51c8984fdfd1566fcee4a36247af0286.ppt
- Количество слайдов: 96
 The physics of Radiation Therapy, pp. 45 - 70 Chapter 4 Clinical Radiation Generators 1
The physics of Radiation Therapy, pp. 45 - 70 Chapter 4 Clinical Radiation Generators 1
 1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 2
1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 2
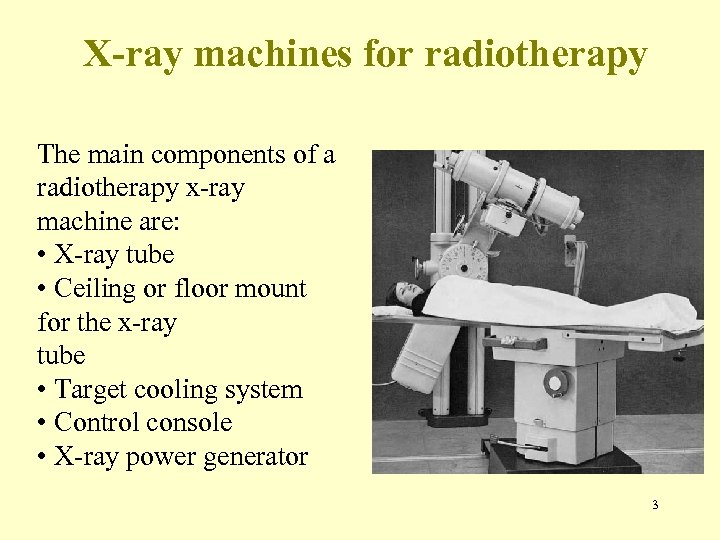 X-ray machines for radiotherapy The main components of a radiotherapy x-ray machine are: • X-ray tube • Ceiling or floor mount for the x-ray tube • Target cooling system • Control console • X-ray power generator 3
X-ray machines for radiotherapy The main components of a radiotherapy x-ray machine are: • X-ray tube • Ceiling or floor mount for the x-ray tube • Target cooling system • Control console • X-ray power generator 3
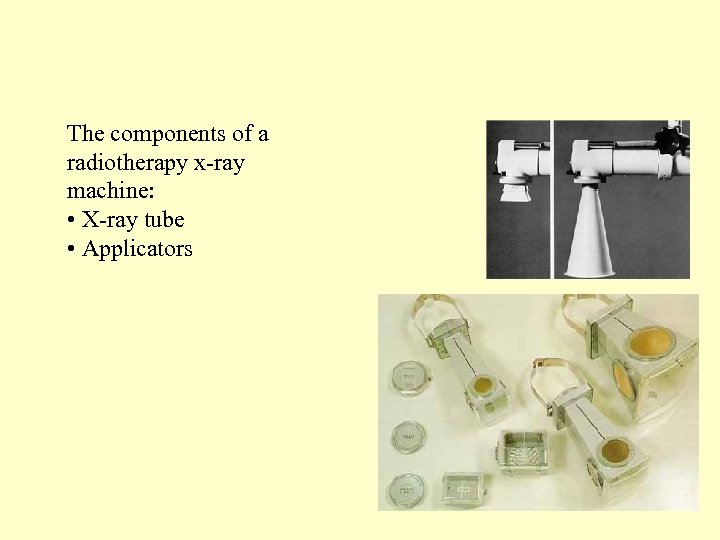 The components of a radiotherapy x-ray machine: • X-ray tube • Applicators 4
The components of a radiotherapy x-ray machine: • X-ray tube • Applicators 4
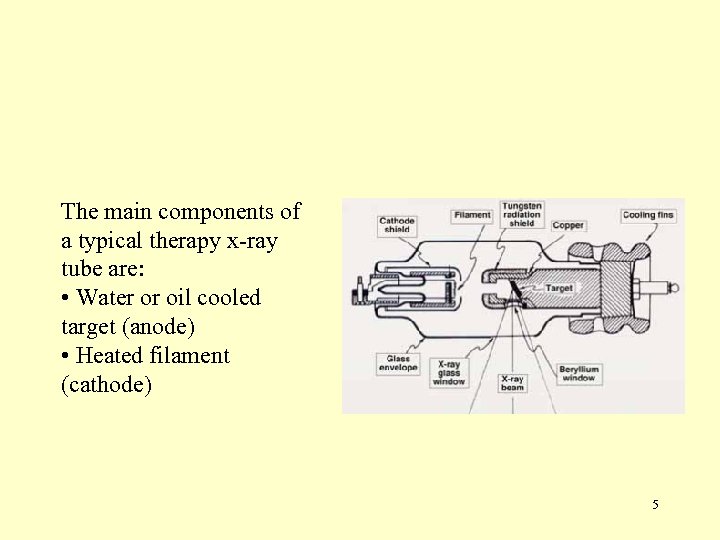 The main components of a typical therapy x-ray tube are: • Water or oil cooled target (anode) • Heated filament (cathode) 5
The main components of a typical therapy x-ray tube are: • Water or oil cooled target (anode) • Heated filament (cathode) 5
 X-ray machines for radiotherapy With x-ray tubes the patient dose is delivered using a timer and the treatment time must incorporate a shutter correction time. comparison with diagnostic radiology x-ray tubes, a In therapy x-ray tube operates: • At about 10% of instantaneous current. • At about 10 times average energy input. • With significantly larger focal spot and a fixed rather than rotating anode. 6
X-ray machines for radiotherapy With x-ray tubes the patient dose is delivered using a timer and the treatment time must incorporate a shutter correction time. comparison with diagnostic radiology x-ray tubes, a In therapy x-ray tube operates: • At about 10% of instantaneous current. • At about 10 times average energy input. • With significantly larger focal spot and a fixed rather than rotating anode. 6
 Kilovoltage Units • Up to above 1950 • X-rays generated at voltages up to 300 k. Vps • Still some use in the present era, esp. treatment of superficial skin lesions • Kilovoltage Therapy – – – Grenz-Ray Therapy Contact Therapy Superficial Therapy Orthovoltage Therapy or Deep Therapy Supervoltage Therapy 7
Kilovoltage Units • Up to above 1950 • X-rays generated at voltages up to 300 k. Vps • Still some use in the present era, esp. treatment of superficial skin lesions • Kilovoltage Therapy – – – Grenz-Ray Therapy Contact Therapy Superficial Therapy Orthovoltage Therapy or Deep Therapy Supervoltage Therapy 7
 Kilovoltage Units • Grenz-Ray Therapy – Energy : < 20 k. V – Very low depth of penetration – No longer used in R/T • Contact Therapy – – – Energy: 40 – 50 k. V Short SSD (< 2 cm) Produces a very rapidly decreasing depth dose Max irradiated tissue : skin surface Application: Tumor not deeper than 1 – 2 mm 8
Kilovoltage Units • Grenz-Ray Therapy – Energy : < 20 k. V – Very low depth of penetration – No longer used in R/T • Contact Therapy – – – Energy: 40 – 50 k. V Short SSD (< 2 cm) Produces a very rapidly decreasing depth dose Max irradiated tissue : skin surface Application: Tumor not deeper than 1 – 2 mm 8
 Kilovoltage Units • Superficial Therapy – – – Energy: 50 – 150 k. V HVLs: 1. 0 - – 8. 0 -mm Al Applicator or cone attached to the diaphragm SSD: 15 – 20 cm Tube current: 5 – 8 m. A Application: tumors confined to about 5 -mm depth 9
Kilovoltage Units • Superficial Therapy – – – Energy: 50 – 150 k. V HVLs: 1. 0 - – 8. 0 -mm Al Applicator or cone attached to the diaphragm SSD: 15 – 20 cm Tube current: 5 – 8 m. A Application: tumors confined to about 5 -mm depth 9
 Kilovoltage Units • Orthovoltage Therapy or Deep Therapy – – Energy: 200 – 300 k. V Tube current: 10 – 20 m. A HVLs: 1 – 4 mm Cu Cones or movable diaphragm (continuous adjustable field size) – SSD: 50 cm – Application: tumor located < 2 – 3 cm in depth – Limitation of the treatment: • skin dose • Depth dose distribution • Increase absorbed dose in bone 10
Kilovoltage Units • Orthovoltage Therapy or Deep Therapy – – Energy: 200 – 300 k. V Tube current: 10 – 20 m. A HVLs: 1 – 4 mm Cu Cones or movable diaphragm (continuous adjustable field size) – SSD: 50 cm – Application: tumor located < 2 – 3 cm in depth – Limitation of the treatment: • skin dose • Depth dose distribution • Increase absorbed dose in bone 10
 Kilovoltage Units • Supervoltage Therapy – Energy: 500 – 1000 k. V – Technical problem • Insulating the high-voltage transformer • Conventional transformer systems were not suitable for producing potential > 300 k. Vp – The problem solved by invention of resonant transformer 11
Kilovoltage Units • Supervoltage Therapy – Energy: 500 – 1000 k. V – Technical problem • Insulating the high-voltage transformer • Conventional transformer systems were not suitable for producing potential > 300 k. Vp – The problem solved by invention of resonant transformer 11
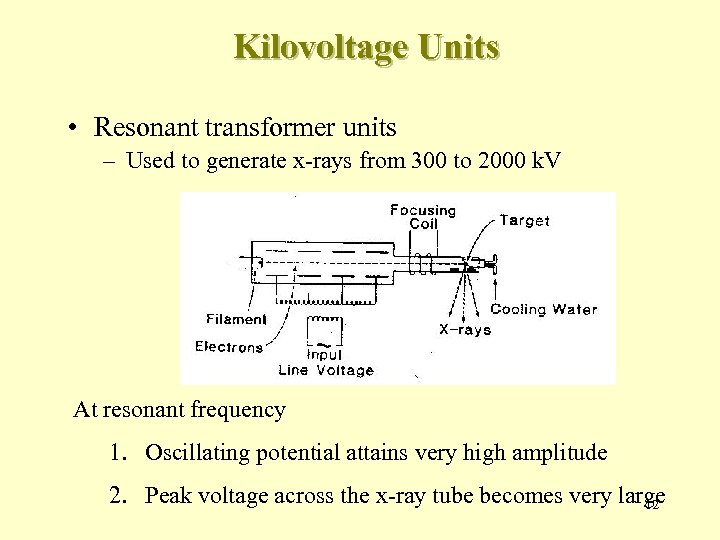 Kilovoltage Units • Resonant transformer units – Used to generate x-rays from 300 to 2000 k. V At resonant frequency 1. Oscillating potential attains very high amplitude 2. Peak voltage across the x-ray tube becomes very large 12
Kilovoltage Units • Resonant transformer units – Used to generate x-rays from 300 to 2000 k. V At resonant frequency 1. Oscillating potential attains very high amplitude 2. Peak voltage across the x-ray tube becomes very large 12
 Megavoltage Therapy • X-ray beams of energy > 1 MV • Accelerators or γray produced by radionuclides • Examples of clinical megavoltage machines – – – Van de Graaff generator Linear accelerator Betatron Microtron Teletherapy γray units (e. g. cobalt-60) 13
Megavoltage Therapy • X-ray beams of energy > 1 MV • Accelerators or γray produced by radionuclides • Examples of clinical megavoltage machines – – – Van de Graaff generator Linear accelerator Betatron Microtron Teletherapy γray units (e. g. cobalt-60) 13
