post_lecture4_ats762_nitrogen.ppt
- Количество слайдов: 30
 THE NITROGEN CYCLE
THE NITROGEN CYCLE
 TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
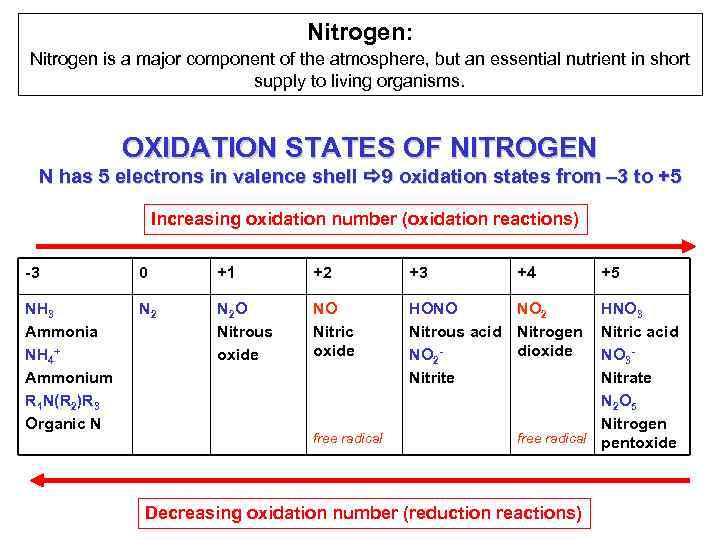 Nitrogen: Nitrogen is a major component of the atmosphere, but an essential nutrient in short supply to living organisms. OXIDATION STATES OF NITROGEN N has 5 electrons in valence shell a 9 oxidation states from – 3 to +5 Increasing oxidation number (oxidation reactions) -3 0 +1 +2 +3 NH 3 Ammonia NH 4+ Ammonium R 1 N(R 2)R 3 Organic N N 2 O Nitrous oxide NO Nitric oxide HONO NO 2 Nitrous acid Nitrogen dioxide NO 2 Nitrite free radical +4 +5 HNO 3 Nitric acid NO 3 Nitrate N 2 O 5 Nitrogen free radical pentoxide Decreasing oxidation number (reduction reactions)
Nitrogen: Nitrogen is a major component of the atmosphere, but an essential nutrient in short supply to living organisms. OXIDATION STATES OF NITROGEN N has 5 electrons in valence shell a 9 oxidation states from – 3 to +5 Increasing oxidation number (oxidation reactions) -3 0 +1 +2 +3 NH 3 Ammonia NH 4+ Ammonium R 1 N(R 2)R 3 Organic N N 2 O Nitrous oxide NO Nitric oxide HONO NO 2 Nitrous acid Nitrogen dioxide NO 2 Nitrite free radical +4 +5 HNO 3 Nitric acid NO 3 Nitrate N 2 O 5 Nitrogen free radical pentoxide Decreasing oxidation number (reduction reactions)
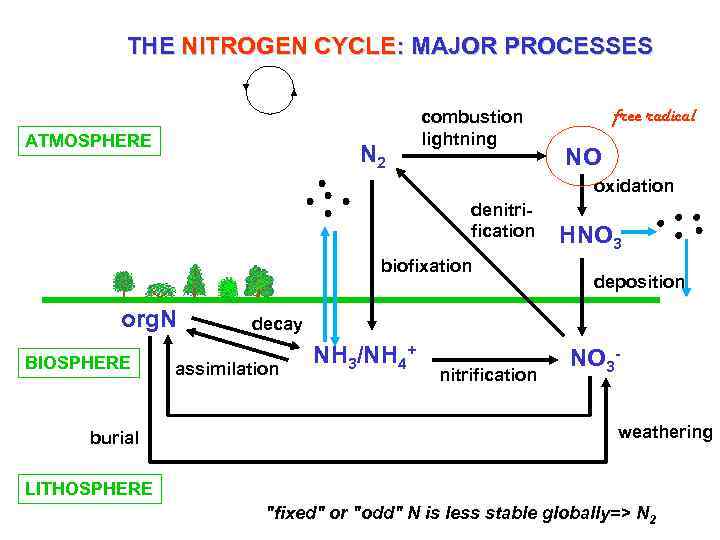 THE NITROGEN CYCLE: MAJOR PROCESSES ATMOSPHERE N 2 combustion lightning free radical NO oxidation denitrification biofixation org. N BIOSPHERE burial HNO 3 deposition decay assimilation NH 3/NH 4+ nitrification NO 3 weathering LITHOSPHERE "fixed" or "odd" N is less stable globally=> N 2
