d71a2deb725402c63f8dc0890b8a50c6.ppt
- Количество слайдов: 33
 The Pharmacogenetics of Asthma Therapy Wayne Anderson, Ph. D. Therapeutic Area Head, Respiratory Translational Medicine and Genetics Glaxo. Smith. Kline
The Pharmacogenetics of Asthma Therapy Wayne Anderson, Ph. D. Therapeutic Area Head, Respiratory Translational Medicine and Genetics Glaxo. Smith. Kline
 Outline • Introduction – Some Genetics Basics – What is Pharmacogenetics • Respiratory Pharmacogenetic Examples – Leukotriene Modifiers – Glucocorticoids – Beta Agonists • The Future Impact of Pharmacogenetics – How does this affect drug discovery – How will this affect clinical practice
Outline • Introduction – Some Genetics Basics – What is Pharmacogenetics • Respiratory Pharmacogenetic Examples – Leukotriene Modifiers – Glucocorticoids – Beta Agonists • The Future Impact of Pharmacogenetics – How does this affect drug discovery – How will this affect clinical practice
 Sir Archibald E. Garrod 1858 -1936 Coined the term “Chemical Instability” “Even against chemical poisons taken by mouth, or by other channels, there are some means of defense. Every active drug is a poison, when taken in large enough doses; and in some subjects a dose which is innocuous to the majority of people has toxic effects, whereas others show exceptional tolerance of the same drug. Some chemical poisons are destroyed in the tissues, provided that the dose given be not too large, and others are combined up with substances to hand, and so rendered innocuous and got rid of. ”
Sir Archibald E. Garrod 1858 -1936 Coined the term “Chemical Instability” “Even against chemical poisons taken by mouth, or by other channels, there are some means of defense. Every active drug is a poison, when taken in large enough doses; and in some subjects a dose which is innocuous to the majority of people has toxic effects, whereas others show exceptional tolerance of the same drug. Some chemical poisons are destroyed in the tissues, provided that the dose given be not too large, and others are combined up with substances to hand, and so rendered innocuous and got rid of. ”