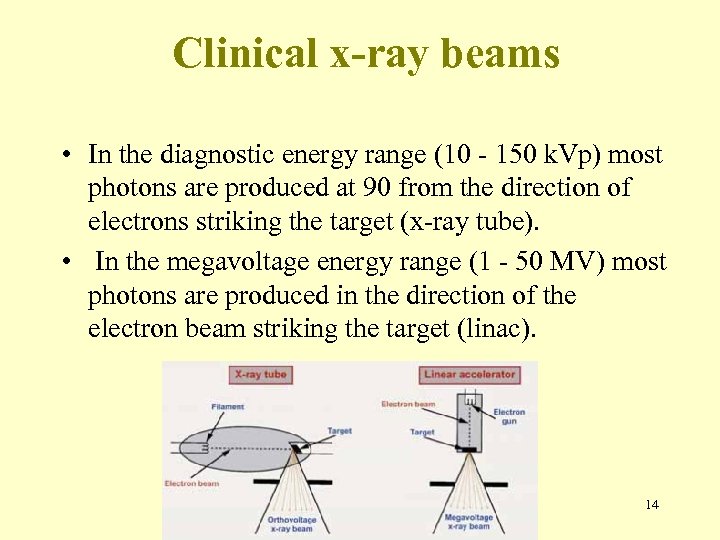 Clinical x-ray beams • In the diagnostic energy range (10 - 150 k. Vp) most photons are produced at 90 from the direction of electrons striking the target (x-ray tube). • In the megavoltage energy range (1 - 50 MV) most photons are produced in the direction of the electron beam striking the target (linac). 14
Clinical x-ray beams • In the diagnostic energy range (10 - 150 k. Vp) most photons are produced at 90 from the direction of electrons striking the target (x-ray tube). • In the megavoltage energy range (1 - 50 MV) most photons are produced in the direction of the electron beam striking the target (linac). 14
 1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 15
1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 15
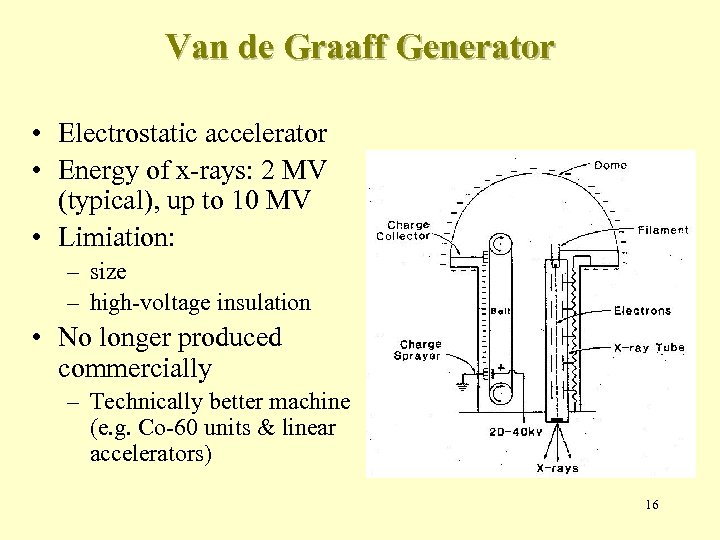 Van de Graaff Generator • Electrostatic accelerator • Energy of x-rays: 2 MV (typical), up to 10 MV • Limiation: – size – high-voltage insulation • No longer produced commercially – Technically better machine (e. g. Co-60 units & linear accelerators) 16
Van de Graaff Generator • Electrostatic accelerator • Energy of x-rays: 2 MV (typical), up to 10 MV • Limiation: – size – high-voltage insulation • No longer produced commercially – Technically better machine (e. g. Co-60 units & linear accelerators) 16
 1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 17
1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 17
 LINACS Medical linacs are cyclic accelerators that accelerate electrons to kinetic energies from 4 to 25 Me. V using microwave radiofrequency fields: • 103 MHz : L band • 2856 MHz: S band • 104 MHz: X band a linac the electrons are accelerated following straight In trajectories in special evacuated structures called accelerating waveguides. 18
LINACS Medical linacs are cyclic accelerators that accelerate electrons to kinetic energies from 4 to 25 Me. V using microwave radiofrequency fields: • 103 MHz : L band • 2856 MHz: S band • 104 MHz: X band a linac the electrons are accelerated following straight In trajectories in special evacuated structures called accelerating waveguides. 18
 Linac generations During the past 40 years medical linacs have gone through five distinct generations, each one increasingly more sophisticated: (1) Low energy x rays (4 -6 MV) (2) Medium energy x rays (10 -15 MV) and electrons (3) High energy x rays (18 -25 MV) and electrons (4) Computer controlled dual energy linac with electrons (5) Computer controlled dual energy linac with electrons combined with intensity modulation 19
Linac generations During the past 40 years medical linacs have gone through five distinct generations, each one increasingly more sophisticated: (1) Low energy x rays (4 -6 MV) (2) Medium energy x rays (10 -15 MV) and electrons (3) High energy x rays (18 -25 MV) and electrons (4) Computer controlled dual energy linac with electrons (5) Computer controlled dual energy linac with electrons combined with intensity modulation 19
 Linear Accelerator • Use high frequency electromagnetic waves to acelerate charged particles (e. g. electrons) to high energies through a linear tube • High-energy electron beam – treating superficial tumors • X-rays – treating deep-seated tumors 20
Linear Accelerator • Use high frequency electromagnetic waves to acelerate charged particles (e. g. electrons) to high energies through a linear tube • High-energy electron beam – treating superficial tumors • X-rays – treating deep-seated tumors 20
 Linear Accelerator • Types of EM wave 1. Traveling EM wave • • Required a terminating (“dummy”) load to absorb the residual power at the end of the structure Prevent backward reflection wave 2. Standing EM wave • • • Combination of forward and reverse traveling waves More efficiency – Axial beam transport cavities and the side cavities can be independently optimized More expensive – Requires installation of a circulator (or insulator) between the power source – the structure prevent reflections from reaching the power source 21
Linear Accelerator • Types of EM wave 1. Traveling EM wave • • Required a terminating (“dummy”) load to absorb the residual power at the end of the structure Prevent backward reflection wave 2. Standing EM wave • • • Combination of forward and reverse traveling waves More efficiency – Axial beam transport cavities and the side cavities can be independently optimized More expensive – Requires installation of a circulator (or insulator) between the power source – the structure prevent reflections from reaching the power source 21
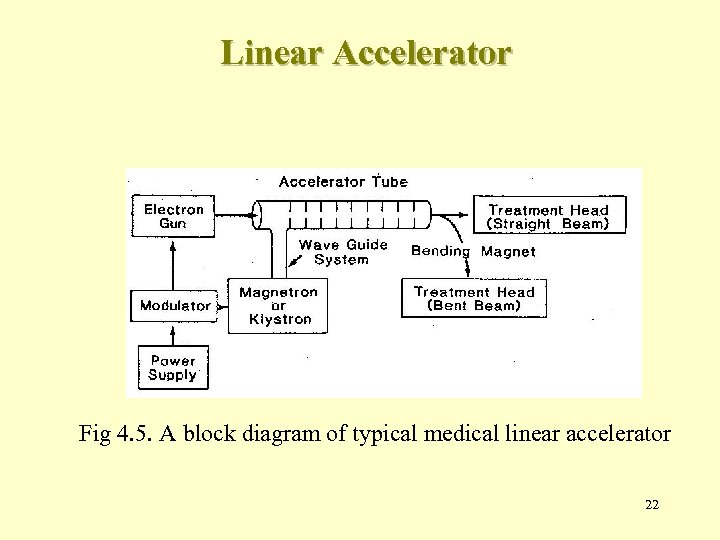 Linear Accelerator Fig 4. 5. A block diagram of typical medical linear accelerator 22
Linear Accelerator Fig 4. 5. A block diagram of typical medical linear accelerator 22
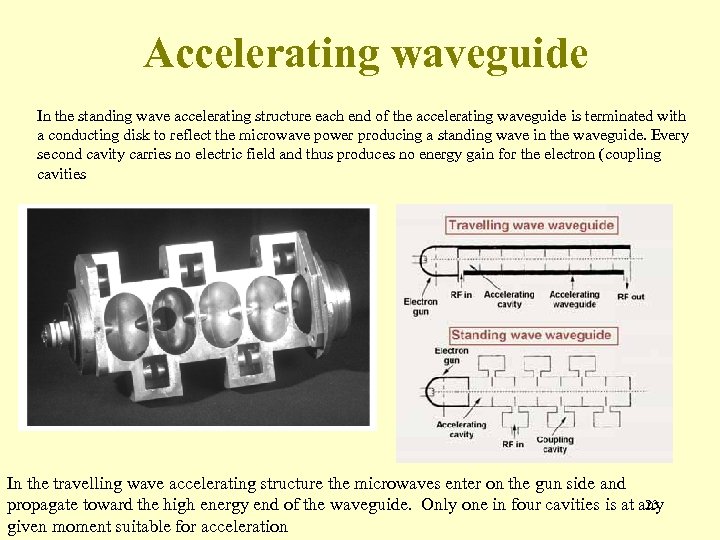 Accelerating waveguide In the standing wave accelerating structure each end of the accelerating waveguide is terminated with a conducting disk to reflect the microwave power producing a standing wave in the waveguide. Every second cavity carries no electric field and thus produces no energy gain for the electron (coupling cavities In the travelling wave accelerating structure the microwaves enter on the gun side and 23 propagate toward the high energy end of the waveguide. Only one in four cavities is at any given moment suitable for acceleration
Accelerating waveguide In the standing wave accelerating structure each end of the accelerating waveguide is terminated with a conducting disk to reflect the microwave power producing a standing wave in the waveguide. Every second cavity carries no electric field and thus produces no energy gain for the electron (coupling cavities In the travelling wave accelerating structure the microwaves enter on the gun side and 23 propagate toward the high energy end of the waveguide. Only one in four cavities is at any given moment suitable for acceleration
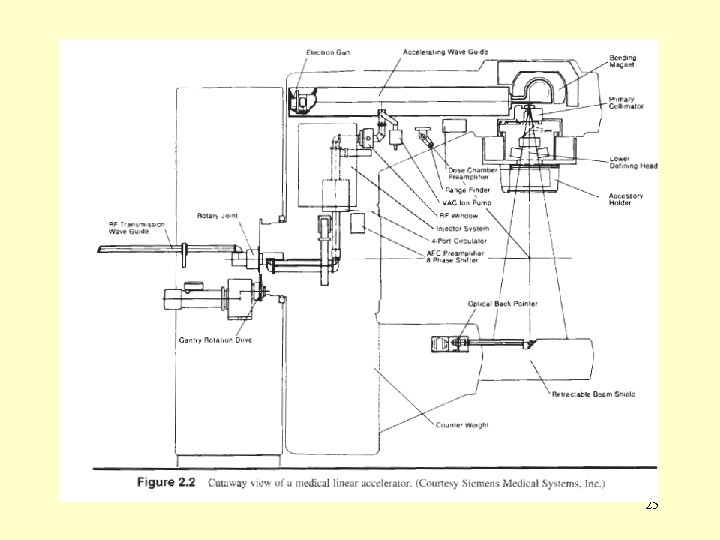
