapse following the first dose, the second dose can be administered without restarting the schedule. CHILDREN LESS THAN 13 YEARS OLD (12 months through 12 years): ONE DOSE ONLY. Pneumococcal Vaccine One dose Influenza Vaccine One dose annually each fall (Flu) season
Vaccine Schedule for Routine Immunizations Vaccine Schedule Tetanus and Diphtheria Toxoids combined and Pertussis Vaccine (DTP) Three doses 4 -6 weeks apart, fourth dose 6 -12 months alter third, fifth dose at 4 -6 years old. if fourth dose given after four years old no need for fifth dose at 4 -6 years old. No need to repeat doses if the schedule is interrupted. Tetanus and Diphtheria Toxoids Combined (Td) Two doses 4 -6 weeks apart, third dose 6 -12 months after the second No need to repeal doses if the schedule is interrupted, Boosters - single dose every 10 years following completion of DTP/DT/Td series Haemophilus influenzae type Conjugated Vaccine Three doses 2 months apart (for Pedvax. HIB [Merck] vaccine 2 doses only) Poliovirus Vaccine: IPV Inactivated vaccine, OPV Oral (live) vaccine OPV or IPV - two doses at 4 -8 week intervals, third dose 6 -12 months after second Measles Vaccine or MR of MMR** At least one dose on or after first birthday Mumps Vaccine or MMR 1 At least one dose on or after first birthday. Rubella Vaccine or MR or MMR** At least one dose on or after first birthday. Hepatitis Vaccine Three doses: first two 1 month apart, third dose 6 months after first. No need to start series over if schedule interrupted Can start series with