THE NITROGEN CYCLE: MAJOR PROCESSES ATMOSPHERE N 2 combustion lightning free radical NO oxidation denitrification biofixation org. N BIOSPHERE burial HNO 3 deposition decay assimilation NH 3/NH 4+ nitrification NO 3 weathering LITHOSPHERE "fixed" or "odd" N is less stable globally=> N 2
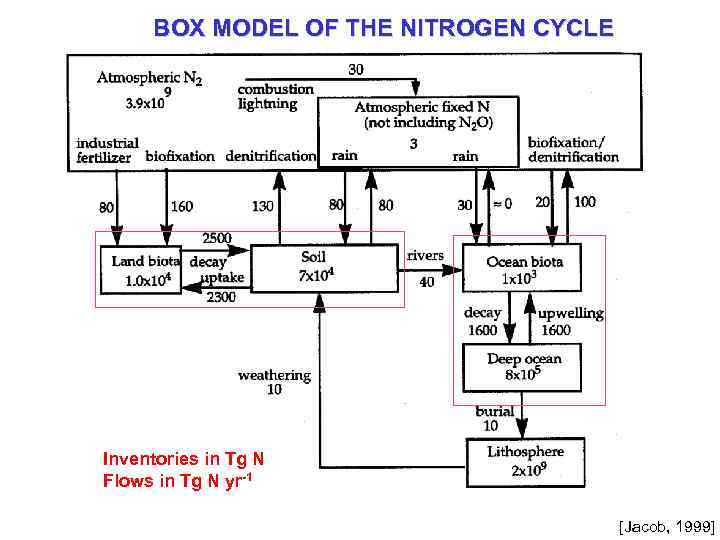 BOX MODEL OF THE NITROGEN CYCLE Inventories in Tg N Flows in Tg N yr-1 [Jacob, 1999]
BOX MODEL OF THE NITROGEN CYCLE Inventories in Tg N Flows in Tg N yr-1 [Jacob, 1999]
 TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
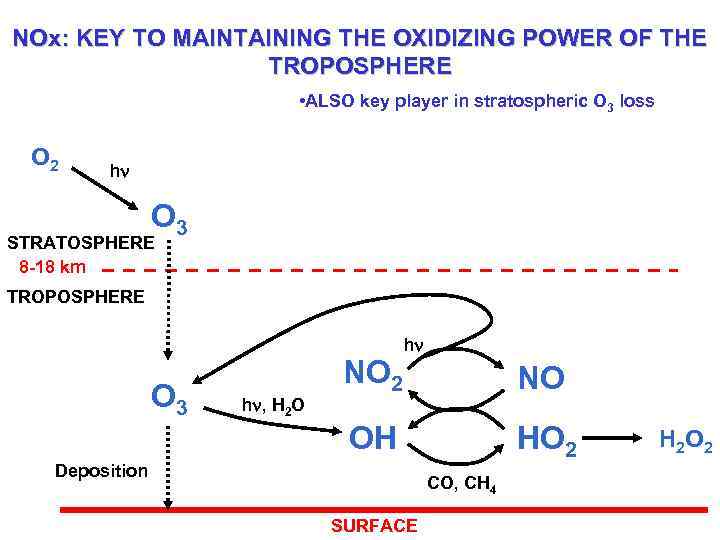 NOx: KEY TO MAINTAINING THE OXIDIZING POWER OF THE TROPOSPHERE • ALSO key player in stratospheric O 3 loss O 2 hn O 3 STRATOSPHERE 8 -18 km TROPOSPHERE O 3 hn, H 2 O NO 2 hn NO OH Deposition HO 2 CO, CH 4 SURFACE H 2 O 2
NOx: KEY TO MAINTAINING THE OXIDIZING POWER OF THE TROPOSPHERE • ALSO key player in stratospheric O 3 loss O 2 hn O 3 STRATOSPHERE 8 -18 km TROPOSPHERE O 3 hn, H 2 O NO 2 hn NO OH Deposition HO 2 CO, CH 4 SURFACE H 2 O 2
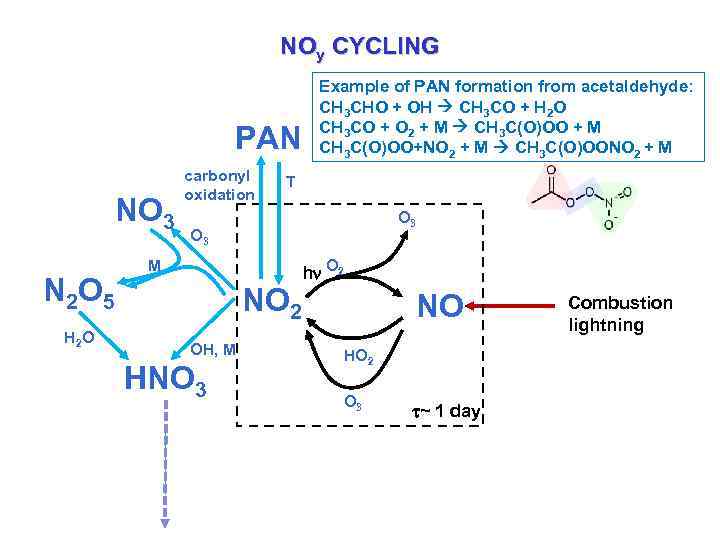 NOy CYCLING PAN NO 3 N 2 O 5 H 2 O carbonyl oxidation Example of PAN formation from acetaldehyde: CH 3 CHO + OH CH 3 CO + H 2 O CH 3 CO + O 2 + M CH 3 C(O)OO + M CH 3 C(O)OO+NO 2 + M CH 3 C(O)OONO 2 + M T O 3 M NO 2 OH, M HNO 3 hn O 2 NO HO 2 O 3 ~ 1 day Combustion lightning