 Microwave power transmission microwave power produced by the RF generator is The carried to the accelerating waveguide through rectangular uniform waveguides usually pressurized with a dielectric gas (freon or sulphur hexafluoride SF 6). Between the RF generator and the accelerating waveguide is a circulator (isolator) which transmits the RF power from the RF generator to the accelerating waveguide but does not transmit microwaves in the opposite direction. 24
Microwave power transmission microwave power produced by the RF generator is The carried to the accelerating waveguide through rectangular uniform waveguides usually pressurized with a dielectric gas (freon or sulphur hexafluoride SF 6). Between the RF generator and the accelerating waveguide is a circulator (isolator) which transmits the RF power from the RF generator to the accelerating waveguide but does not transmit microwaves in the opposite direction. 24
 25
25
 The Magnetron • A device that produces microwaves • Functions as a high-power oscillator • Generating microwave pulses of several microseconds with repetition rate of several hundred pulses per second • Frequency of microwave within each pulse is about 3000 MHz • Peak power output: – 2 MW (for low-energy linacs, 6 MV or less) – 5 MW (for higher-energy linacs, mostly use klystrons) 26
The Magnetron • A device that produces microwaves • Functions as a high-power oscillator • Generating microwave pulses of several microseconds with repetition rate of several hundred pulses per second • Frequency of microwave within each pulse is about 3000 MHz • Peak power output: – 2 MW (for low-energy linacs, 6 MV or less) – 5 MW (for higher-energy linacs, mostly use klystrons) 26
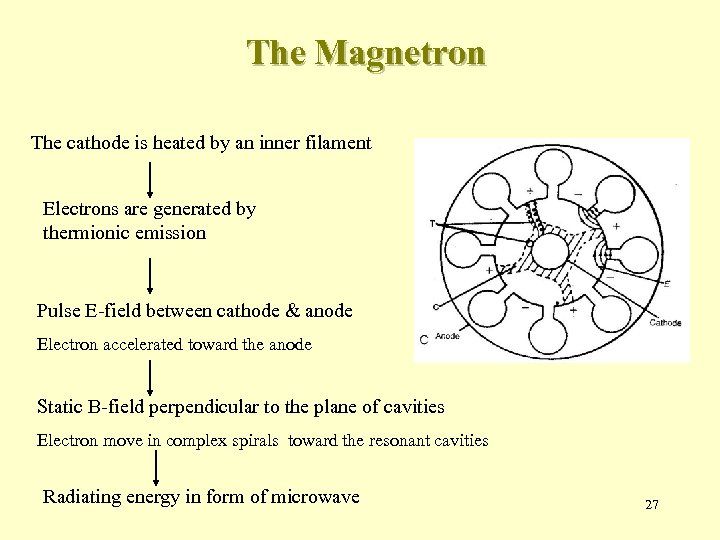 The Magnetron The cathode is heated by an inner filament Electrons are generated by thermionic emission Pulse E-field between cathode & anode Electron accelerated toward the anode Static B-field perpendicular to the plane of cavities Electron move in complex spirals toward the resonant cavities Radiating energy in form of microwave 27
The Magnetron The cathode is heated by an inner filament Electrons are generated by thermionic emission Pulse E-field between cathode & anode Electron accelerated toward the anode Static B-field perpendicular to the plane of cavities Electron move in complex spirals toward the resonant cavities Radiating energy in form of microwave 27
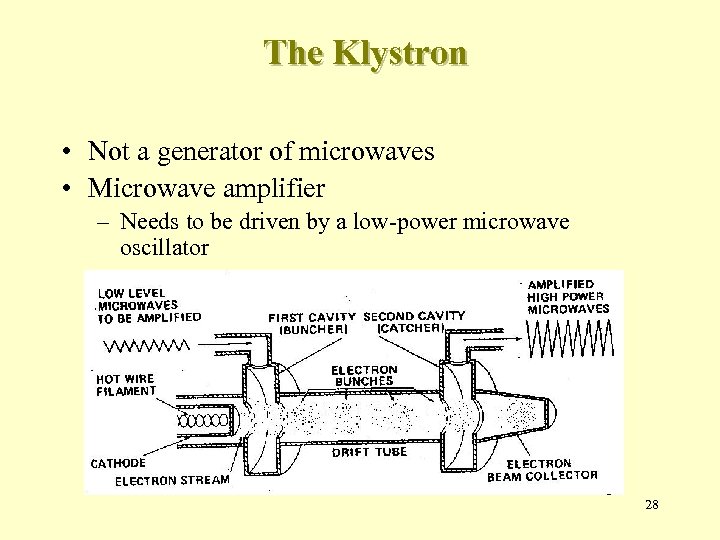 The Klystron • Not a generator of microwaves • Microwave amplifier – Needs to be driven by a low-power microwave oscillator 28
The Klystron • Not a generator of microwaves • Microwave amplifier – Needs to be driven by a low-power microwave oscillator 28
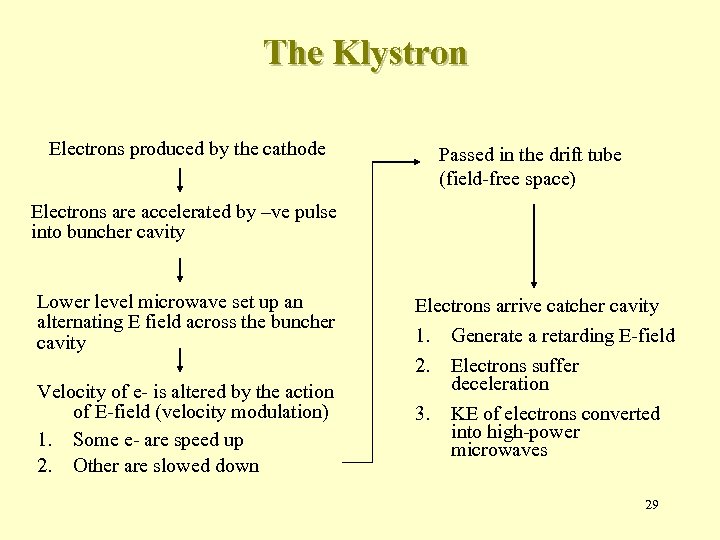 The Klystron Electrons produced by the cathode Passed in the drift tube (field-free space) Electrons are accelerated by –ve pulse into buncher cavity Lower level microwave set up an alternating E field across the buncher cavity Velocity of e- is altered by the action of E-field (velocity modulation) 1. Some e- are speed up 2. Other are slowed down Electrons arrive catcher cavity 1. 2. 3. Generate a retarding E-field Electrons suffer deceleration KE of electrons converted into high-power microwaves 29
The Klystron Electrons produced by the cathode Passed in the drift tube (field-free space) Electrons are accelerated by –ve pulse into buncher cavity Lower level microwave set up an alternating E field across the buncher cavity Velocity of e- is altered by the action of E-field (velocity modulation) 1. Some e- are speed up 2. Other are slowed down Electrons arrive catcher cavity 1. 2. 3. Generate a retarding E-field Electrons suffer deceleration KE of electrons converted into high-power microwaves 29
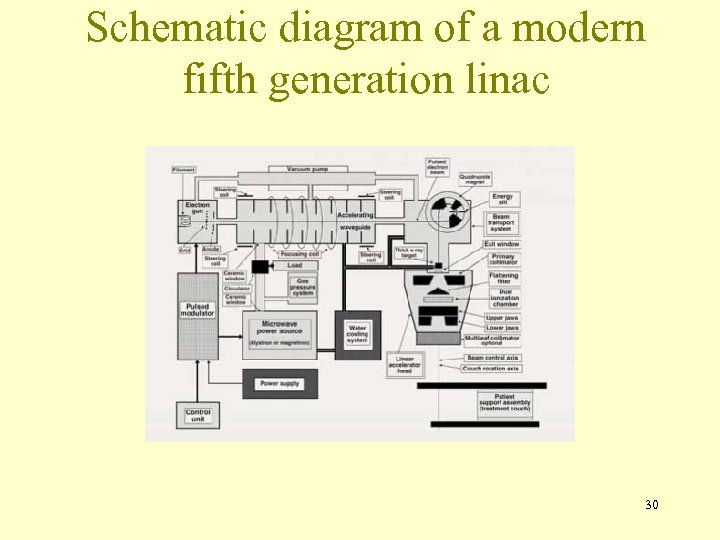 Schematic diagram of a modern fifth generation linac 30
Schematic diagram of a modern fifth generation linac 30
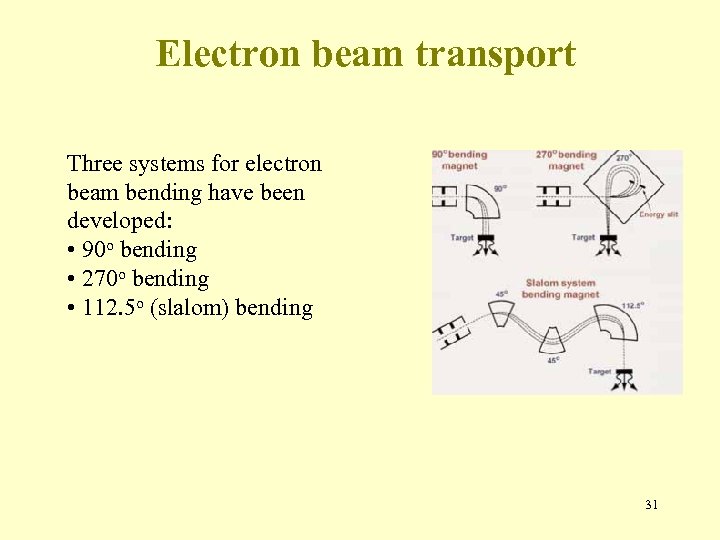 Electron beam transport Three systems for electron beam bending have been developed: • 90 o bending • 270 o bending • 112. 5 o (slalom) bending 31
Electron beam transport Three systems for electron beam bending have been developed: • 90 o bending • 270 o bending • 112. 5 o (slalom) bending 31
 The Linac X-Ray Beam • Production of x-rays – Electrons are incident on a target of a high-Z material (e. g. tungsten) – Target – need water cooled & thick enough to absorb most of the incident electrons – Bremsstrahlung interactions • Electrons energy is converted into a spectrum of x-rays energies • Max energy of x-rays = energy of incident energy of electrons • Average photon energy = 1/3 of max energy of x-rays • Designation of energy of electron beam and x-rays – Electron beam - Me. V (million electron volts, monoenergetic) – X-ray beam – MV (megavolts, voltage across an x-ray tube, hetergeneous in energy) 32
The Linac X-Ray Beam • Production of x-rays – Electrons are incident on a target of a high-Z material (e. g. tungsten) – Target – need water cooled & thick enough to absorb most of the incident electrons – Bremsstrahlung interactions • Electrons energy is converted into a spectrum of x-rays energies • Max energy of x-rays = energy of incident energy of electrons • Average photon energy = 1/3 of max energy of x-rays • Designation of energy of electron beam and x-rays – Electron beam - Me. V (million electron volts, monoenergetic) – X-ray beam – MV (megavolts, voltage across an x-ray tube, hetergeneous in energy) 32
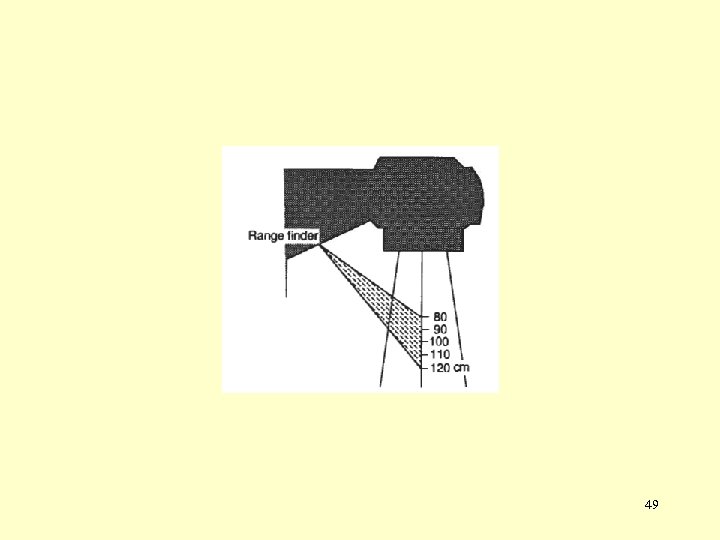
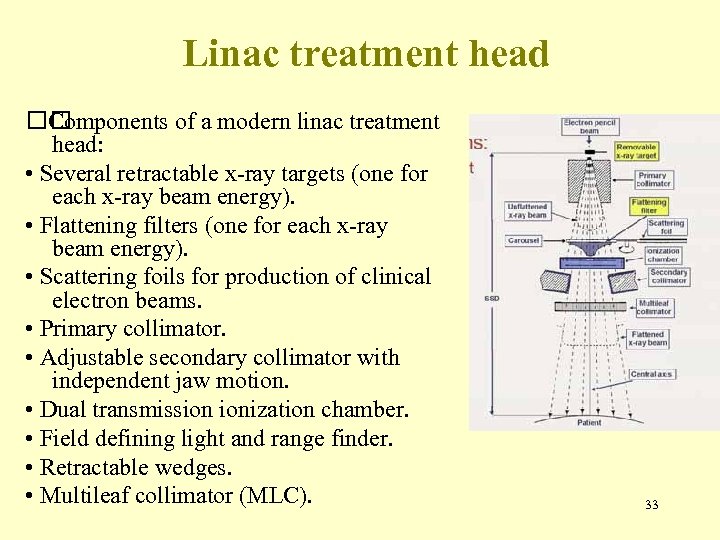 Linac treatment head Components of a modern linac treatment head: • Several retractable x-ray targets (one for each x-ray beam energy). • Flattening filters (one for each x-ray beam energy). • Scattering foils for production of clinical electron beams. • Primary collimator. • Adjustable secondary collimator with independent jaw motion. • Dual transmission ionization chamber. • Field defining light and range finder. • Retractable wedges. • Multileaf collimator (MLC). 33
Linac treatment head Components of a modern linac treatment head: • Several retractable x-ray targets (one for each x-ray beam energy). • Flattening filters (one for each x-ray beam energy). • Scattering foils for production of clinical electron beams. • Primary collimator. • Adjustable secondary collimator with independent jaw motion. • Dual transmission ionization chamber. • Field defining light and range finder. • Retractable wedges. • Multileaf collimator (MLC). 33