one manufacturei's vaccine and finish win another. Vancella Vaccine Two doses separated by 4 -8 weeks. If >8 weeks elapse following the first dose, the second dose can be administered without restarting the schedule. CHILDREN LESS THAN 13 YEARS OLD (12 months through 12 years): ONE DOSE ONLY. Pneumococcal Vaccine One dose Influenza Vaccine One dose annually each fall (Flu) season
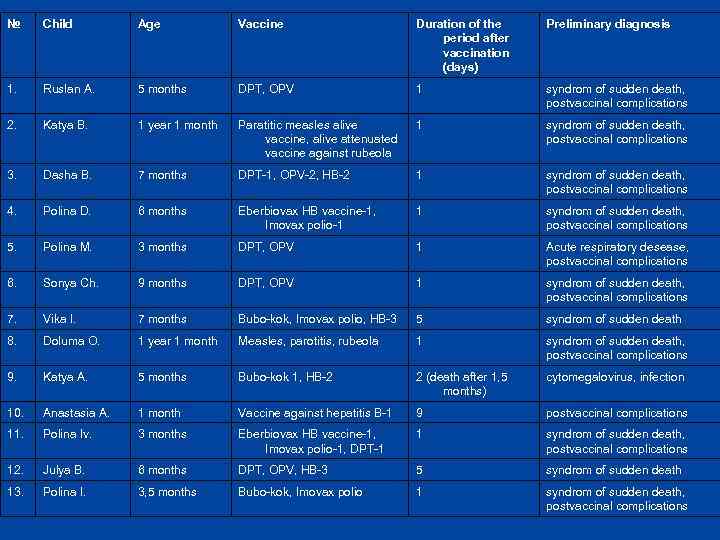 № Child Age Vaccine Duration of the period after vaccination (days) Preliminary diagnosis 1. Ruslan A. 5 months DPT, OPV 1 syndrom of sudden death, postvaccinal complications 2. Katya B. 1 year 1 month Paratitic measles alive vaccine, alive attenuated vaccine against rubeola 1 syndrom of sudden death, postvaccinal complications 3. Dasha B. 7 months DPT-1, OPV-2, HB-2 1 syndrom of sudden death, postvaccinal complications 4. Polina D. 6 months Eberbiovax HB vaccine-1, Imovax polio-1 1 syndrom of sudden death, postvaccinal complications 5. Polina M. 3 months DPT, OPV 1 Acute respiratory desease, postvaccinal complications 6. Sonya Ch. 9 months DPT, OPV 1 syndrom of sudden death, postvaccinal complications 7. Vika I. 7 months Bubo-kok, Imovax polio, HB-3 5 syndrom of sudden death 8. Doluma O. 1 year 1 month Measles, parotitis, rubeola 1 syndrom of sudden death, postvaccinal complications 9. Katya A. 5 months Bubo-kok 1, HB-2 2 (death after 1, 5 months) cytomegalovirus, infection 10. Anastasia A. 1 month Vaccine against hepatitis B-1 9 postvaccinal complications 11. Polina Iv. 3 months Eberbiovax HB