6f03fd202ad42efd3d4d2f30a7f75711.ppt
- Количество слайдов: 33
 The role of chromatography in physico-chemical characterisation Shenaz Nunhuck CASS, GSK
The role of chromatography in physico-chemical characterisation Shenaz Nunhuck CASS, GSK
 Why do we need physchem measurements? · Physicochemical properties of drugs influence · · their absorption and distribution in vivo Systemic absorption of drug involves a number of rate processes: Distribution of the drug in the body Dissolution of the drug in the body fluids Permeation across the cell membranes to reach the site of action. · Key physicochemical parameters influencing these processes are lipophilicity, solubility, p. Ka, permeability
Why do we need physchem measurements? · Physicochemical properties of drugs influence · · their absorption and distribution in vivo Systemic absorption of drug involves a number of rate processes: Distribution of the drug in the body Dissolution of the drug in the body fluids Permeation across the cell membranes to reach the site of action. · Key physicochemical parameters influencing these processes are lipophilicity, solubility, p. Ka, permeability
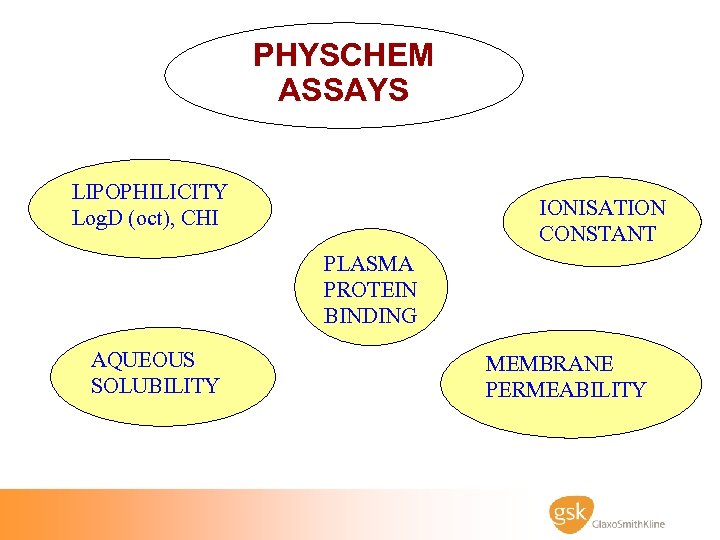 PHYSCHEM ASSAYS LIPOPHILICITY Log. D (oct), CHI IONISATION CONSTANT PLASMA PROTEIN BINDING AQUEOUS SOLUBILITY MEMBRANE PERMEABILITY
PHYSCHEM ASSAYS LIPOPHILICITY Log. D (oct), CHI IONISATION CONSTANT PLASMA PROTEIN BINDING AQUEOUS SOLUBILITY MEMBRANE PERMEABILITY
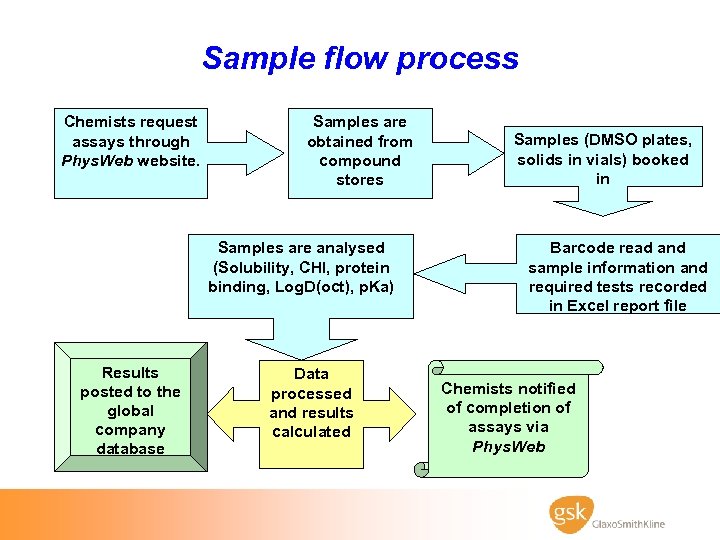 Sample flow process Chemists request assays through Phys. Web website. Samples are obtained from compound stores Samples are analysed (Solubility, CHI, protein binding, Log. D(oct), p. Ka) Results posted to the global company database Data processed and results calculated Samples (DMSO plates, solids in vials) booked in Barcode read and sample information and required tests recorded in Excel report file Chemists notified of completion of assays via Phys. Web
Sample flow process Chemists request assays through Phys. Web website. Samples are obtained from compound stores Samples are analysed (Solubility, CHI, protein binding, Log. D(oct), p. Ka) Results posted to the global company database Data processed and results calculated Samples (DMSO plates, solids in vials) booked in Barcode read and sample information and required tests recorded in Excel report file Chemists notified of completion of assays via Phys. Web
 Lipophilicity measurements · Chromatographic Hydrophobicity Index (CHI) · Immobilised artificial membrane (IAM) partition · Protein binding (human serum albumin, alpha 1 -acid-glycoprotein) · Octanol/water partition coefficient (Log. P/D(oct)
Lipophilicity measurements · Chromatographic Hydrophobicity Index (CHI) · Immobilised artificial membrane (IAM) partition · Protein binding (human serum albumin, alpha 1 -acid-glycoprotein) · Octanol/water partition coefficient (Log. P/D(oct)