 Physical Wedge Beamline 34
Physical Wedge Beamline 34
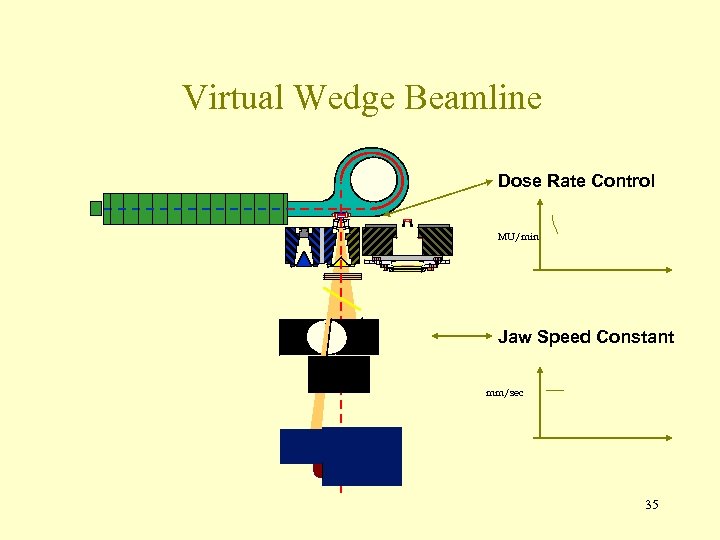 Virtual Wedge Beamline Dose Rate Control MU/min Jaw Speed Constant mm/sec 35
Virtual Wedge Beamline Dose Rate Control MU/min Jaw Speed Constant mm/sec 35
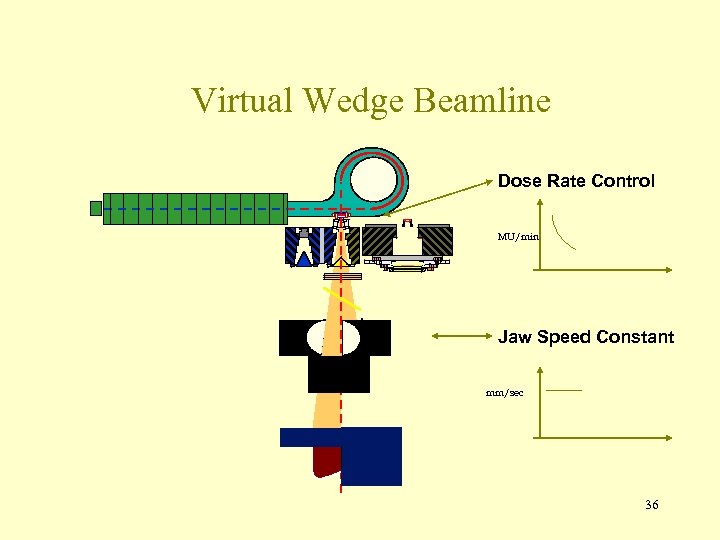 Virtual Wedge Beamline Dose Rate Control MU/min Jaw Speed Constant mm/sec 36
Virtual Wedge Beamline Dose Rate Control MU/min Jaw Speed Constant mm/sec 36
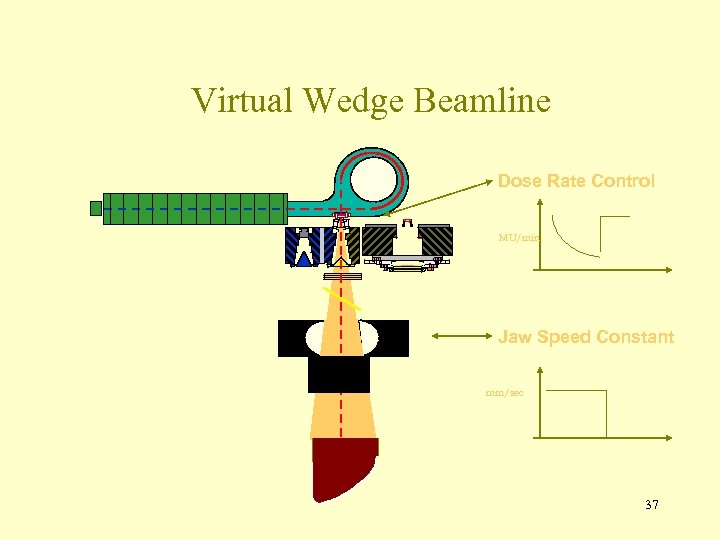 Virtual Wedge Beamline Dose Rate Control MU/min Jaw Speed Constant mm/sec 37
Virtual Wedge Beamline Dose Rate Control MU/min Jaw Speed Constant mm/sec 37
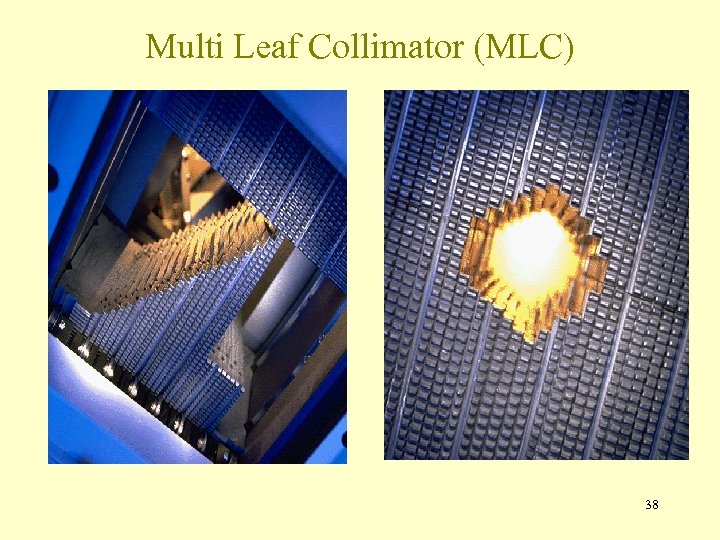 Multi Leaf Collimator (MLC) 38
Multi Leaf Collimator (MLC) 38
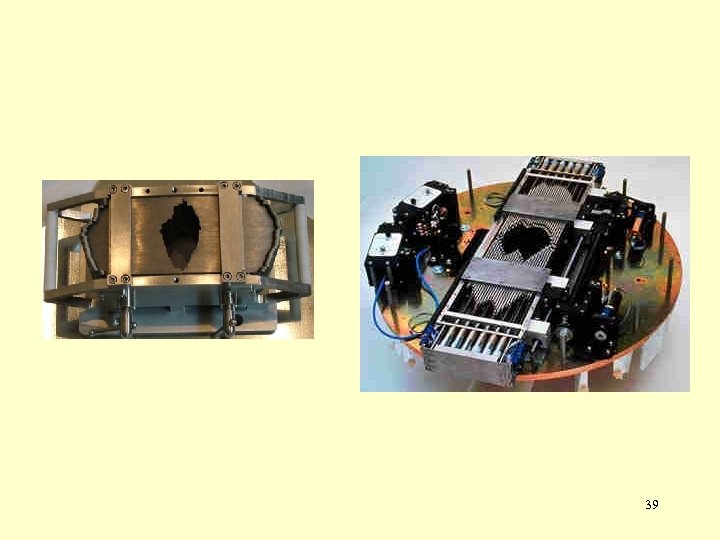 39
39
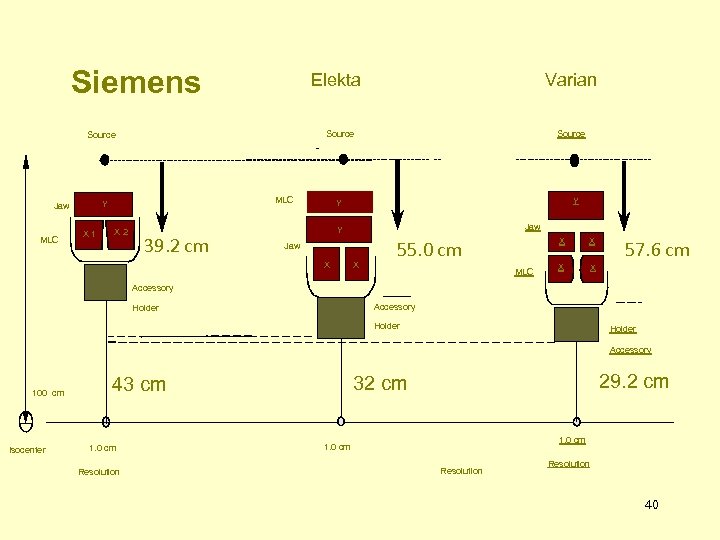 Siemens Elekta Source MLC Y Jaw X 1 X 2 Varian 39. 2 cm Source Y Y Jaw X X X 55. 0 cm MLC X X X 57. 6 cm Accessory Holder Accessory 100 cm Isocenter 1. 0 cm Resolution 29. 2 cm 32 cm 43 cm 1. 0 cm Resolution 40
Siemens Elekta Source MLC Y Jaw X 1 X 2 Varian 39. 2 cm Source Y Y Jaw X X X 55. 0 cm MLC X X X 57. 6 cm Accessory Holder Accessory 100 cm Isocenter 1. 0 cm Resolution 29. 2 cm 32 cm 43 cm 1. 0 cm Resolution 40
 41
41
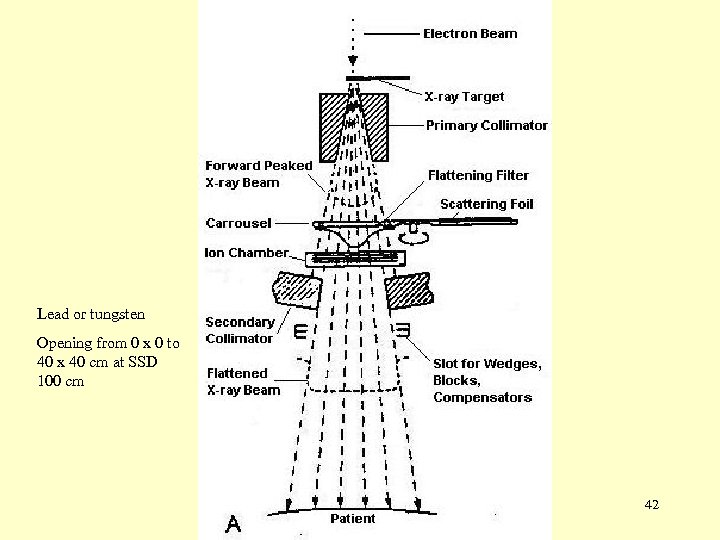 Lead or tungsten Opening from 0 x 0 to 40 x 40 cm at SSD 100 cm 42
Lead or tungsten Opening from 0 x 0 to 40 x 40 cm at SSD 100 cm 42
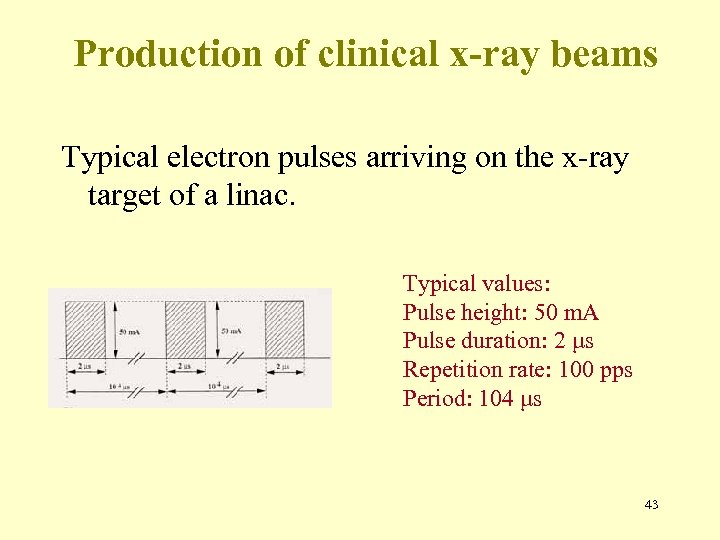 Production of clinical x-ray beams Typical electron pulses arriving on the x-ray target of a linac. Typical values: Pulse height: 50 m. A Pulse duration: 2 μs Repetition rate: 100 pps Period: 104 μs 43
Production of clinical x-ray beams Typical electron pulses arriving on the x-ray target of a linac. Typical values: Pulse height: 50 m. A Pulse duration: 2 μs Repetition rate: 100 pps Period: 104 μs 43
 44
44
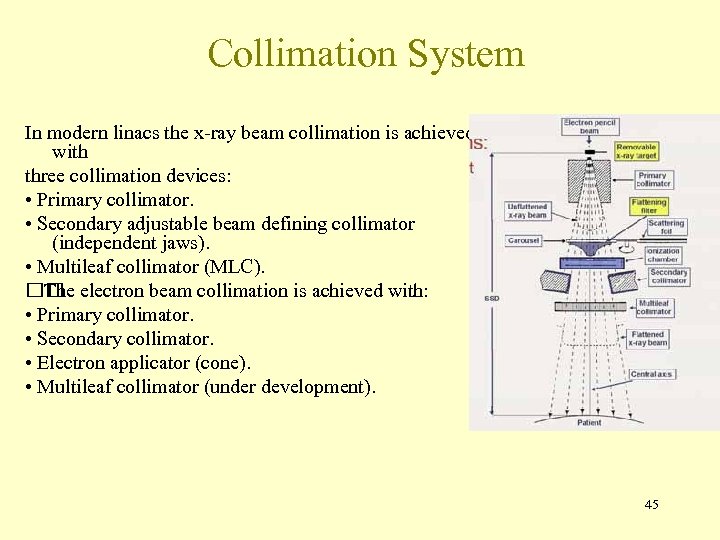 Collimation System In modern linacs the x-ray beam collimation is achieved with three collimation devices: • Primary collimator. • Secondary adjustable beam defining collimator (independent jaws). • Multileaf collimator (MLC). electron beam collimation is achieved with: The • Primary collimator. • Secondary collimator. • Electron applicator (cone). • Multileaf collimator (under development). 45
Collimation System In modern linacs the x-ray beam collimation is achieved with three collimation devices: • Primary collimator. • Secondary adjustable beam defining collimator (independent jaws). • Multileaf collimator (MLC). electron beam collimation is achieved with: The • Primary collimator. • Secondary collimator. • Electron applicator (cone). • Multileaf collimator (under development). 45
 46
46
 47
47
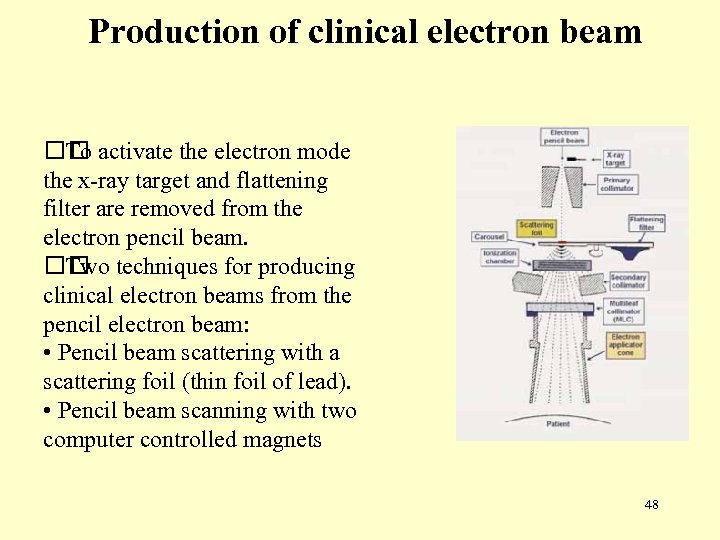 Production of clinical electron beam activate the electron mode To the x-ray target and flattening filter are removed from the electron pencil beam. techniques for producing Two clinical electron beams from the pencil electron beam: • Pencil beam scattering with a scattering foil (thin foil of lead). • Pencil beam scanning with two computer controlled magnets 48
Production of clinical electron beam activate the electron mode To the x-ray target and flattening filter are removed from the electron pencil beam. techniques for producing Two clinical electron beams from the pencil electron beam: • Pencil beam scattering with a scattering foil (thin foil of lead). • Pencil beam scanning with two computer controlled magnets 48
 49
49
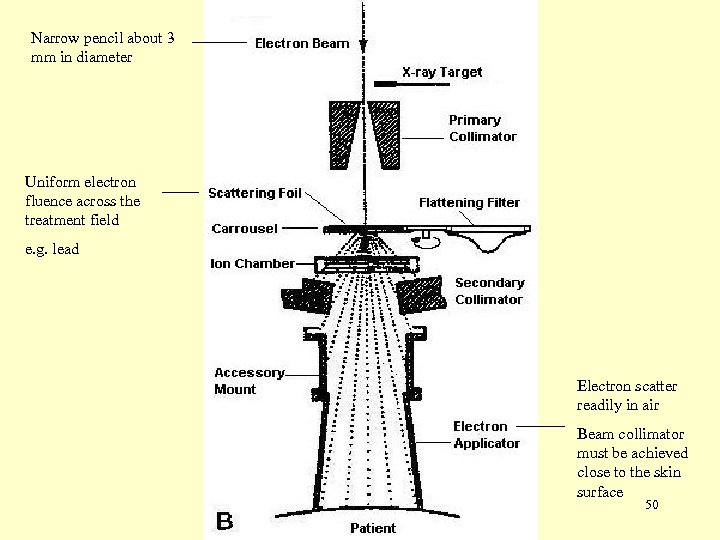 Narrow pencil about 3 mm in diameter Uniform electron fluence across the treatment field e. g. lead Electron scatter readily in air Beam collimator must be achieved close to the skin surface 50
Narrow pencil about 3 mm in diameter Uniform electron fluence across the treatment field e. g. lead Electron scatter readily in air Beam collimator must be achieved close to the skin surface 50
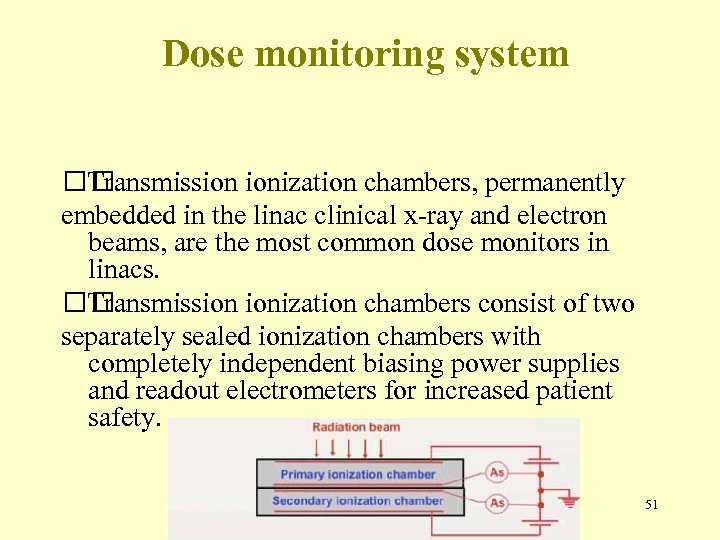 Dose monitoring system Transmission ionization chambers, permanently embedded in the linac clinical x-ray and electron beams, are the most common dose monitors in linacs. Transmission ionization chambers consist of two separately sealed ionization chambers with completely independent biasing power supplies and readout electrometers for increased patient safety. 51
Dose monitoring system Transmission ionization chambers, permanently embedded in the linac clinical x-ray and electron beams, are the most common dose monitors in linacs. Transmission ionization chambers consist of two separately sealed ionization chambers with completely independent biasing power supplies and readout electrometers for increased patient safety. 51
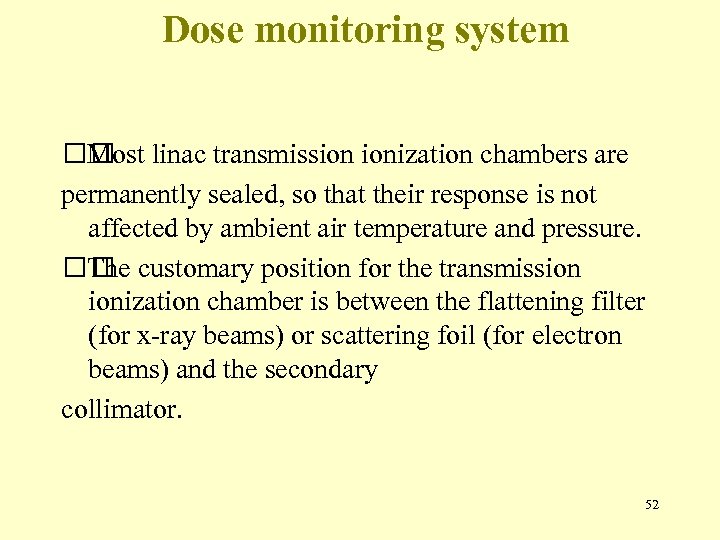 Dose monitoring system linac transmission ionization chambers are Most permanently sealed, so that their response is not affected by ambient air temperature and pressure. customary position for the transmission The ionization chamber is between the flattening filter (for x-ray beams) or scattering foil (for electron beams) and the secondary collimator. 52
Dose monitoring system linac transmission ionization chambers are Most permanently sealed, so that their response is not affected by ambient air temperature and pressure. customary position for the transmission The ionization chamber is between the flattening filter (for x-ray beams) or scattering foil (for electron beams) and the secondary collimator. 52
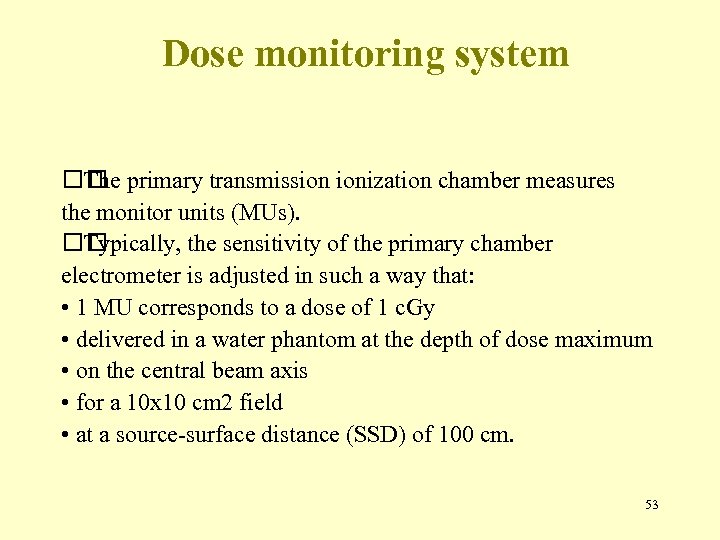 Dose monitoring system primary transmission ionization chamber measures The the monitor units (MUs). Typically, the sensitivity of the primary chamber electrometer is adjusted in such a way that: • 1 MU corresponds to a dose of 1 c. Gy • delivered in a water phantom at the depth of dose maximum • on the central beam axis • for a 10 x 10 cm 2 field • at a source-surface distance (SSD) of 100 cm. 53