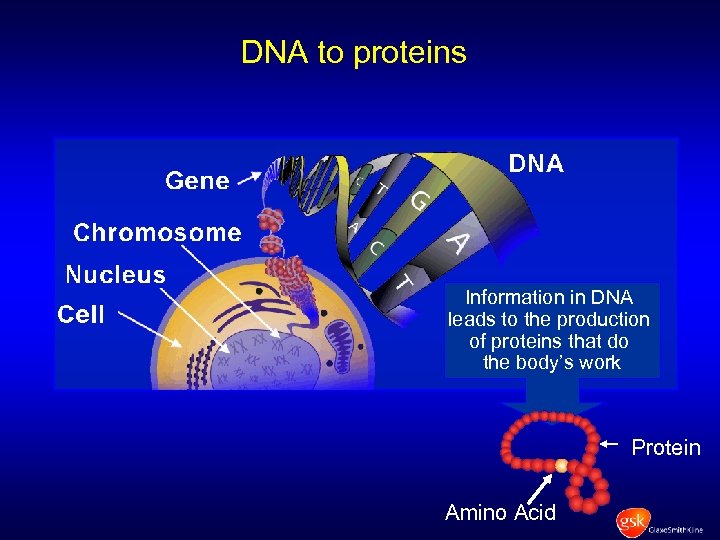 DNA to proteins Information in DNA leads to the production of proteins that do the body’s work Protein Amino Acid
DNA to proteins Information in DNA leads to the production of proteins that do the body’s work Protein Amino Acid
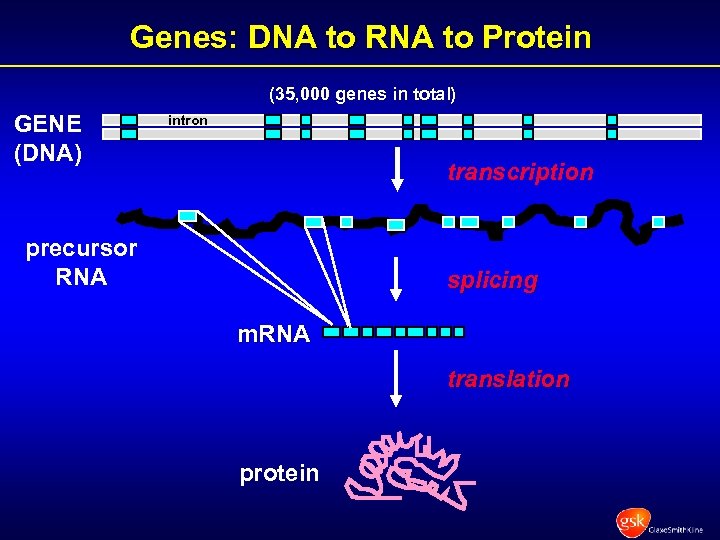 Genes: DNA to RNA to Protein (35, 000 genes in total) GENE (DNA) intron transcription precursor RNA splicing m. RNA translation protein
Genes: DNA to RNA to Protein (35, 000 genes in total) GENE (DNA) intron transcription precursor RNA splicing m. RNA translation protein
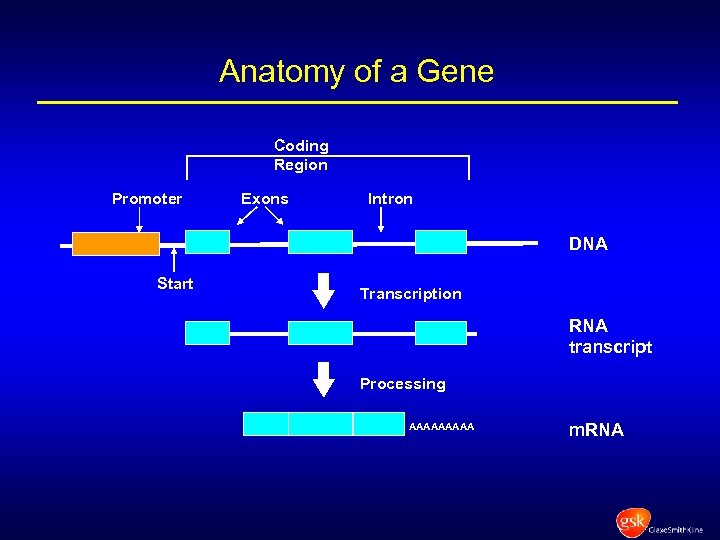 Anatomy of a Gene Coding Region Promoter Exons Intron DNA Start Transcription RNA transcript Processing AAAAA m. RNA
Anatomy of a Gene Coding Region Promoter Exons Intron DNA Start Transcription RNA transcript Processing AAAAA m. RNA
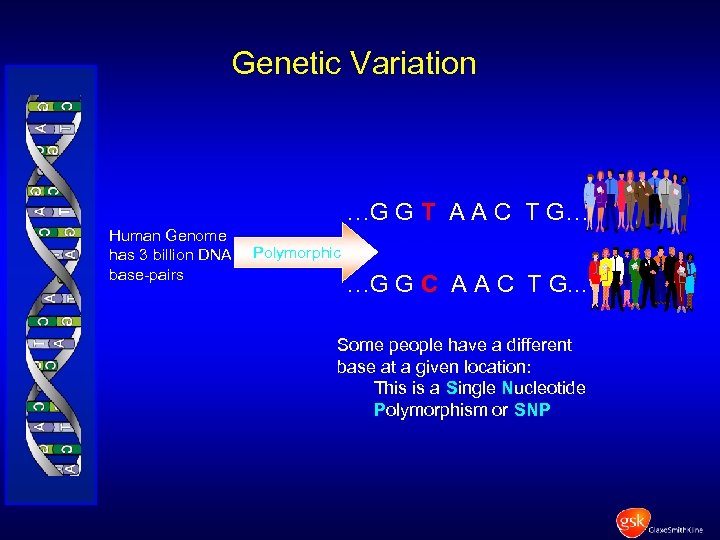 Genetic Variation …G G T A A C T G… Human Genome has 3 billion DNA base-pairs Polymorphic …G G C A A C T G. . . Some people have a different base at a given location: This is a Single Nucleotide Polymorphism or SNP
Genetic Variation …G G T A A C T G… Human Genome has 3 billion DNA base-pairs Polymorphic …G G C A A C T G. . . Some people have a different base at a given location: This is a Single Nucleotide Polymorphism or SNP
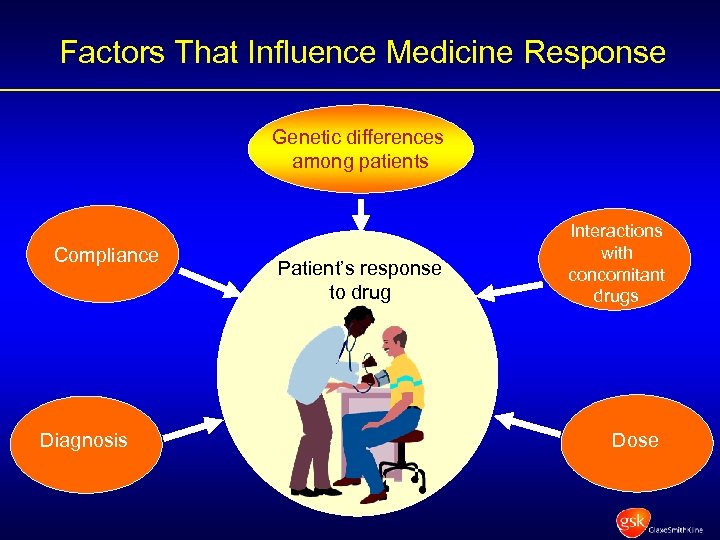 Factors That Influence Medicine Response Genetic differences among patients Compliance Diagnosis Patient’s response to drug Interactions with concomitant drugs Dose
Factors That Influence Medicine Response Genetic differences among patients Compliance Diagnosis Patient’s response to drug Interactions with concomitant drugs Dose
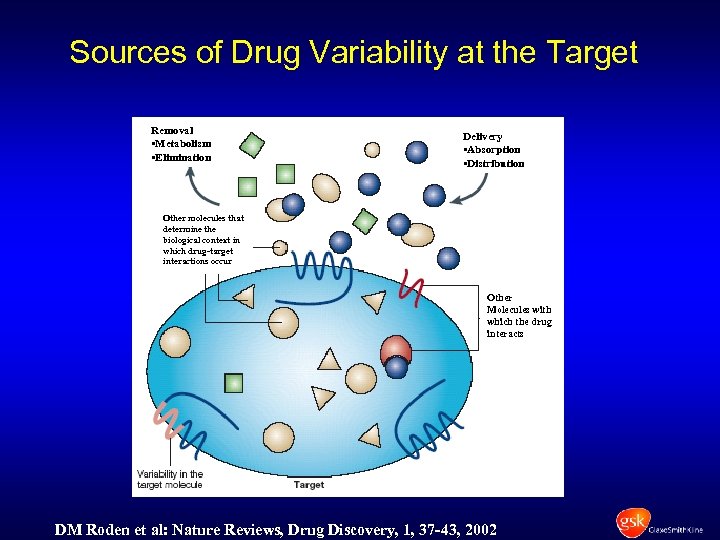 Sources of Drug Variability at the Target Removal • Metabolism • Elimination Delivery • Absorption • Distribution Other molecules that determine the biological context in which drug-target interactions occur Other Molecules with which the drug interacts DM Roden et al: Nature Reviews, Drug Discovery, 1, 37 -43, 2002
