980c7361f9b61c9f5b4b63f01c60baa1.ppt
- Количество слайдов: 55
 The Past, Present and Future of Salmonella Control in Poultry: The Example of Salmonella enteritidis Prepared by Richard K. Gast United States Department of Agriculture Agricultural Research Service Southeast Poultry Research Laboratory Athens, Georgia
The Past, Present and Future of Salmonella Control in Poultry: The Example of Salmonella enteritidis Prepared by Richard K. Gast United States Department of Agriculture Agricultural Research Service Southeast Poultry Research Laboratory Athens, Georgia
 Modified and presented by Prof. Dr. Mohamed Refai Department of Microbiology Faculty of Veterinary Medicine Cairo University, Giza, Egypt At the International Poultry Conference in Cairo
Modified and presented by Prof. Dr. Mohamed Refai Department of Microbiology Faculty of Veterinary Medicine Cairo University, Giza, Egypt At the International Poultry Conference in Cairo
 The genus Salmonella (Lignieres, 1900) n n n n Salmonella choleraesuis ( Salmon, 1885) Salmonella typhi (Schroeter, 1886) Salmonella enteritidis (Gaertner, 1888) Salmonella london, panama, cairo etc Salmonella arizonae (Kauffmann, 1964) Salmonella bongori (Le. Minor, 1985) Salmonella enterica (Le. Minor, 1987) Now we have more than 2300 Salmonellae
The genus Salmonella (Lignieres, 1900) n n n n Salmonella choleraesuis ( Salmon, 1885) Salmonella typhi (Schroeter, 1886) Salmonella enteritidis (Gaertner, 1888) Salmonella london, panama, cairo etc Salmonella arizonae (Kauffmann, 1964) Salmonella bongori (Le. Minor, 1985) Salmonella enterica (Le. Minor, 1987) Now we have more than 2300 Salmonellae
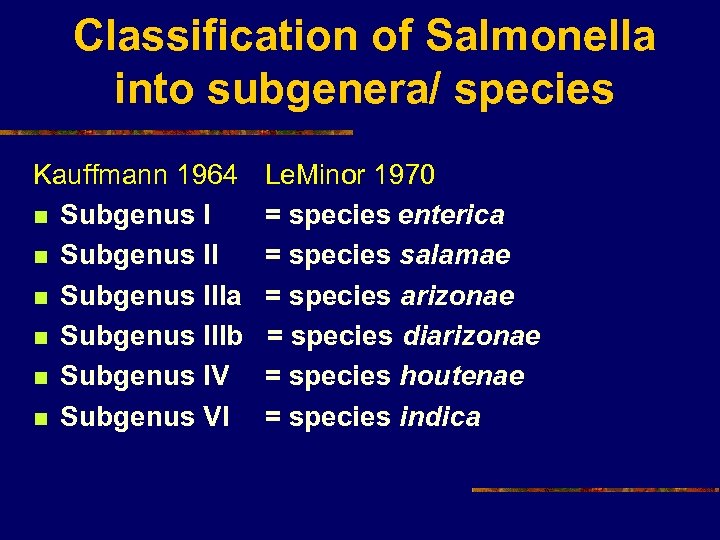 Classification of Salmonella into subgenera/ species Kauffmann 1964 n Subgenus IIIa n Subgenus IIIb n Subgenus IV n Subgenus VI Le. Minor 1970 = species enterica = species salamae = species arizonae = species diarizonae = species houtenae = species indica
Classification of Salmonella into subgenera/ species Kauffmann 1964 n Subgenus IIIa n Subgenus IIIb n Subgenus IV n Subgenus VI Le. Minor 1970 = species enterica = species salamae = species arizonae = species diarizonae = species houtenae = species indica
 Salmonella species n Three species * S. cholerae-suis, S. typhosa, S. kauffmanni * S. cholerae-suis, S. typhi, S. enteritidis One species * S. enteritidis n Two species * S. enterica , S. bongori n
Salmonella species n Three species * S. cholerae-suis, S. typhosa, S. kauffmanni * S. cholerae-suis, S. typhi, S. enteritidis One species * S. enteritidis n Two species * S. enterica , S. bongori n
 Terminology of Salmonella • the complete nomenclature: * S. enterica, subsp. enterica serovar Enteritidis * or Salmonella enterica ser. Enteritidis Salmonella Enteritidis
Terminology of Salmonella • the complete nomenclature: * S. enterica, subsp. enterica serovar Enteritidis * or Salmonella enterica ser. Enteritidis Salmonella Enteritidis
 Antigenic formulae of some serovars of Salmonella enterica Group A Group B Group C Group D 1, 2, 12: a: 1, 5 1, 4, 5, 12: b: 1, 2 1, 4, 5, 12: i: 1, 2 6, 7: c: 1, 5 1, 9, 12: -: 1, 9, 12: g, m: 1, 7 1, 9, 12: g, p: - ser. Paratyphi-A ser. Paratyphi-B ser. Typhimurium ser. Choleraesuis ser. Pullorum ser. Gallinarum ser. Enteritidis ser. Dublin
Antigenic formulae of some serovars of Salmonella enterica Group A Group B Group C Group D 1, 2, 12: a: 1, 5 1, 4, 5, 12: b: 1, 2 1, 4, 5, 12: i: 1, 2 6, 7: c: 1, 5 1, 9, 12: -: 1, 9, 12: g, m: 1, 7 1, 9, 12: g, p: - ser. Paratyphi-A ser. Paratyphi-B ser. Typhimurium ser. Choleraesuis ser. Pullorum ser. Gallinarum ser. Enteritidis ser. Dublin