Dose monitoring system primary transmission ionization chamber measures The the monitor units (MUs). Typically, the sensitivity of the primary chamber electrometer is adjusted in such a way that: • 1 MU corresponds to a dose of 1 c. Gy • delivered in a water phantom at the depth of dose maximum • on the central beam axis • for a 10 x 10 cm 2 field • at a source-surface distance (SSD) of 100 cm. 53
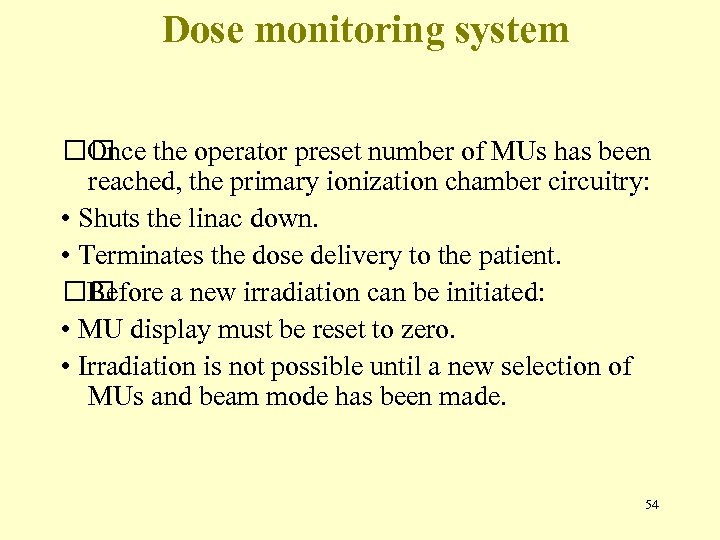 Dose monitoring system the operator preset number of MUs has been Once reached, the primary ionization chamber circuitry: • Shuts the linac down. • Terminates the dose delivery to the patient. Before a new irradiation can be initiated: • MU display must be reset to zero. • Irradiation is not possible until a new selection of MUs and beam mode has been made. 54
Dose monitoring system the operator preset number of MUs has been Once reached, the primary ionization chamber circuitry: • Shuts the linac down. • Terminates the dose delivery to the patient. Before a new irradiation can be initiated: • MU display must be reset to zero. • Irradiation is not possible until a new selection of MUs and beam mode has been made. 54
 1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 55
1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 55
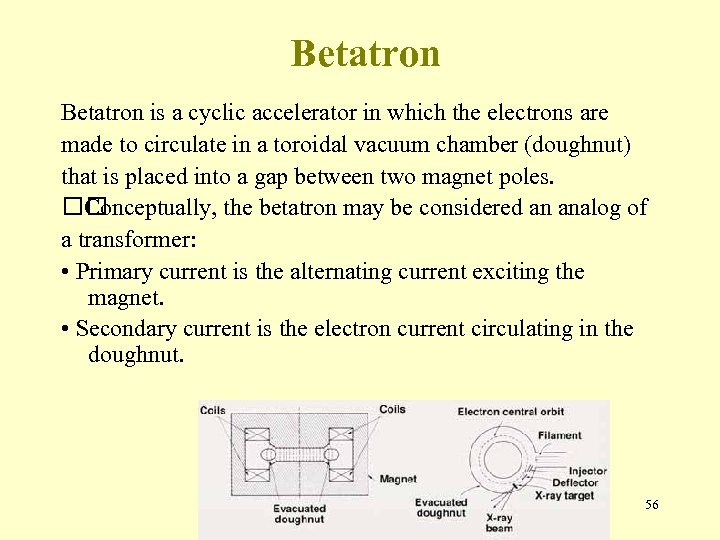 Betatron is a cyclic accelerator in which the electrons are made to circulate in a toroidal vacuum chamber (doughnut) that is placed into a gap between two magnet poles. Conceptually, the betatron may be considered an analog of a transformer: • Primary current is the alternating current exciting the magnet. • Secondary current is the electron current circulating in the doughnut. 56
Betatron is a cyclic accelerator in which the electrons are made to circulate in a toroidal vacuum chamber (doughnut) that is placed into a gap between two magnet poles. Conceptually, the betatron may be considered an analog of a transformer: • Primary current is the alternating current exciting the magnet. • Secondary current is the electron current circulating in the doughnut. 56
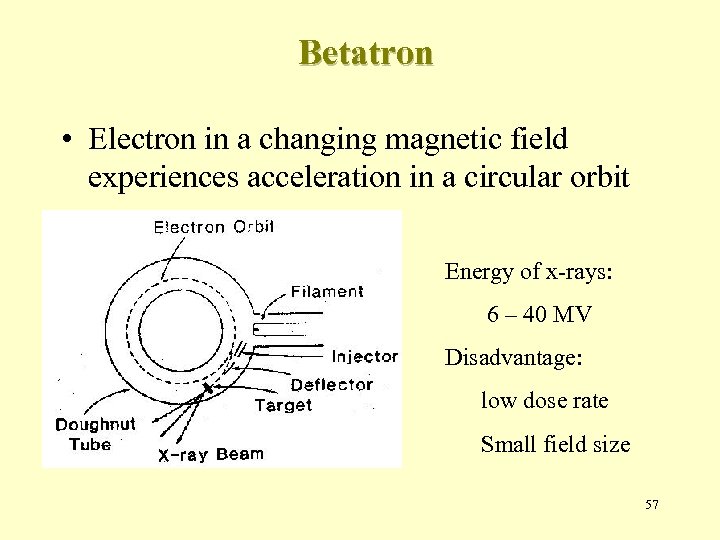 Betatron • Electron in a changing magnetic field experiences acceleration in a circular orbit Energy of x-rays: 6 – 40 MV Disadvantage: low dose rate Small field size 57
Betatron • Electron in a changing magnetic field experiences acceleration in a circular orbit Energy of x-rays: 6 – 40 MV Disadvantage: low dose rate Small field size 57
 1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 58
1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 58
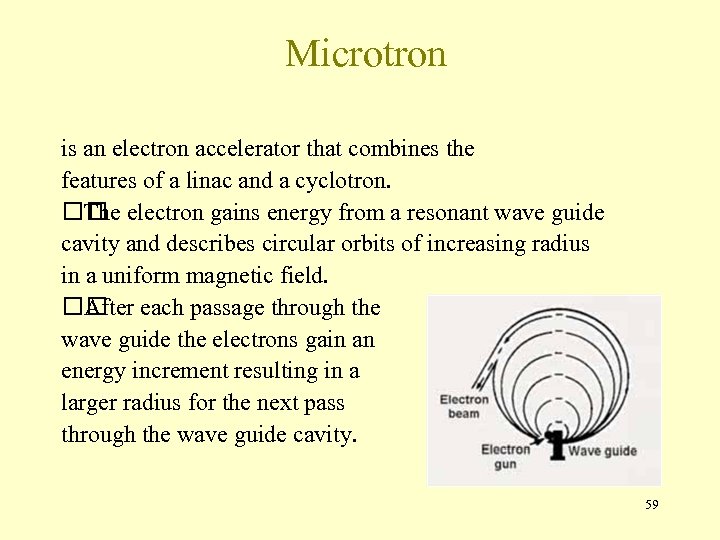 Microtron is an electron accelerator that combines the features of a linac and a cyclotron. electron gains energy from a resonant wave guide The cavity and describes circular orbits of increasing radius in a uniform magnetic field. each passage through the After wave guide the electrons gain an energy increment resulting in a larger radius for the next pass through the wave guide cavity. 59
Microtron is an electron accelerator that combines the features of a linac and a cyclotron. electron gains energy from a resonant wave guide The cavity and describes circular orbits of increasing radius in a uniform magnetic field. each passage through the After wave guide the electrons gain an energy increment resulting in a larger radius for the next pass through the wave guide cavity. 59
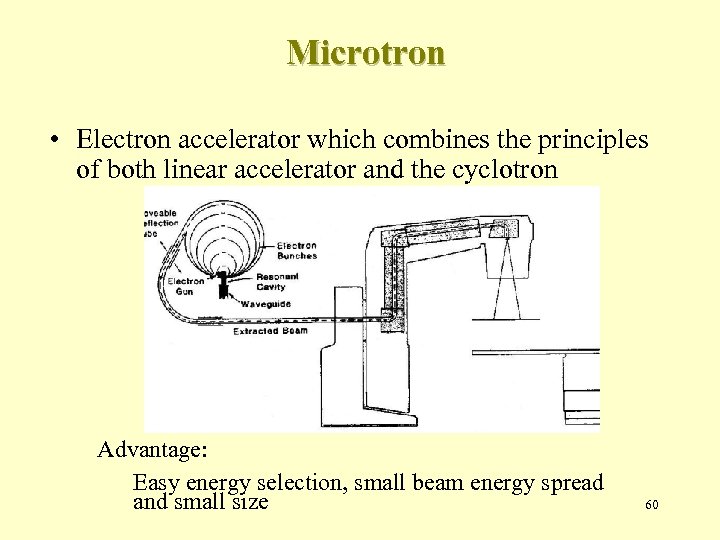 Microtron • Electron accelerator which combines the principles of both linear accelerator and the cyclotron Advantage: Easy energy selection, small beam energy spread and small size 60
Microtron • Electron accelerator which combines the principles of both linear accelerator and the cyclotron Advantage: Easy energy selection, small beam energy spread and small size 60
 1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 61
1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 61
 Cyclotron • Charged particle accelerator • Mainly used for nuclear physics research • As a source of high-energy protons for proton beam therapy • Have been adopted for generating neutron beams recently 62
Cyclotron • Charged particle accelerator • Mainly used for nuclear physics research • As a source of high-energy protons for proton beam therapy • Have been adopted for generating neutron beams recently 62
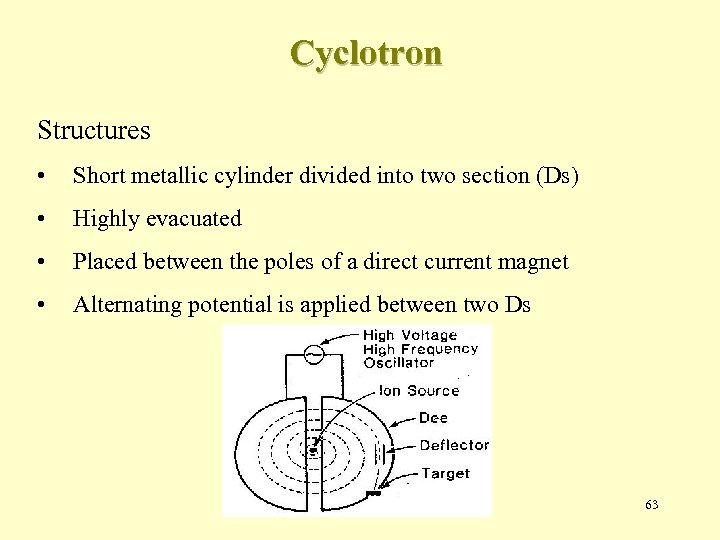 Cyclotron Structures • Short metallic cylinder divided into two section (Ds) • Highly evacuated • Placed between the poles of a direct current magnet • Alternating potential is applied between two Ds 63
Cyclotron Structures • Short metallic cylinder divided into two section (Ds) • Highly evacuated • Placed between the poles of a direct current magnet • Alternating potential is applied between two Ds 63
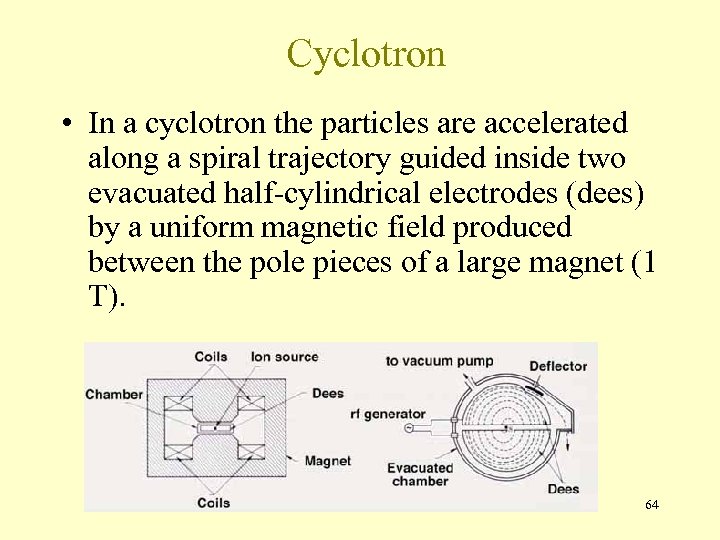 Cyclotron • In a cyclotron the particles are accelerated along a spiral trajectory guided inside two evacuated half-cylindrical electrodes (dees) by a uniform magnetic field produced between the pole pieces of a large magnet (1 T). 64
Cyclotron • In a cyclotron the particles are accelerated along a spiral trajectory guided inside two evacuated half-cylindrical electrodes (dees) by a uniform magnetic field produced between the pole pieces of a large magnet (1 T). 64
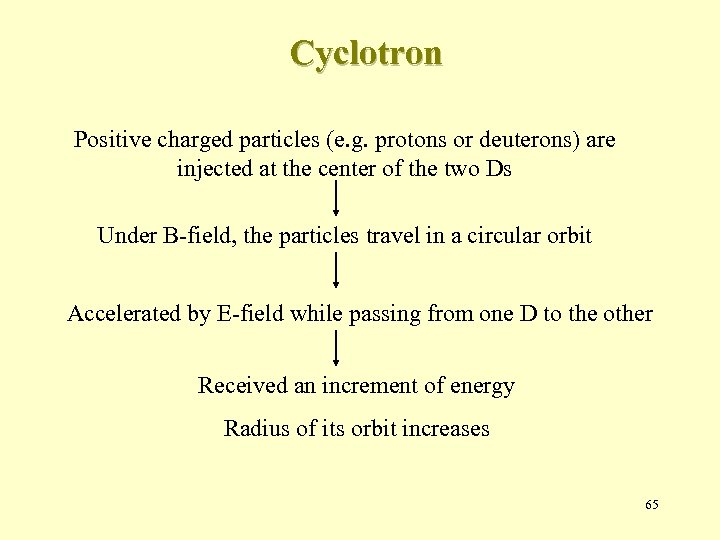 Cyclotron Positive charged particles (e. g. protons or deuterons) are injected at the center of the two Ds Under B-field, the particles travel in a circular orbit Accelerated by E-field while passing from one D to the other Received an increment of energy Radius of its orbit increases 65
Cyclotron Positive charged particles (e. g. protons or deuterons) are injected at the center of the two Ds Under B-field, the particles travel in a circular orbit Accelerated by E-field while passing from one D to the other Received an increment of energy Radius of its orbit increases 65
 1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 66
1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 66