NOy CYCLING PAN NO 3 N 2 O 5 H 2 O carbonyl oxidation Example of PAN formation from acetaldehyde: CH 3 CHO + OH CH 3 CO + H 2 O CH 3 CO + O 2 + M CH 3 C(O)OO + M CH 3 C(O)OO+NO 2 + M CH 3 C(O)OONO 2 + M T O 3 M NO 2 OH, M HNO 3 hn O 2 NO HO 2 O 3 ~ 1 day Combustion lightning
![PEROXYACETYLNITRATE (PAN) AS RESERVOIR FOR LONG-RANGE TRANSPORT OF NOx [Jacob, 1999] PEROXYACETYLNITRATE (PAN) AS RESERVOIR FOR LONG-RANGE TRANSPORT OF NOx [Jacob, 1999]](https://present5.com/presentation/195722153_298576660/image-9.jpg) PEROXYACETYLNITRATE (PAN) AS RESERVOIR FOR LONG-RANGE TRANSPORT OF NOx [Jacob, 1999]
PEROXYACETYLNITRATE (PAN) AS RESERVOIR FOR LONG-RANGE TRANSPORT OF NOx [Jacob, 1999]
 TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
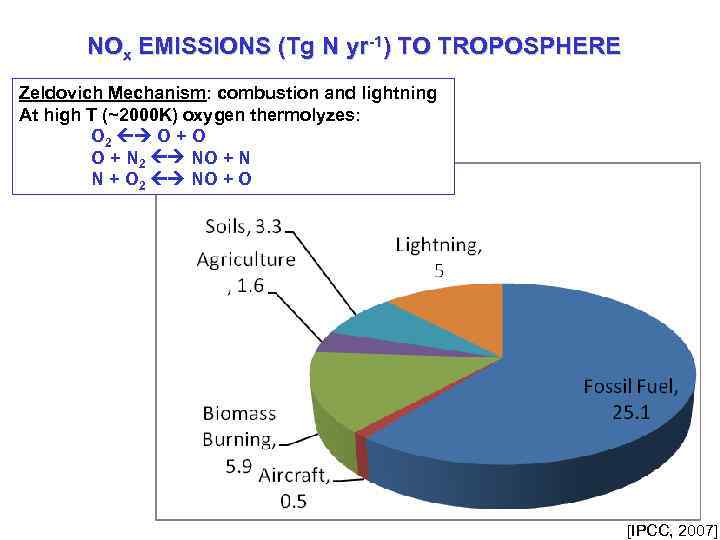 NOx EMISSIONS (Tg N yr-1) TO TROPOSPHERE Zeldovich Mechanism: combustion and lightning At high T (~2000 K) oxygen thermolyzes: O 2 O + O O + N 2 NO + N N + O 2 NO + O [IPCC, 2007]
NOx EMISSIONS (Tg N yr-1) TO TROPOSPHERE Zeldovich Mechanism: combustion and lightning At high T (~2000 K) oxygen thermolyzes: O 2 O + O O + N 2 NO + N N + O 2 NO + O [IPCC, 2007]
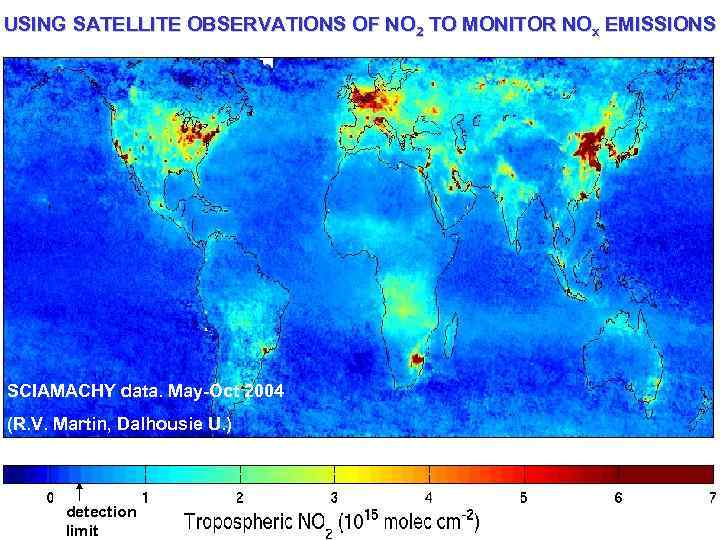 USING SATELLITE OBSERVATIONS OF NO 2 TO MONITOR NOx EMISSIONS SCIAMACHY data. May-Oct 2004 (R. V. Martin, Dalhousie U. ) detection limit
USING SATELLITE OBSERVATIONS OF NO 2 TO MONITOR NOx EMISSIONS SCIAMACHY data. May-Oct 2004 (R. V. Martin, Dalhousie U. ) detection limit
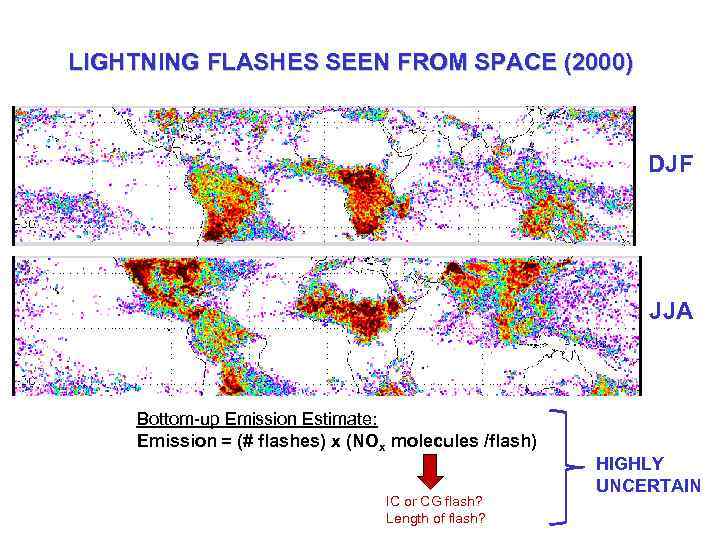 LIGHTNING FLASHES SEEN FROM SPACE (2000) DJF JJA Bottom-up Emission Estimate: Emission = (# flashes) x (NOx molecules /flash) IC or CG flash? Length of flash? HIGHLY UNCERTAIN