Sources of Drug Variability at the Target Removal • Metabolism • Elimination Delivery • Absorption • Distribution Other molecules that determine the biological context in which drug-target interactions occur Other Molecules with which the drug interacts DM Roden et al: Nature Reviews, Drug Discovery, 1, 37 -43, 2002
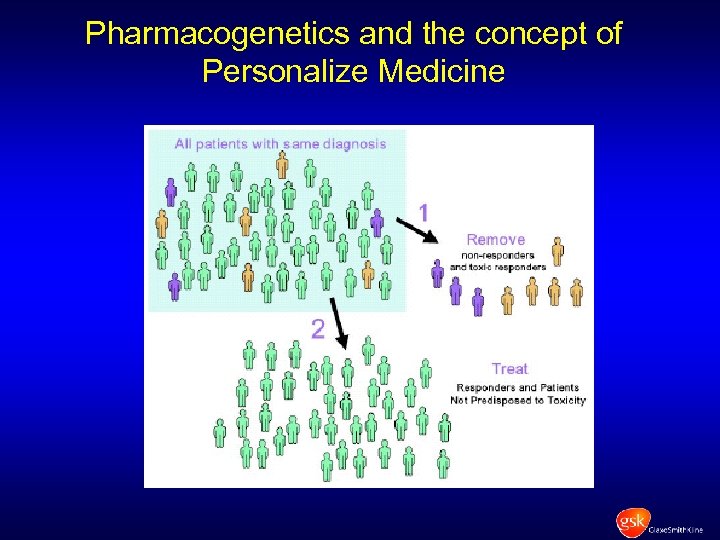 Pharmacogenetics and the concept of Personalize Medicine
Pharmacogenetics and the concept of Personalize Medicine
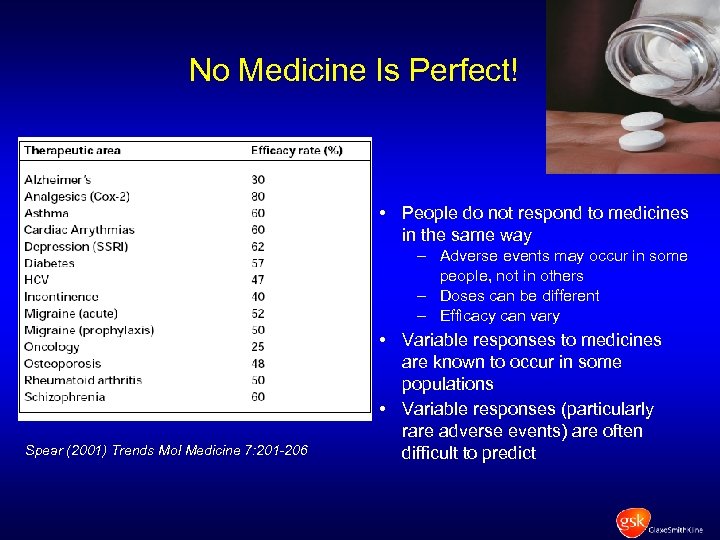 No Medicine Is Perfect! • People do not respond to medicines in the same way – Adverse events may occur in some people, not in others – Doses can be different – Efficacy can vary Spear (2001) Trends Mol Medicine 7: 201 -206 • Variable responses to medicines are known to occur in some populations • Variable responses (particularly rare adverse events) are often difficult to predict
No Medicine Is Perfect! • People do not respond to medicines in the same way – Adverse events may occur in some people, not in others – Doses can be different – Efficacy can vary Spear (2001) Trends Mol Medicine 7: 201 -206 • Variable responses to medicines are known to occur in some populations • Variable responses (particularly rare adverse events) are often difficult to predict
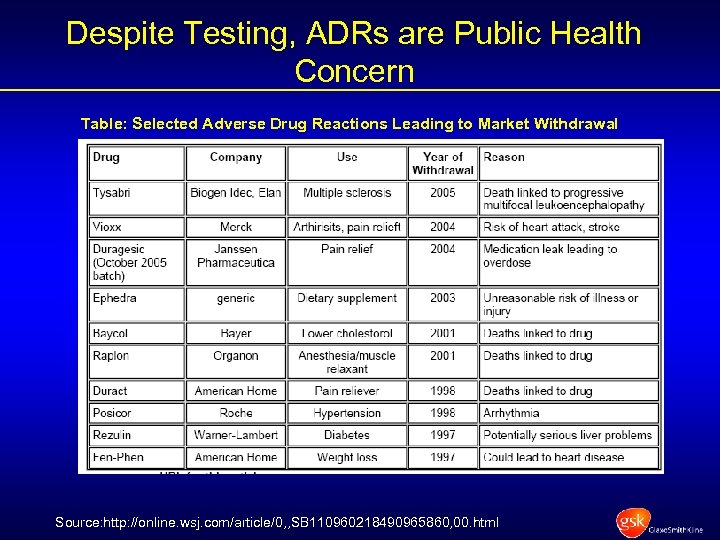 Despite Testing, ADRs are Public Health Concern Table: Selected Adverse Drug Reactions Leading to Market Withdrawal Source: http: //online. wsj. com/article/0, , SB 110960218490965860, 00. html
Despite Testing, ADRs are Public Health Concern Table: Selected Adverse Drug Reactions Leading to Market Withdrawal Source: http: //online. wsj. com/article/0, , SB 110960218490965860, 00. html
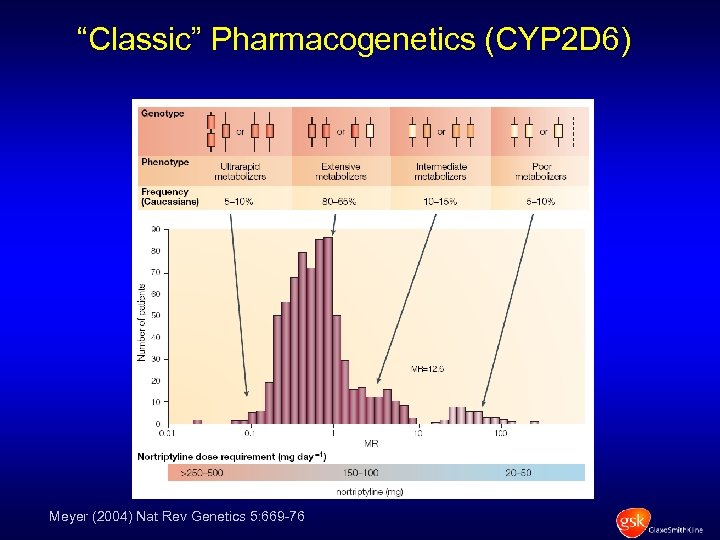 “Classic” Pharmacogenetics (CYP 2 D 6) Meyer (2004) Nat Rev Genetics 5: 669 -76
“Classic” Pharmacogenetics (CYP 2 D 6) Meyer (2004) Nat Rev Genetics 5: 669 -76
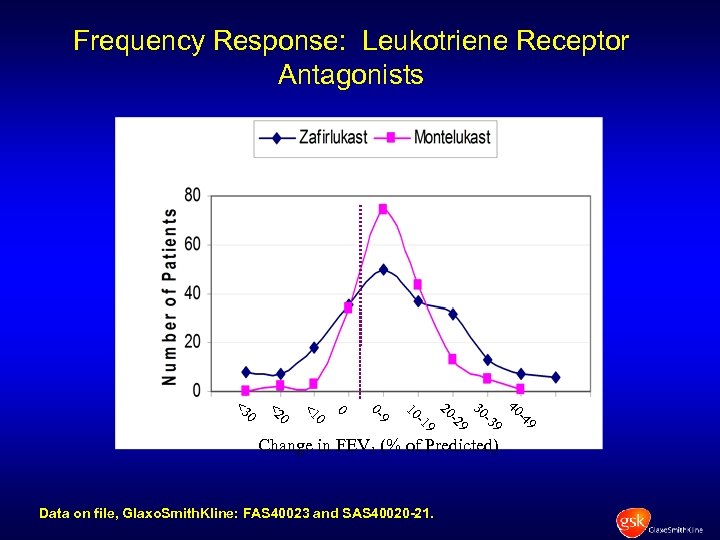 Frequency Response: Leukotriene Receptor Antagonists 9 -4 40 9 -3 30 9 -2 20 9 -1 10 9 0 - 0 0 <1 0 <2 0 <3 Change in FEV 1 (% of Predicted) Data on file, Glaxo. Smith. Kline: FAS 40023 and SAS 40020 -21.