 Salmonella Epidemiological Classification n Group 1. Anthropophilic serovars n Salmonella n Typhi Group 2. Zoophilic serovars n Salmonella Gallinarum poultry n Salmonella Choleraesuis swine n Group 3. Serovars with no particular host n All other serovars, including SE
Salmonella Epidemiological Classification n Group 1. Anthropophilic serovars n Salmonella n Typhi Group 2. Zoophilic serovars n Salmonella Gallinarum poultry n Salmonella Choleraesuis swine n Group 3. Serovars with no particular host n All other serovars, including SE
 Incidence of Salmonella Enteritidis infections in laying flocks n Environmental samples from 7. 1% of commercial laying houses in the USA were positive for Salmonella Enteritidis USDA, 2000
Incidence of Salmonella Enteritidis infections in laying flocks n Environmental samples from 7. 1% of commercial laying houses in the USA were positive for Salmonella Enteritidis USDA, 2000
 Salmonella Enteritidis infection in man in the USA Salmonella Enteritidis constitued 5% in 1976 25% in 1994 of human Salmonella reported to CDC n
Salmonella Enteritidis infection in man in the USA Salmonella Enteritidis constitued 5% in 1976 25% in 1994 of human Salmonella reported to CDC n
 Sources of SE outbreaks in the USA, 1995 -1997 In 110 outbreaks reported by CDC 59% no confirmed vehicle n 34% contaminated shell eggs n 07% other than eggs n
Sources of SE outbreaks in the USA, 1995 -1997 In 110 outbreaks reported by CDC 59% no confirmed vehicle n 34% contaminated shell eggs n 07% other than eggs n
 Salmonella Enteritidis contamination of shell eggs The transmission of Salmonella Enteritidis by eggs has become a leading public health issue in the USA
Salmonella Enteritidis contamination of shell eggs The transmission of Salmonella Enteritidis by eggs has become a leading public health issue in the USA
 Sites of human SE outbreaks in the USA, 1998 -1999 Sites Outbreaks n Commercial food preparers 46 n Private homes 19 n Church/community events 10 n Colleges/schools/camps 7 n Nursing homes 5 n Prisons 2
Sites of human SE outbreaks in the USA, 1998 -1999 Sites Outbreaks n Commercial food preparers 46 n Private homes 19 n Church/community events 10 n Colleges/schools/camps 7 n Nursing homes 5 n Prisons 2
 The problem in the past Before 1970 Cracked or dirty table eggs and processed egg products were often implicated in human salmonella outbreaks attention was directed to: external contamination of eggs
The problem in the past Before 1970 Cracked or dirty table eggs and processed egg products were often implicated in human salmonella outbreaks attention was directed to: external contamination of eggs
 Control of External Contamination of eggs Measures *Stringent regulation for shell eggs inspection *Pasteurization of liquid egg products Results: Eggs were nearly eliminated as significant source of human disease
Control of External Contamination of eggs Measures *Stringent regulation for shell eggs inspection *Pasteurization of liquid egg products Results: Eggs were nearly eliminated as significant source of human disease
 The new problem A dramatic increase in incidence of human Salmonella Enteritidis infection is principally caused by consumption of clean and intact but internally contaminated table eggs Attention was directed to Internal contamination of eggs
The new problem A dramatic increase in incidence of human Salmonella Enteritidis infection is principally caused by consumption of clean and intact but internally contaminated table eggs Attention was directed to Internal contamination of eggs