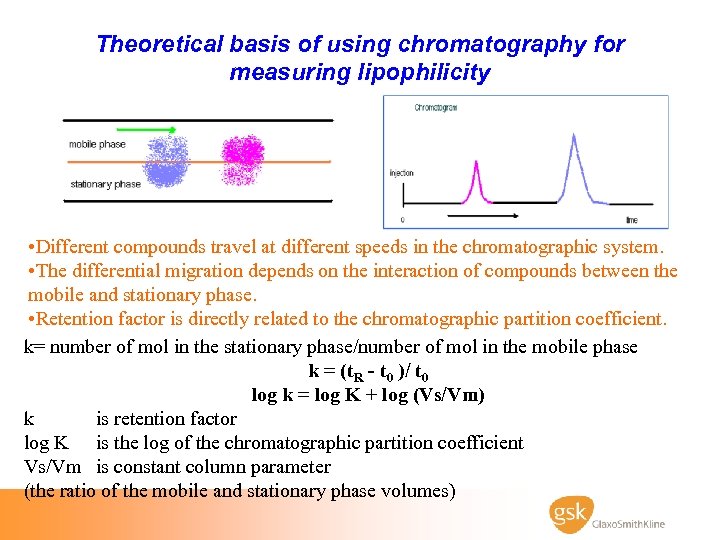 Theoretical basis of using chromatography for measuring lipophilicity • Different compounds travel at different speeds in the chromatographic system. • The differential migration depends on the interaction of compounds between the mobile and stationary phase. • Retention factor is directly related to the chromatographic partition coefficient. k= number of mol in the stationary phase/number of mol in the mobile phase k = (t. R - t 0 )/ t 0 log k = log K + log (Vs/Vm) k is retention factor log K is the log of the chromatographic partition coefficient Vs/Vm is constant column parameter (the ratio of the mobile and stationary phase volumes)
Theoretical basis of using chromatography for measuring lipophilicity • Different compounds travel at different speeds in the chromatographic system. • The differential migration depends on the interaction of compounds between the mobile and stationary phase. • Retention factor is directly related to the chromatographic partition coefficient. k= number of mol in the stationary phase/number of mol in the mobile phase k = (t. R - t 0 )/ t 0 log k = log K + log (Vs/Vm) k is retention factor log K is the log of the chromatographic partition coefficient Vs/Vm is constant column parameter (the ratio of the mobile and stationary phase volumes)
 Chromatographic Hydrophobicity Index, CHI · Fast gradient methods: the gradient retention time is proportional to the compound lipophilicity. · Fast gradient retention time obtained on commercially available C -18 stationary phase converted to Chromatographic Hydrophobicity Index (CHI); this is the chromatographic lipophilicity. · The CHI Indices at three different p. Hs are determined from the gradient retention times obtained by injecting the compound into a HPLC system. · Dynamic range extended by the gradient method. · Can be expressed on a log. P/D scale. (CHIlog. D = 0. 054*CHI -1. 467)
Chromatographic Hydrophobicity Index, CHI · Fast gradient methods: the gradient retention time is proportional to the compound lipophilicity. · Fast gradient retention time obtained on commercially available C -18 stationary phase converted to Chromatographic Hydrophobicity Index (CHI); this is the chromatographic lipophilicity. · The CHI Indices at three different p. Hs are determined from the gradient retention times obtained by injecting the compound into a HPLC system. · Dynamic range extended by the gradient method. · Can be expressed on a log. P/D scale. (CHIlog. D = 0. 054*CHI -1. 467)
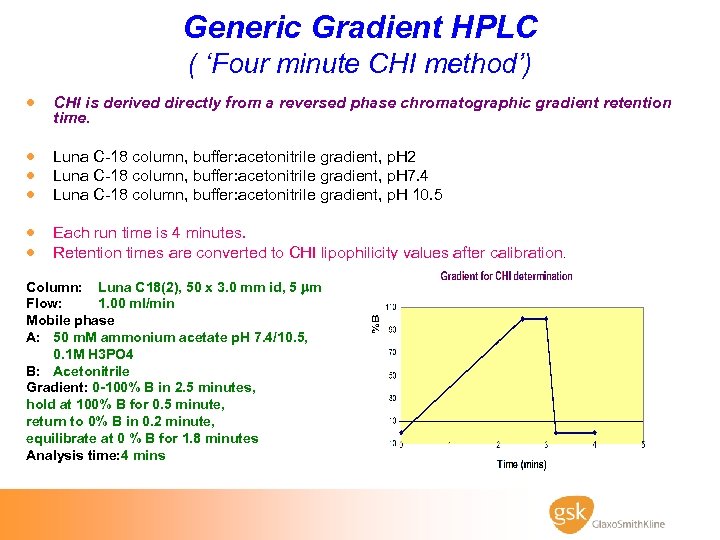 Generic Gradient HPLC ( ‘Four minute CHI method’) · CHI is derived directly from a reversed phase chromatographic gradient retention time. · Luna C-18 column, buffer: acetonitrile gradient, p. H 2 · Luna C-18 column, buffer: acetonitrile gradient, p. H 7. 4 · Luna C-18 column, buffer: acetonitrile gradient, p. H 10. 5 · Each run time is 4 minutes. · Retention times are converted to CHI lipophilicity values after calibration. Column: Luna C 18(2), 50 x 3. 0 mm id, 5 m Flow: 1. 00 ml/min Mobile phase A: 50 m. M ammonium acetate p. H 7. 4/10. 5, 0. 1 M H 3 PO 4 B: Acetonitrile Gradient: 0 -100% B in 2. 5 minutes, hold at 100% B for 0. 5 minute, return to 0% B in 0. 2 minute, equilibrate at 0 % B for 1. 8 minutes Analysis time: 4 mins