Frequency Response: Leukotriene Receptor Antagonists 9 -4 40 9 -3 30 9 -2 20 9 -1 10 9 0 - 0 0 <1 0 <2 0 <3 Change in FEV 1 (% of Predicted) Data on file, Glaxo. Smith. Kline: FAS 40023 and SAS 40020 -21.
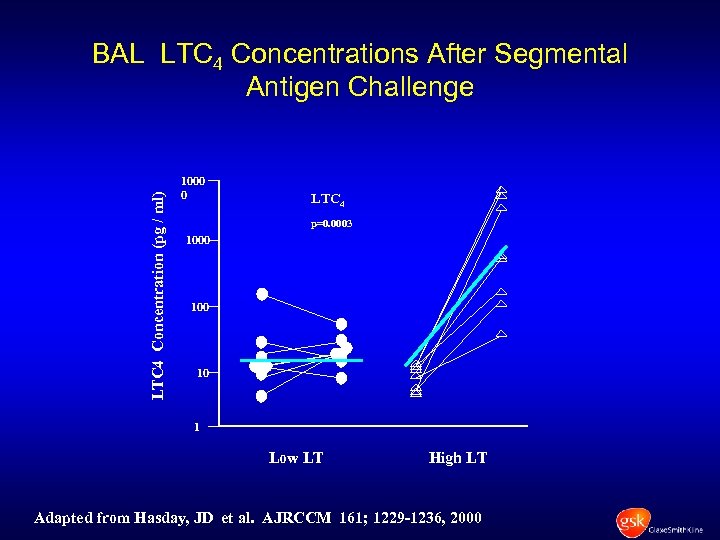 LTC 4 Concentration (pg / ml) BAL LTC 4 Concentrations After Segmental Antigen Challenge 1000 0 LTC 4 p=0. 0003 1000 10 1 Low LT High LT Adapted from Hasday, JD et al. AJRCCM 161; 1229 -1236, 2000
LTC 4 Concentration (pg / ml) BAL LTC 4 Concentrations After Segmental Antigen Challenge 1000 0 LTC 4 p=0. 0003 1000 10 1 Low LT High LT Adapted from Hasday, JD et al. AJRCCM 161; 1229 -1236, 2000
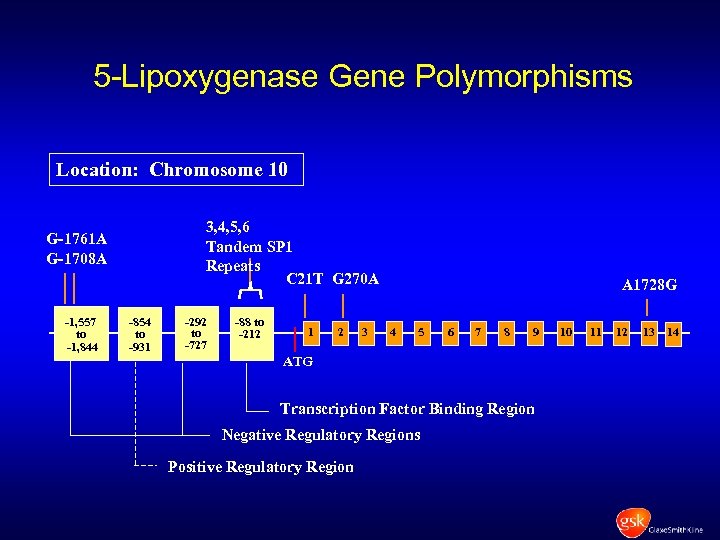 5 -Lipoxygenase Gene Polymorphisms Location: Chromosome 10 3, 4, 5, 6 Tandem SP 1 Repeats C 21 T G 270 A G-1761 A G-1708 A -1, 557 to -1, 844 -854 to -931 -88 to -292 to -212 -727 -88 to -212 1 2 3 A 1728 G 4 5 6 7 8 9 ATG Transcription Factor Binding Region Negative Regulatory Regions Positive Regulatory Region 10 11 12 13 14
5 -Lipoxygenase Gene Polymorphisms Location: Chromosome 10 3, 4, 5, 6 Tandem SP 1 Repeats C 21 T G 270 A G-1761 A G-1708 A -1, 557 to -1, 844 -854 to -931 -88 to -292 to -212 -727 -88 to -212 1 2 3 A 1728 G 4 5 6 7 8 9 ATG Transcription Factor Binding Region Negative Regulatory Regions Positive Regulatory Region 10 11 12 13 14