vaccine-1, Imovax polio-1, DPT-1 1 syndrom of sudden death, postvaccinal complications 12. Julya B. 6 months DPT, OPV, HB-3 5 syndrom of sudden death 13. Polina I. 3, 5 months Bubo-kok, Imovax polio 1 syndrom of sudden death, postvaccinal complications
№ Child Age Vaccine Duration of the period after vaccination (days) Preliminary diagnosis 1. Ruslan A. 5 months DPT, OPV 1 syndrom of sudden death, postvaccinal complications 2. Katya B. 1 year 1 month Paratitic measles alive vaccine, alive attenuated vaccine against rubeola 1 syndrom of sudden death, postvaccinal complications 3. Dasha B. 7 months DPT-1, OPV-2, HB-2 1 syndrom of sudden death, postvaccinal complications 4. Polina D. 6 months Eberbiovax HB vaccine-1, Imovax polio-1 1 syndrom of sudden death, postvaccinal complications 5. Polina M. 3 months DPT, OPV 1 Acute respiratory desease, postvaccinal complications 6. Sonya Ch. 9 months DPT, OPV 1 syndrom of sudden death, postvaccinal complications 7. Vika I. 7 months Bubo-kok, Imovax polio, HB-3 5 syndrom of sudden death 8. Doluma O. 1 year 1 month Measles, parotitis, rubeola 1 syndrom of sudden death, postvaccinal complications 9. Katya A. 5 months Bubo-kok 1, HB-2 2 (death after 1, 5 months) cytomegalovirus, infection 10. Anastasia A. 1 month Vaccine against hepatitis B-1 9 postvaccinal complications 11. Polina Iv. 3 months Eberbiovax HB vaccine-1, Imovax polio-1, DPT-1 1 syndrom of sudden death, postvaccinal complications 12. Julya B. 6 months DPT, OPV, HB-3 5 syndrom of sudden death 13. Polina I. 3, 5 months Bubo-kok, Imovax polio 1 syndrom of sudden death, postvaccinal complications