 The important characteristics of radionuclides useful for external beam radiotherapy are: • High gamma ray energy (of the order of 1 Me. V). • High specific activity (of the order of 100 Ci/g). • Relatively long half life (of the order of several years). • Large specific air kerma rate constant. over 3000 radionuclides known only 3 meet the Of required characteristics and essentially only cobalt-60 is currently used for external beam radiotherapy. 67
The important characteristics of radionuclides useful for external beam radiotherapy are: • High gamma ray energy (of the order of 1 Me. V). • High specific activity (of the order of 100 Ci/g). • Relatively long half life (of the order of several years). • Large specific air kerma rate constant. over 3000 radionuclides known only 3 meet the Of required characteristics and essentially only cobalt-60 is currently used for external beam radiotherapy. 67
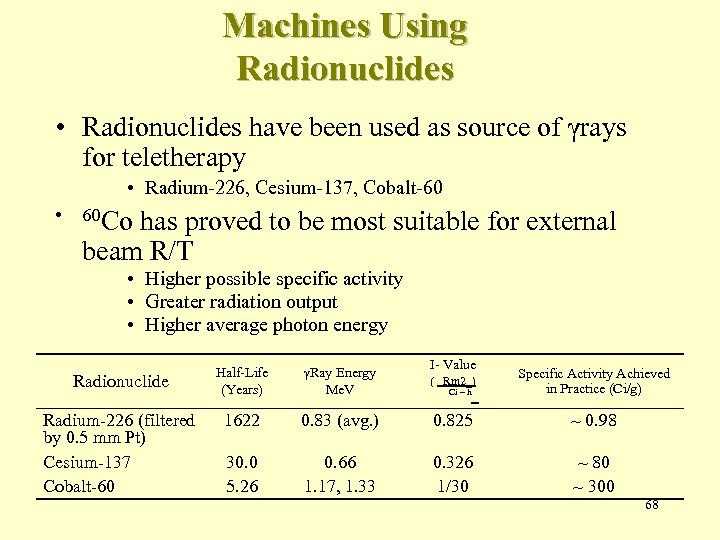 Machines Using Radionuclides • Radionuclides have been used as source of γrays for teletherapy • Radium-226, Cesium-137, Cobalt-60 • 60 Co has proved to be most suitable for external beam R/T • Higher possible specific activity • Greater radiation output • Higher average photon energy I- Value Radionuclide Half-Life (Years) γRay Energy Me. V ( Rm 2_) Specific Activity Achieved in Practice (Ci/g) Radium-226 (filtered by 0. 5 mm Pt) Cesium-137 Cobalt-60 1622 0. 83 (avg. ) 0. 825 ~ 0. 98 30. 0 5. 26 0. 66 1. 17, 1. 33 0. 326 1/30 ~ 80 ~ 300 Ci – h 68
Machines Using Radionuclides • Radionuclides have been used as source of γrays for teletherapy • Radium-226, Cesium-137, Cobalt-60 • 60 Co has proved to be most suitable for external beam R/T • Higher possible specific activity • Greater radiation output • Higher average photon energy I- Value Radionuclide Half-Life (Years) γRay Energy Me. V ( Rm 2_) Specific Activity Achieved in Practice (Ci/g) Radium-226 (filtered by 0. 5 mm Pt) Cesium-137 Cobalt-60 1622 0. 83 (avg. ) 0. 825 ~ 0. 98 30. 0 5. 26 0. 66 1. 17, 1. 33 0. 326 1/30 ~ 80 ~ 300 Ci – h 68
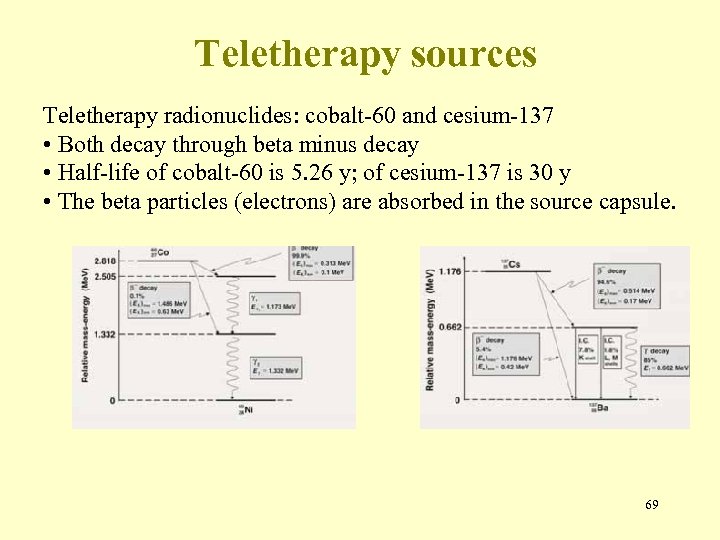 Teletherapy sources Teletherapy radionuclides: cobalt-60 and cesium-137 • Both decay through beta minus decay • Half-life of cobalt-60 is 5. 26 y; of cesium-137 is 30 y • The beta particles (electrons) are absorbed in the source capsule. 69
Teletherapy sources Teletherapy radionuclides: cobalt-60 and cesium-137 • Both decay through beta minus decay • Half-life of cobalt-60 is 5. 26 y; of cesium-137 is 30 y • The beta particles (electrons) are absorbed in the source capsule. 69
 Teletherapy machines Treatment machines used for external beam radiotherapy with gamma ray sources are called teletherapy machines. They are most often mounted isocentrically with SAD of 80 cm or 100 cm. main components of a teletherapy machine are: The • Radioactive source • Source housing, including beam collimator and source movement mechanism. • Gantry and stand. • Patient support assembly. • Machine control console 70
Teletherapy machines Treatment machines used for external beam radiotherapy with gamma ray sources are called teletherapy machines. They are most often mounted isocentrically with SAD of 80 cm or 100 cm. main components of a teletherapy machine are: The • Radioactive source • Source housing, including beam collimator and source movement mechanism. • Gantry and stand. • Patient support assembly. • Machine control console 70
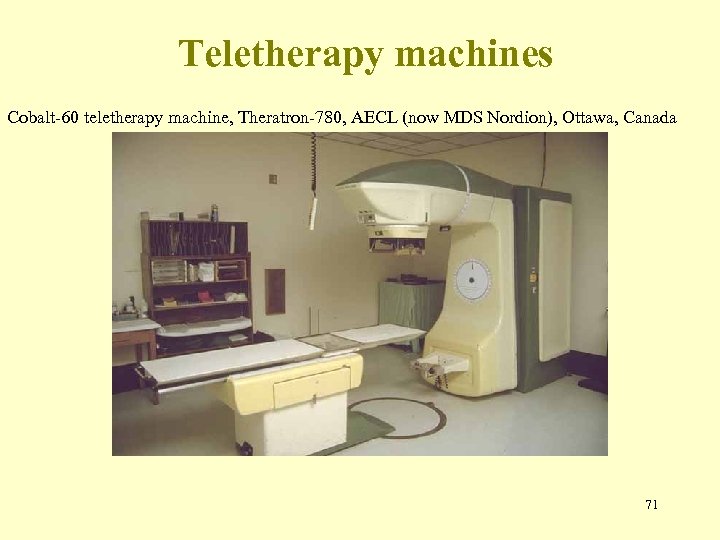 Teletherapy machines Cobalt-60 teletherapy machine, Theratron-780, AECL (now MDS Nordion), Ottawa, Canada 71
Teletherapy machines Cobalt-60 teletherapy machine, Theratron-780, AECL (now MDS Nordion), Ottawa, Canada 71
 Cobalt-60 Unit • Source – From 59 Co(n, γ) nuclear reactor – Stable 59 Co → radioactive 60 Co – In form of solid cylinder, discs, or pallets • Treatment beam 60 Co → 60 Ni + 0β(0. 32 Me. V) + γ(1. 17 & 1. 33 Me. V) • Heterogeneity of the beam – Secondary interactions – βabsorbed by capsule → bremsstrahlung x-rays (0. 1 Me. V) – scattering from the surrounding capsule, the source housing and the collimation system (eletron contamination) 72
Cobalt-60 Unit • Source – From 59 Co(n, γ) nuclear reactor – Stable 59 Co → radioactive 60 Co – In form of solid cylinder, discs, or pallets • Treatment beam 60 Co → 60 Ni + 0β(0. 32 Me. V) + γ(1. 17 & 1. 33 Me. V) • Heterogeneity of the beam – Secondary interactions – βabsorbed by capsule → bremsstrahlung x-rays (0. 1 Me. V) – scattering from the surrounding capsule, the source housing and the collimation system (eletron contamination) 72
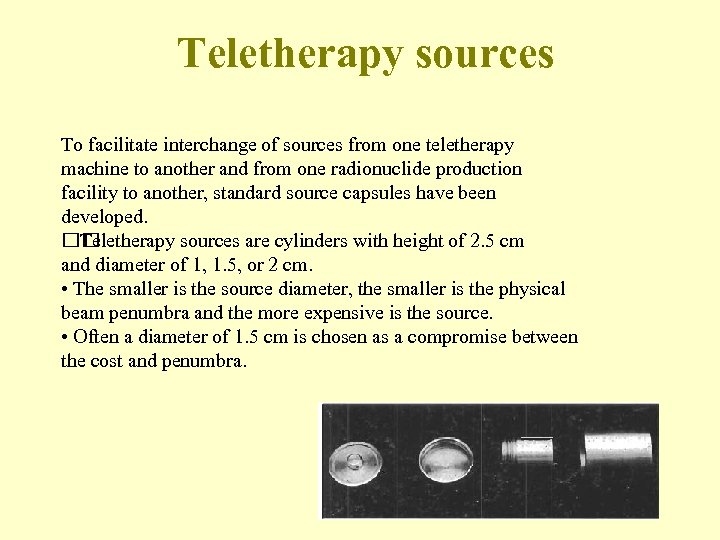 Teletherapy sources To facilitate interchange of sources from one teletherapy machine to another and from one radionuclide production facility to another, standard source capsules have been developed. Teletherapy sources are cylinders with height of 2. 5 cm and diameter of 1, 1. 5, or 2 cm. • The smaller is the source diameter, the smaller is the physical beam penumbra and the more expensive is the source. • Often a diameter of 1. 5 cm is chosen as a compromise between the cost and penumbra. 73
Teletherapy sources To facilitate interchange of sources from one teletherapy machine to another and from one radionuclide production facility to another, standard source capsules have been developed. Teletherapy sources are cylinders with height of 2. 5 cm and diameter of 1, 1. 5, or 2 cm. • The smaller is the source diameter, the smaller is the physical beam penumbra and the more expensive is the source. • Often a diameter of 1. 5 cm is chosen as a compromise between the cost and penumbra. 73
 Teletherapy sources Typical source activity: of the order of 5 000 - 10 000 Ci (185 - 370 TBq). Typical dose rates at 80 cm from source: of the order of 100 - 200 c. Gy/min Teletherapy source is usually replaced within one half-life after it is installed. Financial considerations often result in longer source usage. 74
Teletherapy sources Typical source activity: of the order of 5 000 - 10 000 Ci (185 - 370 TBq). Typical dose rates at 80 cm from source: of the order of 100 - 200 c. Gy/min Teletherapy source is usually replaced within one half-life after it is installed. Financial considerations often result in longer source usage. 74
