d4f3c710879336816462e6fe2cb8b49b.ppt
- Количество слайдов: 66
 The national flu immunisation programme 2016/17 Training for healthcare practitioners
The national flu immunisation programme 2016/17 Training for healthcare practitioners
 2 Key messages • flu immunisation is one of the most effective interventions we can provide to reduce harm from flu and pressures on health and social care services during the winter • it is important to increase flu vaccine uptake in clinical risk groups because of increased risk of death and serious illness if people in these groups catch flu • for a number of years, only around half of patients aged six months to under 65 years in clinical risk groups have been vaccinated • influenza during pregnancy may be associated with perinatal mortality, prematurity, smaller neonatal size, lower birth weight and increased risk of complications for mother • vaccination of health and social care workers protects them and reduces risk of spreading flu to their patients, service users, colleagues and family members • by preventing flu infection through vaccination, secondary bacterial infections such as pneumonia are prevented. This reduces the need for antibiotics and helps prevent antibiotic resistance The national flu immunisation programme 2016/17
2 Key messages • flu immunisation is one of the most effective interventions we can provide to reduce harm from flu and pressures on health and social care services during the winter • it is important to increase flu vaccine uptake in clinical risk groups because of increased risk of death and serious illness if people in these groups catch flu • for a number of years, only around half of patients aged six months to under 65 years in clinical risk groups have been vaccinated • influenza during pregnancy may be associated with perinatal mortality, prematurity, smaller neonatal size, lower birth weight and increased risk of complications for mother • vaccination of health and social care workers protects them and reduces risk of spreading flu to their patients, service users, colleagues and family members • by preventing flu infection through vaccination, secondary bacterial infections such as pneumonia are prevented. This reduces the need for antibiotics and helps prevent antibiotic resistance The national flu immunisation programme 2016/17
 3 Aims of resource The purpose of this training resource is to: • develop the knowledge base of healthcare practitioners regarding the 2016/17 seasonal flu vaccination programme • support healthcare practitioners involved in discussing flu vaccination with those eligible by providing evidence based information • promote high uptake of flu vaccination in those eligible by increasing the knowledge of those involved in delivering the vaccination programme • provide information on the administration of flu vaccines The national flu immunisation programme 2016/17
3 Aims of resource The purpose of this training resource is to: • develop the knowledge base of healthcare practitioners regarding the 2016/17 seasonal flu vaccination programme • support healthcare practitioners involved in discussing flu vaccination with those eligible by providing evidence based information • promote high uptake of flu vaccination in those eligible by increasing the knowledge of those involved in delivering the vaccination programme • provide information on the administration of flu vaccines The national flu immunisation programme 2016/17
 4 Learning outcomes On completion of this resource, healthcare practitioners will be able to: • describe the cause of flu • understand how flu is transmitted and the possible effects of flu • understand the evidence base for the administration of flu vaccination to those aged 65 years and over and those in clinical risk groups • explain which vaccines will be used and the precautions and contraindications to the administration of flu vaccines • explain the sequence of steps in flu vaccine administration • explain the possible side effects from flu vaccines • understand the importance of their role in promoting and providing evidence based information about flu vaccination to patients • identify sources of additional information The national flu immunisation programme 2016/17
4 Learning outcomes On completion of this resource, healthcare practitioners will be able to: • describe the cause of flu • understand how flu is transmitted and the possible effects of flu • understand the evidence base for the administration of flu vaccination to those aged 65 years and over and those in clinical risk groups • explain which vaccines will be used and the precautions and contraindications to the administration of flu vaccines • explain the sequence of steps in flu vaccine administration • explain the possible side effects from flu vaccines • understand the importance of their role in promoting and providing evidence based information about flu vaccination to patients • identify sources of additional information The national flu immunisation programme 2016/17
 5 What is flu? • flu is an acute viral infection of the respiratory tract (nose, mouth, throat, bronchial tubes and lungs) • it is a highly infectious illness which spreads rapidly in closed communities • even people with mild or no symptoms can infect others • most cases in the UK occur during an 8 to 10 week period during the winter The national flu immunisation programme 2014/15 The national flu immunisation programme 2016/17
5 What is flu? • flu is an acute viral infection of the respiratory tract (nose, mouth, throat, bronchial tubes and lungs) • it is a highly infectious illness which spreads rapidly in closed communities • even people with mild or no symptoms can infect others • most cases in the UK occur during an 8 to 10 week period during the winter The national flu immunisation programme 2014/15 The national flu immunisation programme 2016/17
 6 Influenza viruses There are three types of influenza viruses: A viruses • cause outbreaks most years and are the usual cause of epidemics • live and multiply in wildfowl from where they can be transmitted to humans. Also carried by other mammals B viruses • tend to cause less severe disease and smaller outbreaks • burden of disease mostly in children • predominantly found in humans C viruses • minor respiratory illness only The national flu immunisation programme 2016/17
6 Influenza viruses There are three types of influenza viruses: A viruses • cause outbreaks most years and are the usual cause of epidemics • live and multiply in wildfowl from where they can be transmitted to humans. Also carried by other mammals B viruses • tend to cause less severe disease and smaller outbreaks • burden of disease mostly in children • predominantly found in humans C viruses • minor respiratory illness only The national flu immunisation programme 2016/17
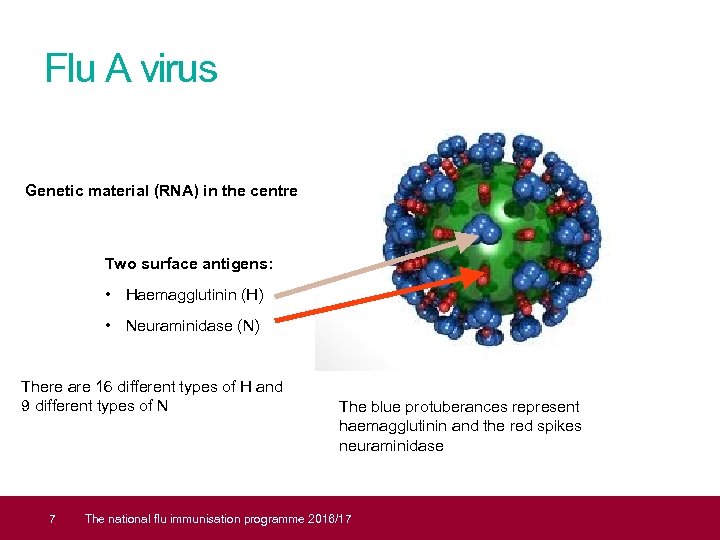 Flu A virus Genetic material (RNA) in the centre Two surface antigens: • Haemagglutinin (H) • Neuraminidase (N) There are 16 different types of H and 9 different types of N 7 The blue protuberances represent haemagglutinin and the red spikes neuraminidase The national flu immunisation programme 2016/17
Flu A virus Genetic material (RNA) in the centre Two surface antigens: • Haemagglutinin (H) • Neuraminidase (N) There are 16 different types of H and 9 different types of N 7 The blue protuberances represent haemagglutinin and the red spikes neuraminidase The national flu immunisation programme 2016/17
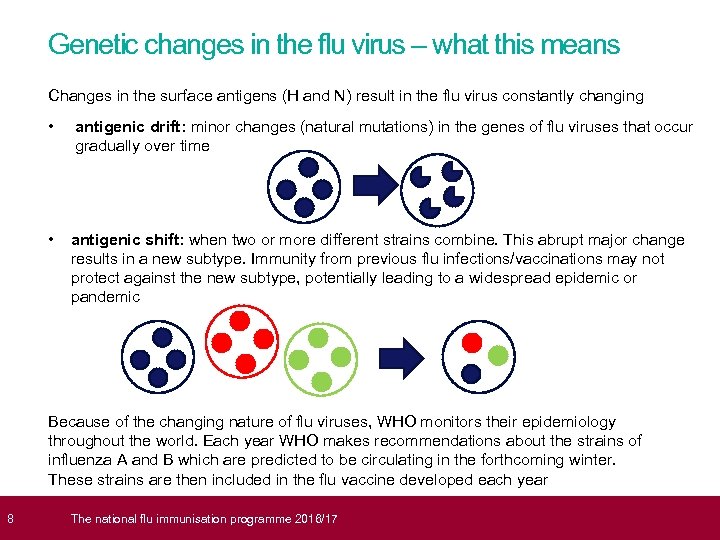 8 Genetic changes in the flu virus – what this means Changes in the surface antigens (H and N) result in the flu virus constantly changing • antigenic drift: minor changes (natural mutations) in the genes of flu viruses that occur gradually over time • antigenic shift: when two or more different strains combine. This abrupt major change results in a new subtype. Immunity from previous flu infections/vaccinations may not protect against the new subtype, potentially leading to a widespread epidemic or pandemic Because of the changing nature of flu viruses, WHO monitors their epidemiology throughout the world. Each year WHO makes recommendations about the strains of influenza A and B which are predicted to be circulating in the forthcoming winter. These strains are then included in the flu vaccine developed each year The national flu immunisation programme 2016/17
8 Genetic changes in the flu virus – what this means Changes in the surface antigens (H and N) result in the flu virus constantly changing • antigenic drift: minor changes (natural mutations) in the genes of flu viruses that occur gradually over time • antigenic shift: when two or more different strains combine. This abrupt major change results in a new subtype. Immunity from previous flu infections/vaccinations may not protect against the new subtype, potentially leading to a widespread epidemic or pandemic Because of the changing nature of flu viruses, WHO monitors their epidemiology throughout the world. Each year WHO makes recommendations about the strains of influenza A and B which are predicted to be circulating in the forthcoming winter. These strains are then included in the flu vaccine developed each year The national flu immunisation programme 2016/17
 9 Flu vaccine effectiveness • efficacy calculated at between 50 -60% for adults aged 18 to 65 years • lower efficacy in elderly although immunisation shown to reduce incidence of severe disease including bronchopneumonia, hospital admissions and mortality • in 2014/15 the flu vaccine only provided limited protection against infection as the main A(H 3 N 2) strain that circulated differed from the A(H 3 N 2) strain selected for the vaccine • however, throughout the last decade, there has generally been a good match between the strains of flu in the vaccine and those that subsequently circulated In 2015/16 , the A(H 1 N 1)strain was the predominant circulating virus for the majority of the season and was well matched to the vaccine strain. The UK provisional vaccine effectiveness was 52. 4% (for all ages) and 57. 6% for the live attenuated vaccine in children aged 2 to 17 years The national flu immunisation programme 2016/17
9 Flu vaccine effectiveness • efficacy calculated at between 50 -60% for adults aged 18 to 65 years • lower efficacy in elderly although immunisation shown to reduce incidence of severe disease including bronchopneumonia, hospital admissions and mortality • in 2014/15 the flu vaccine only provided limited protection against infection as the main A(H 3 N 2) strain that circulated differed from the A(H 3 N 2) strain selected for the vaccine • however, throughout the last decade, there has generally been a good match between the strains of flu in the vaccine and those that subsequently circulated In 2015/16 , the A(H 1 N 1)strain was the predominant circulating virus for the majority of the season and was well matched to the vaccine strain. The UK provisional vaccine effectiveness was 52. 4% (for all ages) and 57. 6% for the live attenuated vaccine in children aged 2 to 17 years The national flu immunisation programme 2016/17