LIGHTNING FLASHES SEEN FROM SPACE (2000) DJF JJA Bottom-up Emission Estimate: Emission = (# flashes) x (NOx molecules /flash) IC or CG flash? Length of flash? HIGHLY UNCERTAIN
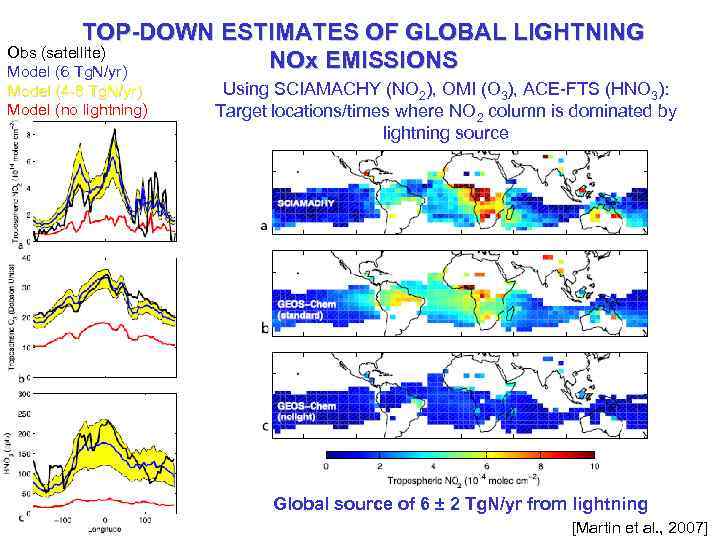 TOP-DOWN ESTIMATES OF GLOBAL LIGHTNING Obs (satellite) NOx EMISSIONS Model (6 Tg. N/yr) Model (4 -8 Tg. N/yr) Model (no lightning) Using SCIAMACHY (NO 2), OMI (O 3), ACE-FTS (HNO 3): Target locations/times where NO 2 column is dominated by lightning source Global source of 6 ± 2 Tg. N/yr from lightning [Martin et al. , 2007]
TOP-DOWN ESTIMATES OF GLOBAL LIGHTNING Obs (satellite) NOx EMISSIONS Model (6 Tg. N/yr) Model (4 -8 Tg. N/yr) Model (no lightning) Using SCIAMACHY (NO 2), OMI (O 3), ACE-FTS (HNO 3): Target locations/times where NO 2 column is dominated by lightning source Global source of 6 ± 2 Tg. N/yr from lightning [Martin et al. , 2007]
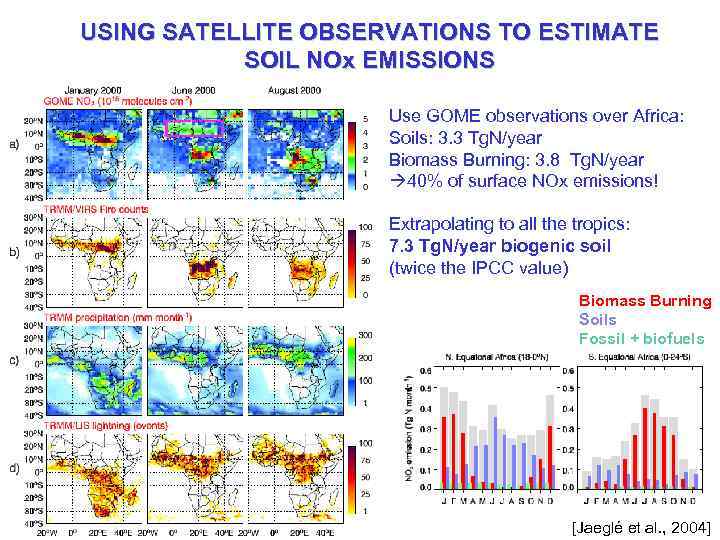 USING SATELLITE OBSERVATIONS TO ESTIMATE SOIL NOx EMISSIONS Use GOME observations over Africa: Soils: 3. 3 Tg. N/year Biomass Burning: 3. 8 Tg. N/year à 40% of surface NOx emissions! Extrapolating to all the tropics: 7. 3 Tg. N/year biogenic soil (twice the IPCC value) Biomass Burning Soils Fossil + biofuels [Jaeglé et al. , 2004]
USING SATELLITE OBSERVATIONS TO ESTIMATE SOIL NOx EMISSIONS Use GOME observations over Africa: Soils: 3. 3 Tg. N/year Biomass Burning: 3. 8 Tg. N/year à 40% of surface NOx emissions! Extrapolating to all the tropics: 7. 3 Tg. N/year biogenic soil (twice the IPCC value) Biomass Burning Soils Fossil + biofuels [Jaeglé et al. , 2004]