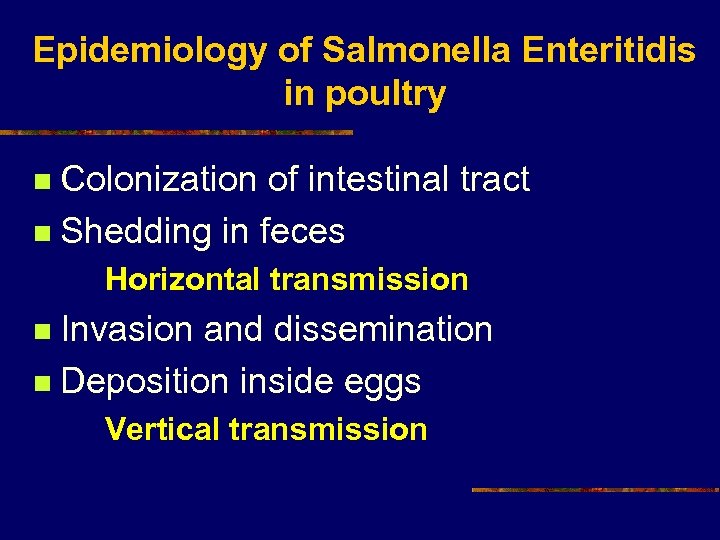 Epidemiology of Salmonella Enteritidis in poultry Colonization of intestinal tract n Shedding in feces n Horizontal transmission Invasion and dissemination n Deposition inside eggs n Vertical transmission
Epidemiology of Salmonella Enteritidis in poultry Colonization of intestinal tract n Shedding in feces n Horizontal transmission Invasion and dissemination n Deposition inside eggs n Vertical transmission
 Internal contamination of eggs • • • Principally before oviposition Fecal contamination and penetration of the shell Contamination during breaking
Internal contamination of eggs • • • Principally before oviposition Fecal contamination and penetration of the shell Contamination during breaking
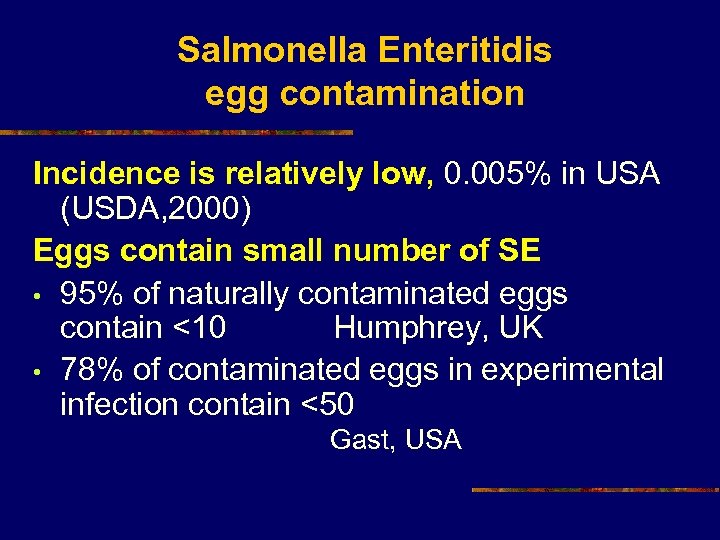 Salmonella Enteritidis egg contamination Incidence is relatively low, 0. 005% in USA (USDA, 2000) Eggs contain small number of SE • 95% of naturally contaminated eggs contain <10 Humphrey, UK • 78% of contaminated eggs in experimental infection contain <50 Gast, USA
Salmonella Enteritidis egg contamination Incidence is relatively low, 0. 005% in USA (USDA, 2000) Eggs contain small number of SE • 95% of naturally contaminated eggs contain <10 Humphrey, UK • 78% of contaminated eggs in experimental infection contain <50 Gast, USA
 Salmonella Enteritidis infection in chickens and egg contamination Consequences Dangerous increase of SE in eggs not before the 3 d week of storage at ambient temperature Humphrey & Whitehead, 1993
Salmonella Enteritidis infection in chickens and egg contamination Consequences Dangerous increase of SE in eggs not before the 3 d week of storage at ambient temperature Humphrey & Whitehead, 1993
 Site of bacterial contamination of eggs * If it is within the nutrient-rich yolk It would lead to rapid and explosive multiplication * If it is in the albumin Multiplication would be restricted by the several inhibitory factors
Site of bacterial contamination of eggs * If it is within the nutrient-rich yolk It would lead to rapid and explosive multiplication * If it is in the albumin Multiplication would be restricted by the several inhibitory factors
 Site of bacterial contamination of eggs in experimentally infected hens (Gast and beard, 1990) *SE was isolated from albumin or entire yolk, including vitelline membrane *SE could not be isolated by sampling only the interior contents of yolk
Site of bacterial contamination of eggs in experimentally infected hens (Gast and beard, 1990) *SE was isolated from albumin or entire yolk, including vitelline membrane *SE could not be isolated by sampling only the interior contents of yolk
 Site of bacterial contamination of eggs Gast and Holt, 2000 • SE can penetrate through the yolk membrane at warm temperature • Instances were reported in which yolk contamination occurred more often than albumin contamination
Site of bacterial contamination of eggs Gast and Holt, 2000 • SE can penetrate through the yolk membrane at warm temperature • Instances were reported in which yolk contamination occurred more often than albumin contamination
 Detection of Salmonella Enteritidis in eggs is difficult • Low incidence of contamination needs large number of eggs to be examined, 10 -30 eggs • Low level of bacterial cells needs long incubation for one or more days
Detection of Salmonella Enteritidis in eggs is difficult • Low incidence of contamination needs large number of eggs to be examined, 10 -30 eggs • Low level of bacterial cells needs long incubation for one or more days