 Features of flu • easily transmitted by large droplets, small-particle aerosols and by hand to mouth/eye contamination from a contaminated surface or respiratory secretions of infected person • people with mild or no symptoms can still infect others • incubation period 1 -5 days (average 2 -3 days) though may be longer especially in people with immune deficiency Common symptoms include: • sudden onset of fever, chills, headache, muscle and joint pain and extreme fatigue • dry cough, sore throat and stuffy nose • in young children gastrointestinal symptoms such as vomiting and diarrhoea may be seen 10 The national flu immunisation programme 2016/17
Features of flu • easily transmitted by large droplets, small-particle aerosols and by hand to mouth/eye contamination from a contaminated surface or respiratory secretions of infected person • people with mild or no symptoms can still infect others • incubation period 1 -5 days (average 2 -3 days) though may be longer especially in people with immune deficiency Common symptoms include: • sudden onset of fever, chills, headache, muscle and joint pain and extreme fatigue • dry cough, sore throat and stuffy nose • in young children gastrointestinal symptoms such as vomiting and diarrhoea may be seen 10 The national flu immunisation programme 2016/17
 11 Possible complications of flu Common: • bronchitis • otitis media (children), sinusitis • secondary bacterial pneumonia Less common: • meningitis, encephalitis, meningoencephalitis • primary influenza pneumonia Risk of most serious illness is higher in children under six months, pregnant women, older people and those with underlying health conditions such as respiratory disease, cardiac disease, long-term neurological conditions or immunosuppression. Flu during pregnancy may be associated with perinatal mortality, prematurity, smaller neonatal size and lower birth weight The national flu immunisation programme 2016/17
11 Possible complications of flu Common: • bronchitis • otitis media (children), sinusitis • secondary bacterial pneumonia Less common: • meningitis, encephalitis, meningoencephalitis • primary influenza pneumonia Risk of most serious illness is higher in children under six months, pregnant women, older people and those with underlying health conditions such as respiratory disease, cardiac disease, long-term neurological conditions or immunosuppression. Flu during pregnancy may be associated with perinatal mortality, prematurity, smaller neonatal size and lower birth weight The national flu immunisation programme 2016/17
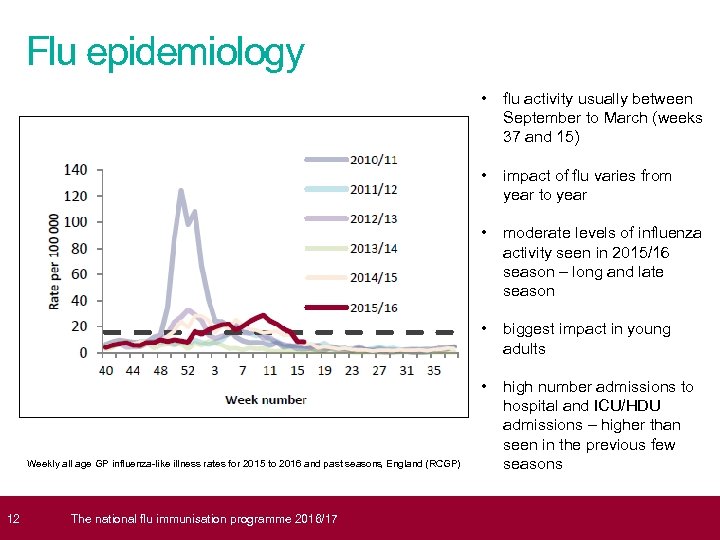 12 Flu epidemiology • • moderate levels of influenza activity seen in 2015/16 season – long and late season • biggest impact in young adults • The national flu immunisation programme 2016/17 impact of flu varies from year to year • Weekly all age GP influenza-like illness rates for 2015 to 2016 and past seasons, England (RCGP) flu activity usually between September to March (weeks 37 and 15) high number admissions to hospital and ICU/HDU admissions – higher than seen in the previous few seasons
12 Flu epidemiology • • moderate levels of influenza activity seen in 2015/16 season – long and late season • biggest impact in young adults • The national flu immunisation programme 2016/17 impact of flu varies from year to year • Weekly all age GP influenza-like illness rates for 2015 to 2016 and past seasons, England (RCGP) flu activity usually between September to March (weeks 37 and 15) high number admissions to hospital and ICU/HDU admissions – higher than seen in the previous few seasons
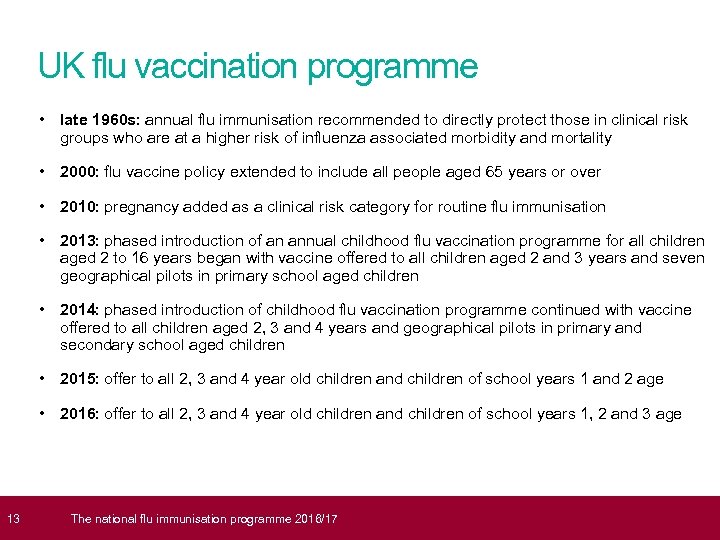 13 UK flu vaccination programme • late 1960 s: annual flu immunisation recommended to directly protect those in clinical risk groups who are at a higher risk of influenza associated morbidity and mortality • 2000: flu vaccine policy extended to include all people aged 65 years or over • 2010: pregnancy added as a clinical risk category for routine flu immunisation • 2013: phased introduction of an annual childhood flu vaccination programme for all children aged 2 to 16 years began with vaccine offered to all children aged 2 and 3 years and seven geographical pilots in primary school aged children • 2014: phased introduction of childhood flu vaccination programme continued with vaccine offered to all children aged 2, 3 and 4 years and geographical pilots in primary and secondary school aged children • 2015: offer to all 2, 3 and 4 year old children and children of school years 1 and 2 age • 2016: offer to all 2, 3 and 4 year old children and children of school years 1, 2 and 3 age The national flu immunisation programme 2016/17
13 UK flu vaccination programme • late 1960 s: annual flu immunisation recommended to directly protect those in clinical risk groups who are at a higher risk of influenza associated morbidity and mortality • 2000: flu vaccine policy extended to include all people aged 65 years or over • 2010: pregnancy added as a clinical risk category for routine flu immunisation • 2013: phased introduction of an annual childhood flu vaccination programme for all children aged 2 to 16 years began with vaccine offered to all children aged 2 and 3 years and seven geographical pilots in primary school aged children • 2014: phased introduction of childhood flu vaccination programme continued with vaccine offered to all children aged 2, 3 and 4 years and geographical pilots in primary and secondary school aged children • 2015: offer to all 2, 3 and 4 year old children and children of school years 1 and 2 age • 2016: offer to all 2, 3 and 4 year old children and children of school years 1, 2 and 3 age The national flu immunisation programme 2016/17
 14 Flu vaccine eligibility: 2016/17 flu season • all children aged two, three and four years on 31 August 2016 • all children of school years 1, 2 and 3 age • all primary school-aged children in former primary school pilot areas • those aged six months to under 65 years in clinical risk groups • all pregnant women (including those who become pregnant during flu season) • those aged 65 years and over (including those becoming 65 years by 31 March 2017 • those living in long-stay residential care homes or other long-stay care facilities • carers and household contacts of immunocompromised individuals Frontline health and social care workers should be provided flu vaccination by their employer. This includes general practice staff The national flu immunisation programme 2016/17
14 Flu vaccine eligibility: 2016/17 flu season • all children aged two, three and four years on 31 August 2016 • all children of school years 1, 2 and 3 age • all primary school-aged children in former primary school pilot areas • those aged six months to under 65 years in clinical risk groups • all pregnant women (including those who become pregnant during flu season) • those aged 65 years and over (including those becoming 65 years by 31 March 2017 • those living in long-stay residential care homes or other long-stay care facilities • carers and household contacts of immunocompromised individuals Frontline health and social care workers should be provided flu vaccination by their employer. This includes general practice staff The national flu immunisation programme 2016/17
 15 Morbidly obese patients • JCVI has advised morbidly obese patients (BMI 40+) could benefit from flu vaccination • those with morbid obesity (BMI>40) found to be at higher risk of hospitalisation and death following pandemic influenza infection • many in this patient group already eligible due to complications of obesity that place them in another risk category • practices need to use clinical judgement to decide whether to vaccinate this group of patients • however, flu vaccinations for morbidly obese patients with no other recognised risk factor will not attract a payment in 2016/17 The national flu immunisation programme 2016/17
15 Morbidly obese patients • JCVI has advised morbidly obese patients (BMI 40+) could benefit from flu vaccination • those with morbid obesity (BMI>40) found to be at higher risk of hospitalisation and death following pandemic influenza infection • many in this patient group already eligible due to complications of obesity that place them in another risk category • practices need to use clinical judgement to decide whether to vaccinate this group of patients • however, flu vaccinations for morbidly obese patients with no other recognised risk factor will not attract a payment in 2016/17 The national flu immunisation programme 2016/17
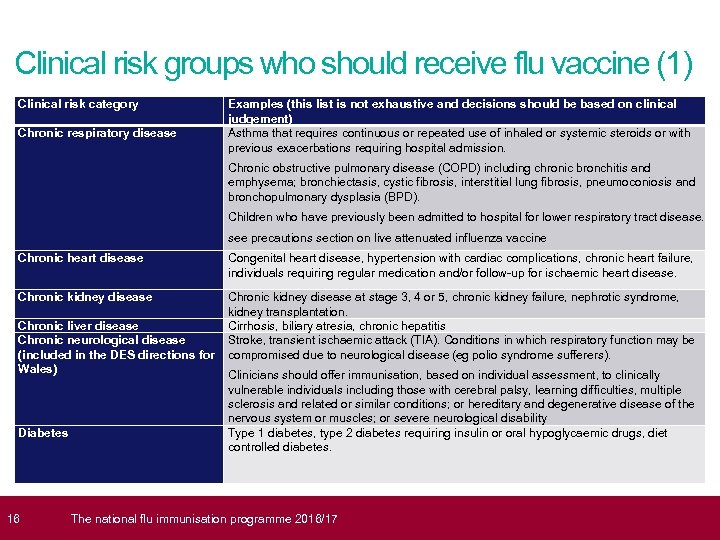 Clinical risk groups who should receive flu vaccine (1) Clinical risk category Chronic respiratory disease Examples (this list is not exhaustive and decisions should be based on clinical judgement) Asthma that requires continuous or repeated use of inhaled or systemic steroids or with previous exacerbations requiring hospital admission. Chronic obstructive pulmonary disease (COPD) including chronic bronchitis and emphysema; bronchiectasis, cystic fibrosis, interstitial lung fibrosis, pneumoconiosis and bronchopulmonary dysplasia (BPD). Children who have previously been admitted to hospital for lower respiratory tract disease. see precautions section on live attenuated influenza vaccine Chronic heart disease Congenital heart disease, hypertension with cardiac complications, chronic heart failure, individuals requiring regular medication and/or follow-up for ischaemic heart disease. Chronic kidney disease at stage 3, 4 or 5, chronic kidney failure, nephrotic syndrome, kidney transplantation. Chronic liver disease Cirrhosis, biliary atresia, chronic hepatitis Chronic neurological disease Stroke, transient ischaemic attack (TIA). Conditions in which respiratory function may be (included in the DES directions for compromised due to neurological disease (eg polio syndrome sufferers). Wales) Clinicians should offer immunisation, based on individual assessment, to clinically Diabetes 16 vulnerable individuals including those with cerebral palsy, learning difficulties, multiple sclerosis and related or similar conditions; or hereditary and degenerative disease of the nervous system or muscles; or severe neurological disability Type 1 diabetes, type 2 diabetes requiring insulin or oral hypoglycaemic drugs, diet controlled diabetes. The national flu immunisation programme 2016/17
Clinical risk groups who should receive flu vaccine (1) Clinical risk category Chronic respiratory disease Examples (this list is not exhaustive and decisions should be based on clinical judgement) Asthma that requires continuous or repeated use of inhaled or systemic steroids or with previous exacerbations requiring hospital admission. Chronic obstructive pulmonary disease (COPD) including chronic bronchitis and emphysema; bronchiectasis, cystic fibrosis, interstitial lung fibrosis, pneumoconiosis and bronchopulmonary dysplasia (BPD). Children who have previously been admitted to hospital for lower respiratory tract disease. see precautions section on live attenuated influenza vaccine Chronic heart disease Congenital heart disease, hypertension with cardiac complications, chronic heart failure, individuals requiring regular medication and/or follow-up for ischaemic heart disease. Chronic kidney disease at stage 3, 4 or 5, chronic kidney failure, nephrotic syndrome, kidney transplantation. Chronic liver disease Cirrhosis, biliary atresia, chronic hepatitis Chronic neurological disease Stroke, transient ischaemic attack (TIA). Conditions in which respiratory function may be (included in the DES directions for compromised due to neurological disease (eg polio syndrome sufferers). Wales) Clinicians should offer immunisation, based on individual assessment, to clinically Diabetes 16 vulnerable individuals including those with cerebral palsy, learning difficulties, multiple sclerosis and related or similar conditions; or hereditary and degenerative disease of the nervous system or muscles; or severe neurological disability Type 1 diabetes, type 2 diabetes requiring insulin or oral hypoglycaemic drugs, diet controlled diabetes. The national flu immunisation programme 2016/17