![GROWING CONTRIBUTION OF AGRICULTURE TO N CYCLE [IPCC, 2007] GROWING CONTRIBUTION OF AGRICULTURE TO N CYCLE [IPCC, 2007]](https://present5.com/presentation/195722153_298576660/image-16.jpg) GROWING CONTRIBUTION OF AGRICULTURE TO N CYCLE [IPCC, 2007]
GROWING CONTRIBUTION OF AGRICULTURE TO N CYCLE [IPCC, 2007]
 TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
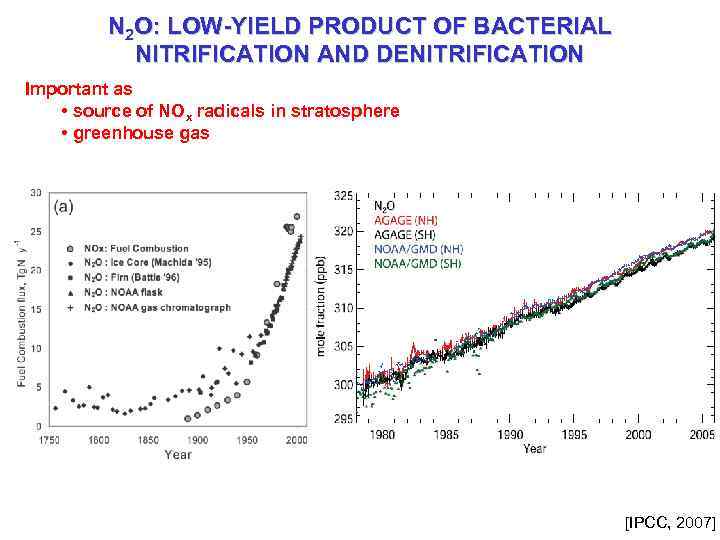 N 2 O: LOW-YIELD PRODUCT OF BACTERIAL NITRIFICATION AND DENITRIFICATION Important as • source of NOx radicals in stratosphere • greenhouse gas [IPCC, 2007]
N 2 O: LOW-YIELD PRODUCT OF BACTERIAL NITRIFICATION AND DENITRIFICATION Important as • source of NOx radicals in stratosphere • greenhouse gas [IPCC, 2007]
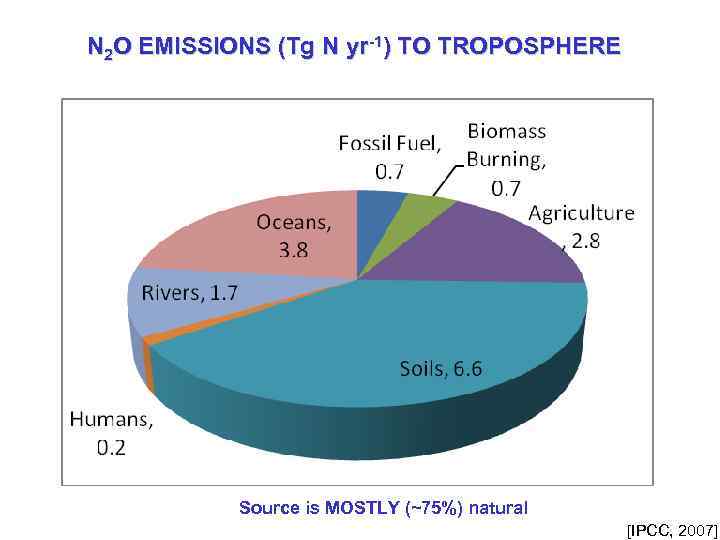 N 2 O EMISSIONS (Tg N yr-1) TO TROPOSPHERE Source is MOSTLY (~75%) natural [IPCC, 2007]
N 2 O EMISSIONS (Tg N yr-1) TO TROPOSPHERE Source is MOSTLY (~75%) natural [IPCC, 2007]
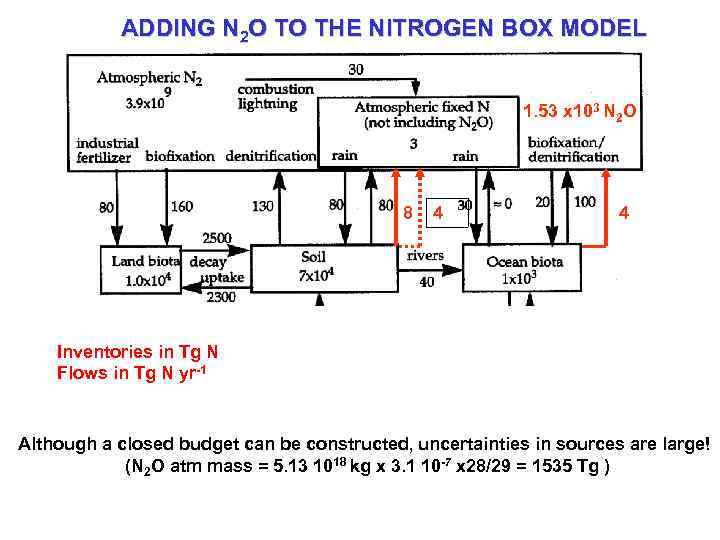 ADDING N 2 O TO THE NITROGEN BOX MODEL 1. 53 x 103 N 2 O 8 4 4 Inventories in Tg N Flows in Tg N yr-1 Although a closed budget can be constructed, uncertainties in sources are large! (N 2 O atm mass = 5. 13 1018 kg x 3. 1 10 -7 x 28/29 = 1535 Tg )
ADDING N 2 O TO THE NITROGEN BOX MODEL 1. 53 x 103 N 2 O 8 4 4 Inventories in Tg N Flows in Tg N yr-1 Although a closed budget can be constructed, uncertainties in sources are large! (N 2 O atm mass = 5. 13 1018 kg x 3. 1 10 -7 x 28/29 = 1535 Tg )
 TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
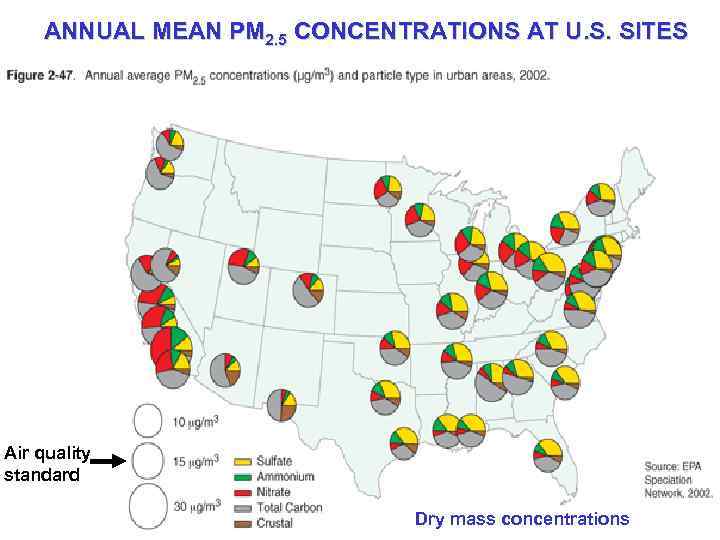 ANNUAL MEAN PM 2. 5 CONCENTRATIONS AT U. S. SITES Air quality standard Dry mass concentrations
ANNUAL MEAN PM 2. 5 CONCENTRATIONS AT U. S. SITES Air quality standard Dry mass concentrations
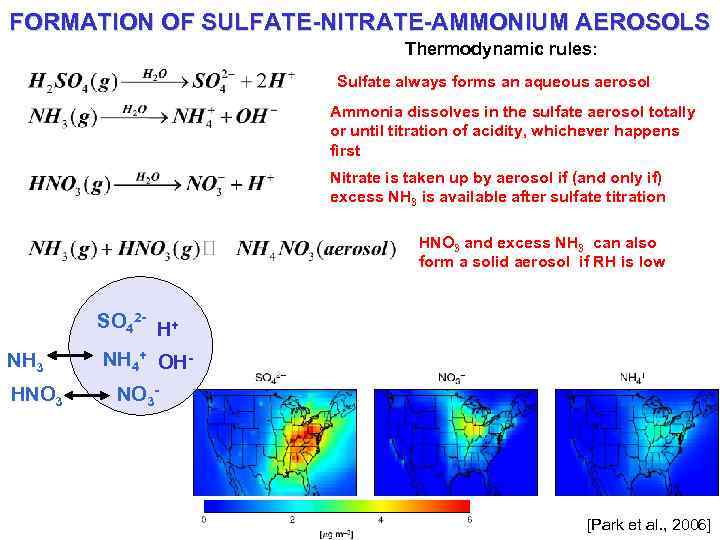 FORMATION OF SULFATE-NITRATE-AMMONIUM AEROSOLS Thermodynamic rules: Sulfate always forms an aqueous aerosol Ammonia dissolves in the sulfate aerosol totally or until titration of acidity, whichever happens first Nitrate is taken up by aerosol if (and only if) excess NH 3 is available after sulfate titration HNO 3 and excess NH 3 can also form a solid aerosol if RH is low SO 42 - H+ NH 3 HNO 3 NH 4+ OHNO 3 - [Park et al. , 2006]
FORMATION OF SULFATE-NITRATE-AMMONIUM AEROSOLS Thermodynamic rules: Sulfate always forms an aqueous aerosol Ammonia dissolves in the sulfate aerosol totally or until titration of acidity, whichever happens first Nitrate is taken up by aerosol if (and only if) excess NH 3 is available after sulfate titration HNO 3 and excess NH 3 can also form a solid aerosol if RH is low SO 42 - H+ NH 3 HNO 3 NH 4+ OHNO 3 - [Park et al. , 2006]
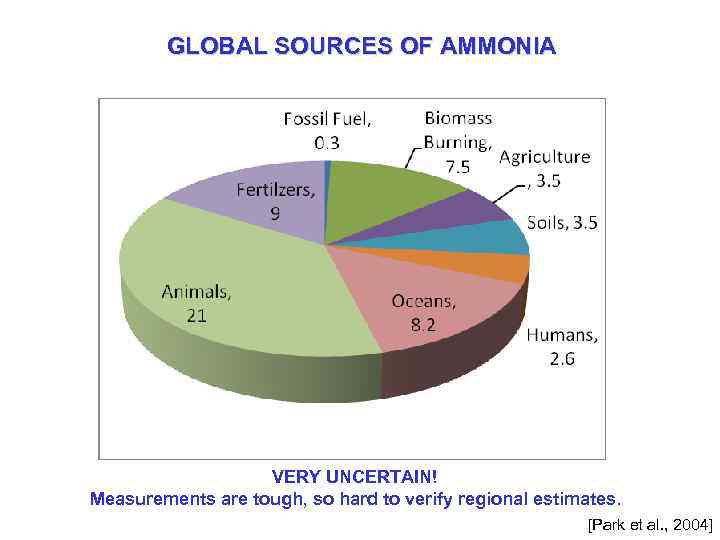 GLOBAL SOURCES OF AMMONIA VERY UNCERTAIN! Measurements are tough, so hard to verify regional estimates. [Park et al. , 2004]
GLOBAL SOURCES OF AMMONIA VERY UNCERTAIN! Measurements are tough, so hard to verify regional estimates. [Park et al. , 2004]