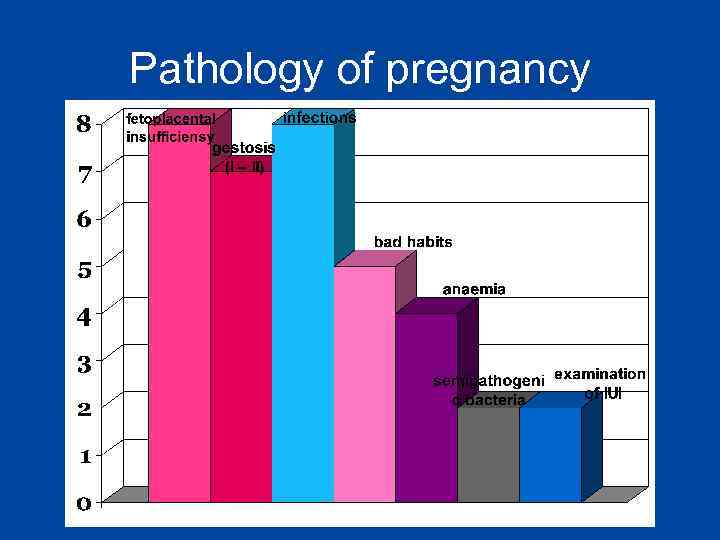 Pathology of pregnancy
Pathology of pregnancy
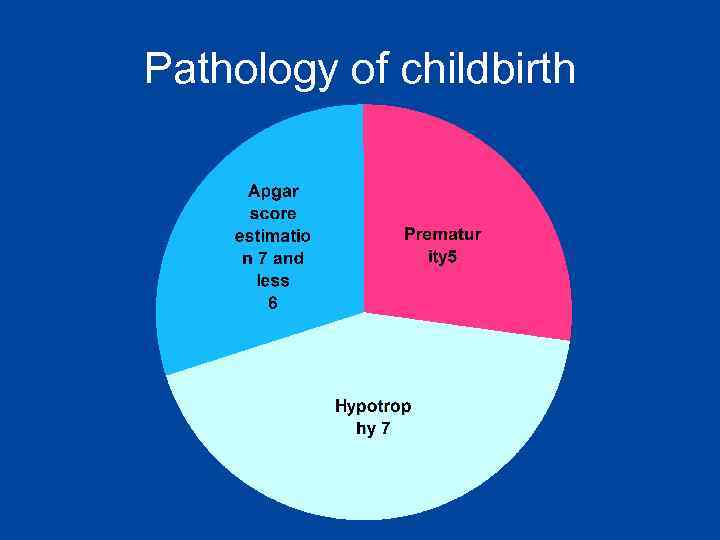 Pathology of childbirth
Pathology of childbirth
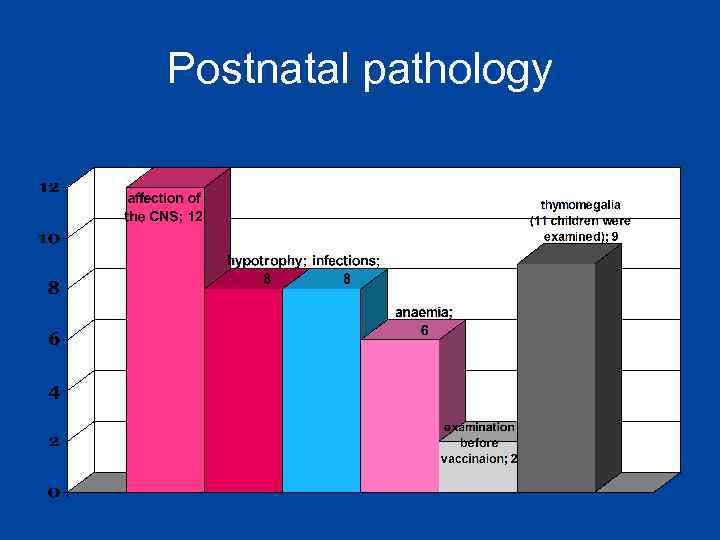 Postnatal pathology
Postnatal pathology
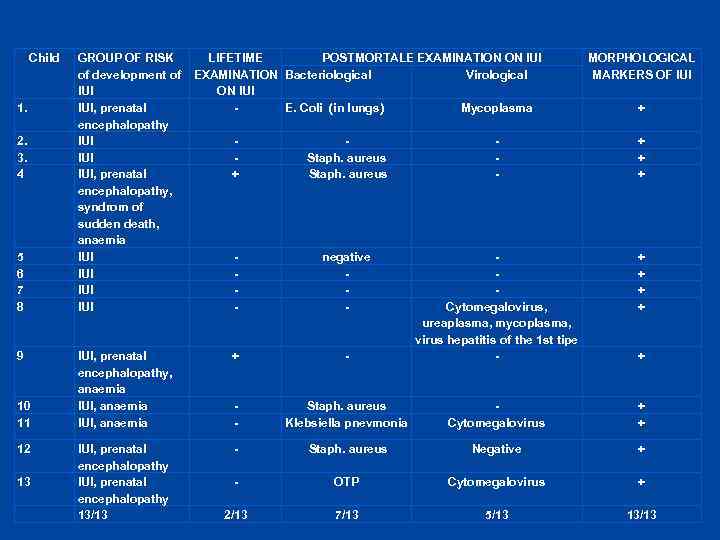 Child 1. 2. 3. 4 5 6 7 8 9 10 11 12 13 GROUP OF RISK of development of IUI, prenatal encephalopathy IUI IUI, prenatal encephalopathy, syndrom of sudden death, anaemia IUI IUI LIFETIME POSTMORTALE EXAMINATION ON IUI Virological EXAMINATION Bacteriological ON IUI E. Coli (in lungs) Mycoplasma MORPHOLOGICAL MARKERS OF IUI + + Staph. aureus - + + + - negative - + + IUI, prenatal encephalopathy, anaemia IUI, anaemia + - Cytomegalovirus, ureaplasma, mycoplasma, virus hepatitis of the 1 st tipe - - Staph. aureus Klebsiella pnevmonia Cytomegalovirus + + IUI, prenatal encephalopathy 13/13 - Staph. aureus Negative + - ОТР Cytomegalovirus + 2/13 7/13 5/13 13/13 +
Child 1. 2. 3. 4 5 6 7 8 9 10 11 12 13 GROUP OF RISK of development of IUI, prenatal encephalopathy IUI IUI, prenatal encephalopathy, syndrom of sudden death, anaemia IUI IUI LIFETIME POSTMORTALE EXAMINATION ON IUI Virological EXAMINATION Bacteriological ON IUI E. Coli (in lungs) Mycoplasma MORPHOLOGICAL MARKERS OF IUI + + Staph. aureus - + + + - negative - + + IUI, prenatal encephalopathy, anaemia IUI, anaemia + - Cytomegalovirus, ureaplasma, mycoplasma, virus hepatitis of the 1 st tipe - - Staph. aureus Klebsiella pnevmonia Cytomegalovirus + + IUI, prenatal encephalopathy 13/13 - Staph. aureus Negative + - ОТР Cytomegalovirus + 2/13 7/13 5/13 13/13 +