 17 Clinical risk groups who should receive flu vaccine (2) Clinical risk category Immunosuppression (see contraindications and precautions section on live attenuated influenza vaccine) Examples (this list is not exhaustive and decisions should be based on clinical judgement) Immunosuppression due to disease or treatment, including patients undergoing chemotherapy leading to immunosuppression, bone marrow transplant, HIV infection at all stages, multiple myeloma or genetic disorders affecting the immune system (eg IRAK-4, NEMO, complement disorders) Individuals treated with or likely to be treated with systemic steroids for more than a month at a dose equivalent to prednisolone at 20 mg or more per day (any age), or for children under 20 kg, a dose of 1 mg or more per kg per day. It is difficult to define at what level of immunosuppression a patient could be considered to be at a greater risk of the serious consequences of influenza and should be offered influenza vaccination. This decision is best made on an individual basis and left to the patient’s clinician. Some immunocompromised patients may have a suboptimal immunological response to the vaccine. Asplenia or dysfunction of the This also includes conditions such as homozygous sickle cell disease and spleen coeliac syndrome that may lead to splenic dysfunction. Pregnant women at any stage of pregnancy (first, second or third trimesters). (see precautions section on live attenuated influenza vaccine) The national flu immunisation programme 2016/17
17 Clinical risk groups who should receive flu vaccine (2) Clinical risk category Immunosuppression (see contraindications and precautions section on live attenuated influenza vaccine) Examples (this list is not exhaustive and decisions should be based on clinical judgement) Immunosuppression due to disease or treatment, including patients undergoing chemotherapy leading to immunosuppression, bone marrow transplant, HIV infection at all stages, multiple myeloma or genetic disorders affecting the immune system (eg IRAK-4, NEMO, complement disorders) Individuals treated with or likely to be treated with systemic steroids for more than a month at a dose equivalent to prednisolone at 20 mg or more per day (any age), or for children under 20 kg, a dose of 1 mg or more per kg per day. It is difficult to define at what level of immunosuppression a patient could be considered to be at a greater risk of the serious consequences of influenza and should be offered influenza vaccination. This decision is best made on an individual basis and left to the patient’s clinician. Some immunocompromised patients may have a suboptimal immunological response to the vaccine. Asplenia or dysfunction of the This also includes conditions such as homozygous sickle cell disease and spleen coeliac syndrome that may lead to splenic dysfunction. Pregnant women at any stage of pregnancy (first, second or third trimesters). (see precautions section on live attenuated influenza vaccine) The national flu immunisation programme 2016/17
 18 Flu immunisation should also be offered to: • those living in long-stay residential care homes or other long-stay care facilities where rapid spread is likely to follow introduction of infection and cause high morbidity and mortality (this does not include prisons, young offender institutions, university halls of residence etc) • those who are in receipt of a carer’s allowance, or those who are the main carer of an elderly or disabled person whose welfare may be at risk if the carer falls ill • household contacts of immunocompromised individuals, specifically those who expect to share living accommodation on most days over the winter and therefore for whom continuing close contact is unavoidable. • health and social care staff in direct contact with patients/service users should be vaccinated as part of an employer’s occupational health obligation The national flu immunisation programme 2016/17
18 Flu immunisation should also be offered to: • those living in long-stay residential care homes or other long-stay care facilities where rapid spread is likely to follow introduction of infection and cause high morbidity and mortality (this does not include prisons, young offender institutions, university halls of residence etc) • those who are in receipt of a carer’s allowance, or those who are the main carer of an elderly or disabled person whose welfare may be at risk if the carer falls ill • household contacts of immunocompromised individuals, specifically those who expect to share living accommodation on most days over the winter and therefore for whom continuing close contact is unavoidable. • health and social care staff in direct contact with patients/service users should be vaccinated as part of an employer’s occupational health obligation The national flu immunisation programme 2016/17
 19 Other groups who should receive flu vaccine • the list of clinical risk groups is not exhaustive • healthcare practitioners should apply clinical judgement to take into account the risk of flu exacerbating any underlying disease as well as the risk of serious illness from flu itself • flu vaccine should be offered to such patients even if the individual is not in the clinical risk groups specified in the risk groups list • child contacts of very severely immunocompromised individuals should be given inactivated vaccine The national flu immunisation programme 2016/17
19 Other groups who should receive flu vaccine • the list of clinical risk groups is not exhaustive • healthcare practitioners should apply clinical judgement to take into account the risk of flu exacerbating any underlying disease as well as the risk of serious illness from flu itself • flu vaccine should be offered to such patients even if the individual is not in the clinical risk groups specified in the risk groups list • child contacts of very severely immunocompromised individuals should be given inactivated vaccine The national flu immunisation programme 2016/17
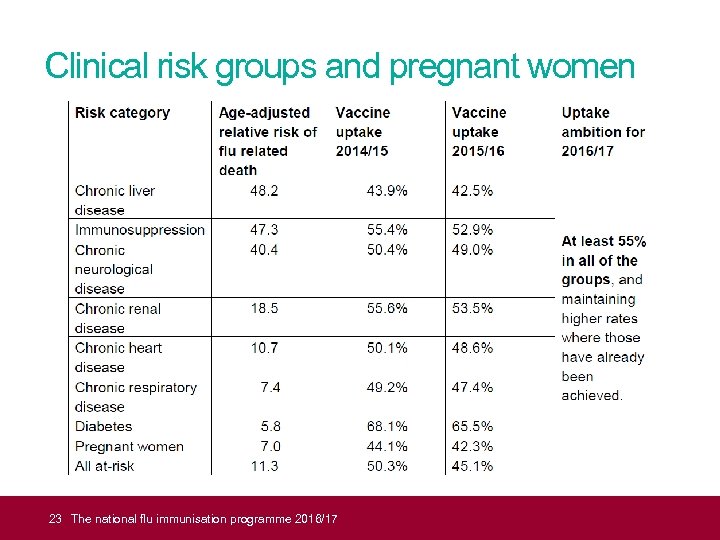
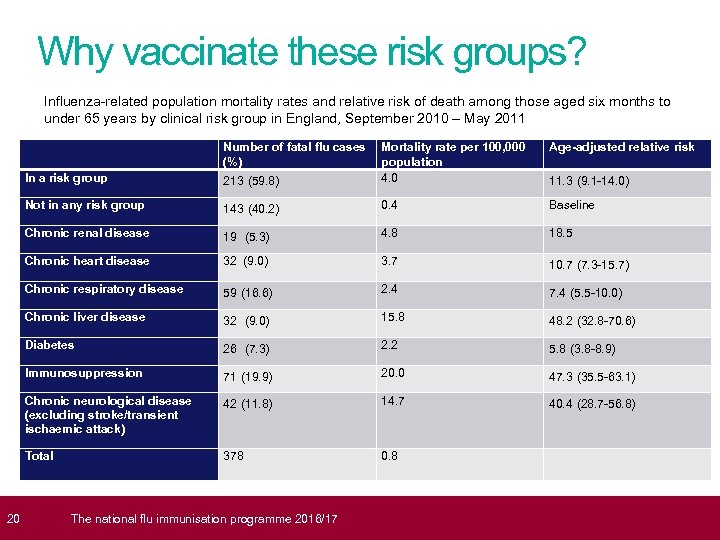 20 Why vaccinate these risk groups? Influenza-related population mortality rates and relative risk of death among those aged six months to under 65 years by clinical risk group in England, September 2010 – May 2011 In a risk group Number of fatal flu cases Mortality rate per 100, 000 (%) population 4. 0 213 (59. 8) Not in any risk group 143 (40. 2) 0. 4 Baseline Chronic renal disease 19 (5. 3) 4. 8 18. 5 Chronic heart disease 32 (9. 0) 3. 7 10. 7 (7. 3 -15. 7) Chronic respiratory disease 59 (16. 6) 2. 4 7. 4 (5. 5 -10. 0) Chronic liver disease 32 (9. 0) 15. 8 48. 2 (32. 8 -70. 6) Diabetes 26 (7. 3) 2. 2 5. 8 (3. 8 -8. 9) Immunosuppression 71 (19. 9) 20. 0 47. 3 (35. 5 -63. 1) Chronic neurological disease (excluding stroke/transient ischaemic attack) 42 (11. 8) 14. 7 40. 4 (28. 7 -56. 8) Total 378 0. 8 The national flu immunisation programme 2016/17 Age-adjusted relative risk 11. 3 (9. 1 -14. 0)
20 Why vaccinate these risk groups? Influenza-related population mortality rates and relative risk of death among those aged six months to under 65 years by clinical risk group in England, September 2010 – May 2011 In a risk group Number of fatal flu cases Mortality rate per 100, 000 (%) population 4. 0 213 (59. 8) Not in any risk group 143 (40. 2) 0. 4 Baseline Chronic renal disease 19 (5. 3) 4. 8 18. 5 Chronic heart disease 32 (9. 0) 3. 7 10. 7 (7. 3 -15. 7) Chronic respiratory disease 59 (16. 6) 2. 4 7. 4 (5. 5 -10. 0) Chronic liver disease 32 (9. 0) 15. 8 48. 2 (32. 8 -70. 6) Diabetes 26 (7. 3) 2. 2 5. 8 (3. 8 -8. 9) Immunosuppression 71 (19. 9) 20. 0 47. 3 (35. 5 -63. 1) Chronic neurological disease (excluding stroke/transient ischaemic attack) 42 (11. 8) 14. 7 40. 4 (28. 7 -56. 8) Total 378 0. 8 The national flu immunisation programme 2016/17 Age-adjusted relative risk 11. 3 (9. 1 -14. 0)
 21 Vaccination of clinical risk groups • increasing flu vaccine uptake in clinical risk groups important because of increased risk of death and serious illness if people in these groups catch flu • for a number of years only around half of patients aged six months to under 65 in clinical risk groups have been vaccinated • despite those with liver disease and chronic neurological disease having some of the highest mortality rates, they have low flu vaccine uptake rate compared with those in other clinical risk groups • vaccine uptake for all those in clinical risk groups needs to improve, but particularly in those with chronic liver and neurological disease, and people with learning disabilities The national flu immunisation programme 2016/17
21 Vaccination of clinical risk groups • increasing flu vaccine uptake in clinical risk groups important because of increased risk of death and serious illness if people in these groups catch flu • for a number of years only around half of patients aged six months to under 65 in clinical risk groups have been vaccinated • despite those with liver disease and chronic neurological disease having some of the highest mortality rates, they have low flu vaccine uptake rate compared with those in other clinical risk groups • vaccine uptake for all those in clinical risk groups needs to improve, but particularly in those with chronic liver and neurological disease, and people with learning disabilities The national flu immunisation programme 2016/17
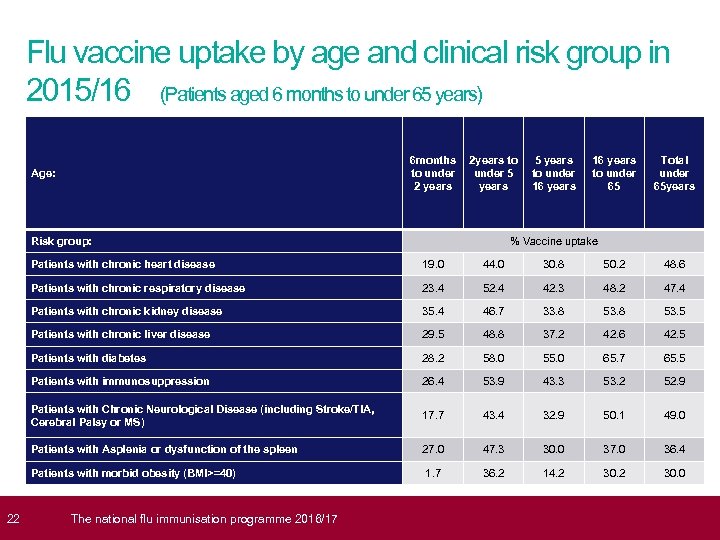 22 Flu vaccine uptake by age and clinical risk group in 2015/16 (Patients aged 6 months to under 65 years) 6 months 2 years to to under 5 2 years Age: Risk group: 5 years to under 16 years to under 65 Total under 65 years % Vaccine uptake Patients with chronic heart disease 19. 0 44. 0 30. 8 50. 2 48. 6 Patients with chronic respiratory disease 23. 4 52. 4 42. 3 48. 2 47. 4 Patients with chronic kidney disease 35. 4 46. 7 33. 8 53. 5 Patients with chronic liver disease 29. 5 48. 8 37. 2 42. 6 42. 5 Patients with diabetes 28. 2 58. 0 55. 0 65. 7 65. 5 Patients with immunosuppression 26. 4 53. 9 43. 3 53. 2 52. 9 Patients with Chronic Neurological Disease (including Stroke/TIA, Cerebral Palsy or MS) 17. 7 43. 4 32. 9 50. 1 49. 0 Patients with Asplenia or dysfunction of the spleen 27. 0 47. 3 30. 0 37. 0 36. 4 Patients with morbid obesity (BMI>=40) 1. 7 36. 2 14. 2 30. 0 The national flu immunisation programme 2016/17