Generic Gradient HPLC ( ‘Four minute CHI method’) · CHI is derived directly from a reversed phase chromatographic gradient retention time. · Luna C-18 column, buffer: acetonitrile gradient, p. H 2 · Luna C-18 column, buffer: acetonitrile gradient, p. H 7. 4 · Luna C-18 column, buffer: acetonitrile gradient, p. H 10. 5 · Each run time is 4 minutes. · Retention times are converted to CHI lipophilicity values after calibration. Column: Luna C 18(2), 50 x 3. 0 mm id, 5 m Flow: 1. 00 ml/min Mobile phase A: 50 m. M ammonium acetate p. H 7. 4/10. 5, 0. 1 M H 3 PO 4 B: Acetonitrile Gradient: 0 -100% B in 2. 5 minutes, hold at 100% B for 0. 5 minute, return to 0% B in 0. 2 minute, equilibrate at 0 % B for 1. 8 minutes Analysis time: 4 mins
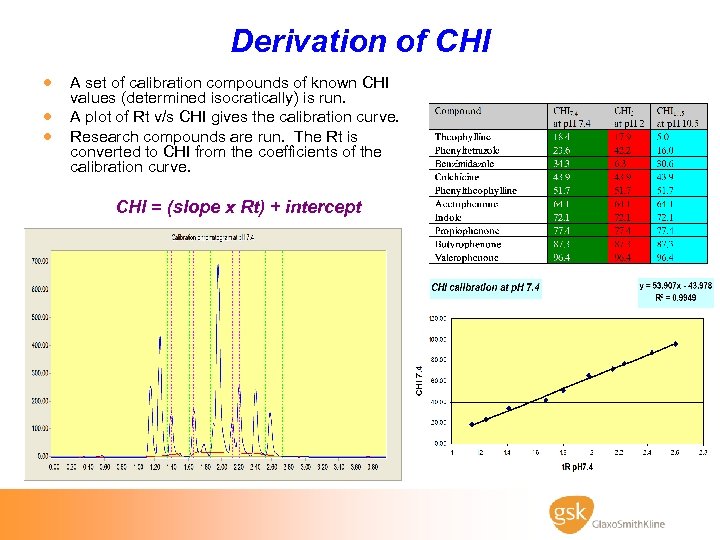 Derivation of CHI · A set of calibration compounds of known CHI · · values (determined isocratically) is run. A plot of Rt v/s CHI gives the calibration curve. Research compounds are run. The Rt is converted to CHI from the coefficients of the calibration curve. CHI = (slope x Rt) + intercept
Derivation of CHI · A set of calibration compounds of known CHI · · values (determined isocratically) is run. A plot of Rt v/s CHI gives the calibration curve. Research compounds are run. The Rt is converted to CHI from the coefficients of the calibration curve. CHI = (slope x Rt) + intercept
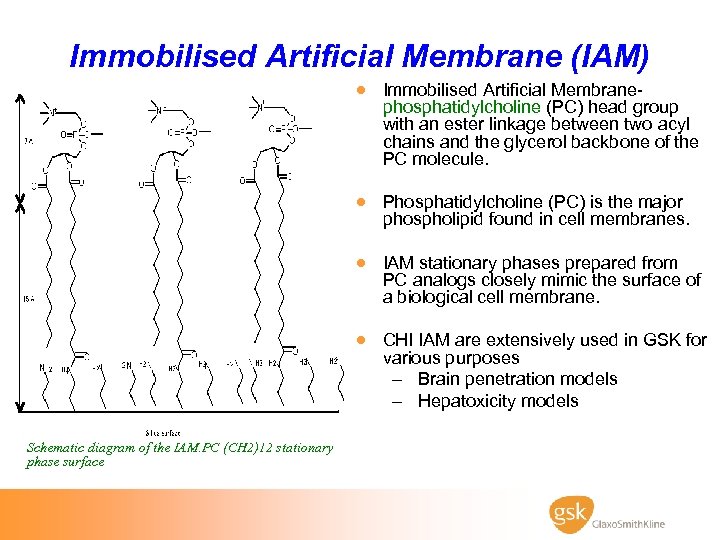 Immobilised Artificial Membrane (IAM) · Immobilised Artificial Membrane- phosphatidylcholine (PC) head group with an ester linkage between two acyl chains and the glycerol backbone of the PC molecule. · Phosphatidylcholine (PC) is the major phospholipid found in cell membranes. · IAM stationary phases prepared from PC analogs closely mimic the surface of a biological cell membrane. · CHI IAM are extensively used in GSK for various purposes – Brain penetration models – Hepatoxicity models Schematic diagram of the IAM. PC (CH 2)12 stationary phase surface
Immobilised Artificial Membrane (IAM) · Immobilised Artificial Membrane- phosphatidylcholine (PC) head group with an ester linkage between two acyl chains and the glycerol backbone of the PC molecule. · Phosphatidylcholine (PC) is the major phospholipid found in cell membranes. · IAM stationary phases prepared from PC analogs closely mimic the surface of a biological cell membrane. · CHI IAM are extensively used in GSK for various purposes – Brain penetration models – Hepatoxicity models Schematic diagram of the IAM. PC (CH 2)12 stationary phase surface
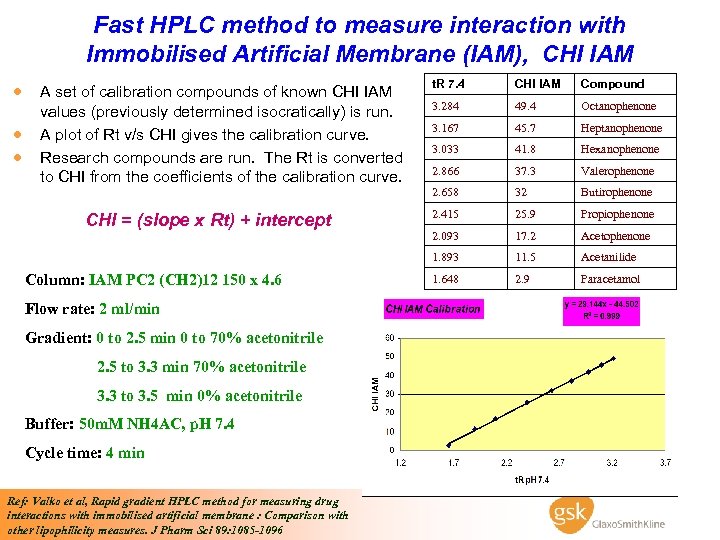 Fast HPLC method to measure interaction with Immobilised Artificial Membrane (IAM), CHI IAM · A set of calibration compounds of known CHI IAM Column: IAM PC 2 (CH 2)12 150 x 4. 6 Flow rate: 2 ml/min Gradient: 0 to 2. 5 min 0 to 70% acetonitrile 2. 5 to 3. 3 min 70% acetonitrile 3. 3 to 3. 5 min 0% acetonitrile Buffer: 50 m. M NH 4 AC, p. H 7. 4 Cycle time: 4 min Ref: Valko et al, Rapid gradient HPLC method for measuring drug interactions with immobilised artificial membrane : Comparison with other lipophilicity measures. J Pharm Sci 89: 1085 -1096 Compound 3. 284 49. 4 Octanophenone 3. 167 45. 7 Heptanophenone 3. 033 41. 8 Hexanophenone 2. 866 37. 3 Valerophenone 32 Butirophenone 2. 415 25. 9 Propiophenone 2. 093 17. 2 Acetophenone 1. 893 CHI = (slope x Rt) + intercept CHI IAM 2. 658 · · values (previously determined isocratically) is run. A plot of Rt v/s CHI gives the calibration curve. Research compounds are run. The Rt is converted to CHI from the coefficients of the calibration curve. t. R 7. 4 11. 5 Acetanilide 1. 648 2. 9 Paracetamol
