Презентация детей.ppt
- Количество слайдов: 21
 The physiochemical method to compare effectiveness of drugs applied to treat urolithiasis Ilya Rusak, Ivan Sergienko Gomel State Medical University, Belarus
The physiochemical method to compare effectiveness of drugs applied to treat urolithiasis Ilya Rusak, Ivan Sergienko Gomel State Medical University, Belarus
 General information Urolithiasis is the process of forming stones in the kidney, bladder, and/or urethra (urinary tract). Kidney stones occur in 1 in 20 people at some time in their life. Passage of a urinary stone is the most common cause of acute ureteral obstruction and affects as many as 12% of the population.
General information Urolithiasis is the process of forming stones in the kidney, bladder, and/or urethra (urinary tract). Kidney stones occur in 1 in 20 people at some time in their life. Passage of a urinary stone is the most common cause of acute ureteral obstruction and affects as many as 12% of the population.
 The pain may be some of the most severe pain that humans experience, and complications of stone disease may result in severe infection; renal failure; or, in rare cases, death.
The pain may be some of the most severe pain that humans experience, and complications of stone disease may result in severe infection; renal failure; or, in rare cases, death.
 General information The development of the stones is related to decreased urine volume or increased excretion of stoneforming components such as calcium, oxalate, urate, cystine, xanthine, and phosphate. The stones form in the urine collecting area (the pelvis) of the kidney and may range in size from tiny to staghorn stones the size of the renal pelvis itself.
General information The development of the stones is related to decreased urine volume or increased excretion of stoneforming components such as calcium, oxalate, urate, cystine, xanthine, and phosphate. The stones form in the urine collecting area (the pelvis) of the kidney and may range in size from tiny to staghorn stones the size of the renal pelvis itself.
 General information Calcium stones account for 7585% of urinary stones. Approximately one half of calcium stones are composed of a mixture of calcium oxalate and calcium phosphate. Approximately three eighths of calcium stones are formed of only calcium oxalate dihydrate. The remaining one eighth of stones are composed of calcium phosphate (apatite) or calcium monohydrate. Uric acid stones account for 5 -10% of urinary stones. They are small and smooth.
General information Calcium stones account for 7585% of urinary stones. Approximately one half of calcium stones are composed of a mixture of calcium oxalate and calcium phosphate. Approximately three eighths of calcium stones are formed of only calcium oxalate dihydrate. The remaining one eighth of stones are composed of calcium phosphate (apatite) or calcium monohydrate. Uric acid stones account for 5 -10% of urinary stones. They are small and smooth.
 Key problem The colloidal chemistry defines urolithiasis as the result of decrease in colloidal stability of calcium salts dispersions in urea. Effects of some therapeutic agents can be explained in the following terms: they stabilize calcium oxalates, phosphates and urates in biological fluids thus preventing their crystallization.
Key problem The colloidal chemistry defines urolithiasis as the result of decrease in colloidal stability of calcium salts dispersions in urea. Effects of some therapeutic agents can be explained in the following terms: they stabilize calcium oxalates, phosphates and urates in biological fluids thus preventing their crystallization.
 Purpose The aim of our investigation was to develop the new physiochemical method to compare effectiveness of medicines that are applied in clinical practice to prevent the formation of kidney stones. The experiments were fulfilled in vitro thus a proposed model is a simplified model of those processes proceeding in vivo. Practical application The results made possible to give quantitative characteristics to effectiveness of some drugs that are applied to prevent and treat urolithiasis.
Purpose The aim of our investigation was to develop the new physiochemical method to compare effectiveness of medicines that are applied in clinical practice to prevent the formation of kidney stones. The experiments were fulfilled in vitro thus a proposed model is a simplified model of those processes proceeding in vivo. Practical application The results made possible to give quantitative characteristics to effectiveness of some drugs that are applied to prevent and treat urolithiasis.
 Materials and methods The medicines on study were subdivided into several categories: • α-amino acids (methionine), • vitamins A, E, C (antioxicaps), • medicines of plant origin (prolyte and cyston), • allopurinol.
Materials and methods The medicines on study were subdivided into several categories: • α-amino acids (methionine), • vitamins A, E, C (antioxicaps), • medicines of plant origin (prolyte and cyston), • allopurinol.
 Materials and methods We added the daily therapeutic doses of the medicines to the colloidal solutions prepared by ultrasonic degradation of kidney stones, and studied the dynamics of their coagulation initiated by the electrolyte (Na 2 HPO 4).