22 Flu vaccine uptake by age and clinical risk group in 2015/16 (Patients aged 6 months to under 65 years) 6 months 2 years to to under 5 2 years Age: Risk group: 5 years to under 16 years to under 65 Total under 65 years % Vaccine uptake Patients with chronic heart disease 19. 0 44. 0 30. 8 50. 2 48. 6 Patients with chronic respiratory disease 23. 4 52. 4 42. 3 48. 2 47. 4 Patients with chronic kidney disease 35. 4 46. 7 33. 8 53. 5 Patients with chronic liver disease 29. 5 48. 8 37. 2 42. 6 42. 5 Patients with diabetes 28. 2 58. 0 55. 0 65. 7 65. 5 Patients with immunosuppression 26. 4 53. 9 43. 3 53. 2 52. 9 Patients with Chronic Neurological Disease (including Stroke/TIA, Cerebral Palsy or MS) 17. 7 43. 4 32. 9 50. 1 49. 0 Patients with Asplenia or dysfunction of the spleen 27. 0 47. 3 30. 0 37. 0 36. 4 Patients with morbid obesity (BMI>=40) 1. 7 36. 2 14. 2 30. 0 The national flu immunisation programme 2016/17
 Clinical risk groups and pregnant women 23 The national flu immunisation programme 2016/17
Clinical risk groups and pregnant women 23 The national flu immunisation programme 2016/17
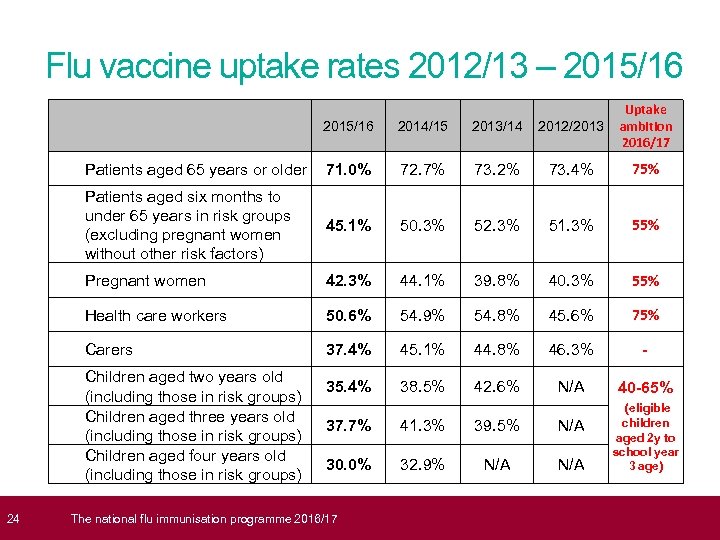 24 Flu vaccine uptake rates 2012/13 – 2015/16 2014/15 2013/14 2012/2013 Uptake ambition 2016/17 Patients aged 65 years or older 71. 0% 72. 7% 73. 2% 73. 4% 75% Patients aged six months to under 65 years in risk groups (excluding pregnant women without other risk factors) 45. 1% 50. 3% 52. 3% 51. 3% 55% Pregnant women 42. 3% 44. 1% 39. 8% 40. 3% 55% Health care workers 50. 6% 54. 9% 54. 8% 45. 6% 75% Carers 37. 4% 45. 1% 44. 8% 46. 3% - 38. 5% 42. 6% N/A 40 -65% (eligible 41. 3% 39. 5% N/A 32. 9% N/A children aged 2 y to school year 3 age) Children aged two years old 35. 4% (including those in risk groups) Children aged three years old 37. 7% (including those in risk groups) Children aged four years old 30. 0% (including those in risk groups) T he national flu immunisation programme 2016/17
24 Flu vaccine uptake rates 2012/13 – 2015/16 2014/15 2013/14 2012/2013 Uptake ambition 2016/17 Patients aged 65 years or older 71. 0% 72. 7% 73. 2% 73. 4% 75% Patients aged six months to under 65 years in risk groups (excluding pregnant women without other risk factors) 45. 1% 50. 3% 52. 3% 51. 3% 55% Pregnant women 42. 3% 44. 1% 39. 8% 40. 3% 55% Health care workers 50. 6% 54. 9% 54. 8% 45. 6% 75% Carers 37. 4% 45. 1% 44. 8% 46. 3% - 38. 5% 42. 6% N/A 40 -65% (eligible 41. 3% 39. 5% N/A 32. 9% N/A children aged 2 y to school year 3 age) Children aged two years old 35. 4% (including those in risk groups) Children aged three years old 37. 7% (including those in risk groups) Children aged four years old 30. 0% (including those in risk groups) T he national flu immunisation programme 2016/17
 25 Pregnant women All pregnant women are recommended to receive the inactivated flu vaccine irrespective of their stage of pregnancy • pregnant women at increased risk from complications if they contract flu • having flu during pregnancy may be associated with premature birth and smaller birth size and weight • flu vaccination during pregnancy provides passive immunity against flu to infants in the first few months of life • studies on safety of flu vaccine in pregnancy show that inactivated flu vaccine can be safely and effectively administered during any trimester of pregnancy • no study to date has demonstrated an increased risk of either maternal complications or adverse fetal outcomes associated with inactivated flu vaccine • women should be offered the vaccine every time they are pregnant The national flu immunisation programme 2016/17
25 Pregnant women All pregnant women are recommended to receive the inactivated flu vaccine irrespective of their stage of pregnancy • pregnant women at increased risk from complications if they contract flu • having flu during pregnancy may be associated with premature birth and smaller birth size and weight • flu vaccination during pregnancy provides passive immunity against flu to infants in the first few months of life • studies on safety of flu vaccine in pregnancy show that inactivated flu vaccine can be safely and effectively administered during any trimester of pregnancy • no study to date has demonstrated an increased risk of either maternal complications or adverse fetal outcomes associated with inactivated flu vaccine • women should be offered the vaccine every time they are pregnant The national flu immunisation programme 2016/17
 26 Why vaccinate children against flu? Extension of the seasonal flu vaccination programme to all children aims to appreciably lower the public health impact of flu by: • providing direct protection thus preventing a large number of cases of flu infection in children • providing indirect protection by lowering flu transmission from children: • to other children • to adults • to those in the clinical risk groups of any age Reducing flu transmission in the community will avert many cases of severe flu and flu-related deaths in older adults and people with clinical risk factors. Annual administration of flu vaccine to children is expected to substantially reduce flu -related illness, GP consultations, hospital admissions and deaths. The national flu immunisation programme 2016/17
26 Why vaccinate children against flu? Extension of the seasonal flu vaccination programme to all children aims to appreciably lower the public health impact of flu by: • providing direct protection thus preventing a large number of cases of flu infection in children • providing indirect protection by lowering flu transmission from children: • to other children • to adults • to those in the clinical risk groups of any age Reducing flu transmission in the community will avert many cases of severe flu and flu-related deaths in older adults and people with clinical risk factors. Annual administration of flu vaccine to children is expected to substantially reduce flu -related illness, GP consultations, hospital admissions and deaths. The national flu immunisation programme 2016/17
 27 Health and social care workers • frontline health and social care workers have a duty of care to protect their patients and service users from infection • vaccination of health and social care workers protects them and reduces risk of spreading flu to their patients, service users, colleagues and family members • evidence vaccination significantly lowers rates of flu-like illness, hospitalisation and mortality in vulnerable patients in long-term healthcare settings • reduces transmission of flu to vulnerable patients, some of whom may have impaired immunity that may not respond well to immunisation • vaccination of frontline workers also helps reduce sickness absences and contributes to keeping the NHS and care services running through winter pressures The national flu immunisation programme 2016/17
27 Health and social care workers • frontline health and social care workers have a duty of care to protect their patients and service users from infection • vaccination of health and social care workers protects them and reduces risk of spreading flu to their patients, service users, colleagues and family members • evidence vaccination significantly lowers rates of flu-like illness, hospitalisation and mortality in vulnerable patients in long-term healthcare settings • reduces transmission of flu to vulnerable patients, some of whom may have impaired immunity that may not respond well to immunisation • vaccination of frontline workers also helps reduce sickness absences and contributes to keeping the NHS and care services running through winter pressures The national flu immunisation programme 2016/17
 28 Health and social care workers (cont) • NHS and social care bodies have a responsibility to ensure, as far as is reasonably practicable, that health and social care workers are free of, and are protected from exposure to infections that can be caught at work (Health and Social Care Act 2008, Code of Practice on the prevention and control of infections) • responsibility for funding and administering seasonal flu vaccine to staff lies with employers • trusts/employers must ensure that health and social care staff directly involved in delivering care encouraged to be immunised and that processes are in place to facilitate this • overall level of flu vaccine uptake in health care workers is still below the 75% aspiration • see NHS Employers flu fighter campaign www. nhsemployers. org/flu The national flu immunisation programme 2016/17
28 Health and social care workers (cont) • NHS and social care bodies have a responsibility to ensure, as far as is reasonably practicable, that health and social care workers are free of, and are protected from exposure to infections that can be caught at work (Health and Social Care Act 2008, Code of Practice on the prevention and control of infections) • responsibility for funding and administering seasonal flu vaccine to staff lies with employers • trusts/employers must ensure that health and social care staff directly involved in delivering care encouraged to be immunised and that processes are in place to facilitate this • overall level of flu vaccine uptake in health care workers is still below the 75% aspiration • see NHS Employers flu fighter campaign www. nhsemployers. org/flu The national flu immunisation programme 2016/17
 29 Key messages to health and social care workers • duty of care as professionals to patients or residents to do everything in your power to protect them against infection, including being immunised against flu • getting vaccinated against flu can help protect you, your patients and family • everyone is susceptible to flu, even if you are in good health and eat well • you can be infected with the virus and have no symptoms but can still pass flu virus to others including patients or residents • good infection control measures reduce spread of flu and other acute respiratory infections in healthcare settings but are not sufficient alone to prevent them • impact of flu on frail and vulnerable patients can be fatal and outbreaks can cause severe disruption in communities, care homes and hospitals • flu vaccine has a good safety record and will help protect you. It cannot give you flu. Having the vaccination can encourage your colleagues to do likewise • throughout the last ten years there has generally been a good to moderate match between the strains of flu virus in the vaccine and those that subsequently circulated • staff act as positive role models for patients aged 65 and over, those with long-term health conditions and pregnant women to take up the offer too The national flu immunisation programme 2016/17
29 Key messages to health and social care workers • duty of care as professionals to patients or residents to do everything in your power to protect them against infection, including being immunised against flu • getting vaccinated against flu can help protect you, your patients and family • everyone is susceptible to flu, even if you are in good health and eat well • you can be infected with the virus and have no symptoms but can still pass flu virus to others including patients or residents • good infection control measures reduce spread of flu and other acute respiratory infections in healthcare settings but are not sufficient alone to prevent them • impact of flu on frail and vulnerable patients can be fatal and outbreaks can cause severe disruption in communities, care homes and hospitals • flu vaccine has a good safety record and will help protect you. It cannot give you flu. Having the vaccination can encourage your colleagues to do likewise • throughout the last ten years there has generally been a good to moderate match between the strains of flu virus in the vaccine and those that subsequently circulated • staff act as positive role models for patients aged 65 and over, those with long-term health conditions and pregnant women to take up the offer too The national flu immunisation programme 2016/17