 Human Salmonella Enteritidis outbreaks Human infection requires: * Ambient storage temperature that allow multiplication of SE * Cross-contamination of kitchen surfaces and foods * Improper food handling and preparation practices
Human Salmonella Enteritidis outbreaks Human infection requires: * Ambient storage temperature that allow multiplication of SE * Cross-contamination of kitchen surfaces and foods * Improper food handling and preparation practices
 Problems of Salmonella control in poultry n n n Infections can be inapparent Newly hatched poultry are highly susceptible to Salmonella colonization Salmonellae have a very wide host range Salmonella can persist in the environment Manure and dust are present in large quantities in poultry houses
Problems of Salmonella control in poultry n n n Infections can be inapparent Newly hatched poultry are highly susceptible to Salmonella colonization Salmonellae have a very wide host range Salmonella can persist in the environment Manure and dust are present in large quantities in poultry houses
 Salmonella Enteritidis Control Strategies Principal objectives *To reduce incidence of infection in egg-laying flocks *To improve the microbial safety of processing, storage and preparation practices for egg and egg-containing foods
Salmonella Enteritidis Control Strategies Principal objectives *To reduce incidence of infection in egg-laying flocks *To improve the microbial safety of processing, storage and preparation practices for egg and egg-containing foods
 Reducing egg contamination Prevention of infection: A. B. Elimination of sources and reservoirs of SE in poultry flocks and facilities Control of transmission of SE within and between flocks
Reducing egg contamination Prevention of infection: A. B. Elimination of sources and reservoirs of SE in poultry flocks and facilities Control of transmission of SE within and between flocks
 A. Elimination of sources and reservoirs of Salmonella enteritidis Sources of contamination • Replacement chicks themselves • Environment of the poultry house, • Rodents, feeds, etc n Measures. Using uninfected chicks • Hygiene (cleaning, disinfection, etc) • Rodent control n
A. Elimination of sources and reservoirs of Salmonella enteritidis Sources of contamination • Replacement chicks themselves • Environment of the poultry house, • Rodents, feeds, etc n Measures. Using uninfected chicks • Hygiene (cleaning, disinfection, etc) • Rodent control n
 Cleaning and disinfection eliminated SE from about 50% of environmentally positive houses Henzler et al. , 1998, Schlosser et al. , 1999
Cleaning and disinfection eliminated SE from about 50% of environmentally positive houses Henzler et al. , 1998, Schlosser et al. , 1999
 Rodent Control Rodent control was the only practice that correlated well with successful control of SE in poultry houses Henzler et al. , 1998, Schlosser et al. , 1999
Rodent Control Rodent control was the only practice that correlated well with successful control of SE in poultry houses Henzler et al. , 1998, Schlosser et al. , 1999
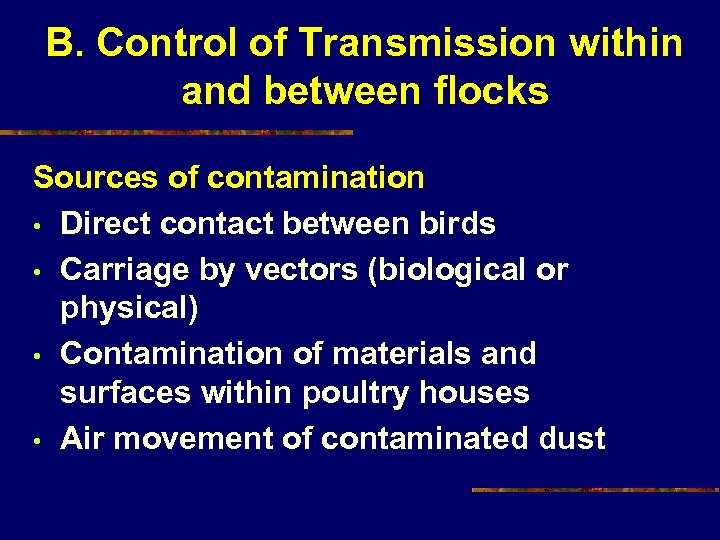 B. Control of Transmission within and between flocks Sources of contamination • Direct contact between birds • Carriage by vectors (biological or physical) • Contamination of materials and surfaces within poultry houses • Air movement of contaminated dust