Materials and methods We added the daily therapeutic doses of the medicines to the colloidal solutions prepared by ultrasonic degradation of kidney stones, and studied the dynamics of their coagulation initiated by the electrolyte (Na 2 HPO 4).
 Materials and methods The coagulating process was examined by photometric measuring of turbidity in solutions after the addition of definite amounts of a coagulator. To some extent the turbidity coefficient characterized the rate of calcium phosphates, urates and oxalates sedimentation.
Materials and methods The coagulating process was examined by photometric measuring of turbidity in solutions after the addition of definite amounts of a coagulator. To some extent the turbidity coefficient characterized the rate of calcium phosphates, urates and oxalates sedimentation.
 Materials and methods We represented the turbidity coefficient as a function of the amount of electrolytecoagulator and, using this graph, calculated the critical coagulation concentrations of a coagulator (c. c. c) and the rate constants of the slow step of coagulation. C. c. c is a minimal amount of an electrolyte which starts coagulation in 1 L of a sol.
Materials and methods We represented the turbidity coefficient as a function of the amount of electrolytecoagulator and, using this graph, calculated the critical coagulation concentrations of a coagulator (c. c. c) and the rate constants of the slow step of coagulation. C. c. c is a minimal amount of an electrolyte which starts coagulation in 1 L of a sol.
 Materials and methods • с ×V • c. c. c = • Vs • с – concentration of an electrolyte (mol/L) • V – volume of an electrolyte solution (L) • Vs – volume of a sol (L)
Materials and methods • с ×V • c. c. c = • Vs • с – concentration of an electrolyte (mol/L) • V – volume of an electrolyte solution (L) • Vs – volume of a sol (L)
 Materials and methods Both parameters can be used as the creteria for colloidal stability of solutions that are simplified models of biological fluids (blood and urea). The greater c. c. c and smaller rate constants of coagulation the higher is colloidal stability of solutions, and lower is a thread of stones precipitation.
Materials and methods Both parameters can be used as the creteria for colloidal stability of solutions that are simplified models of biological fluids (blood and urea). The greater c. c. c and smaller rate constants of coagulation the higher is colloidal stability of solutions, and lower is a thread of stones precipitation.
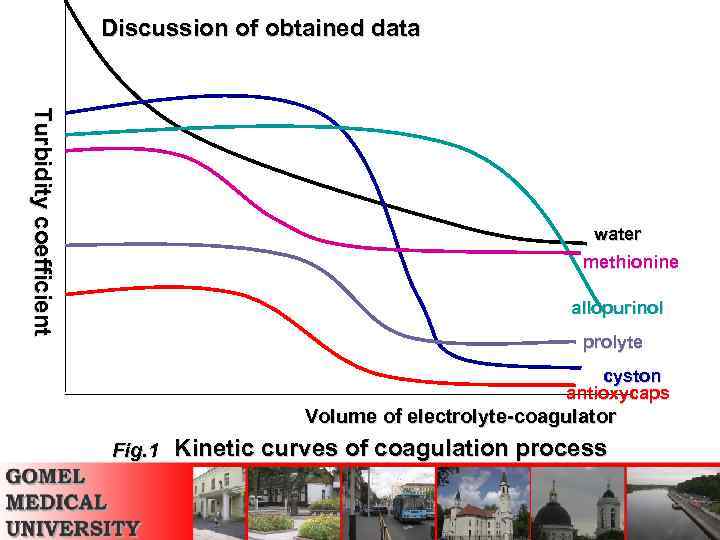 Discussion of obtained data Turbidity coefficient water methionine allopurinol prolyte cyston antioxycaps Volume of electrolyte-coagulator Fig. 1 Kinetic curves of coagulation process
Discussion of obtained data Turbidity coefficient water methionine allopurinol prolyte cyston antioxycaps Volume of electrolyte-coagulator Fig. 1 Kinetic curves of coagulation process
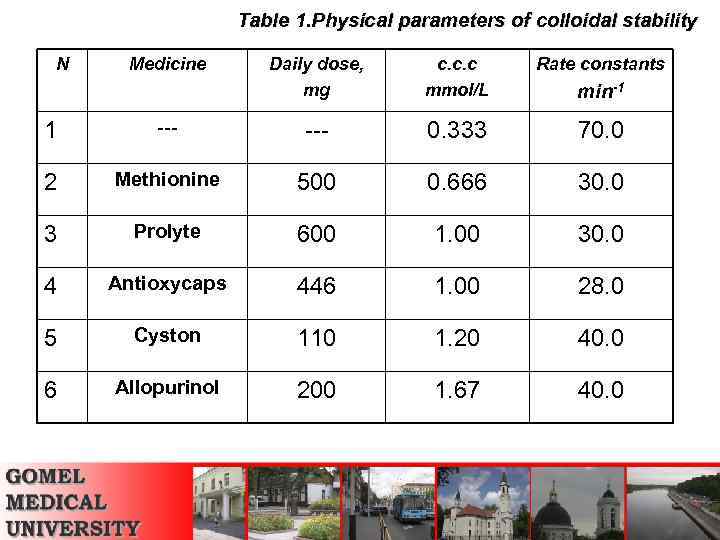 Table 1. Physical parameters of colloidal stability N Medicine Daily dose, mg c. c. c mmol/L Rate constants min-1 1 --- 0. 333 70. 0 2 Methionine 500 0. 666 30. 0 3 Prolyte 600 1. 00 30. 0 4 Antioxycaps 446 1. 00 28. 0 5 Cyston 110 1. 20 40. 0 6 Allopurinol 200 1. 67 40. 0
Table 1. Physical parameters of colloidal stability N Medicine Daily dose, mg c. c. c mmol/L Rate constants min-1 1 --- 0. 333 70. 0 2 Methionine 500 0. 666 30. 0 3 Prolyte 600 1. 00 30. 0 4 Antioxycaps 446 1. 00 28. 0 5 Cyston 110 1. 20 40. 0 6 Allopurinol 200 1. 67 40. 0