 30 When to vaccinate • those eligible should be given flu vaccination as soon as vaccine is available so that people are protected when flu begins to circulate in the community • ideally most vaccination should be completed before the end of December before flu circulation usually peaks • flu can circulate considerably later than this however so clinical judgement should be applied to assess needs of individual patients and whether it is appropriate to continue to offer vaccination from January to March • this decision should take into account level of flu-like illness in community and fact that the immune response following flu vaccination takes about two weeks to develop fully • protection afforded by the vaccine thought to last at least one influenza season • however, as antibody levels likely to reduce in subsequent seasons and may be changes to circulating strains from one season to next, annual revaccination is important The national flu immunisation programme 2016/17
30 When to vaccinate • those eligible should be given flu vaccination as soon as vaccine is available so that people are protected when flu begins to circulate in the community • ideally most vaccination should be completed before the end of December before flu circulation usually peaks • flu can circulate considerably later than this however so clinical judgement should be applied to assess needs of individual patients and whether it is appropriate to continue to offer vaccination from January to March • this decision should take into account level of flu-like illness in community and fact that the immune response following flu vaccination takes about two weeks to develop fully • protection afforded by the vaccine thought to last at least one influenza season • however, as antibody levels likely to reduce in subsequent seasons and may be changes to circulating strains from one season to next, annual revaccination is important The national flu immunisation programme 2016/17
 31 Which flu vaccine should be used? The national flu immunisation programme 2016/17
31 Which flu vaccine should be used? The national flu immunisation programme 2016/17
 32 Types of flu vaccines Two main types of vaccine available: • inactivated – by injection • live attenuated – by nasal application None of the flu vaccines can cause clinical influenza in those that can be vaccinated Ø Trivalent: flu vaccines contain two subtypes of Influenza A and one type B virus Ø Quadrivalent vaccines contain two subtypes of Influenza A and both B virus types* As quadrivalent vaccines contain both lineages of B viruses and therefore may provide better protection against the circulating B strain(s) than trivalent flu vaccines, the live intranasal vaccine offered to children aged 2 years and over is a quadrivalent vaccine, as is the inactivated vaccine recommended for children aged 3 years and above who cannot receive live attenuated vaccine. *Quadrivalent inactivated flu vaccine only authorised for children aged 3 years and older. The national flu immunisation programme 2016/17
32 Types of flu vaccines Two main types of vaccine available: • inactivated – by injection • live attenuated – by nasal application None of the flu vaccines can cause clinical influenza in those that can be vaccinated Ø Trivalent: flu vaccines contain two subtypes of Influenza A and one type B virus Ø Quadrivalent vaccines contain two subtypes of Influenza A and both B virus types* As quadrivalent vaccines contain both lineages of B viruses and therefore may provide better protection against the circulating B strain(s) than trivalent flu vaccines, the live intranasal vaccine offered to children aged 2 years and over is a quadrivalent vaccine, as is the inactivated vaccine recommended for children aged 3 years and above who cannot receive live attenuated vaccine. *Quadrivalent inactivated flu vaccine only authorised for children aged 3 years and older. The national flu immunisation programme 2016/17
 33 Live attenuated influenza vaccine (LAIV) • a live attenuated intranasal spray is the recommended vaccine for the childhood flu programme • the live attenuated influenza vaccine (LAIV) has been shown to be more effective in children compared with inactivated influenza vaccines • it may offer some protection against strains not contained in the vaccine as well as to those that are and has the potential to offer better protection against virus strains that have undergone antigenic drift • since this vaccine is comprised of weakened whole live virus, it replicates natural infection which induces better immune memory (thereby offering better long-term protection to children than from the inactivated vaccines) • in addition to being attenuated (weakened), the live viruses in LAIV have been adapted to cold so that they cannot replicate efficiently at body temperature • LAIV has a good safety profile in children aged two years and older The national flu immunisation programme 2016/17
33 Live attenuated influenza vaccine (LAIV) • a live attenuated intranasal spray is the recommended vaccine for the childhood flu programme • the live attenuated influenza vaccine (LAIV) has been shown to be more effective in children compared with inactivated influenza vaccines • it may offer some protection against strains not contained in the vaccine as well as to those that are and has the potential to offer better protection against virus strains that have undergone antigenic drift • since this vaccine is comprised of weakened whole live virus, it replicates natural infection which induces better immune memory (thereby offering better long-term protection to children than from the inactivated vaccines) • in addition to being attenuated (weakened), the live viruses in LAIV have been adapted to cold so that they cannot replicate efficiently at body temperature • LAIV has a good safety profile in children aged two years and older The national flu immunisation programme 2016/17
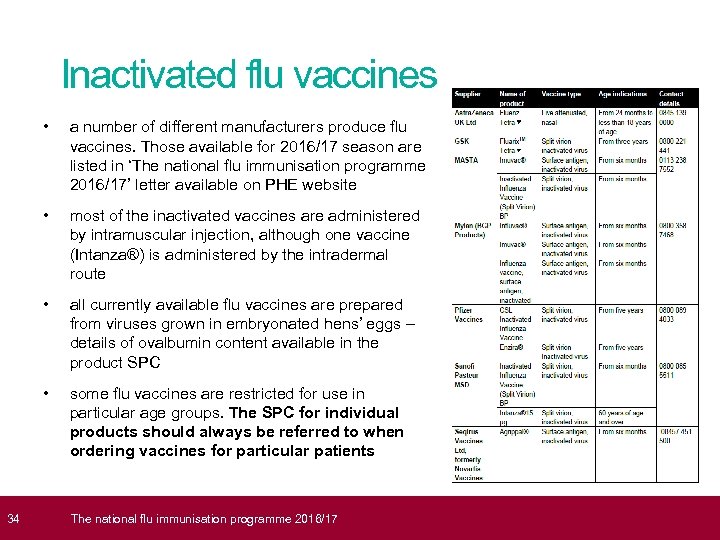 34 Inactivated flu vaccines • a number of different manufacturers produce flu vaccines. Those available for 2016/17 season are listed in ‘The national flu immunisation programme 2016/17’ letter available on PHE website • most of the inactivated vaccines are administered by intramuscular injection, although one vaccine (Intanza®) is administered by the intradermal route • all currently available flu vaccines are prepared from viruses grown in embryonated hens’ eggs – details of ovalbumin content available in the product SPC • some flu vaccines are restricted for use in particular age groups. The SPC for individual products should always be referred to when ordering vaccines for particular patients The national flu immunisation programme 2016/17
34 Inactivated flu vaccines • a number of different manufacturers produce flu vaccines. Those available for 2016/17 season are listed in ‘The national flu immunisation programme 2016/17’ letter available on PHE website • most of the inactivated vaccines are administered by intramuscular injection, although one vaccine (Intanza®) is administered by the intradermal route • all currently available flu vaccines are prepared from viruses grown in embryonated hens’ eggs – details of ovalbumin content available in the product SPC • some flu vaccines are restricted for use in particular age groups. The SPC for individual products should always be referred to when ordering vaccines for particular patients The national flu immunisation programme 2016/17
 35 Storage of flu vaccine Efficacy, safety and quality may be adversely affected if vaccines are not stored at the temperatures specified in the licence Flu vaccines must be stored in accordance with manufacturer’s instructions: • store between +2⁰C and +8⁰C • do not freeze • store in original packaging • protect from light Check expiry dates regularly: • the LAIV has an expiry date 18 weeks after manufacture – this is much shorter than inactivated flu vaccines • it is important that the expiry date on the nasal spray applicator is checked before use In the event of cold chain failure involving LAIV, refer to the document ‘Responding to cold chain failures involving the live attenuated intra-nasal influenza vaccine (LAIV)’ available on PHE Immunisation gov. uk website The national flu immunisation programme 2016/17
35 Storage of flu vaccine Efficacy, safety and quality may be adversely affected if vaccines are not stored at the temperatures specified in the licence Flu vaccines must be stored in accordance with manufacturer’s instructions: • store between +2⁰C and +8⁰C • do not freeze • store in original packaging • protect from light Check expiry dates regularly: • the LAIV has an expiry date 18 weeks after manufacture – this is much shorter than inactivated flu vaccines • it is important that the expiry date on the nasal spray applicator is checked before use In the event of cold chain failure involving LAIV, refer to the document ‘Responding to cold chain failures involving the live attenuated intra-nasal influenza vaccine (LAIV)’ available on PHE Immunisation gov. uk website The national flu immunisation programme 2016/17
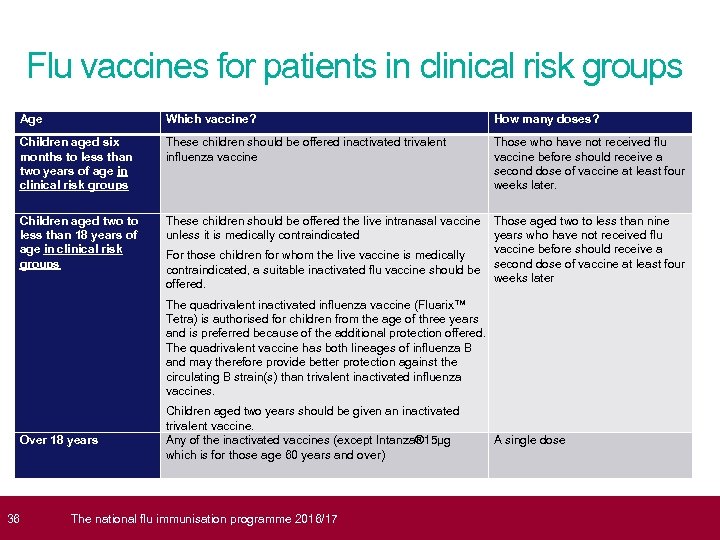 Flu vaccines for patients in clinical risk groups Age Which vaccine? How many doses? Children aged six months to less than two years of age in clinical risk groups These children should be offered inactivated trivalent influenza vaccine Those who have not received flu vaccine before should receive a second dose of vaccine at least four weeks later. Children aged two to less than 18 years of age in clinical risk groups These children should be offered the live intranasal vaccine Those aged two to less than nine unless it is medically contraindicated years who have not received flu vaccine before should receive a For those children for whom the live vaccine is medically contraindicated, a suitable inactivated flu vaccine should be second dose of vaccine at least four weeks later offered. The quadrivalent inactivated influenza vaccine (Fluarix™ Tetra) is authorised for children from the age of three years and is preferred because of the additional protection offered. The quadrivalent vaccine has both lineages of influenza B and may therefore provide better protection against the circulating B strain(s) than trivalent inactivated influenza vaccines. Over 18 years 36 Children aged two years should be given an inactivated trivalent vaccine. Any of the inactivated vaccines (except Intanza® 15μg which is for those age 60 years and over) The national flu immunisation programme 2016/17 A single dose