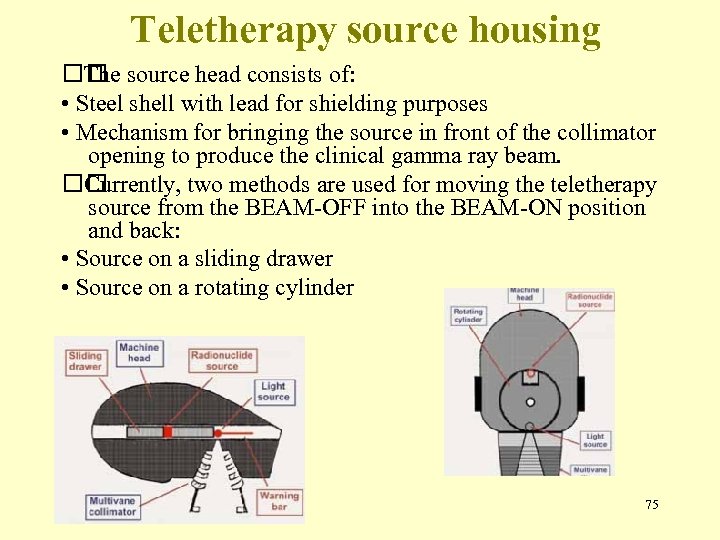 Teletherapy source housing source head consists of: The • Steel shell with lead for shielding purposes • Mechanism for bringing the source in front of the collimator opening to produce the clinical gamma ray beam. Currently, two methods are used for moving the teletherapy source from the BEAM-OFF into the BEAM-ON position and back: • Source on a sliding drawer • Source on a rotating cylinder 75
Teletherapy source housing source head consists of: The • Steel shell with lead for shielding purposes • Mechanism for bringing the source in front of the collimator opening to produce the clinical gamma ray beam. Currently, two methods are used for moving the teletherapy source from the BEAM-OFF into the BEAM-ON position and back: • Source on a sliding drawer • Source on a rotating cylinder 75
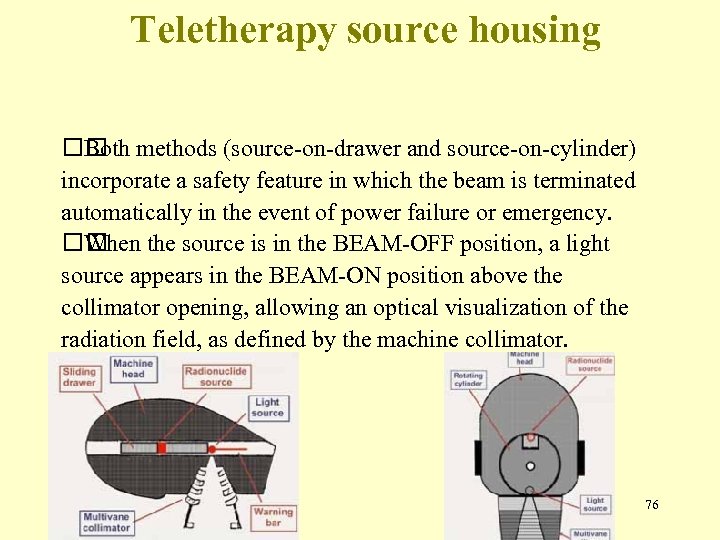 Teletherapy source housing methods (source-on-drawer and source-on-cylinder) Both incorporate a safety feature in which the beam is terminated automatically in the event of power failure or emergency. When the source is in the BEAM-OFF position, a light source appears in the BEAM-ON position above the collimator opening, allowing an optical visualization of the radiation field, as defined by the machine collimator. 76
Teletherapy source housing methods (source-on-drawer and source-on-cylinder) Both incorporate a safety feature in which the beam is terminated automatically in the event of power failure or emergency. When the source is in the BEAM-OFF position, a light source appears in the BEAM-ON position above the collimator opening, allowing an optical visualization of the radiation field, as defined by the machine collimator. 76
 Teletherapy source housing radiation (leakage radiation) will escape from the Some teletherapy machine even when the source is in the BEAM-OFF position. leakage typically amounts to less than 1 m. R/h Head (0. 01 m. Sv/h) at 1 m from the source. International regulations require that the average leakage of a teletherapy machine head be less than 2 m. R/h (0. 02 m. Sv/h). 77
Teletherapy source housing radiation (leakage radiation) will escape from the Some teletherapy machine even when the source is in the BEAM-OFF position. leakage typically amounts to less than 1 m. R/h Head (0. 01 m. Sv/h) at 1 m from the source. International regulations require that the average leakage of a teletherapy machine head be less than 2 m. R/h (0. 02 m. Sv/h). 77
 Collimator and penumbra Collimators of teletherapy machines provide square and rectangular radiation fields typically ranging from 5 x 5 to 35 x 35 cm 2 at 80 cm from the source. 78
Collimator and penumbra Collimators of teletherapy machines provide square and rectangular radiation fields typically ranging from 5 x 5 to 35 x 35 cm 2 at 80 cm from the source. 78
 Penumbra • The region, at the edge of a radiation beam, over which the dose rate changes rapidly as function of distance from the beam axis 1. Transmission penumbra 2. Geometric penumbra 79
Penumbra • The region, at the edge of a radiation beam, over which the dose rate changes rapidly as function of distance from the beam axis 1. Transmission penumbra 2. Geometric penumbra 79
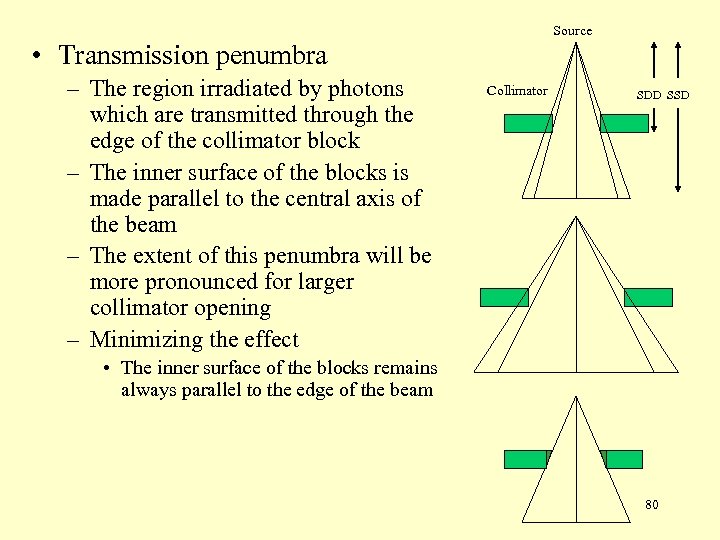 Source • Transmission penumbra – The region irradiated by photons which are transmitted through the edge of the collimator block – The inner surface of the blocks is made parallel to the central axis of the beam – The extent of this penumbra will be more pronounced for larger collimator opening – Minimizing the effect Collimator SDD SSD • The inner surface of the blocks remains always parallel to the edge of the beam 80
Source • Transmission penumbra – The region irradiated by photons which are transmitted through the edge of the collimator block – The inner surface of the blocks is made parallel to the central axis of the beam – The extent of this penumbra will be more pronounced for larger collimator opening – Minimizing the effect Collimator SDD SSD • The inner surface of the blocks remains always parallel to the edge of the beam 80
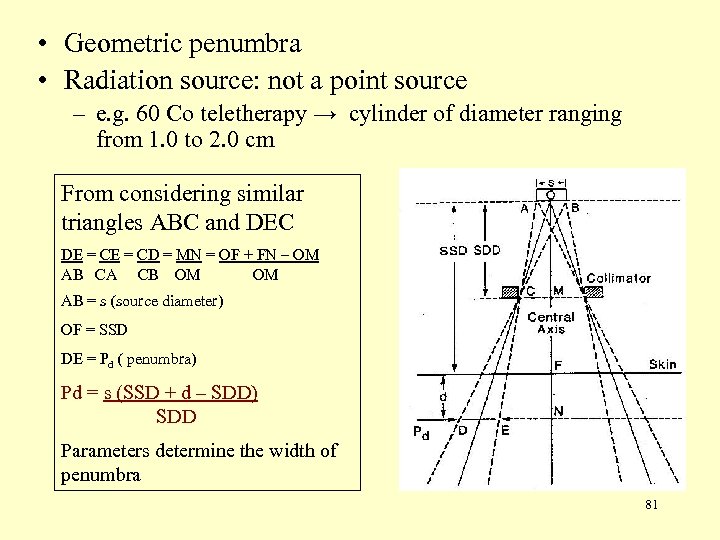 • Geometric penumbra • Radiation source: not a point source – e. g. 60 Co teletherapy → cylinder of diameter ranging from 1. 0 to 2. 0 cm From considering similar triangles ABC and DEC DE = CD = MN = OF + FN – OM AB CA CB OM OM AB = s (source diameter) OF = SSD DE = Pd ( penumbra) Pd = s (SSD + d – SDD) SDD Parameters determine the width of penumbra 81
• Geometric penumbra • Radiation source: not a point source – e. g. 60 Co teletherapy → cylinder of diameter ranging from 1. 0 to 2. 0 cm From considering similar triangles ABC and DEC DE = CD = MN = OF + FN – OM AB CA CB OM OM AB = s (source diameter) OF = SSD DE = Pd ( penumbra) Pd = s (SSD + d – SDD) SDD Parameters determine the width of penumbra 81
 • Geometric penumbra (con’t) – Solutions • Extendable penumbra trimmer – Heavy metal bars to attenuate the beam in the penumbra region • Secondary blocks – Placed closed to the patient for redifining the field – Should not be placed < 15 – 20 cm, excessive electron contaminants – Definition of physical penumbra in dosimetry • Lateral distance between two specified isodose curves at a specified depth – At a depth in the patient, dose variation at the field border – Geometric, transmission penumbras + scattered radiation produced in the patient 82
• Geometric penumbra (con’t) – Solutions • Extendable penumbra trimmer – Heavy metal bars to attenuate the beam in the penumbra region • Secondary blocks – Placed closed to the patient for redifining the field – Should not be placed < 15 – 20 cm, excessive electron contaminants – Definition of physical penumbra in dosimetry • Lateral distance between two specified isodose curves at a specified depth – At a depth in the patient, dose variation at the field border – Geometric, transmission penumbras + scattered radiation produced in the patient 82
 1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 83
1. Kilovoltage Units 2. Van de Graaff Generator 3. Linear Accelerator 4. Betatron 5. Microtron 6. Cyclotron 7. Machine Using Radionuclides 8. Heavy Particle Beams 83