 The obtained data revealed that: • All examined medicines increased c. c. c of coagulating agents thus they all raise the colloidal stability of calcium salts in the model solutions. For example, the c. c. c of colloidal solution which was not stabilized by a medicine is less than 0. 33 mmol/L, while the c. c. c values for solutions that contained medicines were in the range 0. 67 -1. 67 mmol/L.
The obtained data revealed that: • All examined medicines increased c. c. c of coagulating agents thus they all raise the colloidal stability of calcium salts in the model solutions. For example, the c. c. c of colloidal solution which was not stabilized by a medicine is less than 0. 33 mmol/L, while the c. c. c values for solutions that contained medicines were in the range 0. 67 -1. 67 mmol/L.
 The obtained data revealed that: • Allopurinol exhibited the maximum c. c. c value (1. 67 mmol/L) thus being the most effective in preventing kidney stones precipitation, and methionine was proved to be less effective (0. 67 mmol/L) but still the stability of its solutions is twice greater than that in a not stabilized systems.
The obtained data revealed that: • Allopurinol exhibited the maximum c. c. c value (1. 67 mmol/L) thus being the most effective in preventing kidney stones precipitation, and methionine was proved to be less effective (0. 67 mmol/L) but still the stability of its solutions is twice greater than that in a not stabilized systems.
 The obtained data revealed that: The rate constants of coagulation took the following values (min-1): allopurinol and cyston – 40; prolyte and methionine – 30; antioxycaps – 28. The rate constant of a process that occurred without a medicine is much greater and is equal to 70 min-1. Thus we came to belief that anti urolithiasis medicines retard the rate of coagulation preventing the formation of kidney stones.
The obtained data revealed that: The rate constants of coagulation took the following values (min-1): allopurinol and cyston – 40; prolyte and methionine – 30; antioxycaps – 28. The rate constant of a process that occurred without a medicine is much greater and is equal to 70 min-1. Thus we came to belief that anti urolithiasis medicines retard the rate of coagulation preventing the formation of kidney stones.
 The obtained data revealed that: There is a linkage between c. c. c of an electrolyte-coagulator and rate constants of the coagulating process: the medicines that increase c. c. c simultaneously decrease the rate of stones coagulation from model solutions. Thus the anti urolithiasis medicines on study proved their effectiveness in prevention of stones in kidneys.
The obtained data revealed that: There is a linkage between c. c. c of an electrolyte-coagulator and rate constants of the coagulating process: the medicines that increase c. c. c simultaneously decrease the rate of stones coagulation from model solutions. Thus the anti urolithiasis medicines on study proved their effectiveness in prevention of stones in kidneys.
 Conclusion The new physiochemical method to compare effectiveness of drugs applied to treat urolithiasis was developed. The photometric determination of turbidity in colloidal solutions after their coagulation gave an opportunity to calculate the physical parameters that characterize the ability of medicines to prevent the kidney stones formation.
Conclusion The new physiochemical method to compare effectiveness of drugs applied to treat urolithiasis was developed. The photometric determination of turbidity in colloidal solutions after their coagulation gave an opportunity to calculate the physical parameters that characterize the ability of medicines to prevent the kidney stones formation.
 Thank you very much!!!
Thank you very much!!!