Fast HPLC method to measure interaction with Immobilised Artificial Membrane (IAM), CHI IAM · A set of calibration compounds of known CHI IAM Column: IAM PC 2 (CH 2)12 150 x 4. 6 Flow rate: 2 ml/min Gradient: 0 to 2. 5 min 0 to 70% acetonitrile 2. 5 to 3. 3 min 70% acetonitrile 3. 3 to 3. 5 min 0% acetonitrile Buffer: 50 m. M NH 4 AC, p. H 7. 4 Cycle time: 4 min Ref: Valko et al, Rapid gradient HPLC method for measuring drug interactions with immobilised artificial membrane : Comparison with other lipophilicity measures. J Pharm Sci 89: 1085 -1096 Compound 3. 284 49. 4 Octanophenone 3. 167 45. 7 Heptanophenone 3. 033 41. 8 Hexanophenone 2. 866 37. 3 Valerophenone 32 Butirophenone 2. 415 25. 9 Propiophenone 2. 093 17. 2 Acetophenone 1. 893 CHI = (slope x Rt) + intercept CHI IAM 2. 658 · · values (previously determined isocratically) is run. A plot of Rt v/s CHI gives the calibration curve. Research compounds are run. The Rt is converted to CHI from the coefficients of the calibration curve. t. R 7. 4 11. 5 Acetanilide 1. 648 2. 9 Paracetamol
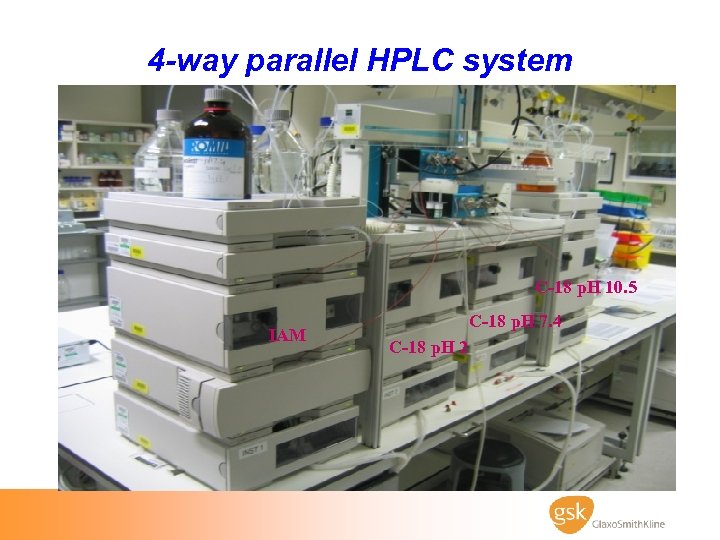 4 -way parallel HPLC system C-18 p. H 10. 5 IAM C-18 p. H 7. 4 C-18 p. H 2
4 -way parallel HPLC system C-18 p. H 10. 5 IAM C-18 p. H 7. 4 C-18 p. H 2
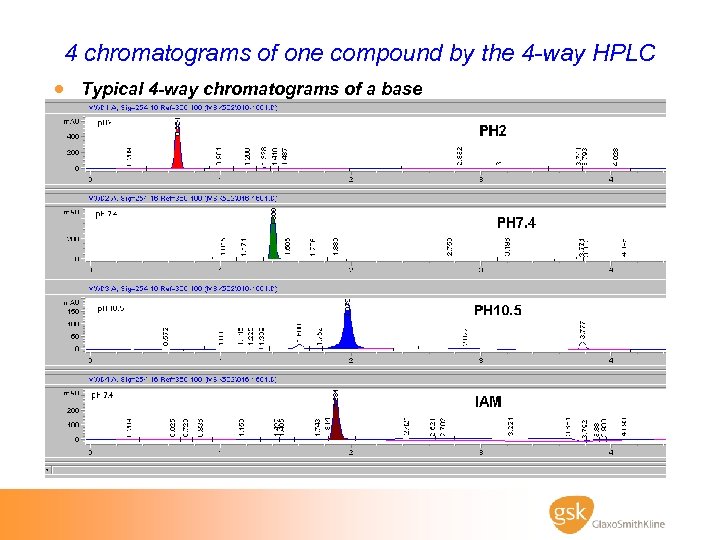 4 chromatograms of one compound by the 4 -way HPLC · Typical 4 -way chromatograms of a base
4 chromatograms of one compound by the 4 -way HPLC · Typical 4 -way chromatograms of a base
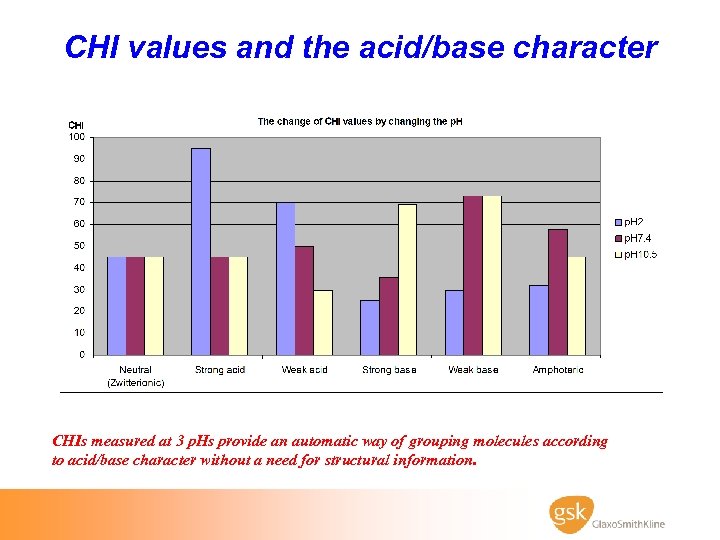 CHI values and the acid/base character CHIs measured at 3 p. Hs provide an automatic way of grouping molecules according to acid/base character without a need for structural information.
CHI values and the acid/base character CHIs measured at 3 p. Hs provide an automatic way of grouping molecules according to acid/base character without a need for structural information.
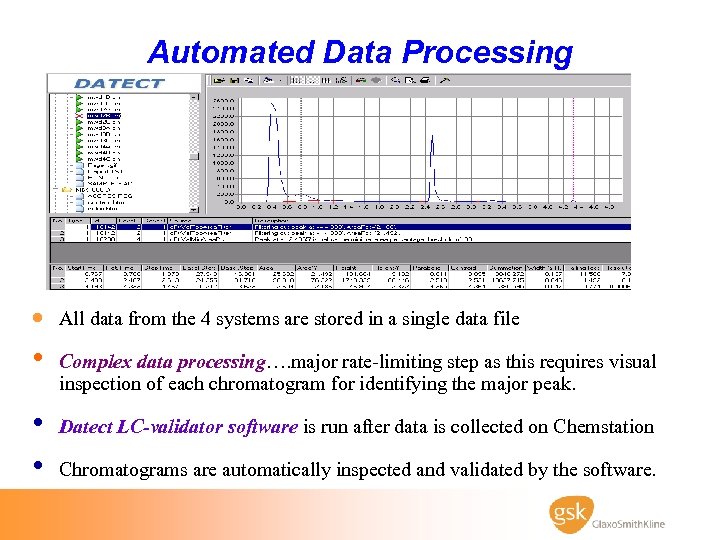 Automated Data Processing · All data from the 4 systems are stored in a single data file • • • Complex data processing…. major rate-limiting step as this requires visual inspection of each chromatogram for identifying the major peak. Datect LC-validator software is run after data is collected on Chemstation Chromatograms are automatically inspected and validated by the software.
Automated Data Processing · All data from the 4 systems are stored in a single data file • • • Complex data processing…. major rate-limiting step as this requires visual inspection of each chromatogram for identifying the major peak. Datect LC-validator software is run after data is collected on Chemstation Chromatograms are automatically inspected and validated by the software.