 Heavy Particle Beams • Advantage – Dose localization – Therapeutic gain (greater effect on tumor than on normal tissue) • Including – neutrons, protons, deuterons, αparticles, negative pions, and heavy ions • Still experimental • Few institutions because of the enormous cost 84
Heavy Particle Beams • Advantage – Dose localization – Therapeutic gain (greater effect on tumor than on normal tissue) • Including – neutrons, protons, deuterons, αparticles, negative pions, and heavy ions • Still experimental • Few institutions because of the enormous cost 84
 Neutrons • Sources of high energy neutron beams – D-T generator, cyclotrons, or linear accelerators • D-T generators 2 H 1 4 + 3 H → 2 He + 01 n + 17. 6 Me. V 1 – Monoenergetic (14 Me. V) – Isotropic (same yield in all directions) – Major problem • Lack of sufficient dose rate at the treatment distance • 15 c. Gy/min at 1 m – Advantage • Its size is small enough to allow isocentric mounting on gantry 85
Neutrons • Sources of high energy neutron beams – D-T generator, cyclotrons, or linear accelerators • D-T generators 2 H 1 4 + 3 H → 2 He + 01 n + 17. 6 Me. V 1 – Monoenergetic (14 Me. V) – Isotropic (same yield in all directions) – Major problem • Lack of sufficient dose rate at the treatment distance • 15 c. Gy/min at 1 m – Advantage • Its size is small enough to allow isocentric mounting on gantry 85
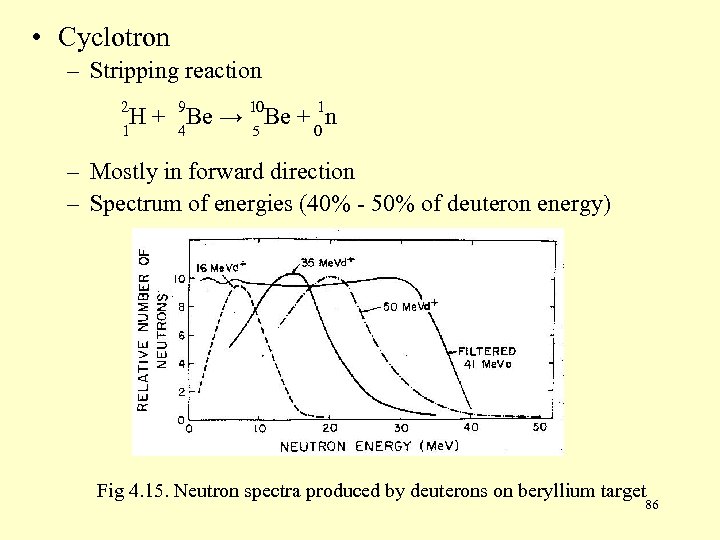 • Cyclotron – Stripping reaction 2 H 1 1 + 9 Be → 10 Be + 0 n 4 5 – Mostly in forward direction – Spectrum of energies (40% - 50% of deuteron energy) Fig 4. 15. Neutron spectra produced by deuterons on beryllium target 86
• Cyclotron – Stripping reaction 2 H 1 1 + 9 Be → 10 Be + 0 n 4 5 – Mostly in forward direction – Spectrum of energies (40% - 50% of deuteron energy) Fig 4. 15. Neutron spectra produced by deuterons on beryllium target 86
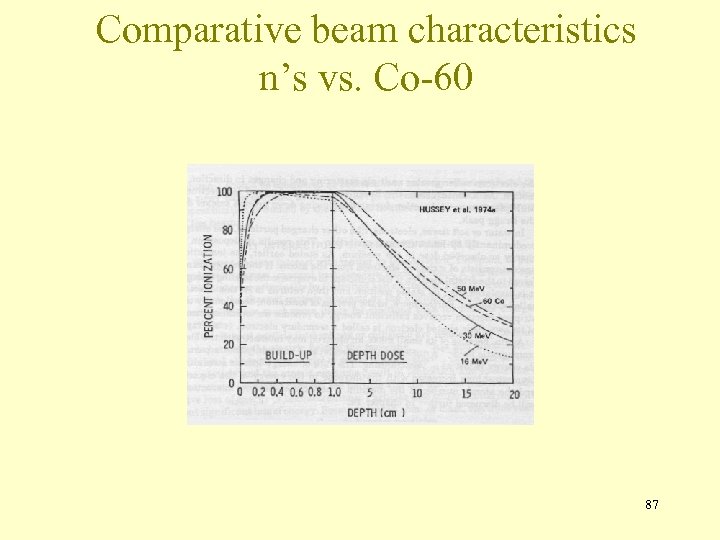 Comparative beam characteristics n’s vs. Co-60 87
Comparative beam characteristics n’s vs. Co-60 87
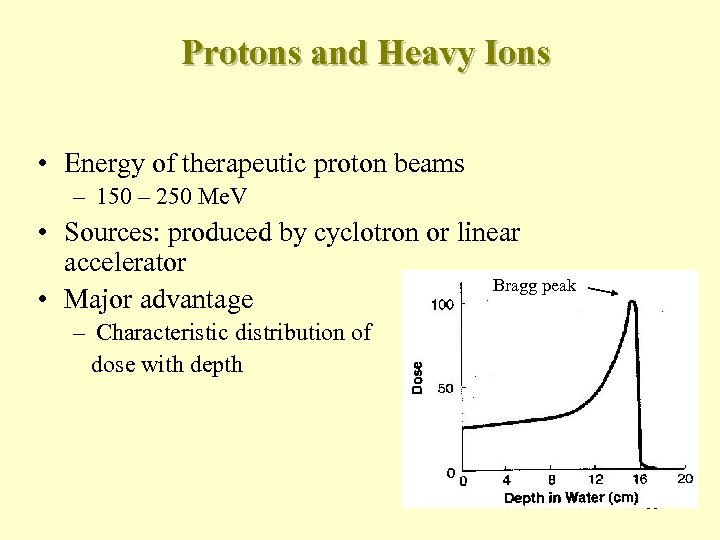 Protons and Heavy Ions • Energy of therapeutic proton beams – 150 – 250 Me. V • Sources: produced by cyclotron or linear accelerator Bragg peak • Major advantage – Characteristic distribution of dose with depth 88
Protons and Heavy Ions • Energy of therapeutic proton beams – 150 – 250 Me. V • Sources: produced by cyclotron or linear accelerator Bragg peak • Major advantage – Characteristic distribution of dose with depth 88
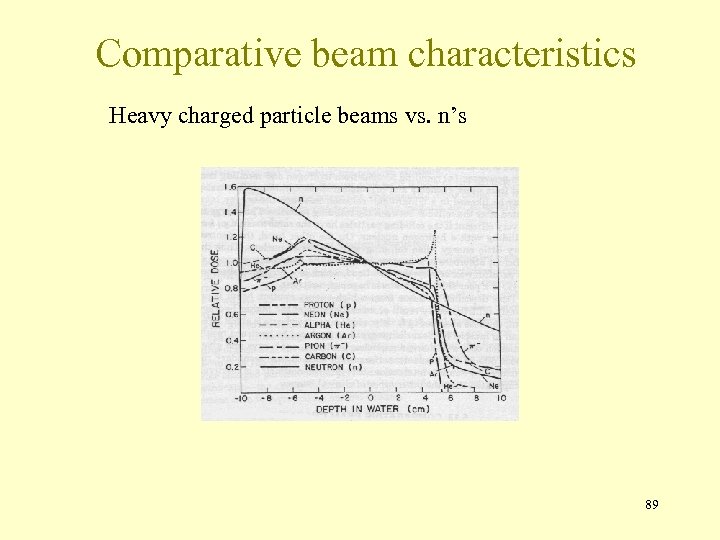 Comparative beam characteristics Heavy charged particle beams vs. n’s 89
Comparative beam characteristics Heavy charged particle beams vs. n’s 89
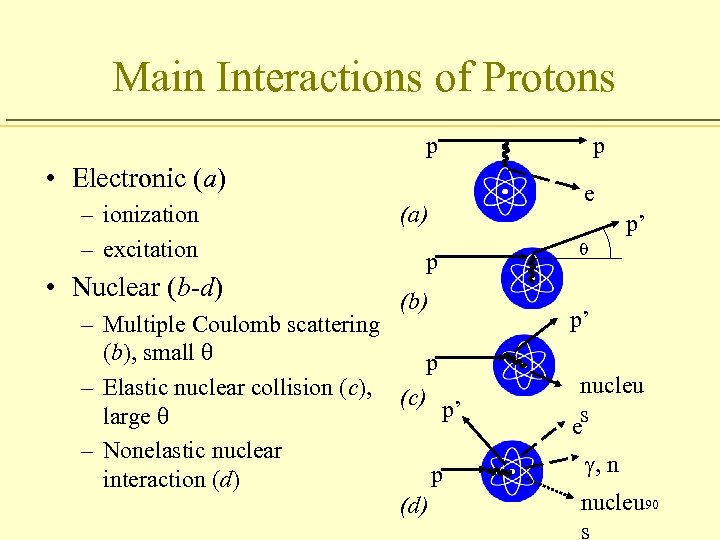 Main Interactions of Protons p • Electronic (a) – ionization – excitation • Nuclear (b-d) (a) p (b) – Multiple Coulomb scattering (b), small q p – Elastic nuclear collision (c), (c) p’ large q – Nonelastic nuclear p interaction (d) p e p’ q p’ nucleu es g, n nucleu 90 s
Main Interactions of Protons p • Electronic (a) – ionization – excitation • Nuclear (b-d) (a) p (b) – Multiple Coulomb scattering (b), small q p – Elastic nuclear collision (c), (c) p’ large q – Nonelastic nuclear p interaction (d) p e p’ q p’ nucleu es g, n nucleu 90 s
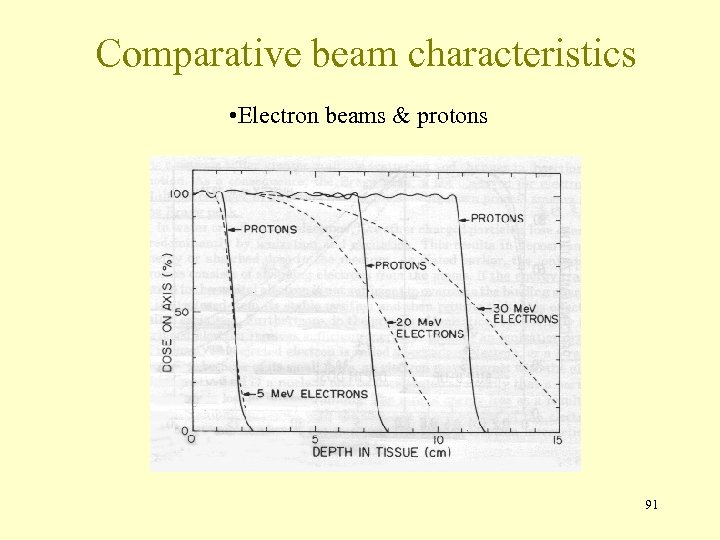 Comparative beam characteristics • Electron beams & protons 91
Comparative beam characteristics • Electron beams & protons 91
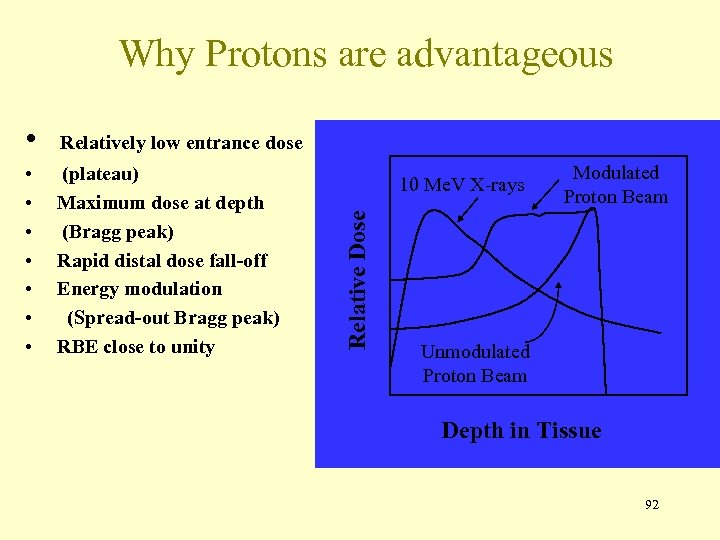 Why Protons are advantageous Relatively low entrance dose • • (plateau) Maximum dose at depth (Bragg peak) Rapid distal dose fall-off Energy modulation (Spread-out Bragg peak) RBE close to unity 10 Me. V X-rays Relative Dose • Modulated Proton Beam Unmodulated Proton Beam Depth in Tissue 92
Why Protons are advantageous Relatively low entrance dose • • (plateau) Maximum dose at depth (Bragg peak) Rapid distal dose fall-off Energy modulation (Spread-out Bragg peak) RBE close to unity 10 Me. V X-rays Relative Dose • Modulated Proton Beam Unmodulated Proton Beam Depth in Tissue 92
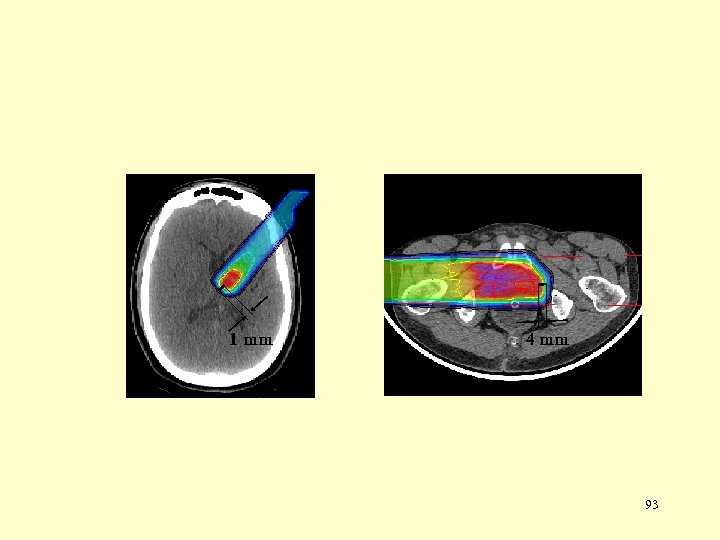 1 mm 4 mm 93
1 mm 4 mm 93
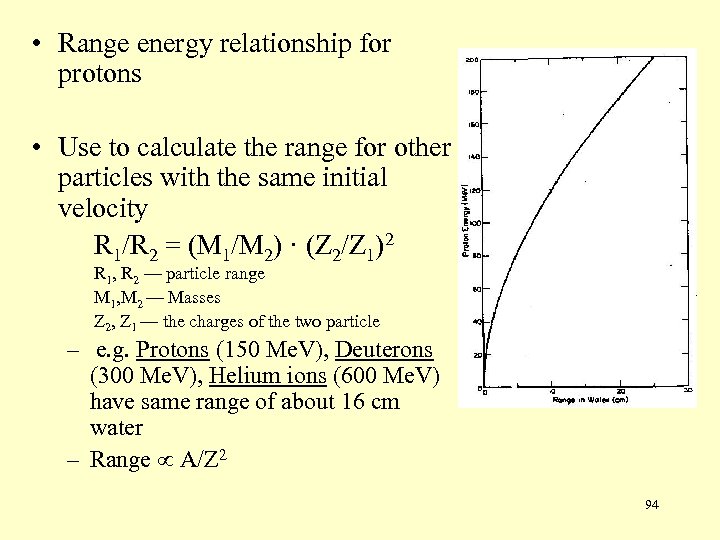 • Range energy relationship for protons • Use to calculate the range for other particles with the same initial velocity R 1/R 2 = (M 1/M 2) · (Z 2/Z 1)2 R 1, R 2 — particle range M 1, M 2 — Masses Z 2, Z 1 — the charges of the two particle – e. g. Protons (150 Me. V), Deuterons (300 Me. V), Helium ions (600 Me. V) have same range of about 16 cm water – Range A/Z 2 94
• Range energy relationship for protons • Use to calculate the range for other particles with the same initial velocity R 1/R 2 = (M 1/M 2) · (Z 2/Z 1)2 R 1, R 2 — particle range M 1, M 2 — Masses Z 2, Z 1 — the charges of the two particle – e. g. Protons (150 Me. V), Deuterons (300 Me. V), Helium ions (600 Me. V) have same range of about 16 cm water – Range A/Z 2 94
 Negative Pions • Pi meson (pion, π) – Protons and neutrons are held together by a mutual exchange of pi mesons – Mass : 237 x of electron – Charge : π+, π-, π0 – Decay: π+ → μ+ + ν (mean life: 2. 54 x 10 -18) π- → μ- + ν (mean life: 2. 54 x 10 -18) π0 → hν 1 + hν 2(mean life: 2. 54 x 10 -18) μ— mesons; ν — neutrinos 95
Negative Pions • Pi meson (pion, π) – Protons and neutrons are held together by a mutual exchange of pi mesons – Mass : 237 x of electron – Charge : π+, π-, π0 – Decay: π+ → μ+ + ν (mean life: 2. 54 x 10 -18) π- → μ- + ν (mean life: 2. 54 x 10 -18) π0 → hν 1 + hν 2(mean life: 2. 54 x 10 -18) μ— mesons; ν — neutrinos 95
 Negative Pions • Pi meson (pion, π) (con’t) – Sources: • nuclear reactor • Cyclotron or linear accelerator with protons (400 – 800 Me. V) and beryllium as target material – Energy range of pion interest in R/T — 100 Me. V – Range in water about — 24 cm – Problems • Low dose rates • Beam contamination • High cost 96
Negative Pions • Pi meson (pion, π) (con’t) – Sources: • nuclear reactor • Cyclotron or linear accelerator with protons (400 – 800 Me. V) and beryllium as target material – Energy range of pion interest in R/T — 100 Me. V – Range in water about — 24 cm – Problems • Low dose rates • Beam contamination • High cost 96