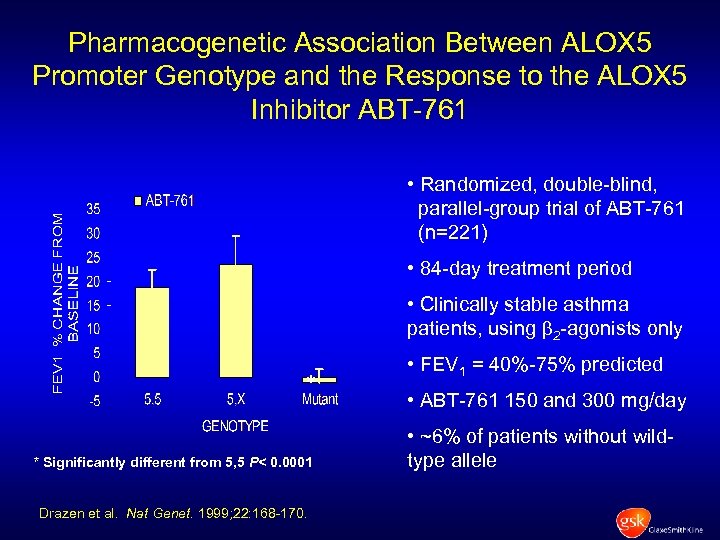 Pharmacogenetic Association Between ALOX 5 Promoter Genotype and the Response to the ALOX 5 Inhibitor ABT-761 • Randomized, double-blind, parallel-group trial of ABT-761 (n=221) • 84 -day treatment period • Clinically stable asthma patients, using 2 -agonists only * * Significantly different from 5, 5 P< 0. 0001 Drazen et al. Nat Genet. 1999; 22: 168 -170. • FEV 1 = 40%-75% predicted • ABT-761 150 and 300 mg/day • ~6% of patients without wildtype allele
Pharmacogenetic Association Between ALOX 5 Promoter Genotype and the Response to the ALOX 5 Inhibitor ABT-761 • Randomized, double-blind, parallel-group trial of ABT-761 (n=221) • 84 -day treatment period • Clinically stable asthma patients, using 2 -agonists only * * Significantly different from 5, 5 P< 0. 0001 Drazen et al. Nat Genet. 1999; 22: 168 -170. • FEV 1 = 40%-75% predicted • ABT-761 150 and 300 mg/day • ~6% of patients without wildtype allele
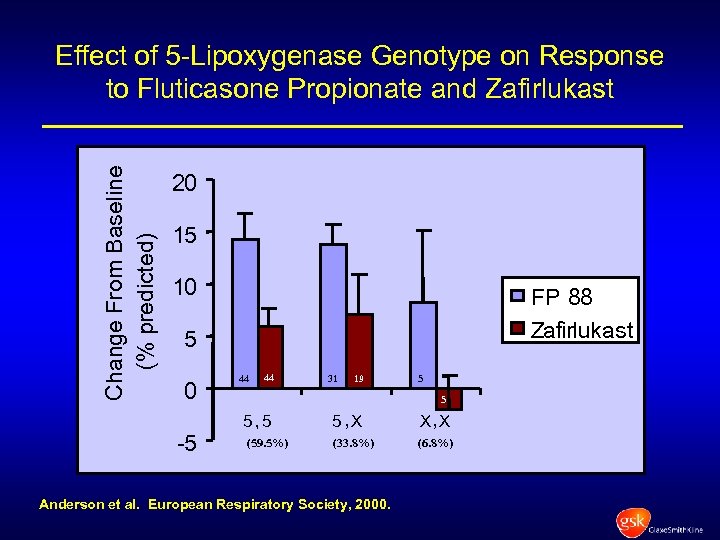 Change From Baseline (% predicted) Effect of 5 -Lipoxygenase Genotype on Response to Fluticasone Propionate and Zafirlukast 20 15 10 FP 88 Zafirlukast 5 0 -5 44 44 31 19 5 5 5, 5 (59. 5%) 5 , X X, X (33. 8%) (6. 8%) Anderson et al. European Respiratory Society, 2000.
Change From Baseline (% predicted) Effect of 5 -Lipoxygenase Genotype on Response to Fluticasone Propionate and Zafirlukast 20 15 10 FP 88 Zafirlukast 5 0 -5 44 44 31 19 5 5 5, 5 (59. 5%) 5 , X X, X (33. 8%) (6. 8%) Anderson et al. European Respiratory Society, 2000.
