bf48a3d135e7ca321e469a63af0ec95c.ppt
- Количество слайдов: 28
The Power of Information Technology Battery used in Portable Device
How important? l Tell you a Story What a fancy watch!
Wonderful!!! Only $100!!
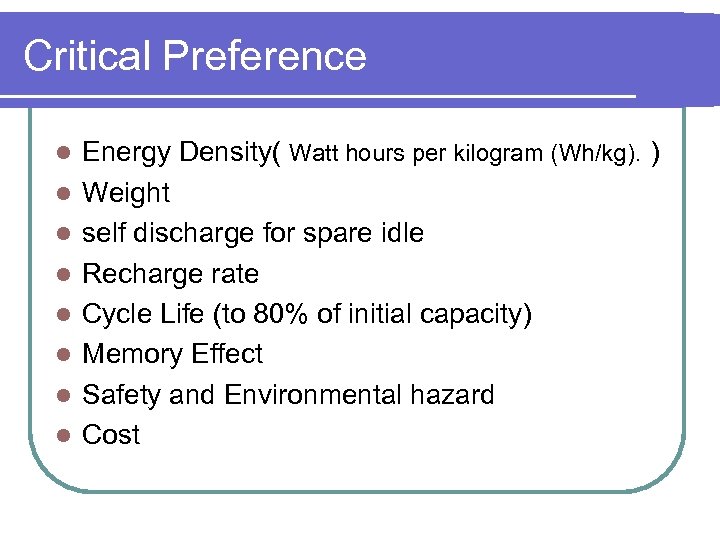
Critical Preference l l l l Energy Density( Watt hours per kilogram (Wh/kg). ) Weight self discharge for spare idle Recharge rate Cycle Life (to 80% of initial capacity) Memory Effect Safety and Environmental hazard Cost
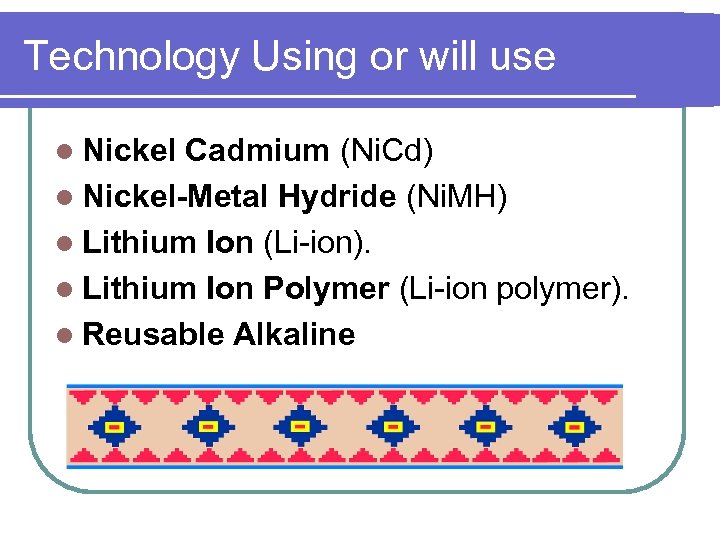
Technology Using or will use l Nickel Cadmium (Ni. Cd) l Nickel-Metal Hydride (Ni. MH) l Lithium Ion (Li-ion). l Lithium Ion Polymer (Li-ion polymer). l Reusable Alkaline
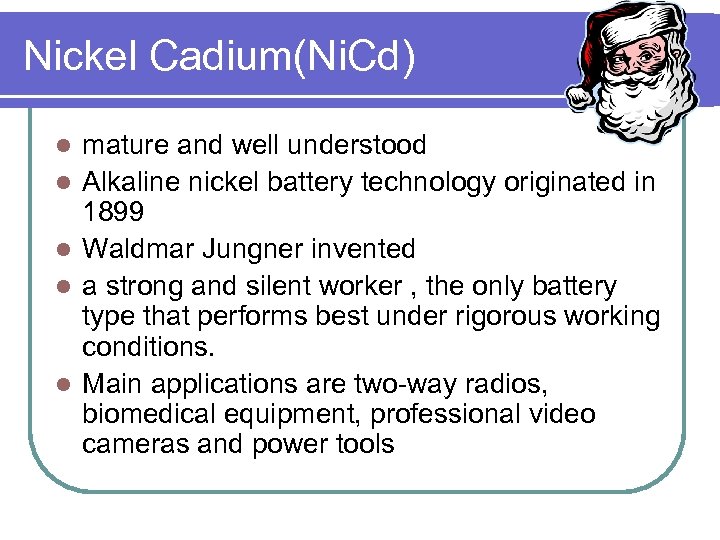
Nickel Cadium(Ni. Cd) l l l mature and well understood Alkaline nickel battery technology originated in 1899 Waldmar Jungner invented a strong and silent worker , the only battery type that performs best under rigorous working conditions. Main applications are two-way radios, biomedical equipment, professional video cameras and power tools

Nickel Cadium(Ni. Cd) l Tysonic AA Ni. Cd Battery
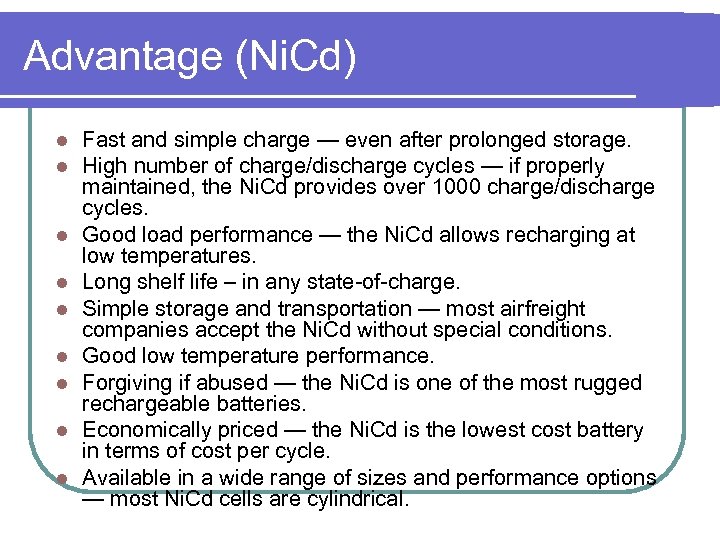