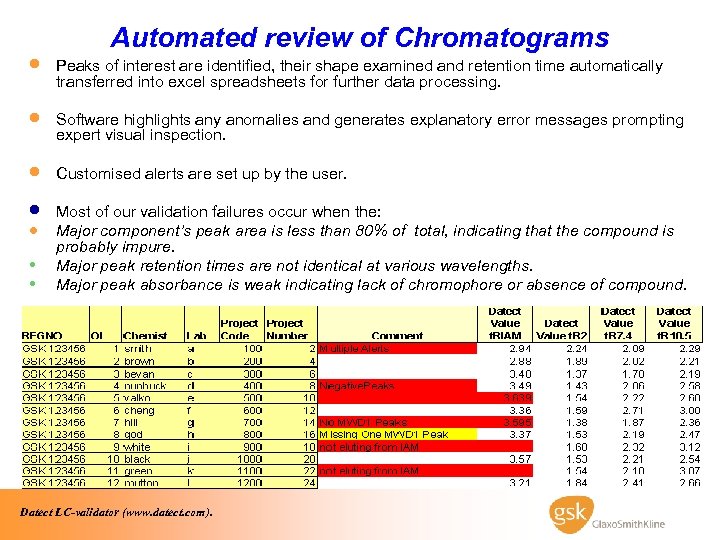 · · · Automated review of Chromatograms Peaks of interest are identified, their shape examined and retention time automatically transferred into excel spreadsheets for further data processing. Software highlights any anomalies and generates explanatory error messages prompting expert visual inspection. Customised alerts are set up by the user. · · • • Most of our validation failures occur when the: Major component’s peak area is less than 80% of total, indicating that the compound is probably impure. Major peak retention times are not identical at various wavelengths. Major peak absorbance is weak indicating lack of chromophore or absence of compound. Datect LC-validator (www. datect. com).
· · · Automated review of Chromatograms Peaks of interest are identified, their shape examined and retention time automatically transferred into excel spreadsheets for further data processing. Software highlights any anomalies and generates explanatory error messages prompting expert visual inspection. Customised alerts are set up by the user. · · • • Most of our validation failures occur when the: Major component’s peak area is less than 80% of total, indicating that the compound is probably impure. Major peak retention times are not identical at various wavelengths. Major peak absorbance is weak indicating lack of chromophore or absence of compound. Datect LC-validator (www. datect. com).
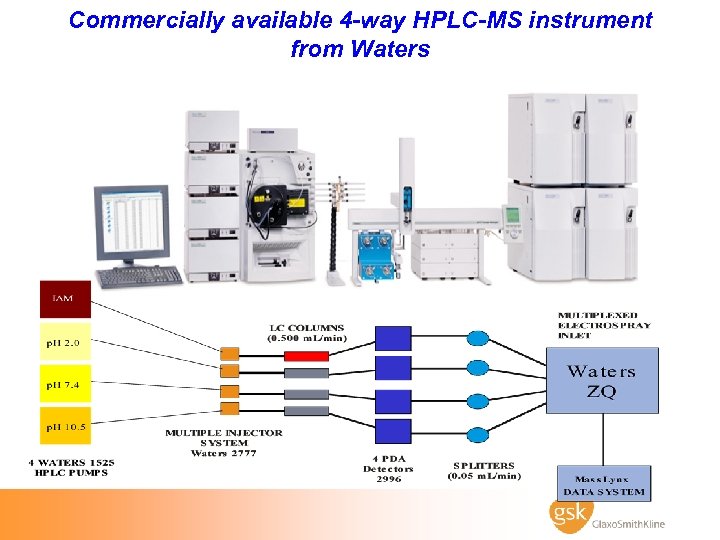 Commercially available 4 -way HPLC-MS instrument from Waters
Commercially available 4 -way HPLC-MS instrument from Waters
 Biomimetic hplc stationary phases (HSA, RSA, AGP) · Used to measure the binding affinity of · · compounds to proteins. Plasma protein binding affects the unbound (free) drug concentration available to diffuse from the blood and reach the target tissue. Commercially available human and rat serum albumin and α-acid glycoprotein hplc stationary phases (available from Chrom. Tech Ltd)
Biomimetic hplc stationary phases (HSA, RSA, AGP) · Used to measure the binding affinity of · · compounds to proteins. Plasma protein binding affects the unbound (free) drug concentration available to diffuse from the blood and reach the target tissue. Commercially available human and rat serum albumin and α-acid glycoprotein hplc stationary phases (available from Chrom. Tech Ltd)
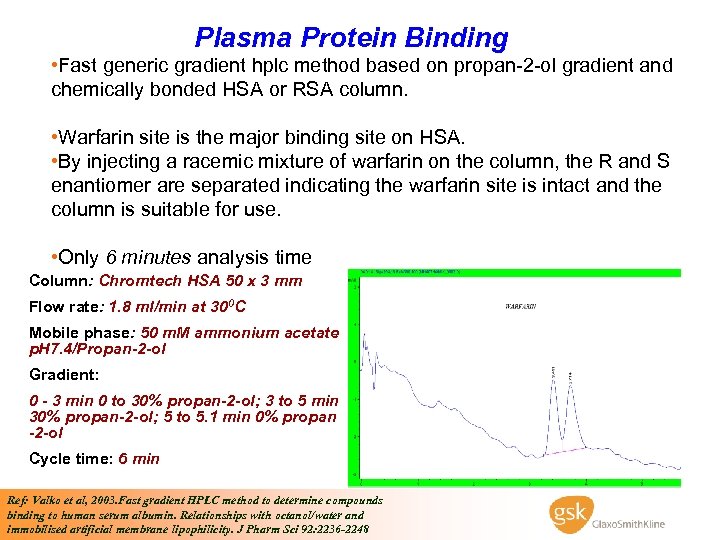 Plasma Protein Binding • Fast generic gradient hplc method based on propan-2 -ol gradient and chemically bonded HSA or RSA column. • Warfarin site is the major binding site on HSA. • By injecting a racemic mixture of warfarin on the column, the R and S enantiomer are separated indicating the warfarin site is intact and the column is suitable for use. • Only 6 minutes analysis time Column: Chromtech HSA 50 x 3 mm Flow rate: 1. 8 ml/min at 300 C Mobile phase: 50 m. M ammonium acetate p. H 7. 4/Propan-2 -ol Gradient: 0 - 3 min 0 to 30% propan-2 -ol; 3 to 5 min 30% propan-2 -ol; 5 to 5. 1 min 0% propan -2 -ol Cycle time: 6 min Ref: Valko et al, 2003. Fast gradient HPLC method to determine compounds binding to human serum albumin. Relationships with octanol/water and immobilised artificial membrane lipophilicity. J Pharm Sci 92: 2236 -2248
Plasma Protein Binding • Fast generic gradient hplc method based on propan-2 -ol gradient and chemically bonded HSA or RSA column. • Warfarin site is the major binding site on HSA. • By injecting a racemic mixture of warfarin on the column, the R and S enantiomer are separated indicating the warfarin site is intact and the column is suitable for use. • Only 6 minutes analysis time Column: Chromtech HSA 50 x 3 mm Flow rate: 1. 8 ml/min at 300 C Mobile phase: 50 m. M ammonium acetate p. H 7. 4/Propan-2 -ol Gradient: 0 - 3 min 0 to 30% propan-2 -ol; 3 to 5 min 30% propan-2 -ol; 5 to 5. 1 min 0% propan -2 -ol Cycle time: 6 min Ref: Valko et al, 2003. Fast gradient HPLC method to determine compounds binding to human serum albumin. Relationships with octanol/water and immobilised artificial membrane lipophilicity. J Pharm Sci 92: 2236 -2248