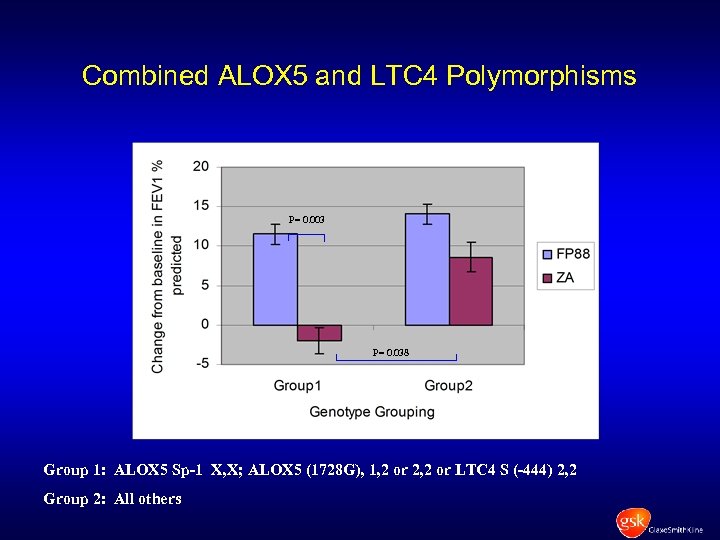 Combined ALOX 5 and LTC 4 Polymorphisms P= 0. 003 P= 0. 038 Group 1: ALOX 5 Sp-1 X, X; ALOX 5 (1728 G), 1, 2 or 2, 2 or LTC 4 S (-444) 2, 2 Group 2: All others
Combined ALOX 5 and LTC 4 Polymorphisms P= 0. 003 P= 0. 038 Group 1: ALOX 5 Sp-1 X, X; ALOX 5 (1728 G), 1, 2 or 2, 2 or LTC 4 S (-444) 2, 2 Group 2: All others
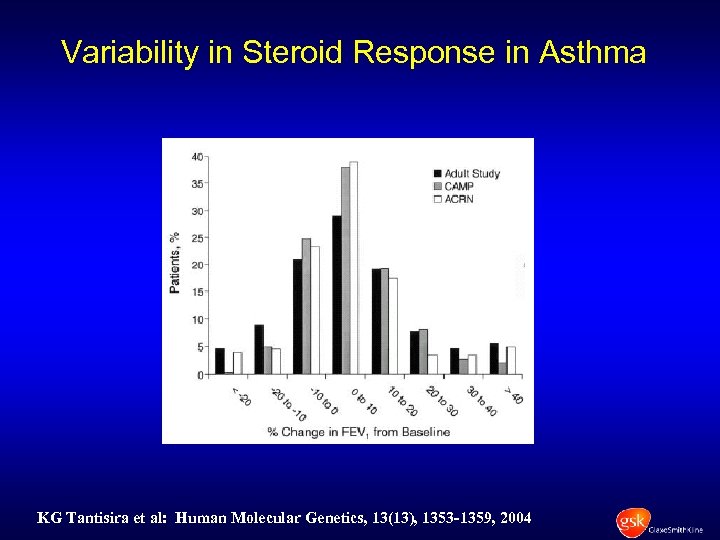 Variability in Steroid Response in Asthma KG Tantisira et al: Human Molecular Genetics, 13(13), 1353 -1359, 2004
Variability in Steroid Response in Asthma KG Tantisira et al: Human Molecular Genetics, 13(13), 1353 -1359, 2004
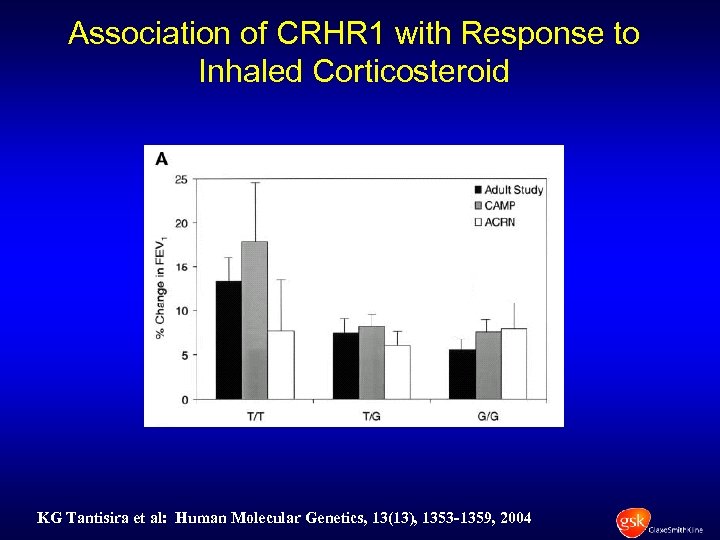 Association of CRHR 1 with Response to Inhaled Corticosteroid KG Tantisira et al: Human Molecular Genetics, 13(13), 1353 -1359, 2004
Association of CRHR 1 with Response to Inhaled Corticosteroid KG Tantisira et al: Human Molecular Genetics, 13(13), 1353 -1359, 2004
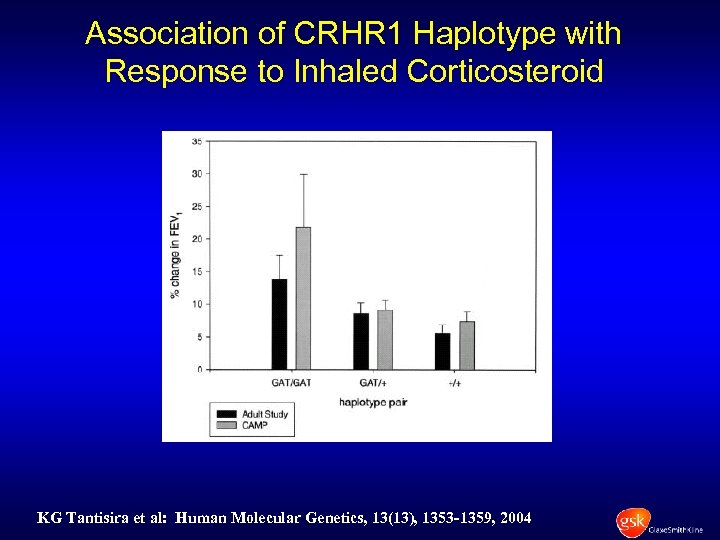 Association of CRHR 1 Haplotype with Response to Inhaled Corticosteroid KG Tantisira et al: Human Molecular Genetics, 13(13), 1353 -1359, 2004
Association of CRHR 1 Haplotype with Response to Inhaled Corticosteroid KG Tantisira et al: Human Molecular Genetics, 13(13), 1353 -1359, 2004
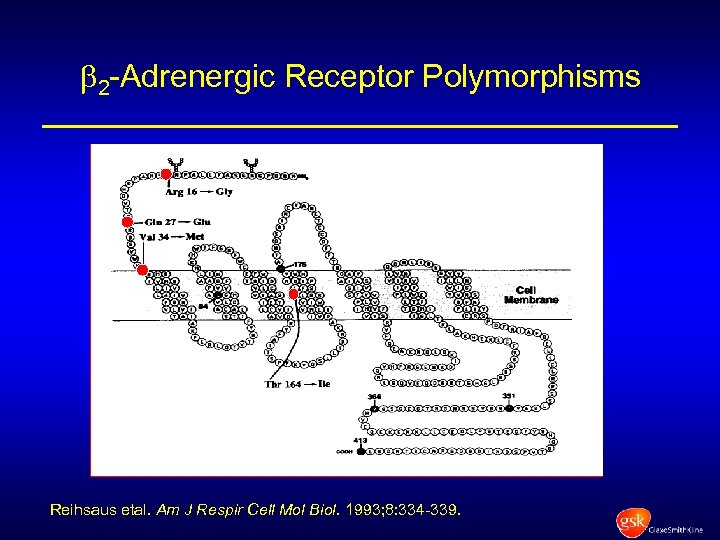 2 -Adrenergic Receptor Polymorphisms Reihsaus etal. Am J Respir Cell Mol Biol. 1993; 8: 334 -339.
2 -Adrenergic Receptor Polymorphisms Reihsaus etal. Am J Respir Cell Mol Biol. 1993; 8: 334 -339.