 Morphological markers of intrauterine infections
Morphological markers of intrauterine infections
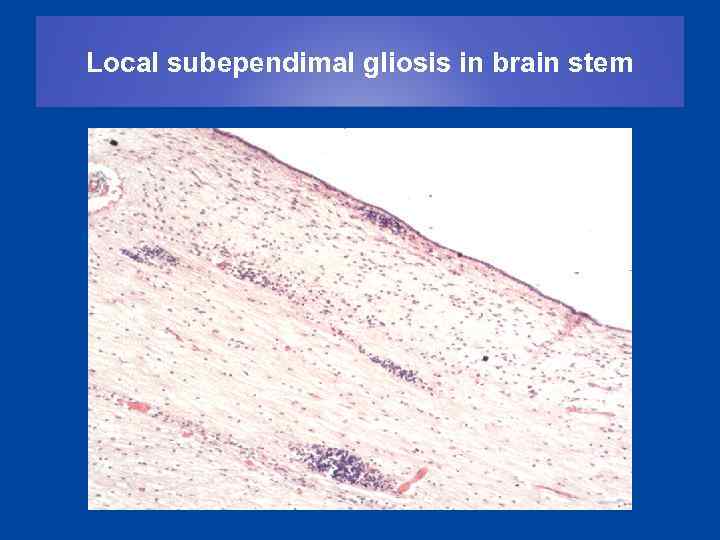 Local subependimal gliosis in brain stem
Local subependimal gliosis in brain stem
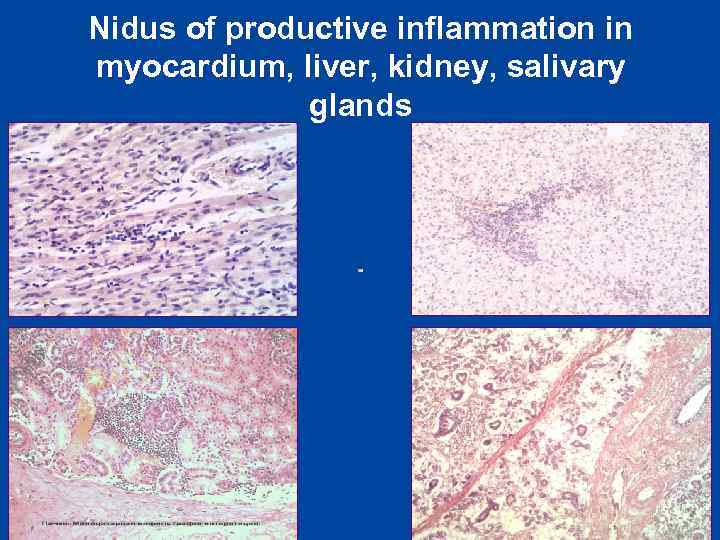 Nidus of productive inflammation in myocardium, liver, kidney, salivary glands
Nidus of productive inflammation in myocardium, liver, kidney, salivary glands
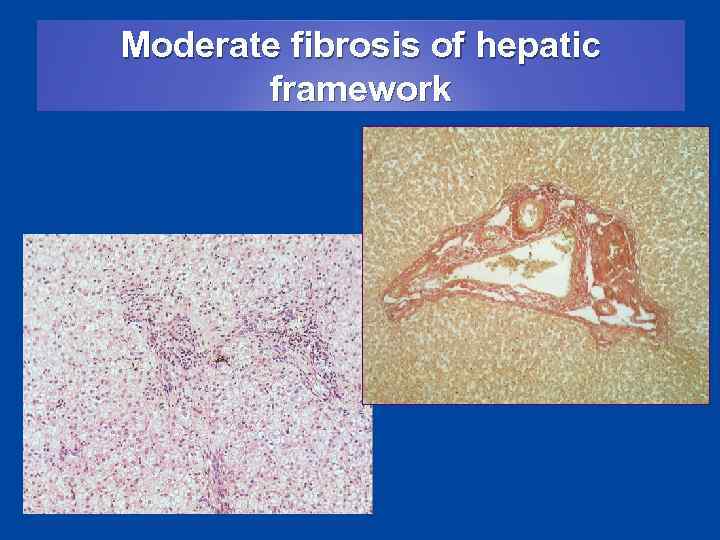 Moderate fibrosis of hepatic framework
Moderate fibrosis of hepatic framework
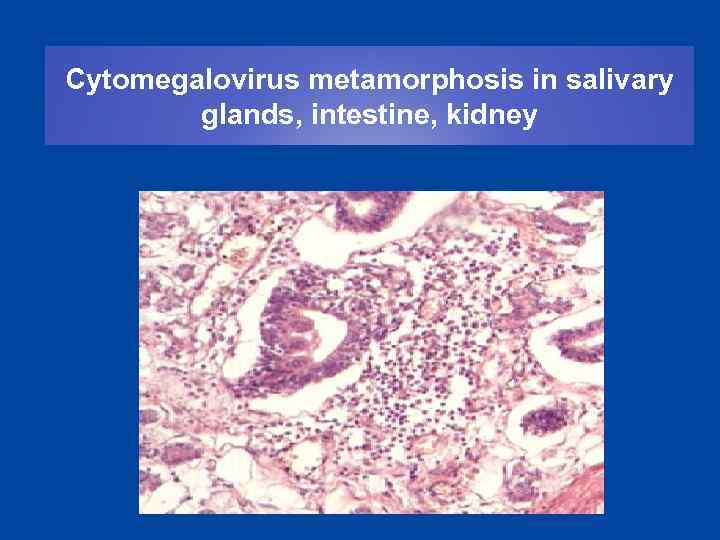 Cytomegalovirus metamorphosis in salivary glands, intestine, kidney
Cytomegalovirus metamorphosis in salivary glands, intestine, kidney
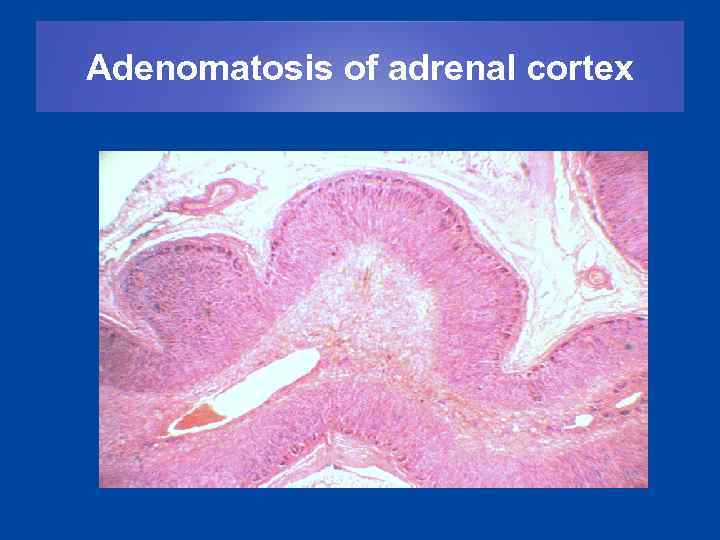 Adenomatosis of adrenal cortex
Adenomatosis of adrenal cortex
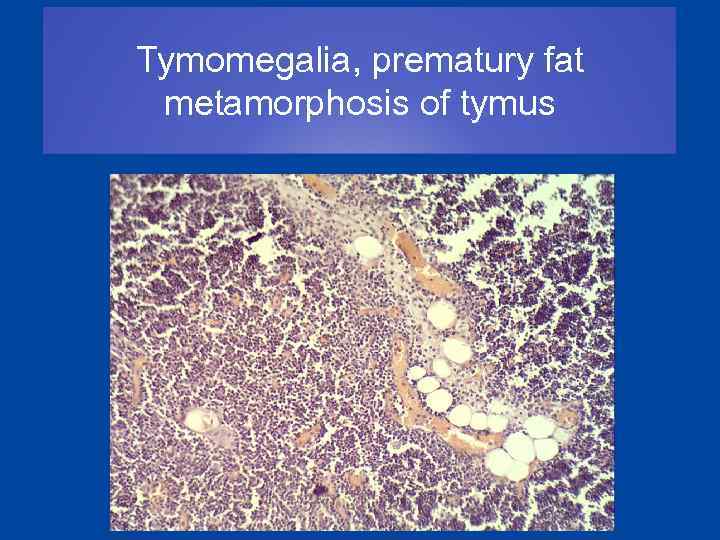 Tymomegalia, prematury fat metamorphosis of tymus
Tymomegalia, prematury fat metamorphosis of tymus
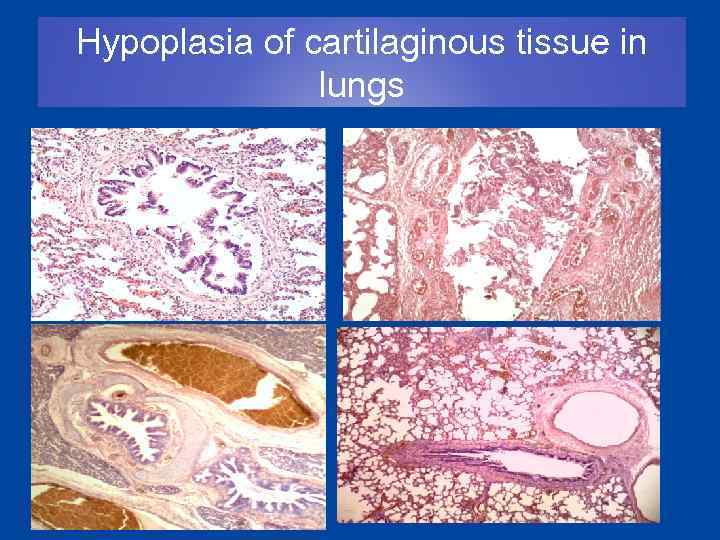 Hypoplasia of cartilaginous tissue in lungs
Hypoplasia of cartilaginous tissue in lungs
 Conclusion • In this 13 cases vaccine was not the cause of death. An intrauterine infection was the cause. • Women should be examined during the pregnancy to find out if they have a risk of intrauterine infections. • The immunity of girls is stronger than the immunity of boys.
Conclusion • In this 13 cases vaccine was not the cause of death. An intrauterine infection was the cause. • Women should be examined during the pregnancy to find out if they have a risk of intrauterine infections. • The immunity of girls is stronger than the immunity of boys.
 Algorithm of examination of mothers and children having high risk of intrauterine infections • careful collecting of anamnoses data (pregnancy, childbirth) • full clinical and laboratory examination of children and mothers with high risk of infections • verification of infection by not less than two methods (virological, cytological, seroimmunological, PCR) • in case of revelation of intrauterine infection, the child must complete a course of etiotropic therapy before the vaccination • the vaccination should be done only after negative results of the second examination, according to an individual plan
Algorithm of examination of mothers and children having high risk of intrauterine infections • careful collecting of anamnoses data (pregnancy, childbirth) • full clinical and laboratory examination of children and mothers with high risk of infections • verification of infection by not less than two methods (virological, cytological, seroimmunological, PCR) • in case of revelation of intrauterine infection, the child must complete a course of etiotropic therapy before the vaccination • the vaccination should be done only after negative results of the second examination, according to an individual plan
 Thank you for your attention!
Thank you for your attention!