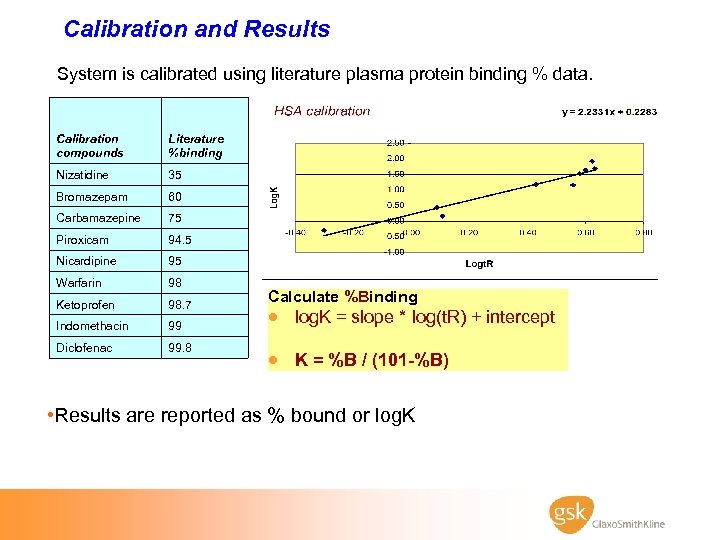 Calibration and Results System is calibrated using literature plasma protein binding % data. Calibration compounds Literature %binding Nizatidine 35 Bromazepam 60 Carbamazepine 75 Piroxicam 94. 5 Nicardipine 95 Warfarin 98 Ketoprofen 98. 7 Indomethacin 99 Diclofenac 99. 8 Calculate %Binding · log. K = slope * log(t. R) + intercept · K = %B / (101 -%B) • Results are reported as % bound or log. K
Calibration and Results System is calibrated using literature plasma protein binding % data. Calibration compounds Literature %binding Nizatidine 35 Bromazepam 60 Carbamazepine 75 Piroxicam 94. 5 Nicardipine 95 Warfarin 98 Ketoprofen 98. 7 Indomethacin 99 Diclofenac 99. 8 Calculate %Binding · log. K = slope * log(t. R) + intercept · K = %B / (101 -%B) • Results are reported as % bound or log. K
 Plasma Protein Binding • Reproducibility on the column is very good; the gradient retention time is within 0. 1 min from day to day • The hplc method is fast, simple and is easily automated. • The use of calibration compensates for any changes in the column properties and hence increases the accuracy of the determination. • The hplc procedure can discriminate easily in the high binding region (better than the traditional ultrafiltration or equilibrium dialysis methods) as the percentage of drug bound to the protein is measured and not the free drug. • Approximately 400 injections per column
Plasma Protein Binding • Reproducibility on the column is very good; the gradient retention time is within 0. 1 min from day to day • The hplc method is fast, simple and is easily automated. • The use of calibration compensates for any changes in the column properties and hence increases the accuracy of the determination. • The hplc procedure can discriminate easily in the high binding region (better than the traditional ultrafiltration or equilibrium dialysis methods) as the percentage of drug bound to the protein is measured and not the free drug. • Approximately 400 injections per column
 Chromatographic methods for quantitative assays · HPLC is a powerful technique for separation and quantification · Suitable approach for determination of compound concentration · Applied as end-point for log. P(octanol) and solubility determination
Chromatographic methods for quantitative assays · HPLC is a powerful technique for separation and quantification · Suitable approach for determination of compound concentration · Applied as end-point for log. P(octanol) and solubility determination
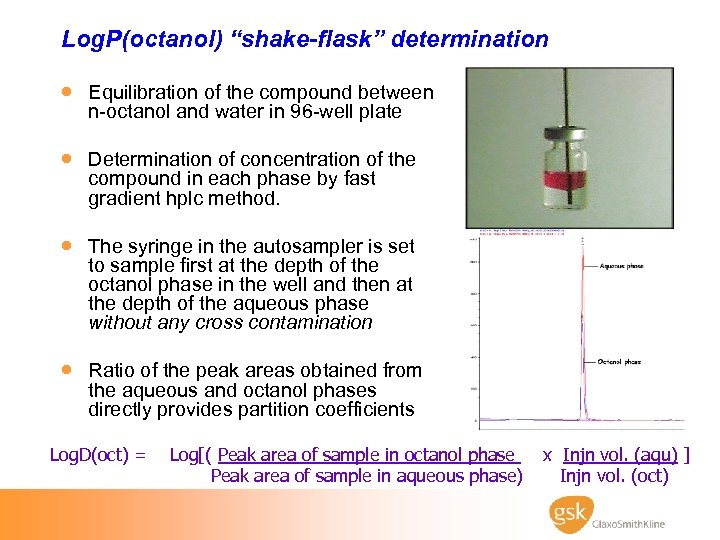 Log. P(octanol) “shake-flask” determination · Equilibration of the compound between n-octanol and water in 96 -well plate · Determination of concentration of the compound in each phase by fast gradient hplc method. · The syringe in the autosampler is set to sample first at the depth of the octanol phase in the well and then at the depth of the aqueous phase without any cross contamination · Ratio of the peak areas obtained from the aqueous and octanol phases directly provides partition coefficients Log. D(oct) = Log[( Peak area of sample in octanol phase Peak area of sample in aqueous phase) x Injn vol. (aqu) ] Injn vol. (oct)
Log. P(octanol) “shake-flask” determination · Equilibration of the compound between n-octanol and water in 96 -well plate · Determination of concentration of the compound in each phase by fast gradient hplc method. · The syringe in the autosampler is set to sample first at the depth of the octanol phase in the well and then at the depth of the aqueous phase without any cross contamination · Ratio of the peak areas obtained from the aqueous and octanol phases directly provides partition coefficients Log. D(oct) = Log[( Peak area of sample in octanol phase Peak area of sample in aqueous phase) x Injn vol. (aqu) ] Injn vol. (oct)
 Why is aqueous solubility important in early drug discovery? · Solubility is a key property for gastrointestinal · · absorption of orally administered drugs. Affects bioavailability Helpful in drug formulation stages for optimal drug delivery route and optimization Insoluble compounds may compromise screening results. Various solubilities • DMSO precipitative solubility • Solubility from solids • Solubility in simulated intestinal fluid (SIF)