B. Control of Transmission within and between flocks Sources of contamination • Direct contact between birds • Carriage by vectors (biological or physical) • Contamination of materials and surfaces within poultry houses • Air movement of contaminated dust
 B. Control of Transmission within and between flocks Measures n Reducing the concentration of the circulating particles by negative ionization has reduced experimental horizontal transmission of SE in chicks Gast et al. , 1999
B. Control of Transmission within and between flocks Measures n Reducing the concentration of the circulating particles by negative ionization has reduced experimental horizontal transmission of SE in chicks Gast et al. , 1999
 Control of Transmission within and between flocks 2. Reducing the susceptibility of chicks to SE infection by vaccination of pullets or hens can significantly reduce fecal shedding, organ invasion and egg contamination (Gast et al. , 1992, Zhang-Barber et al. , 1999)
Control of Transmission within and between flocks 2. Reducing the susceptibility of chicks to SE infection by vaccination of pullets or hens can significantly reduce fecal shedding, organ invasion and egg contamination (Gast et al. , 1992, Zhang-Barber et al. , 1999)
 Control of Transmission within and between flocks Vaccination does not create impenetrable barrier against infection n Immunity is not solid and protection is insignificant Davison et al. , 1999 n
Control of Transmission within and between flocks Vaccination does not create impenetrable barrier against infection n Immunity is not solid and protection is insignificant Davison et al. , 1999 n
 Control of Transmission within and between flocks n n Prophylactic administration of probiotic bacterial cultures for competitive exclusion of pathogens from the intestinal tract prevents colonization This approach is less useful in protecting mature hens against environmentally acquired SE
Control of Transmission within and between flocks n n Prophylactic administration of probiotic bacterial cultures for competitive exclusion of pathogens from the intestinal tract prevents colonization This approach is less useful in protecting mature hens against environmentally acquired SE
 Controversial susceptibility issue Forced molting of laying hens by feed deprivation can increase frequency, transmission and severity of SE infection (Holt, 1993, 1995)
Controversial susceptibility issue Forced molting of laying hens by feed deprivation can increase frequency, transmission and severity of SE infection (Holt, 1993, 1995)
 Back to the title of the lecture The Past, Present and Future of Salmonella Control in Poultry
Back to the title of the lecture The Past, Present and Future of Salmonella Control in Poultry
 Control of Salmonella Enteritidis in the USA: Past efforts, 90 -95 Trace-back Testing Program When eggs are implicated as source of human SE infection *Laying flocks are identified *Environmental samples, if + then *Internal tissues are cultured
Control of Salmonella Enteritidis in the USA: Past efforts, 90 -95 Trace-back Testing Program When eggs are implicated as source of human SE infection *Laying flocks are identified *Environmental samples, if + then *Internal tissues are cultured
 Trace-back Testing Program In case of SE positive results: • Selling shell eggs is restricted • Producers have to choose between *pasteurization of eggs or *depopulating affected flocks
Trace-back Testing Program In case of SE positive results: • Selling shell eggs is restricted • Producers have to choose between *pasteurization of eggs or *depopulating affected flocks
