735dc1effafb0cc660015996cedc6baa.ppt
- Количество слайдов: 1
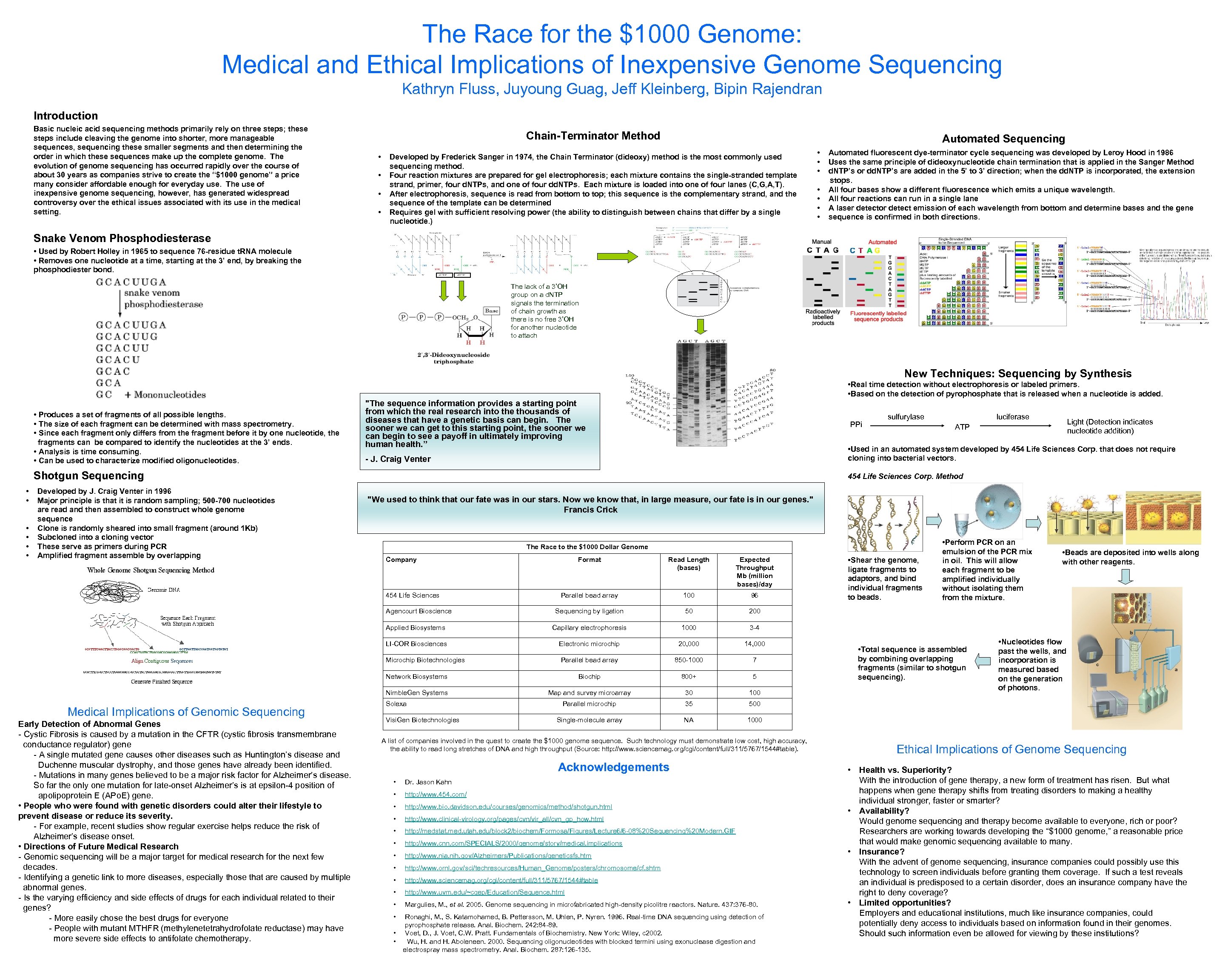 The Race for the $1000 Genome: Medical and Ethical Implications of Inexpensive Genome Sequencing Kathryn Fluss, Juyoung Guag, Jeff Kleinberg, Bipin Rajendran Introduction Basic nucleic acid sequencing methods primarily rely on three steps; these steps include cleaving the genome into shorter, more manageable sequences, sequencing these smaller segments and then determining the order in which these sequences make up the complete genome. The evolution of genome sequencing has occurred rapidly over the course of about 30 years as companies strive to create the “$1000 genome” a price many consider affordable enough for everyday use. The use of inexpensive genome sequencing, however, has generated widespread controversy over the ethical issues associated with its use in the medical setting. Chain-Terminator Method • • Automated Sequencing Developed by Frederick Sanger in 1974, the Chain Terminator (dideoxy) method is the most commonly used sequencing method. Four reaction mixtures are prepared for gel electrophoresis; each mixture contains the single-stranded template strand, primer, four d. NTPs, and one of four dd. NTPs. Each mixture is loaded into one of four lanes (C, G, A, T). After electrophoresis, sequence is read from bottom to top; this sequence is the complementary strand, and the sequence of the template can be determined Requires gel with sufficient resolving power (the ability to distinguish between chains that differ by a single nucleotide. ) • • Automated fluorescent dye-terminator cycle sequencing was developed by Leroy Hood in 1986 Uses the same principle of dideoxynucleotide chain termination that is applied in the Sanger Method d. NTP’s or dd. NTP’s are added in the 5’ to 3’ direction; when the dd. NTP is incorporated, the extension stops. All four bases show a different fluorescence which emits a unique wavelength. All four reactions can run in a single lane A laser detector detect emission of each wavelength from bottom and determine bases and the gene sequence is confirmed in both directions. Snake Venom Phosphodiesterase • Used by Robert Holley in 1965 to sequence 76 -residue t. RNA molecule • Removes one nucleotide at a time, starting at the 3’ end, by breaking the phosphodiester bond. The lack of a 3’OH group on a d. NTP signals the termination of chain growth as there is no free 3’OH for another nucleotide to attach New Techniques: Sequencing by Synthesis • Real time detection without electrophoresis or labeled primers. • Based on the detection of pyrophosphate that is released when a nucleotide is added. • Produces a set of fragments of all possible lengths. • The size of each fragment can be determined with mass spectrometry. • Since each fragment only differs from the fragment before it by one nucleotide, the fragments can be compared to identify the nucleotides at the 3’ ends. • Analysis is time