Why is aqueous solubility important in early drug discovery? · Solubility is a key property for gastrointestinal · · absorption of orally administered drugs. Affects bioavailability Helpful in drug formulation stages for optimal drug delivery route and optimization Insoluble compounds may compromise screening results. Various solubilities • DMSO precipitative solubility • Solubility from solids • Solubility in simulated intestinal fluid (SIF)
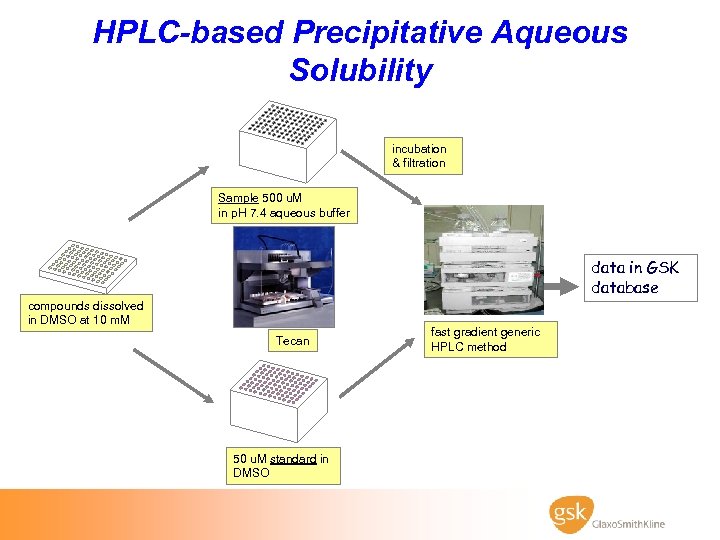 HPLC-based Precipitative Aqueous Solubility incubation & filtration Sample 500 u. M in p. H 7. 4 aqueous buffer data in GSK database compounds dissolved in DMSO at 10 m. M Tecan 50 u. M standard in DMSO fast gradient generic HPLC method
HPLC-based Precipitative Aqueous Solubility incubation & filtration Sample 500 u. M in p. H 7. 4 aqueous buffer data in GSK database compounds dissolved in DMSO at 10 m. M Tecan 50 u. M standard in DMSO fast gradient generic HPLC method
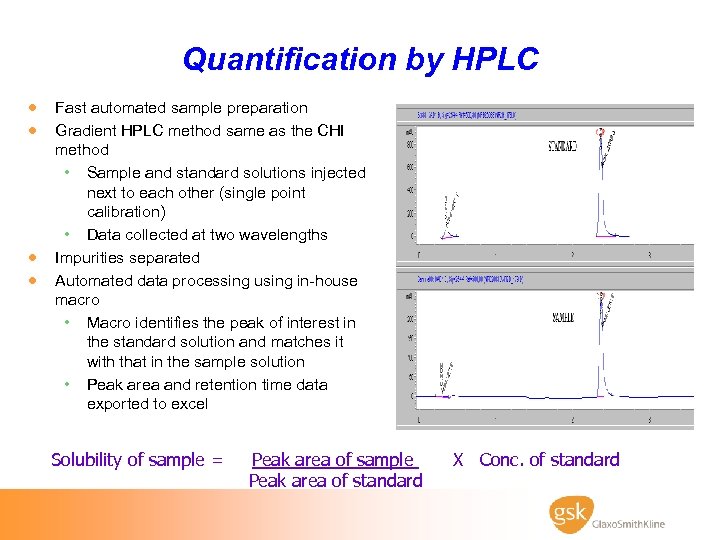 Quantification by HPLC · Fast automated sample preparation · Gradient HPLC method same as the CHI · · method • Sample and standard solutions injected next to each other (single point calibration) • Data collected at two wavelengths Impurities separated Automated data processing using in-house macro • Macro identifies the peak of interest in the standard solution and matches it with that in the sample solution • Peak area and retention time data exported to excel Solubility of sample = Peak area of sample Peak area of standard X Conc. of standard
Quantification by HPLC · Fast automated sample preparation · Gradient HPLC method same as the CHI · · method • Sample and standard solutions injected next to each other (single point calibration) • Data collected at two wavelengths Impurities separated Automated data processing using in-house macro • Macro identifies the peak of interest in the standard solution and matches it with that in the sample solution • Peak area and retention time data exported to excel Solubility of sample = Peak area of sample Peak area of standard X Conc. of standard
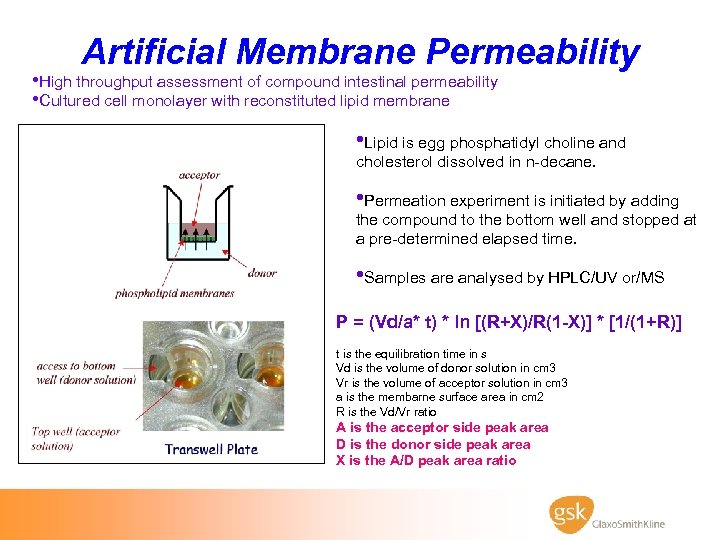 Artificial Membrane Permeability • High throughput assessment of compound intestinal permeability • Cultured cell monolayer with reconstituted lipid membrane • Lipid is egg phosphatidyl choline and cholesterol dissolved in n-decane. • Permeation experiment is initiated by adding the compound to the bottom well and stopped at a pre-determined elapsed time. • Samples are analysed by HPLC/UV or/MS P = (Vd/a* t) * ln [(R+X)/R(1 -X)] * [1/(1+R)] t is the equilibration time in s Vd is the volume of donor solution in cm 3 Vr is the volume of acceptor solution in cm 3 a is the membarne surface area in cm 2 R is the Vd/Vr ratio A is the acceptor side peak area D is the donor side peak area X is the A/D peak area ratio
Artificial Membrane Permeability • High throughput assessment of compound intestinal permeability • Cultured cell monolayer with reconstituted lipid membrane • Lipid is egg phosphatidyl choline and cholesterol dissolved in n-decane. • Permeation experiment is initiated by adding the compound to the bottom well and stopped at a pre-determined elapsed time. • Samples are analysed by HPLC/UV or/MS P = (Vd/a* t) * ln [(R+X)/R(1 -X)] * [1/(1+R)] t is the equilibration time in s Vd is the volume of donor solution in cm 3 Vr is the volume of acceptor solution in cm 3 a is the membarne surface area in cm 2 R is the Vd/Vr ratio A is the acceptor side peak area D is the donor side peak area X is the A/D peak area ratio
 Advantages of using HPLC technique · The compounds retention time can be directly related to the distribution between the stationary and the mobile phase, there is no need for concentration determination. · By changing the stationary phases and the mobile phase composition various types of lipophilic interactions can be investigated. · Impurities do not affect results as they are separated from the main peak and the compound of interest can be identified.