Advantage (Ni. Cd) l l l l l Fast and simple charge — even after prolonged storage. High number of charge/discharge cycles — if properly maintained, the Ni. Cd provides over 1000 charge/discharge cycles. Good load performance — the Ni. Cd allows recharging at low temperatures. Long shelf life – in any state-of-charge. Simple storage and transportation — most airfreight companies accept the Ni. Cd without special conditions. Good low temperature performance. Forgiving if abused — the Ni. Cd is one of the most rugged rechargeable batteries. Economically priced — the Ni. Cd is the lowest cost battery in terms of cost per cycle. Available in a wide range of sizes and performance options — most Ni. Cd cells are cylindrical.
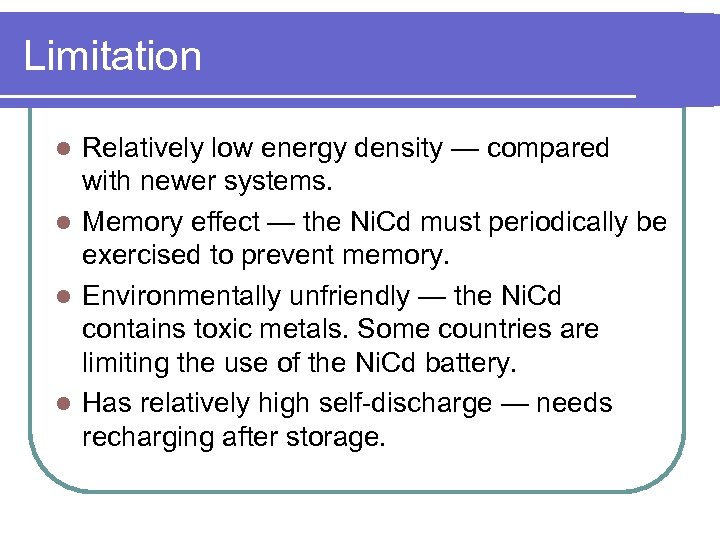
Limitation Relatively low energy density — compared with newer systems. l Memory effect — the Ni. Cd must periodically be exercised to prevent memory. l Environmentally unfriendly — the Ni. Cd contains toxic metals. Some countries are limiting the use of the Ni. Cd battery. l Has relatively high self-discharge — needs recharging after storage. l
Nickel-Metal Hydride (Ni. MH) l l l Ni. MH system started in the 1970 s Higher energy density than Ni. Cd At the expense of reduced cycle life (1/5 -1/3 of Ni. Cd) No toxic metals Similar cost of Ni. Cd mainly used for satellite , applications include mobile phones and laptop computers.