consuming. • Can be used to characterize modified oligonucleotides. "The sequence information provides a starting point from which the real research into the thousands of diseases that have a genetic basis can begin. The sooner we can get to this starting point, the sooner we can begin to see a payoff in ultimately improving human health. ” PPi • • Developed by J. Craig Venter in 1996 Major principle is that it is random sampling; 500 -700 nucleotides are read and then assembled to construct whole genome sequence Clone is randomly sheared into small fragment (around 1 Kb) Subcloned into a cloning vector These serve as primers during PCR Amplified fragment assemble by overlapping - J. Craig Venter "We used to think that our fate was in our stars. Now we know that, in large measure, our fate is in our genes. " Francis Crick The Race to the $1000 Dollar Genome Company Format Read Length (bases) Expected Throughput Mb (million bases)/day Parallel bead array 100 96 Sequencing by ligation 50 Applied Biosystems Capillary electrophoresis 1000 Electronic microchip 20, 000 14, 000 Microchip Biotechnologies Parallel bead array 850 -1000 7 Network Biosystems Biochip 800+ 5 Nimble. Gen Systems Map and survey microarray 30 100 Parallel microchip 35 500 Single-molecule array NA • Beads are deposited into wells along with other reagents. 3 -4 LI-COR Biosciences • Shear the genome, ligate fragments to adaptors, and bind individual fragments to beads. • Perform PCR on an emulsion of the PCR mix in oil. This will allow each fragment to be amplified individually without isolating them from the mixture. 200 1000 Agencourt Bioscience Early Detection of Abnormal Genes - Cystic Fibrosis is caused by a mutation in the CFTR (cystic fibrosis transmembrane conductance regulator) gene - A single mutated gene causes other diseases such as Huntington’s disease and Duchenne muscular dystrophy, and those genes have already been identified. - Mutations in many genes believed to be a major risk factor for Alzheimer’s disease. So far the only one mutation for late-onset Alzheimer’s is at epsilon-4 position of apolipoprotein E (APo. E) gene. • People who were found with genetic disorders could alter their lifestyle to prevent disease or reduce its severity. - For example, recent studies show regular exercise helps reduce the risk of Alzheimer’s disease onset. • Directions of Future Medical Research - Genomic sequencing will be a major target for medical research for the next few decades. - Identifying a genetic link to more diseases, especially those that are caused by multiple abnormal genes. - Is the varying efficiency and side effects of drugs for each individual related to their genes? - More easily chose the best drugs for everyone - People with mutant MTHFR (methylenetetrahydrofolate reductase) may have more severe side effects to antifolate chemotherapy. Light (Detection indicates nucleotide addition) ATP 454 Life Sciences Corp. Method 454 Life