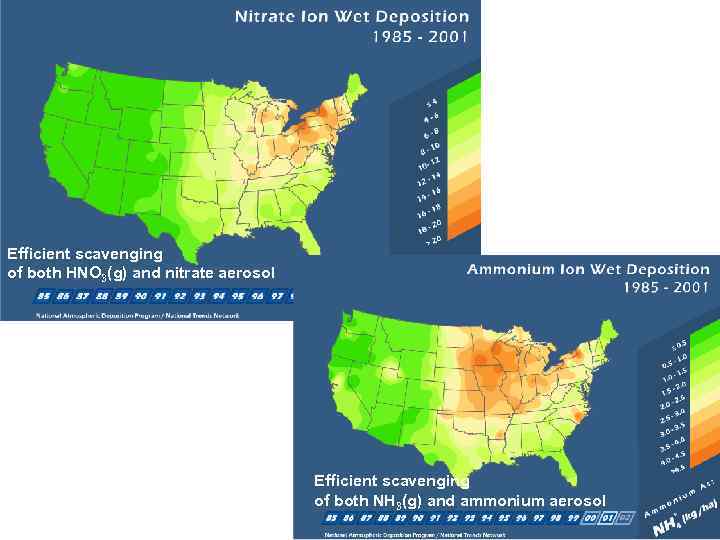 Efficient scavenging of both HNO 3(g) and nitrate aerosol Efficient scavenging of both NH 3(g) and ammonium aerosol
Efficient scavenging of both HNO 3(g) and nitrate aerosol Efficient scavenging of both NH 3(g) and ammonium aerosol
 TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
TOPICS FOR TODAY 1. The Nitrogen Cycle 2. Fixed Nitrogen in the Atmosphere 3. Sources of NOx 4. What about N 2 O? 5. Nitrogen Cycle: on the particle side 6. How might the nitrogen cycle be affected by climate change?
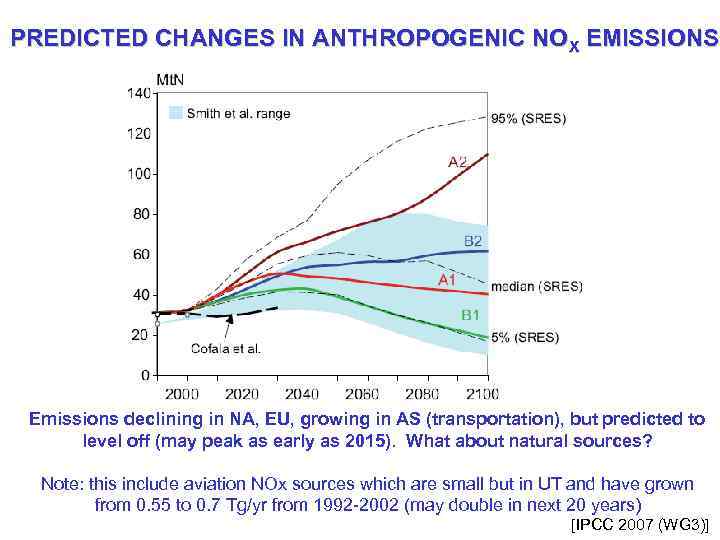 PREDICTED CHANGES IN ANTHROPOGENIC NOX EMISSIONS Emissions declining in NA, EU, growing in AS (transportation), but predicted to level off (may peak as early as 2015). What about natural sources? Note: this include aviation NOx sources which are small but in UT and have grown from 0. 55 to 0. 7 Tg/yr from 1992 -2002 (may double in next 20 years) [IPCC 2007 (WG 3)]
PREDICTED CHANGES IN ANTHROPOGENIC NOX EMISSIONS Emissions declining in NA, EU, growing in AS (transportation), but predicted to level off (may peak as early as 2015). What about natural sources? Note: this include aviation NOx sources which are small but in UT and have grown from 0. 55 to 0. 7 Tg/yr from 1992 -2002 (may double in next 20 years) [IPCC 2007 (WG 3)]
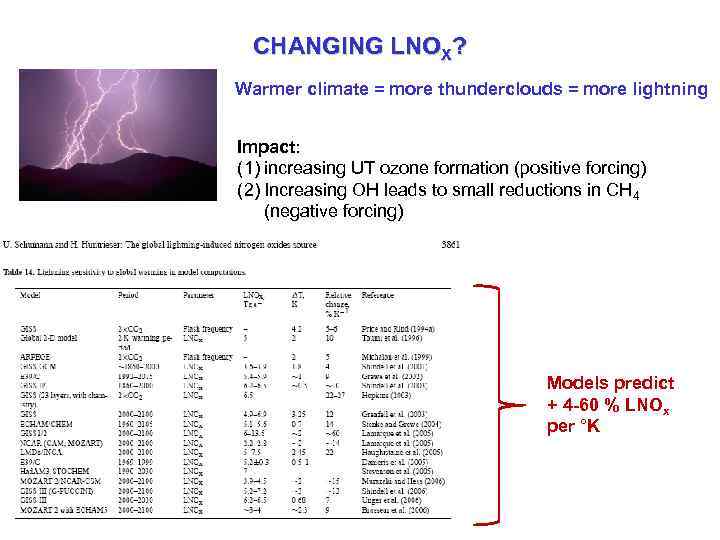 CHANGING LNOX? Warmer climate = more thunderclouds = more lightning Impact: (1) increasing UT ozone formation (positive forcing) (2) Increasing OH leads to small reductions in CH 4 (negative forcing) Models predict + 4 -60 % LNOx per °K