Nickel-Metal Hydride (Ni. MH) l For Nokia 100 Category: Nimh Capacity: 1200 mah Voltage : 4. 8 V Stand by: 52120 h
Advantage 30 – 40 percent higher capacity over a standard Ni. Cd. The Ni. MH has potential for yet higher energy densities. l Less prone to memory than the Ni. Cd. Periodic exercise cycles are required less often. l Simple storage and transportation — transportation conditions are not subject to regulatory control. l Environmentally friendly — contains only mild toxins; profitable for recycling. l
Limitation l l l l Limited service life — if repeatedly deep cycled, especially at high load currents, the performance starts to deteriorate after 200 to 300 cycles. Shallow rather than deep discharge cycles are preferred. Limited discharge current — although a Ni. MH battery is capable of delivering high discharge currents, repeated discharges with high load currents reduces the battery’s cycle life. Best results are achieved with load currents of 0. 2 C to 0. 5 C (one-fifth to one-half of the rated capacity). More complex charge algorithm needed — the Ni. MH generates more heat during charge and requires a longer charge time than the Ni. Cd. The trickle charge is critical and must be controlled carefully. High self-discharge — the Ni. MH has about 50 percent higher self-discharge compared to the Ni. Cd. New chemical additives improve the self-discharge but at the expense of lower energy density. Performance degrades if stored at elevated temperatures — the Ni. MH should be stored in a cool place and at a state-of-charge of about 40 percent. High maintenance — battery requires regular full discharge to prevent crystalline formation. About 20 percent more expensive than Ni. Cd — Ni. MH batteries designed for high current draw are more expensive than the regular version.
Lithium Ion (Li-ion) l l l Pioneer work with the lithium battery began in 1912 under G. N. Lewis early 1970 s that the first non-rechargeable lithium batteries became commercially available. fastest growing battery system Li-ion is used where high-energy density and light weight is of prime importance more expensive than other systems and must follow strict guidelines to assure safety. Applications include notebook computers and cellular phones.

l For Nokia 3310/3390 Category: Li-ion Capacity: 950 mah Voltage : 3. 6 V Stand by: 72 -120 h
Advantage l High energy density — potential for yet higher capacities. l Relatively low self-discharge — selfdischarge is less than half that of Ni. Cd and Ni. MH. l Low Maintenance — no periodic discharge is needed; no memory.
Limitation l l l Requires protection circuit — protection circuit limits voltage and current. Battery is safe if not provoked. Subject to aging, even if not in use — storing the battery in a cool place and at 40 percent state-of-charge reduces the aging effect. Moderate discharge current. Subject to transportation regulations — shipment of larger quantities of Li-ion batteries may be subject to regulatory control. This restriction does not apply to personal carry-on batteries. Expensive to manufacture — about 40 percent higher in cost than Ni. Cd. Better manufacturing techniques and replacement of rare metals with lower cost alternatives will likely reduce the price. Not fully mature — changes in metal and chemical combinations affect battery test results, especially with some quick test methods.
The Lithium Polymer Battery differentiates itself from other battery systems in the type of electrolyte used l The polymer electrolyte replaces the traditional porous separator, which is soaked with electrolyte l no danger of flammability , suffers from poor conductivity l A dry solid Li-polymer version is expected to be commercially available by 2005. l
The Lithium Polymer Battery (Cont. ) some Li-polymers are used as standby batteries in hot climates l unique in that it uses a solid electrolyte, replacing the porous separator. The gelled electrolyte is simply added to enhance ion conductivity. l postponement, as some critics argue, is due to ‘cashing in’ on the Li-ion battery l compelled mobile phone manufacturers to use this promising technology for their new generation handsets. l

The Lithium Polymer Battery (Cont. ) l Animal Cam From Ultralife
Advantage l l l l Very low profile — batteries that resemble the profile of a credit card are feasible. Flexible form factor — manufacturers are not bound by standard cell formats. With high volume, any reasonable size can be produced economically. Light weight – gelled rather than liquid electrolytes enable simplified packaging, in some cases eliminating the metal shell. Improved safety — more resistant to overcharge; less chance for electrolyte leakage Leakage proof (no free electrolyte) High energy density Long cycle life Environmentally friendly
Limitation l Lower energy density and decreased cycle count compared to Li-ion — potential for improvements exist. l Expensive to manufacture — once massproduced, the Li-ion polymer has the potential for lower cost. Reduced control circuit offsets higher manufacturing costs.