Advantages of using HPLC technique · The compounds retention time can be directly related to the distribution between the stationary and the mobile phase, there is no need for concentration determination. · By changing the stationary phases and the mobile phase composition various types of lipophilic interactions can be investigated. · Impurities do not affect results as they are separated from the main peak and the compound of interest can be identified.
 Advantages of using HPLC technique · Only small amount of material is needed. · Parallel systems can be used to lower cost and increase throughput. · With generic gradient hplc method, one method can be used with a variety of compounds; there is no need for individual customised method development. · Provides an excellent platform for computer controlled automated measurements with computerised data acquisition.
Advantages of using HPLC technique · Only small amount of material is needed. · Parallel systems can be used to lower cost and increase throughput. · With generic gradient hplc method, one method can be used with a variety of compounds; there is no need for individual customised method development. · Provides an excellent platform for computer controlled automated measurements with computerised data acquisition.
 CONCLUSIONS · HPLC provides an excellent generic platform for measuring lipophilicity, acid/base character and bio-mimetic partition properties. · With the application of gradient methods and system calibration with known compounds, large amounts of reproducible data are obtained covering a wide dynamic range of the property. · The extensive application of automated platforms and parallelised chromatography has enabled hundreds of thousands of determinations to be made per annum with a minimum of labour. · The data are suitable to build local and general models to predict developability properties in early stages of drug discovery.
CONCLUSIONS · HPLC provides an excellent generic platform for measuring lipophilicity, acid/base character and bio-mimetic partition properties. · With the application of gradient methods and system calibration with known compounds, large amounts of reproducible data are obtained covering a wide dynamic range of the property. · The extensive application of automated platforms and parallelised chromatography has enabled hundreds of thousands of determinations to be made per annum with a minimum of labour. · The data are suitable to build local and general models to predict developability properties in early stages of drug discovery.
 Acknowledgements · · Klara Valko Chris Bevan Alan Hill Pat Mc. Donough
Acknowledgements · · Klara Valko Chris Bevan Alan Hill Pat Mc. Donough
 Physicochemical Scientist - GSK Location: Harlow, Essex, Southeast England Salary: Attractive Pay & Benefits Start Date: 07/12/2005 Duration: Permanent Reference: 103002 -82072 Glaxo. Smith. Kline is currently recruiting for a Physicochemical Scientist in Harlow - Essex, Southeast England. At Glaxo. Smith. Kline (GSK), one of the world's leading healthcare businesses, we discover, develop and produce products that help people live longer, do more and feel better. Minimum Requirements: You will have a BSc or equivalent experience in Chemistry, Analytical Chemistry or related discipline and have experience within an analytical laboratory environment.
Physicochemical Scientist - GSK Location: Harlow, Essex, Southeast England Salary: Attractive Pay & Benefits Start Date: 07/12/2005 Duration: Permanent Reference: 103002 -82072 Glaxo. Smith. Kline is currently recruiting for a Physicochemical Scientist in Harlow - Essex, Southeast England. At Glaxo. Smith. Kline (GSK), one of the world's leading healthcare businesses, we discover, develop and produce products that help people live longer, do more and feel better. Minimum Requirements: You will have a BSc or equivalent experience in Chemistry, Analytical Chemistry or related discipline and have experience within an analytical laboratory environment.
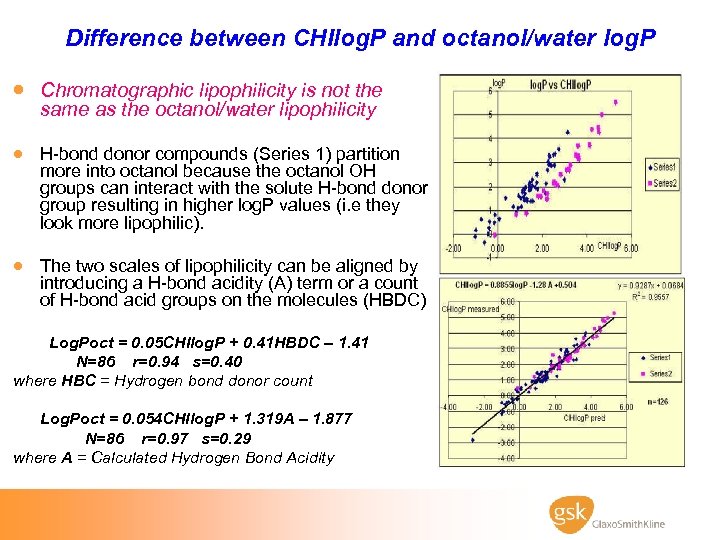 Difference between CHIlog. P and octanol/water log. P · Chromatographic lipophilicity is not the same as the octanol/water lipophilicity · H-bond donor compounds (Series 1) partition more into octanol because the octanol OH groups can interact with the solute H-bond donor group resulting in higher log. P values (i. e they look more lipophilic). · The two scales of lipophilicity can be aligned by introducing a H-bond acidity (A) term or a count of H-bond acid groups on the molecules (HBDC) Log. Poct = 0. 05 CHIlog. P + 0. 41 HBDC – 1. 41 N=86 r=0. 94 s=0. 40 where HBC = Hydrogen bond donor count Log. Poct = 0. 054 CHIlog. P + 1. 319 A – 1. 877 N=86 r=0. 97 s=0. 29 where A = Calculated Hydrogen Bond Acidity
Difference between CHIlog. P and octanol/water log. P · Chromatographic lipophilicity is not the same as the octanol/water lipophilicity · H-bond donor compounds (Series 1) partition more into octanol because the octanol OH groups can interact with the solute H-bond donor group resulting in higher log. P values (i. e they look more lipophilic). · The two scales of lipophilicity can be aligned by introducing a H-bond acidity (A) term or a count of H-bond acid groups on the molecules (HBDC) Log. Poct = 0. 05 CHIlog. P + 0. 41 HBDC – 1. 41 N=86 r=0. 94 s=0. 40 where HBC = Hydrogen bond donor count Log. Poct = 0. 054 CHIlog. P + 1. 319 A – 1. 877 N=86 r=0. 97 s=0. 29 where A = Calculated Hydrogen Bond Acidity