Sciences Medical Implications of Genomic Sequencing luciferase • Used in an automated system developed by 454 Life Sciences Corp. that does not require cloning into bacterial vectors. Shotgun Sequencing • • sulfurylase Solexa Visi. Gen Biotechnologies A list of companies involved in the quest to create the $1000 genome sequence. Such technology must demonstrate low cost, high accuracy, the ability to read long stretches of DNA and high throughput (Source: http: //www. sciencemag. org/cgi/content/full/311/5767/1544#table). Acknowledgements • Dr. Jason Kahn • http: //www. 454. com/ • http: //www. bio. davidson. edu/courses/genomics/method/shotgun. html • http: //www. clinical-virology. org/pages/cvn/vir_all/cvn_gp_how. html • http: //medstat. med. utah. edu/block 2/biochem/Formosa/Figures/Lecture 6/6 -08%20 Sequencing%20 Modern. GIF • http: //www. cnn. com/SPECIALS/2000/genome/story/medical. implications • http: //www. nia. nih. gov/Alzheimers/Publications/geneticsfs. htm • http: //www. ornl. gov/sci/techresources/Human_Genome/posters/chromosome/cf. shtm • http: //www. sciencemag. org/cgi/content/full/311/5767/1544#table • http: //www. uvm. edu/~cgep/Education/Sequence. html • Margulies, M. , et al. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 437: 376 -80. • Ronaghi, M. , S. Katamohamed, B. Pettersson, M. Uhlen, P. Nyren. 1996. Real-time DNA sequencing using detection of pyrophosphate release. Anal. Biochem. 242: 84 -89. Voet, D. , J. Voet, C. W. Pratt. Fundamentals of Biochemistry. New York: Wiley, c 2002. Wu, H. and H. Aboleneen. 2000. Sequencing oligonucleotides with blocked termini using exonuclease digestion and electrospray mass spectrometry. Anal. Biochem. 287: 126 -135. • • • Total sequence is assembled by combining overlapping fragments (similar to shotgun sequencing). • Nucleotides flow past the wells, and incorporation is measured based on the generation of photons. Ethical Implications of Genome Sequencing • Health vs. Superiority? With the introduction of gene therapy, a new form of treatment has risen. But what happens when gene therapy shifts from treating disorders to making a healthy individual stronger, faster or smarter? • Availability? Would genome sequencing and therapy become available to everyone, rich or poor? Researchers are working towards developing the “$1000 genome, ” a reasonable price that would make genomic sequencing available to many. • Insurance? With the advent of genome sequencing, insurance companies could possibly use this technology to screen individuals before granting them coverage. If such a test reveals an individual is predisposed to a certain disorder, does an insurance company have the right to deny coverage? • Limited opportunities? Employers and educational institutions, much like insurance companies, could potentially deny access to individuals based on information found in their genomes. Should such information even be allowed for viewing by these institutions?