CHANGING LNOX? Warmer climate = more thunderclouds = more lightning Impact: (1) increasing UT ozone formation (positive forcing) (2) Increasing OH leads to small reductions in CH 4 (negative forcing) Models predict + 4 -60 % LNOx per °K
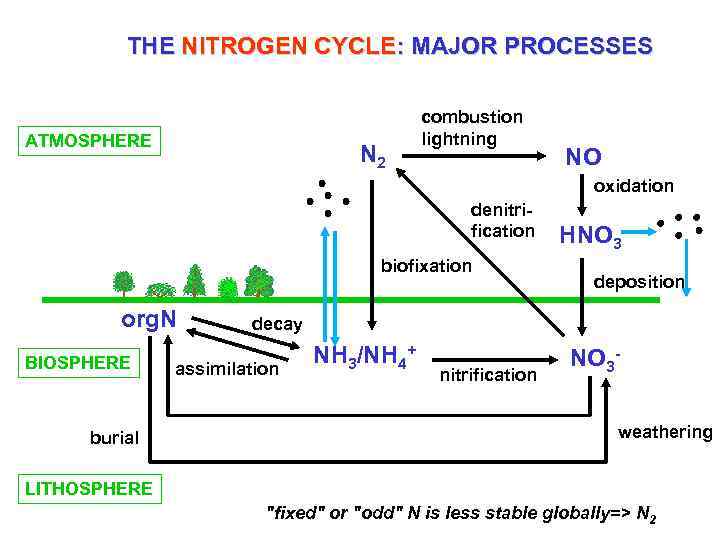 THE NITROGEN CYCLE: MAJOR PROCESSES ATMOSPHERE N 2 combustion lightning NO oxidation denitrification biofixation org. N BIOSPHERE burial HNO 3 deposition decay assimilation NH 3/NH 4+ nitrification NO 3 weathering LITHOSPHERE "fixed" or "odd" N is less stable globally=> N 2
THE NITROGEN CYCLE: MAJOR PROCESSES ATMOSPHERE N 2 combustion lightning NO oxidation denitrification biofixation org. N BIOSPHERE burial HNO 3 deposition decay assimilation NH 3/NH 4+ nitrification NO 3 weathering LITHOSPHERE "fixed" or "odd" N is less stable globally=> N 2
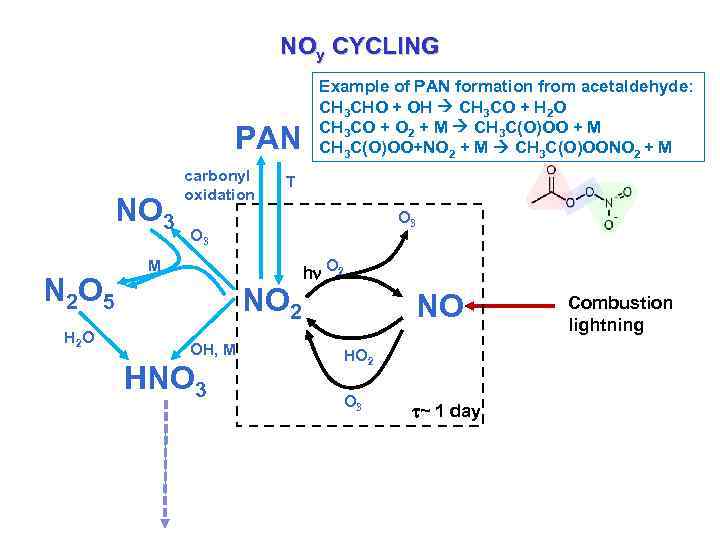 NOy CYCLING PAN NO 3 N 2 O 5 H 2 O carbonyl oxidation Example of PAN formation from acetaldehyde: CH 3 CHO + OH CH 3 CO + H 2 O CH 3 CO + O 2 + M CH 3 C(O)OO + M CH 3 C(O)OO+NO 2 + M CH 3 C(O)OONO 2 + M T O 3 M NO 2 OH, M HNO 3 hn O 2 NO HO 2 O 3 ~ 1 day Combustion lightning
NOy CYCLING PAN NO 3 N 2 O 5 H 2 O carbonyl oxidation Example of PAN formation from acetaldehyde: CH 3 CHO + OH CH 3 CO + H 2 O CH 3 CO + O 2 + M CH 3 C(O)OO + M CH 3 C(O)OO+NO 2 + M CH 3 C(O)OONO 2 + M T O 3 M NO 2 OH, M HNO 3 hn O 2 NO HO 2 O 3 ~ 1 day Combustion lightning