 Trace-back Testing Program Evaluation: • • • During this program 304 SE outbreaks were reported 96 outbreaks were due to eggs 38 flocks were implicated 9 million layers were depopulated I billion eggs were diverted for pasteurization
Trace-back Testing Program Evaluation: • • • During this program 304 SE outbreaks were reported 96 outbreaks were due to eggs 38 flocks were implicated 9 million layers were depopulated I billion eggs were diverted for pasteurization
 Trace-back Testing Program Evaluation: • • During this program SE in cecal samples from hens at slaughter increased from 27% in 1991 to 45% in 1995 SE in unpasteurized liquid eggs increased from 13% to 19%
Trace-back Testing Program Evaluation: • • During this program SE in cecal samples from hens at slaughter increased from 27% in 1991 to 45% in 1995 SE in unpasteurized liquid eggs increased from 13% to 19%
 Trace-back Testing Program Conclusion: Evident failure due to : * Eliminating a presumably small number of infected flocks *Potentially continuous reintroduction of SE into flocks from diverse environmental sources
Trace-back Testing Program Conclusion: Evident failure due to : * Eliminating a presumably small number of infected flocks *Potentially continuous reintroduction of SE into flocks from diverse environmental sources
 Control of Salmonella Enteritidis in the USA: Present efforts Risk Reduction Program Microbiological Quality Assurance Implemented by federal, state and poultry industry
Control of Salmonella Enteritidis in the USA: Present efforts Risk Reduction Program Microbiological Quality Assurance Implemented by federal, state and poultry industry
 Risk Reduction Program * Use certified SE-free chicks * Control pests, especially rodents * Thorough cleaning & disinfection * Heightened biosecurity * Washing & refrigeration of eggs
Risk Reduction Program * Use certified SE-free chicks * Control pests, especially rodents * Thorough cleaning & disinfection * Heightened biosecurity * Washing & refrigeration of eggs
 Risk Reduction Program • • • Intensive testing approach * qualifying serological tests * series of environmental tests Certification of negative flocks Diversion of eggs from + flocks to pasteurization
Risk Reduction Program • • • Intensive testing approach * qualifying serological tests * series of environmental tests Certification of negative flocks Diversion of eggs from + flocks to pasteurization
 Pennsylvania Egg Quality Assurance Program n n n Purchase chicks from uninfected breeder flocks Maintain rodent control and biosecurity programs Keep eggs under refrigeration Culture environmental samples from chicks, pullets and layers for SE If +, culture eggs, if +, divert eggs, clean and disinfect thoroughly between flocks
Pennsylvania Egg Quality Assurance Program n n n Purchase chicks from uninfected breeder flocks Maintain rodent control and biosecurity programs Keep eggs under refrigeration Culture environmental samples from chicks, pullets and layers for SE If +, culture eggs, if +, divert eggs, clean and disinfect thoroughly between flocks
 National Poultry Improvement Plan, monitoring breeding flocks n n n Chicks must originate from participating flocks Feed must be free of SE Hatching eggs must be promptly collected and sanitized or fumigated Blood samples from 300 birds are tested for antibodies, if + culture for SE Environmental samples are taken at 2 -4 w and every 30 days. If + do blood testing