The Race for the $1000 Genome: Medical and Ethical Implications of Inexpensive Genome Sequencing Kathryn Fluss, Juyoung Guag, Jeff Kleinberg, Bipin Rajendran Introduction Basic nucleic acid sequencing methods primarily rely on three steps; these steps include cleaving the genome into shorter, more manageable sequences, sequencing these smaller segments and then determining the order in which these sequences make up the complete genome. The evolution of genome sequencing has occurred rapidly over the course of about 30 years as companies strive to create the “$1000 genome” a price many consider affordable enough for everyday use. The use of inexpensive genome sequencing, however, has generated widespread controversy over the ethical issues associated with its use in the medical setting. Chain-Terminator Method • • Automated Sequencing Developed by Frederick Sanger in 1974, the Chain Terminator (dideoxy) method is the most commonly used sequencing method. Four reaction mixtures are prepared for gel electrophoresis; each mixture contains the single-stranded template strand, primer, four d. NTPs, and one of four dd. NTPs. Each mixture is loaded into one of four lanes (C, G, A, T). After electrophoresis, sequence is read from bottom to top; this sequence is the complementary strand, and the sequence of the template can be determined Requires gel with sufficient resolving power (the ability to distinguish between chains that differ by a single nucleotide. ) • • Automated fluorescent dye-terminator cycle sequencing was developed by Leroy Hood in 1986 Uses the same principle of dideoxynucleotide chain termination that is applied in the Sanger Method d. NTP’s or dd. NTP’s are added in the 5’ to 3’ direction; when the dd. NTP is incorporated, the extension stops. All four bases show a different fluorescence which emits a unique wavelength. All four reactions can run in a single lane A laser detector detect emission of each wavelength from bottom and determine bases and the gene sequence is confirmed in both directions. Snake Venom Phosphodiesterase • Used by Robert Holley in 1965 to sequence 76 -residue t. RNA molecule • Removes one nucleotide at a time, starting at the 3’ end, by breaking the phosphodiester bond. The lack of a 3’OH group on a d. NTP signals the termination of chain growth as there is no free 3’OH for another nucleotide to attach New Techniques: Sequencing by Synthesis • Real time detection without electrophoresis or labeled primers. • Based on the detection of pyrophosphate that is released when a nucleotide is added. • Produces a set of fragments of all possible lengths. • The size of each fragment can be determined with mass spectrometry. • Since each fragment only differs from the fragment before it by one nucleotide, the fragments can be compared to identify the nucleotides at the 3’ ends. • Analysis is time consuming. • Can be used to characterize modified oligonucleotides. "The sequence information provides a starting point from which the real research into the thousands of diseases that have a genetic basis can begin. The sooner we can get to this starting point, the sooner we can begin to see a payoff in ultimately improving human health. ” PPi • • Developed by J. Craig Venter in 1996 Major principle is that it is random sampling; 500 -700 nucleotides are read and then assembled to construct whole genome sequence Clone is randomly sheared into small fragment (around 1 Kb) Subcloned into a cloning vector These serve as primers during PCR Amplified fragment assemble by overlapping - J. Craig Venter "We used to think that our fate was in our stars. Now we know that, in large measure, our fate is in our genes. " Francis Crick The Race to the $1000 Dollar Genome Company Format Read Length (bases) Expected Throughput Mb (million bases)/day Parallel bead array 100 96 Sequencing by ligation 50 Applied Biosystems Capillary electrophoresis 1000 Electronic microchip 20, 000 14, 000 Microchip Biotechnologies Parallel bead array 850 -1000 7 Network Biosystems Biochip 800+ 5 Nimble. Gen Systems Map and survey microarray 30 100 Parallel microchip 35 500 Single-molecule array NA • Beads are deposited into wells along with other reagents. 3 -4 LI-COR Biosciences • Shear the genome, ligate fragments to adaptors, and bind individual fragments to beads. • Perform PCR on an emulsion of the PCR mix in oil. This will allow each fragment to be amplified individually without isolating them from the mixture. 