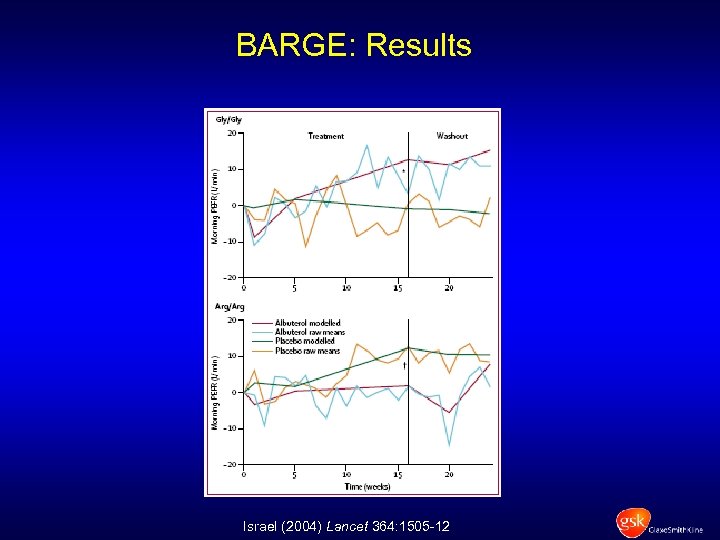 BARGE: Results Israel (2004) Lancet 364: 1505 -12
BARGE: Results Israel (2004) Lancet 364: 1505 -12
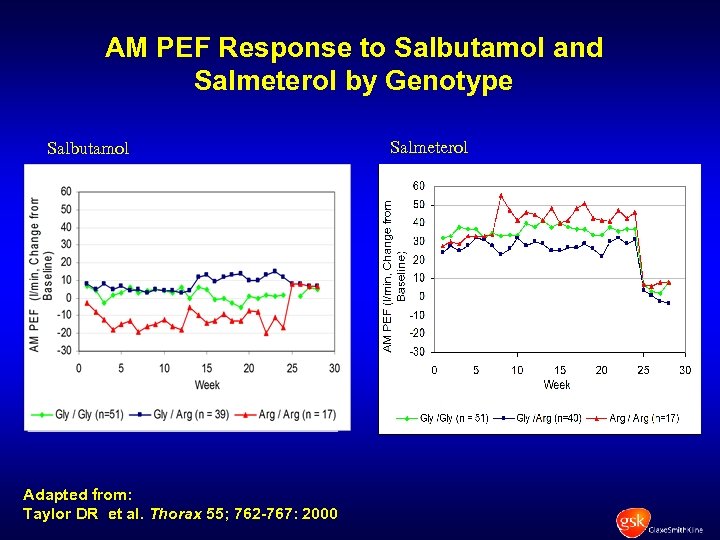 AM PEF Response to Salbutamol and Salmeterol by Genotype Salbutamol Adapted from: Taylor DR et al. Thorax 55; 762 -767: 2000 Salmeterol
AM PEF Response to Salbutamol and Salmeterol by Genotype Salbutamol Adapted from: Taylor DR et al. Thorax 55; 762 -767: 2000 Salmeterol
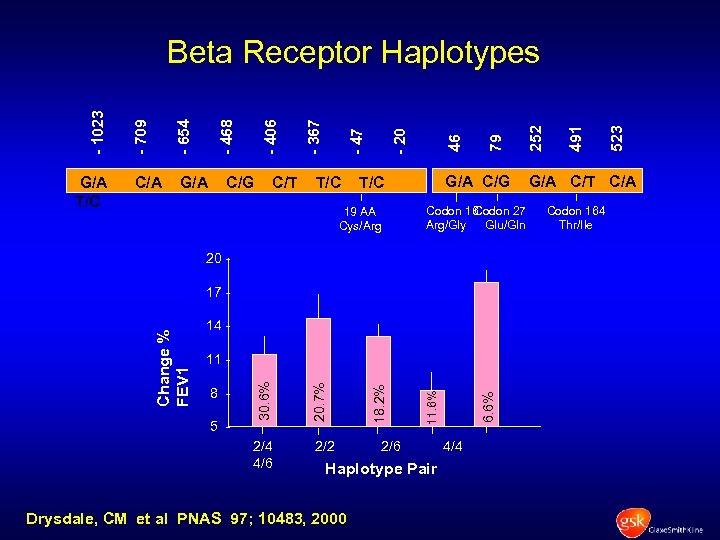 G/A T/C C/A G/A C/G C/T T/C G/A C/G T/C 19 AA Cys/Arg Codon 16 Codon 27 Arg/Gly Glu/Gln 20 - 14 - 2/6 Haplotype Pair Drysdale, CM et al PNAS 97; 10483, 2000 6. 6% 2/2 11. 6% 5 - 18. 2% 2/4 4/6 8 - 20. 7% 11 30. 6% Change % FEV 1 17 - 4/4 523 491 252 79 46 - 20 - 47 - 367 - 406 - 468 - 654 - 709 - 1023 Beta Receptor Haplotypes G/A C/T C/A Codon 164 Thr/Ile
G/A T/C C/A G/A C/G C/T T/C G/A C/G T/C 19 AA Cys/Arg Codon 16 Codon 27 Arg/Gly Glu/Gln 20 - 14 - 2/6 Haplotype Pair Drysdale, CM et al PNAS 97; 10483, 2000 6. 6% 2/2 11. 6% 5 - 18. 2% 2/4 4/6 8 - 20. 7% 11 30. 6% Change % FEV 1 17 - 4/4 523 491 252 79 46 - 20 - 47 - 367 - 406 - 468 - 654 - 709 - 1023 Beta Receptor Haplotypes G/A C/T C/A Codon 164 Thr/Ile
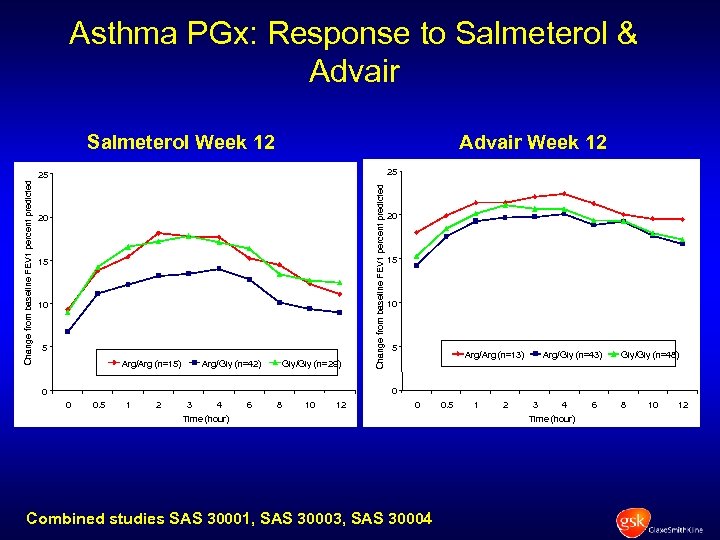 Asthma PGx: Response to Salmeterol & Advair Salmeterol Week 12 Advair Week 12 25 20 15 10 5 Arg/Arg (n=15) Arg/Gly (n=42) Gly/Gly (n=29) Change from baseline FEV 1 percent predicted 25 20 15 10 5 Arg/Arg (n=13) Arg/Gly (n=43) Gly/Gly (n=48) 0 0. 5 1 2 3 4 6 8 10 12 0 Time (hour) Combined studies SAS 30001, SAS 30003, SAS 30004 0. 5 1 2 3 4 Time (hour) 6 8 10 12
Asthma PGx: Response to Salmeterol & Advair Salmeterol Week 12 Advair Week 12 25 20 15 10 5 Arg/Arg (n=15) Arg/Gly (n=42) Gly/Gly (n=29) Change from baseline FEV 1 percent predicted 25 20 15 10 5 Arg/Arg (n=13) Arg/Gly (n=43) Gly/Gly (n=48) 0 0. 5 1 2 3 4 6 8 10 12 0 Time (hour) Combined studies SAS 30001, SAS 30003, SAS 30004 0. 5 1 2 3 4 Time (hour) 6 8 10 12