National Poultry Improvement Plan, monitoring breeding flocks n n n Chicks must originate from participating flocks Feed must be free of SE Hatching eggs must be promptly collected and sanitized or fumigated Blood samples from 300 birds are tested for antibodies, if + culture for SE Environmental samples are taken at 2 -4 w and every 30 days. If + do blood testing
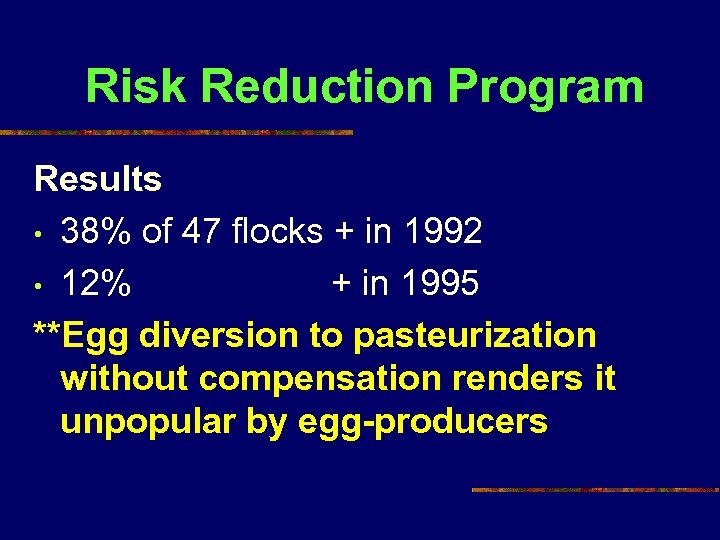 Risk Reduction Program Results • 38% of 47 flocks + in 1992 • 12% + in 1995 **Egg diversion to pasteurization without compensation renders it unpopular by egg-producers
Risk Reduction Program Results • 38% of 47 flocks + in 1992 • 12% + in 1995 **Egg diversion to pasteurization without compensation renders it unpopular by egg-producers
 Risk Reduction Program Alternative program • • Single environmental test Positive result requires: * extra cleaning and disinfection * overall review of control program implementation
Risk Reduction Program Alternative program • • Single environmental test Positive result requires: * extra cleaning and disinfection * overall review of control program implementation
 United Egg Producers 5 -Star Quality Assurance Program • • Cleaning & disinfecftion of poultry houses Rodent and pest elimination Proper egg washing Biosecurity Egg refrigeration from packing to delivery Environmental testing Positive results trigger extra cleaning and disinfection plus review of program implementation
United Egg Producers 5 -Star Quality Assurance Program • • Cleaning & disinfecftion of poultry houses Rodent and pest elimination Proper egg washing Biosecurity Egg refrigeration from packing to delivery Environmental testing Positive results trigger extra cleaning and disinfection plus review of program implementation
 Control of Salmonella Enteritidis in the USA: The future n The most effective and sustainable approaches to control food–borne disease involve risk reduction practices that address a broad spectrum of current and prospective pathogens
Control of Salmonella Enteritidis in the USA: The future n The most effective and sustainable approaches to control food–borne disease involve risk reduction practices that address a broad spectrum of current and prospective pathogens
 Conclusions The main aim of SE control is the consumer protection which can be achieved by: n Short-term measures ensure that eggs are promptly refrigerated, processed, stored, handled, prepared safely and cooked adequately n Long-term measures patient and persistent participation in risk reduction programs of verified efficacy
Conclusions The main aim of SE control is the consumer protection which can be achieved by: n Short-term measures ensure that eggs are promptly refrigerated, processed, stored, handled, prepared safely and cooked adequately n Long-term measures patient and persistent participation in risk reduction programs of verified efficacy
 Final Concluion Efforts to prevent or reduce Salmonella Enteritidis infections in poultry illustrate the evolution of strategies for salmonella control in general which probably lead to control of other food-borne diseases
Final Concluion Efforts to prevent or reduce Salmonella Enteritidis infections in poultry illustrate the evolution of strategies for salmonella control in general which probably lead to control of other food-borne diseases
 Thank you for your attention Prof. Dr. Mohamed Refai Department of Microbiology Faculty of Veterinary Medicine Cairo University Mohrefai@yahoo. com
Thank you for your attention Prof. Dr. Mohamed Refai Department of Microbiology Faculty of Veterinary Medicine Cairo University Mohrefai@yahoo. com