200 1000 Agencourt Bioscience Early Detection of Abnormal Genes - Cystic Fibrosis is caused by a mutation in the CFTR (cystic fibrosis transmembrane conductance regulator) gene - A single mutated gene causes other diseases such as Huntington’s disease and Duchenne muscular dystrophy, and those genes have already been identified. - Mutations in many genes believed to be a major risk factor for Alzheimer’s disease. So far the only one mutation for late-onset Alzheimer’s is at epsilon-4 position of apolipoprotein E (APo. E) gene. • People who were found with genetic disorders could alter their lifestyle to prevent disease or reduce its severity. - For example, recent studies show regular exercise helps reduce the risk of Alzheimer’s disease onset. • Directions of Future Medical Research - Genomic sequencing will be a major target for medical research for the next few decades. - Identifying a genetic link to more diseases, especially those that are caused by multiple abnormal genes. - Is the varying efficiency and side effects of drugs for each individual related to their genes? - More easily chose the best drugs for everyone - People with mutant MTHFR (methylenetetrahydrofolate reductase) may have more severe side effects to antifolate chemotherapy. Light (Detection indicates nucleotide addition) ATP 454 Life Sciences Corp. Method 454 Life Sciences Medical Implications of Genomic Sequencing luciferase • Used in an automated system developed by 454 Life Sciences Corp. that does not require cloning into bacterial vectors. Shotgun Sequencing • • sulfurylase Solexa Visi. Gen Biotechnologies A list of companies involved in the quest to create the $1000 genome sequence. Such technology must demonstrate low cost, high accuracy, the ability to read long stretches of DNA and high throughput (Source: http: //www. sciencemag. org/cgi/content/full/311/5767/1544#table). Acknowledgements • Dr. Jason Kahn • http: //www. 454. com/ • http: //www. bio. davidson. edu/courses/genomics/method/shotgun. html • http: //www. clinical-virology. org/pages/cvn/vir_all/cvn_gp_how. html • http: //medstat. med. utah. edu/block 2/biochem/Formosa/Figures/Lecture 6/6 -08%20 Sequencing%20 Modern. GIF • http: //www. cnn. com/SPECIALS/2000/genome/story/medical. implications • http: //www. nia. nih. gov/Alzheimers/Publications/geneticsfs. htm • http: //www. ornl. gov/sci/techresources/Human_Genome/posters/chromosome/cf. shtm • http: //www. sciencemag. org/cgi/content/full/311/5767/1544#table • http: //www. uvm. edu/~cgep/Education/Sequence. html • Margulies, M. , et al. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 437: 376 -80. • Ronaghi, M. , S. Katamohamed, B. Pettersson, M. Uhlen, P. Nyren. 1996. Real-time DNA sequencing using detection of pyrophosphate release. Anal. Biochem. 242: 84 -89. Voet, D. , J. Voet, C. W. Pratt. Fundamentals of Biochemistry. New York: Wiley, c 2002. Wu, H. and H. Aboleneen. 2000. Sequencing oligonucleotides with blocked termini using exonuclease digestion and electrospray mass spectrometry. Anal. Biochem. 287: 126 -135. • • • Total sequence is assembled by combining overlapping fragments (similar to shotgun sequencing). • Nucleotides flow past the wells, and incorporation is measured based on the generation of photons. Ethical Implications of Genome Sequencing • Health vs. Superiority? With the introduction of gene therapy, a new form of treatment has risen. But what happens when gene therapy shifts from treating disorders to making a healthy individual stronger, faster or smarter? • Availability? Would genome sequencing and therapy become available to everyone, rich or poor? Researchers are working towards developing the “$1000 genome, ” a reasonable price that would make genomic sequencing available to many. • Insurance? With the advent of genome sequencing, insurance companies could possibly use this technology to screen individuals before granting them coverage. If such a test reveals an individual is predisposed to a certain disorder, does an insurance company have the right to deny coverage? • Limited opportunities? Employers and educational institutions, much like insurance companies, could potentially deny access to individuals based on information found in their genomes. Should such information even be allowed for viewing by these institutions?