Flu vaccines for patients in clinical risk groups Age Which vaccine? How many doses? Children aged six months to less than two years of age in clinical risk groups These children should be offered inactivated trivalent influenza vaccine Those who have not received flu vaccine before should receive a second dose of vaccine at least four weeks later. Children aged two to less than 18 years of age in clinical risk groups These children should be offered the live intranasal vaccine Those aged two to less than nine unless it is medically contraindicated years who have not received flu vaccine before should receive a For those children for whom the live vaccine is medically contraindicated, a suitable inactivated flu vaccine should be second dose of vaccine at least four weeks later offered. The quadrivalent inactivated influenza vaccine (Fluarix™ Tetra) is authorised for children from the age of three years and is preferred because of the additional protection offered. The quadrivalent vaccine has both lineages of influenza B and may therefore provide better protection against the circulating B strain(s) than trivalent inactivated influenza vaccines. Over 18 years 36 Children aged two years should be given an inactivated trivalent vaccine. Any of the inactivated vaccines (except Intanza® 15μg which is for those age 60 years and over) The national flu immunisation programme 2016/17 A single dose
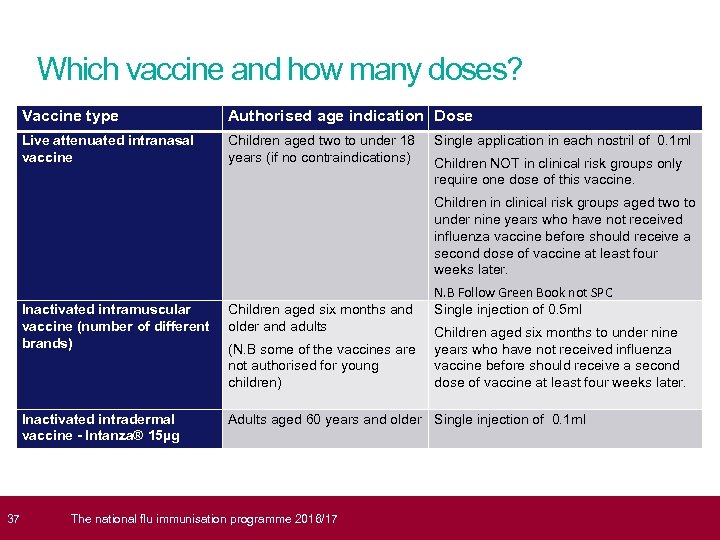 37 Which vaccine and how many doses? Vaccine type Authorised age indication Dose Live attenuated intranasal vaccine Children aged two to under 18 years (if no contraindications) Single application in each nostril of 0. 1 ml Children NOT in clinical risk groups only require one dose of this vaccine. Children in clinical risk groups aged two to under nine years who have not received influenza vaccine before should receive a second dose of vaccine at least four weeks later. N. B Follow Green Book not SPC Single injection of 0. 5 ml Inactivated intramuscular vaccine (number of different brands) Children aged six months and older and adults Inactivated intradermal vaccine - Intanza® 15µg Adults aged 60 years and older Single injection of 0. 1 ml (N. B some of the vaccines are not authorised for young children) The national flu immunisation programme 2016/17 Children aged six months to under nine years who have not received influenza vaccine before should receive a second dose of vaccine at least four weeks later.
37 Which vaccine and how many doses? Vaccine type Authorised age indication Dose Live attenuated intranasal vaccine Children aged two to under 18 years (if no contraindications) Single application in each nostril of 0. 1 ml Children NOT in clinical risk groups only require one dose of this vaccine. Children in clinical risk groups aged two to under nine years who have not received influenza vaccine before should receive a second dose of vaccine at least four weeks later. N. B Follow Green Book not SPC Single injection of 0. 5 ml Inactivated intramuscular vaccine (number of different brands) Children aged six months and older and adults Inactivated intradermal vaccine - Intanza® 15µg Adults aged 60 years and older Single injection of 0. 1 ml (N. B some of the vaccines are not authorised for young children) The national flu immunisation programme 2016/17 Children aged six months to under nine years who have not received influenza vaccine before should receive a second dose of vaccine at least four weeks later.
 38 Flu vaccine composition 2016/17 Trivalent vaccines will contain the following three viruses: • an A/California/7/2009 (H 1 N 1)pdm 09 -like virus • an A/Hong Kong/4801/2014 (H 3 N 2)-like virus • a B/Brisbane/60/2008 -like virus In addition to the above, the quadrivalent vaccine will also contain: B/Phuket/3073/2013 -like virus None of the influenza vaccines for the 2016/17 season contain thiomersal as an added preservative. More detailed information on the characteristics of the available vaccines, including age indications can be found in the Influenza chapter of the Green Book (Immunisation against infectious disease) and the product SPCs. The national flu immunisation programme 2015/16
38 Flu vaccine composition 2016/17 Trivalent vaccines will contain the following three viruses: • an A/California/7/2009 (H 1 N 1)pdm 09 -like virus • an A/Hong Kong/4801/2014 (H 3 N 2)-like virus • a B/Brisbane/60/2008 -like virus In addition to the above, the quadrivalent vaccine will also contain: B/Phuket/3073/2013 -like virus None of the influenza vaccines for the 2016/17 season contain thiomersal as an added preservative. More detailed information on the characteristics of the available vaccines, including age indications can be found in the Influenza chapter of the Green Book (Immunisation against infectious disease) and the product SPCs. The national flu immunisation programme 2015/16
 39 Flu vaccine presentation and dosage • inactivated flu vaccines for intramuscular (IM) administration supplied as suspensions in pre-filled syringes containing a 0. 5 ml dose • if SPC for IM inactivated flu vaccine states young children can be given either a 0. 25 ml or a 0. 5 ml dose, give 0. 5 ml dose • Intanza®, the intradermal vaccine, is supplied in a micro-needle injection system • the live intranasal flu vaccine is supplied as a nasal spray suspension in a special single use, pre-filled, nasal applicator. No reconstitution or dilution required. Each applicator contains 0. 2 ml (administered as 0. 1 ml per nostril) The national flu immunisation programme 2016/17
39 Flu vaccine presentation and dosage • inactivated flu vaccines for intramuscular (IM) administration supplied as suspensions in pre-filled syringes containing a 0. 5 ml dose • if SPC for IM inactivated flu vaccine states young children can be given either a 0. 25 ml or a 0. 5 ml dose, give 0. 5 ml dose • Intanza®, the intradermal vaccine, is supplied in a micro-needle injection system • the live intranasal flu vaccine is supplied as a nasal spray suspension in a special single use, pre-filled, nasal applicator. No reconstitution or dilution required. Each applicator contains 0. 2 ml (administered as 0. 1 ml per nostril) The national flu immunisation programme 2016/17
 40 Vaccine administration (inactivated vaccines) Intramuscular flu vaccines should be given into the upper arm (or anterolateral thigh in infants under one year of age). Individuals with a bleeding disorder should be given vaccine by deep subcutaneous injection to reduce the risk of bleeding Intradermal: Intanza® is supplied in a micro-needle injection system that should be held at right-angles to the skin. The device allows intradermal vaccination to be performed without the need for additional training • both inactivated and live flu vaccines can be given at the same time as, or at any interval before or after, other live and inactivated vaccines • different vaccines should be given at separate sites, preferably in a different limb. If given in the same limb, they should be given at least 2. 5 cm apart The national flu immunisation programme 2016/17
40 Vaccine administration (inactivated vaccines) Intramuscular flu vaccines should be given into the upper arm (or anterolateral thigh in infants under one year of age). Individuals with a bleeding disorder should be given vaccine by deep subcutaneous injection to reduce the risk of bleeding Intradermal: Intanza® is supplied in a micro-needle injection system that should be held at right-angles to the skin. The device allows intradermal vaccination to be performed without the need for additional training • both inactivated and live flu vaccines can be given at the same time as, or at any interval before or after, other live and inactivated vaccines • different vaccines should be given at separate sites, preferably in a different limb. If given in the same limb, they should be given at least 2. 5 cm apart The national flu immunisation programme 2016/17
 41 Administration of LAIV • LAIV is different from other flu vaccines – it is a live attenuated nasal vaccine and must not be injected • LAIV can be administered at the same time as, or at any interval from other vaccines including live vaccines • patient should breathe normally – no need to actively inhale or sniff • the vaccine is rapidly absorbed so no need to repeat either half of dose if patient sneezes, blows their nose or their nose drips following administration The national flu immunisation programme 2016/17
41 Administration of LAIV • LAIV is different from other flu vaccines – it is a live attenuated nasal vaccine and must not be injected • LAIV can be administered at the same time as, or at any interval from other vaccines including live vaccines • patient should breathe normally – no need to actively inhale or sniff • the vaccine is rapidly absorbed so no need to repeat either half of dose if patient sneezes, blows their nose or their nose drips following administration The national flu immunisation programme 2016/17
 42 Supply and administration of flu vaccines A range of mechanisms can be used for the supply and administration of vaccines, including: • patient specific prescription written manually or electronically by a registered medical practitioner or other authorised prescriber • Patient Specific Direction (PSD) • Patient Group Direction (PGD) PGD templates for the administration of the live and inactivated flu vaccines are available on the PHE website: https: //www. gov. uk/government/collections/immunisation-patient-group-direction-pgd NB Local authorisation is required before PHE PGD templates can be used The national flu immunisation programme 2016/17
42 Supply and administration of flu vaccines A range of mechanisms can be used for the supply and administration of vaccines, including: • patient specific prescription written manually or electronically by a registered medical practitioner or other authorised prescriber • Patient Specific Direction (PSD) • Patient Group Direction (PGD) PGD templates for the administration of the live and inactivated flu vaccines are available on the PHE website: https: //www. gov. uk/government/collections/immunisation-patient-group-direction-pgd NB Local authorisation is required before PHE PGD templates can be used The national flu immunisation programme 2016/17
 43 Contraindications There are very few individuals who cannot receive any flu vaccine Where there is doubt, expert advice should be sought promptly so that the period the individual is left unvaccinated is minimised For children aged 2 up to their 18 th birthday, where live flu vaccine cannot be given, it is likely that inactivated vaccine could be given instead The national flu immunisation programme 2016/17
43 Contraindications There are very few individuals who cannot receive any flu vaccine Where there is doubt, expert advice should be sought promptly so that the period the individual is left unvaccinated is minimised For children aged 2 up to their 18 th birthday, where live flu vaccine cannot be given, it is likely that inactivated vaccine could be given instead The national flu immunisation programme 2016/17