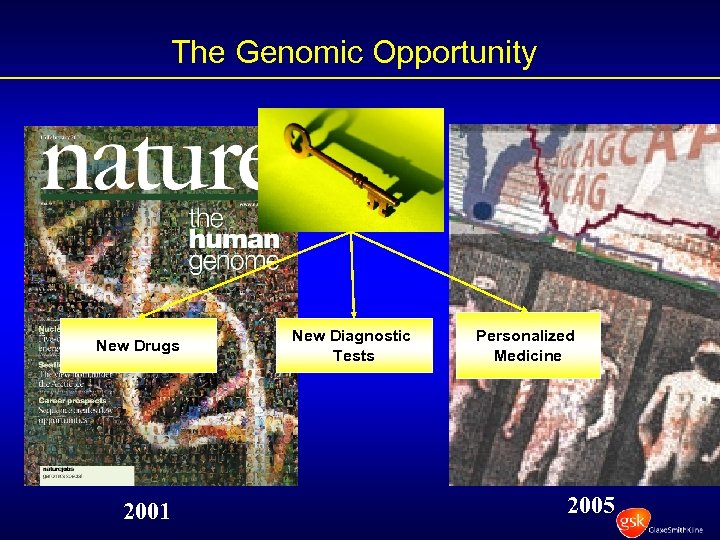 The Genomic Opportunity New Drugs 2001 New Diagnostic Tests Personalized Medicine 2005
The Genomic Opportunity New Drugs 2001 New Diagnostic Tests Personalized Medicine 2005
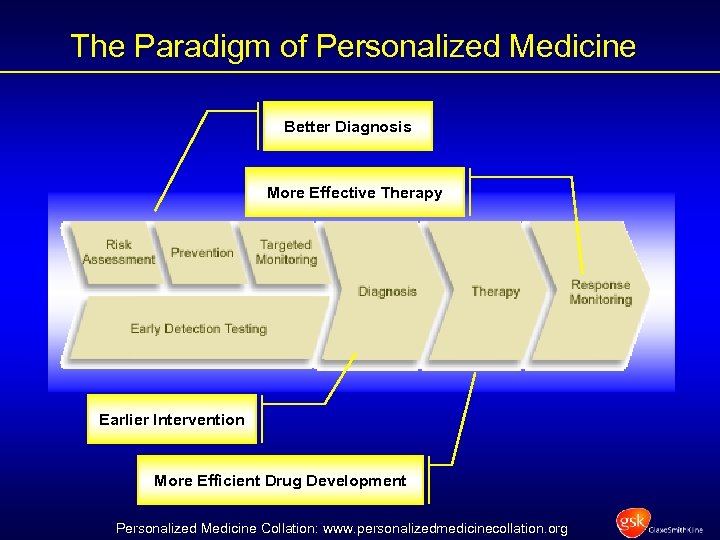 The Paradigm of Personalized Medicine Better Diagnosis More Effective Therapy Earlier Intervention More Efficient Drug Development Personalized Medicine Collation: www. personalizedmedicinecollation. org
The Paradigm of Personalized Medicine Better Diagnosis More Effective Therapy Earlier Intervention More Efficient Drug Development Personalized Medicine Collation: www. personalizedmedicinecollation. org
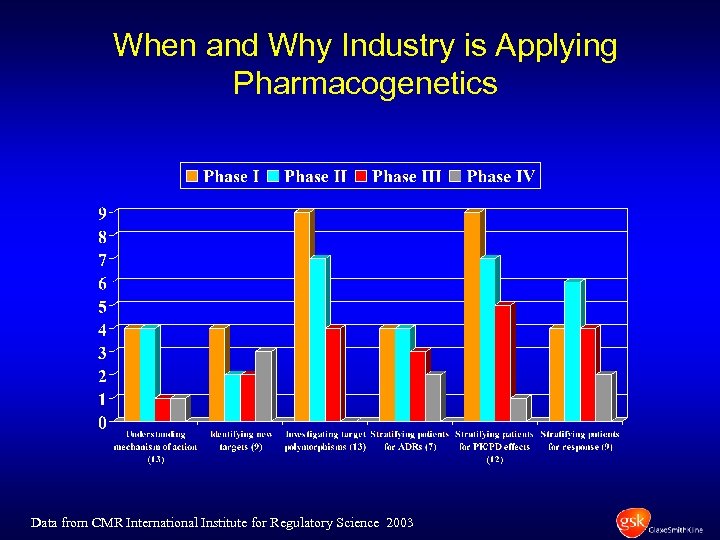 When and Why Industry is Applying Pharmacogenetics Data from CMR International Institute for Regulatory Science 2003
When and Why Industry is Applying Pharmacogenetics Data from CMR International Institute for Regulatory Science 2003
 Public Policy Issues • • • Intellectual Property Regulatory Oversight Privacy and Ethics Reimbursement Genetic Discrimination (Insurance, employment) Patient Education Physician Education Hospital system infrastructure R&D Incentives
Public Policy Issues • • • Intellectual Property Regulatory Oversight Privacy and Ethics Reimbursement Genetic Discrimination (Insurance, employment) Patient Education Physician Education Hospital system infrastructure R&D Incentives
 Personalized Medicine: The Right Drug, for the Right Patient, at the Right Time Risk Prediction • Begin Colonoscopy @ 40 yrs of age • Avoid high fat in diet Pharmacogenomics • Drug dose selected by drug metabolism genetic profile New Therapies • Targeted gene-based therapy with improved efficacy and decreased AEs
Personalized Medicine: The Right Drug, for the Right Patient, at the Right Time Risk Prediction • Begin Colonoscopy @ 40 yrs of age • Avoid high fat in diet Pharmacogenomics • Drug dose selected by drug metabolism genetic profile New Therapies • Targeted gene-based therapy with improved efficacy and decreased AEs