Reusable Alkaline Batteries l replaces disposable household batteries; l suitable for low-power applications. l Its limited cycle life is compensated by low self-discharge, l ideal for portable entertainment devices and flashlights.

Reusable Alkaline Batteries(Cont. ) l Renewal Reusable Alkaline Batteries • Long life alkaline power that is reusable— 25 times or more! • The most environmentally responsible battery available • Charge in Rayovac Renewal Battery Rechargers (RAYPS 1 R/RAYPS 3 R) sold separately
Advantage l l l Inexpensive and readily available — can be used as a direct replacement of non-rechargeable (primary) cells. More economical than non-rechargeable – allows several recharges. Low self-discharge — can be stored as a standby battery for up to 10 years. Environmentally friendly — no toxic metals used, fewer batteries are discarded, reduces landfill. Maintenance free — no need for cycling; no memory
Limitation l Limited current handling — suited for light-duty applications like portable home entertainment, flashlights. l Limited cycle life — for best results, recharge before the battery gets too low.
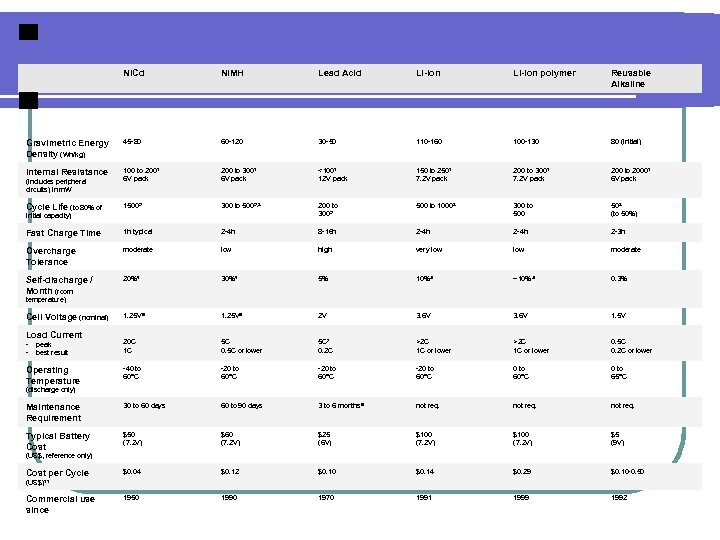
Ni. Cd Ni. MH Lead Acid Li-ion polymer Reusable Alkaline Gravimetric Energy Density (Wh/kg) 45 -80 60 -120 30 -50 110 -160 100 -130 80 (initial) Internal Resistance 100 to 2001 6 V pack 200 to 3001 6 V pack <1001 12 V pack 150 to 2501 7. 2 V pack 200 to 3001 7. 2 V pack 200 to 20001 6 V pack 15002 300 to 5002, 3 200 to 3002 500 to 10003 300 to 500 503 (to 50%) Fast Charge Time 1 h typical 2 -4 h 8 -16 h 2 -4 h 2 -3 h Overcharge Tolerance moderate low high very low moderate Self-discharge / Month (room 20%4 30%4 5% 10%5 ~10%5 0. 3% 1. 25 V 6 2 V 3. 6 V 1. 5 V - peak - best result 20 C 1 C 5 C 0. 5 C or lower 5 C 7 0. 2 C >2 C 1 C or lower 0. 5 C 0. 2 C or lower Operating Temperature -40 to 60°C -20 to 60°C 0 to 65°C Maintenance Requirement 30 to 60 days 60 to 90 days 3 to 6 months 9 not req. Typical Battery Cost $50 (7. 2 V) $60 (7. 2 V) $25 (6 V) $100 (7. 2 V) $5 (9 V) $0. 04 $0. 12 $0. 10 $0. 14 $0. 29 $0. 10 -0. 50 1990 1970 1991 1999 1992 (includes peripheral circuits) in m. W Cycle Life (to 80% of initial capacity) temperature) Cell Voltage (nominal) Load Current (discharge only) (US$, reference only) Cost per Cycle (US$)11 Commercial use since

l. Thank You
bf48a3d135e7ca321e469a63af0ec95c.ppt