 44 Contraindications to flu vaccines None of the influenza vaccines should be given to those who have had: • confirmed anaphylactic reaction to a previous dose of the vaccine • confirmed anaphylactic reaction to any component of the vaccine The live attenuated flu vaccine should not be given to children who are: • clinically severely immunodeficient due to conditions or immunosuppressive therapy: § acute and chronic leukaemias § lymphoma § HIV infection not on highly active antiretroviral therapy § cellular immune deficiencies § high dose corticosteroids • receiving salicylate therapy • known to be pregnant The national flu immunisation programme 2014/15 The national flu immunisation programme 2016/17
44 Contraindications to flu vaccines None of the influenza vaccines should be given to those who have had: • confirmed anaphylactic reaction to a previous dose of the vaccine • confirmed anaphylactic reaction to any component of the vaccine The live attenuated flu vaccine should not be given to children who are: • clinically severely immunodeficient due to conditions or immunosuppressive therapy: § acute and chronic leukaemias § lymphoma § HIV infection not on highly active antiretroviral therapy § cellular immune deficiencies § high dose corticosteroids • receiving salicylate therapy • known to be pregnant The national flu immunisation programme 2014/15 The national flu immunisation programme 2016/17
 45 Precautions to flu vaccines Acutely unwell: • defer until recovered Heavy nasal congestion: • defer live intranasal vaccine until resolved or, if the child is in a risk group, consider inactivated flu vaccine to provide protection without delay Use with antiviral agents against flu: • LAIV should not be administered at the same time or within 48 hours of cessation of treatment with flu antiviral agents • administration of flu antiviral agents within two weeks of administration of LAIV may adversely affect the effectiveness of the vaccine The national flu immunisation programme 2016/17
45 Precautions to flu vaccines Acutely unwell: • defer until recovered Heavy nasal congestion: • defer live intranasal vaccine until resolved or, if the child is in a risk group, consider inactivated flu vaccine to provide protection without delay Use with antiviral agents against flu: • LAIV should not be administered at the same time or within 48 hours of cessation of treatment with flu antiviral agents • administration of flu antiviral agents within two weeks of administration of LAIV may adversely affect the effectiveness of the vaccine The national flu immunisation programme 2016/17
 46 Severe asthma or active wheezing • live flu vaccine is not recommended for children and adolescents with severe asthma or active wheezing eg those who are currently taking or have been prescribed oral steroids for respiratory disease in the last 14 days • children currently taking a high dose inhaled steroid - Budesonide >800 mcg/day or equivalent (eg Fluticasone > 500 mcg/day) should only be given live flu vaccine on the advice of their specialist As these children are a defined flu risk group, those who cannot receive LAIV should receive an inactivated flu vaccine • vaccination with LAIV should be deferred in children with a history of active wheezing in the past 72 hours or those who have increased use of bronchodilators in the previous 72 hours. If condition not improved after a further 72 hours then inactivated flu vaccine should be offered to avoid delaying protection in this high-risk group The national flu immunisation programme 2016/17
46 Severe asthma or active wheezing • live flu vaccine is not recommended for children and adolescents with severe asthma or active wheezing eg those who are currently taking or have been prescribed oral steroids for respiratory disease in the last 14 days • children currently taking a high dose inhaled steroid - Budesonide >800 mcg/day or equivalent (eg Fluticasone > 500 mcg/day) should only be given live flu vaccine on the advice of their specialist As these children are a defined flu risk group, those who cannot receive LAIV should receive an inactivated flu vaccine • vaccination with LAIV should be deferred in children with a history of active wheezing in the past 72 hours or those who have increased use of bronchodilators in the previous 72 hours. If condition not improved after a further 72 hours then inactivated flu vaccine should be offered to avoid delaying protection in this high-risk group The national flu immunisation programme 2016/17
 47 Egg allergy – adults • most flu vaccines are prepared from flu viruses grown in embryonated hens’ eggs – the final vaccine products contains varying amounts of egg (as ovalbumin) • adults with egg allergy can be immunised in any setting using an inactivated flu vaccine with an ovalbumin content less than 0. 12 µg/ml (equivalent to <0. 06µg for 0. 5 ml dose) • adults with severe anaphylaxis to egg that has previously required intensive care should be referred to specialists for immunisation in hospital • there is no ovalbumin-free vaccine available for the 2016/17 flu season The national flu immunisation programme 2016/17
47 Egg allergy – adults • most flu vaccines are prepared from flu viruses grown in embryonated hens’ eggs – the final vaccine products contains varying amounts of egg (as ovalbumin) • adults with egg allergy can be immunised in any setting using an inactivated flu vaccine with an ovalbumin content less than 0. 12 µg/ml (equivalent to <0. 06µg for 0. 5 ml dose) • adults with severe anaphylaxis to egg that has previously required intensive care should be referred to specialists for immunisation in hospital • there is no ovalbumin-free vaccine available for the 2016/17 flu season The national flu immunisation programme 2016/17
 48 Egg allergy – children • children with an egg allergy can be safely vaccinated with the LAIV in any setting (including primary care and schools) • those with both egg allergy and clinical risk factors* that contraindicate LAIV (eg immunosuppression) should be offered an inactivated flu vaccine with a very low ovalbumin content (less than 0. 12μg/ml) • children with a history of severe anaphylaxis to egg that has previously required intensive care, should be referred to specialists for immunisation in hospital • LAIV is not otherwise contraindicated in children with egg allergy. Eggallergic children with asthma can receive LAIV if their asthma is wellcontrolled (see previous slide on severe asthma) *Children in a clinical risk group and aged under nine years who have not been previously vaccinated against influenza will require a second dose whether given LAIV or inactivated vaccine The national flu immunisation programme 2016/17
48 Egg allergy – children • children with an egg allergy can be safely vaccinated with the LAIV in any setting (including primary care and schools) • those with both egg allergy and clinical risk factors* that contraindicate LAIV (eg immunosuppression) should be offered an inactivated flu vaccine with a very low ovalbumin content (less than 0. 12μg/ml) • children with a history of severe anaphylaxis to egg that has previously required intensive care, should be referred to specialists for immunisation in hospital • LAIV is not otherwise contraindicated in children with egg allergy. Eggallergic children with asthma can receive LAIV if their asthma is wellcontrolled (see previous slide on severe asthma) *Children in a clinical risk group and aged under nine years who have not been previously vaccinated against influenza will require a second dose whether given LAIV or inactivated vaccine The national flu immunisation programme 2016/17
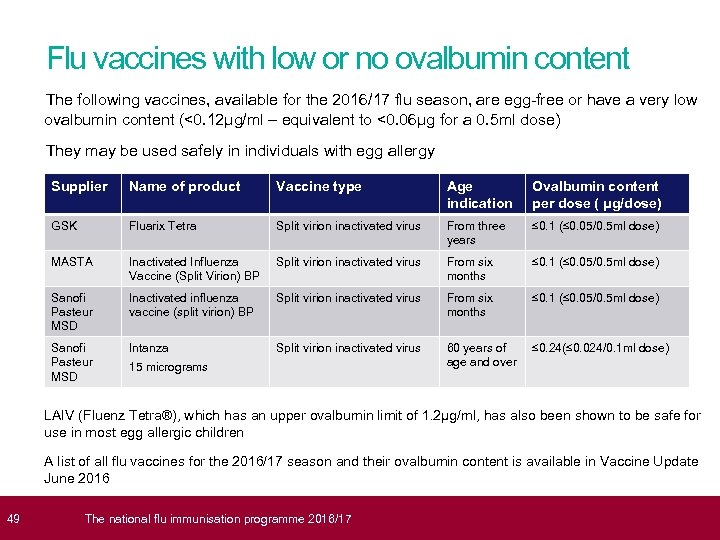 49 Flu vaccines with low or no ovalbumin content The following vaccines, available for the 2016/17 flu season, are egg-free or have a very low ovalbumin content (<0. 12μg/ml – equivalent to <0. 06μg for a 0. 5 ml dose) They may be used safely in individuals with egg allergy Supplier Name of product Vaccine type Age indication Ovalbumin content per dose ( μg/dose) GSK Fluarix Tetra Split virion inactivated virus From three years ≤ 0. 1 (≤ 0. 05/0. 5 ml dose) MASTA Inactivated Influenza Vaccine (Split Virion) BP Split virion inactivated virus From six months ≤ 0. 1 (≤ 0. 05/0. 5 ml dose) Sanofi Pasteur MSD Inactivated influenza vaccine (split virion) BP Split virion inactivated virus From six months ≤ 0. 1 (≤ 0. 05/0. 5 ml dose) Sanofi Pasteur MSD Intanza Split virion inactivated virus 60 years of age and over ≤ 0. 24(≤ 0. 024/0. 1 ml dose) 15 micrograms LAIV (Fluenz Tetra®), which has an upper ovalbumin limit of 1. 2μg/ml, has also been shown to be safe for use in most egg allergic children A list of all flu vaccines for the 2016/17 season and their ovalbumin content is available in Vaccine Update June 2016 The national flu immunisation programme 2016/17
49 Flu vaccines with low or no ovalbumin content The following vaccines, available for the 2016/17 flu season, are egg-free or have a very low ovalbumin content (<0. 12μg/ml – equivalent to <0. 06μg for a 0. 5 ml dose) They may be used safely in individuals with egg allergy Supplier Name of product Vaccine type Age indication Ovalbumin content per dose ( μg/dose) GSK Fluarix Tetra Split virion inactivated virus From three years ≤ 0. 1 (≤ 0. 05/0. 5 ml dose) MASTA Inactivated Influenza Vaccine (Split Virion) BP Split virion inactivated virus From six months ≤ 0. 1 (≤ 0. 05/0. 5 ml dose) Sanofi Pasteur MSD Inactivated influenza vaccine (split virion) BP Split virion inactivated virus From six months ≤ 0. 1 (≤ 0. 05/0. 5 ml dose) Sanofi Pasteur MSD Intanza Split virion inactivated virus 60 years of age and over ≤ 0. 24(≤ 0. 024/0. 1 ml dose) 15 micrograms LAIV (Fluenz Tetra®), which has an upper ovalbumin limit of 1. 2μg/ml, has also been shown to be safe for use in most egg allergic children A list of all flu vaccines for the 2016/17 season and their ovalbumin content is available in Vaccine Update June 2016 The national flu immunisation programme 2016/17
 50 Risk of transmission of live vaccine virus • theoretical potential for transmission of live attenuated virus to immunocompromised contacts • risk is for one to two weeks following vaccination • extensive use of the live attenuated influenza vaccine in US – no reported instances of illness or infections from the vaccine virus among immunocompromised patients inadvertently exposed to vaccinated children • however, where close contact with very severely immunocompromised patients (eg bone marrow transplant patients requiring isolation) is likely or unavoidable (eg household members) consider an appropriate inactivated flu vaccine instead The national flu immunisation programme 2016/17
50 Risk of transmission of live vaccine virus • theoretical potential for transmission of live attenuated virus to immunocompromised contacts • risk is for one to two weeks following vaccination • extensive use of the live attenuated influenza vaccine in US – no reported instances of illness or infections from the vaccine virus among immunocompromised patients inadvertently exposed to vaccinated children • however, where close contact with very severely immunocompromised patients (eg bone marrow transplant patients requiring isolation) is likely or unavoidable (eg household members) consider an appropriate inactivated flu vaccine instead The national flu immunisation programme 2016/17
 51 Exposure of healthcare workers to live attenuated influenza vaccine viruses • theoretically there may be some low level exposure to the vaccine viruses for those administering LAIV and/or from recently vaccinated patients • in the US, where there has been extensive use of LAIV, no reported instances of illness or infections from the vaccine virus among healthcare workers inadvertently exposed • risk of acquiring vaccine viruses from the environment is unknown but probably low • the vaccine viruses are cold-adapted and attenuated and therefore unlikely to cause symptomatic influenza • as a precaution, very severely immunosuppressed individuals should not administer LAIV • other healthcare workers who have less severe immunosuppression or are pregnant, should follow normal clinical practice to avoid inhaling the vaccine and ensure that they themselves are appropriately vaccinated The national flu immunisation programme 2016/17
51 Exposure of healthcare workers to live attenuated influenza vaccine viruses • theoretically there may be some low level exposure to the vaccine viruses for those administering LAIV and/or from recently vaccinated patients • in the US, where there has been extensive use of LAIV, no reported instances of illness or infections from the vaccine virus among healthcare workers inadvertently exposed • risk of acquiring vaccine viruses from the environment is unknown but probably low • the vaccine viruses are cold-adapted and attenuated and therefore unlikely to cause symptomatic influenza • as a precaution, very severely immunosuppressed individuals should not administer LAIV • other healthcare workers who have less severe immunosuppression or are pregnant, should follow normal clinical practice to avoid inhaling the vaccine and ensure that they themselves are appropriately vaccinated The national flu immunisation programme 2016/17
 52 Inadvertent administration of LAIV • if an immunocompromised individual receives LAIV, the degree of immunosuppression should be assessed • if patient is severely immunocompromised, antiviral prophylaxis should be considered • otherwise they should be advised to seek medical advice if they develop flu-like symptoms in the four days following administration of the vaccine • if antivirals are used for prophylaxis or treatment, patient should also be offered inactivated flu vaccine in order to maximise their protection in the forthcoming flu season (this can be given straight away) The national flu immunisation programme 2016/17
52 Inadvertent administration of LAIV • if an immunocompromised individual receives LAIV, the degree of immunosuppression should be assessed • if patient is severely immunocompromised, antiviral prophylaxis should be considered • otherwise they should be advised to seek medical advice if they develop flu-like symptoms in the four days following administration of the vaccine • if antivirals are used for prophylaxis or treatment, patient should also be offered inactivated flu vaccine in order to maximise their protection in the forthcoming flu season (this can be given straight away) The national flu immunisation programme 2016/17
 53 Commonly reported adverse reactions Following inactivated flu vaccine: • pain, swelling or redness at the injection site, low grade fever, malaise, shivering, fatigue, headache, myalgia and arthralgia • a small painless nodule (induration) may also form at the injection site • these symptoms usually disappear within one to two days without treatment Following live attenuated flu vaccine: • nasal congestion/rhinorrhoea, reduced appetite, weakness and headache Rarely, after live or inactivated vaccine, immediate reactions such as urticaria, angiooedema, bronchospasm and anaphylaxis can occur The national flu immunisation programme 2016/17
53 Commonly reported adverse reactions Following inactivated flu vaccine: • pain, swelling or redness at the injection site, low grade fever, malaise, shivering, fatigue, headache, myalgia and arthralgia • a small painless nodule (induration) may also form at the injection site • these symptoms usually disappear within one to two days without treatment Following live attenuated flu vaccine: • nasal congestion/rhinorrhoea, reduced appetite, weakness and headache Rarely, after live or inactivated vaccine, immediate reactions such as urticaria, angiooedema, bronchospasm and anaphylaxis can occur The national flu immunisation programme 2016/17
 54 Reporting suspected adverse reactions All serious suspected reactions following flu vaccination should be reported to the Medicines and Healthcare products Regulatory Agency using the Yellow Card scheme at http: //yellowcard. mhra. gov. uk/ Fluenz Tetra® and Fluarix™ Tetra carry a black triangle symbol (▼) (as do all vaccines during the earlier stages of their introduction). This is to encourage reporting of all suspected adverse reactions The national flu immunisation programme 2016/17
54 Reporting suspected adverse reactions All serious suspected reactions following flu vaccination should be reported to the Medicines and Healthcare products Regulatory Agency using the Yellow Card scheme at http: //yellowcard. mhra. gov. uk/ Fluenz Tetra® and Fluarix™ Tetra carry a black triangle symbol (▼) (as do all vaccines during the earlier stages of their introduction). This is to encourage reporting of all suspected adverse reactions The national flu immunisation programme 2016/17
 55 Vaccine ordering • all flu vaccines for children (both live and inactivated) are purchased centrally by PHE for: • all children aged 2, 3 and 4 years, and of school years 1, 2 and 3 age and • all children in clinical risk groups aged 6 months to less than 18 years ie PHE will supply LAIV for those who can receive it and inactivated flu vaccine for those children for whom LAIV is contraindicated. • flu vaccines for children can be ordered through the Imm. Form website as for other centrally purchased vaccines (www. immform. dh. gov. uk) • providers are responsible for ordering sufficient flu vaccine for all other eligible patients aged 18 years and older directly from manufacturers • ordering from more than one supplier is recommended in case of supplier delays or difficulties in the manufacture or delivery of the vaccine The national flu immunisation programme 2016/17
55 Vaccine ordering • all flu vaccines for children (both live and inactivated) are purchased centrally by PHE for: • all children aged 2, 3 and 4 years, and of school years 1, 2 and 3 age and • all children in clinical risk groups aged 6 months to less than 18 years ie PHE will supply LAIV for those who can receive it and inactivated flu vaccine for those children for whom LAIV is contraindicated. • flu vaccines for children can be ordered through the Imm. Form website as for other centrally purchased vaccines (www. immform. dh. gov. uk) • providers are responsible for ordering sufficient flu vaccine for all other eligible patients aged 18 years and older directly from manufacturers • ordering from more than one supplier is recommended in case of supplier delays or difficulties in the manufacture or delivery of the vaccine The national flu immunisation programme 2016/17
 56 Inactivated influenza vaccine for children contraindicated to receive LAIV • children for whom LAIV is contraindicated should be offered a suitable alternative inactivated flu vaccine • some inactivated flu vaccines have been associated with high rates of febrile convulsions in children • some inactivated flu vaccines contain too much ovalbumin for egg allergic children • check SPC for vaccine suitability before administration Guidance on which vaccines to use for those children who cannot receive LAIV can be found in the Green Book Influenza chapter • Fluarix Tetra® is the preferred vaccine for children aged ≥ 3 years who cannot receive Fluenz Tetra® • children 6 m to <3 years should be given inactivated influenza vaccine (Split Virion) BP® The national flu immunisation programme 2016/17
56 Inactivated influenza vaccine for children contraindicated to receive LAIV • children for whom LAIV is contraindicated should be offered a suitable alternative inactivated flu vaccine • some inactivated flu vaccines have been associated with high rates of febrile convulsions in children • some inactivated flu vaccines contain too much ovalbumin for egg allergic children • check SPC for vaccine suitability before administration Guidance on which vaccines to use for those children who cannot receive LAIV can be found in the Green Book Influenza chapter • Fluarix Tetra® is the preferred vaccine for children aged ≥ 3 years who cannot receive Fluenz Tetra® • children 6 m to <3 years should be given inactivated influenza vaccine (Split Virion) BP® The national flu immunisation programme 2016/17
 57 Beware of product confusion! Fluarix® Tetra is an inactivated vaccine licensed from three years of age that can be given to children who cannot receive the live intranasal flu vaccine, the 65 and overs, the under 65 s at risk, pregnant women and healthcare workers Care must be taken not to confuse the two ‘Tetra’ brands. One way of remembering which vaccine is which is: • Fluenz is the nazal flu vaccine • Fluarix is the arm injected vaccine The national flu immunisation programme 2016/17
57 Beware of product confusion! Fluarix® Tetra is an inactivated vaccine licensed from three years of age that can be given to children who cannot receive the live intranasal flu vaccine, the 65 and overs, the under 65 s at risk, pregnant women and healthcare workers Care must be taken not to confuse the two ‘Tetra’ brands. One way of remembering which vaccine is which is: • Fluenz is the nazal flu vaccine • Fluarix is the arm injected vaccine The national flu immunisation programme 2016/17
 58 Recording of flu vaccine given As a wide variety of influenza vaccines are on the UK market each year, it is especially important that the following information be recorded: • vaccine name, product name, batch number and expiry date • dose administered • date immunisation given • route/site used • name and signature of vaccinator This information should be recorded in: • patient's GP record (or other patient record, depending on location) • personal Child Health Record (the ‘Red Book’) if a child • practice computer system • Child Health Information System The national flu immunisation programme 2016/17
58 Recording of flu vaccine given As a wide variety of influenza vaccines are on the UK market each year, it is especially important that the following information be recorded: • vaccine name, product name, batch number and expiry date • dose administered • date immunisation given • route/site used • name and signature of vaccinator This information should be recorded in: • patient's GP record (or other patient record, depending on location) • personal Child Health Record (the ‘Red Book’) if a child • practice computer system • Child Health Information System The national flu immunisation programme 2016/17
 59 Data collection • flu vaccine uptake data is collected via the web-based Imm. Form system (www. immform. dh. gov. uk) where it is managed and published by PHE • over 90% GP practices are able to make automated data returns where the number of their patients vaccinated is directly extracted from their IT system and put into Imm. Form • for data to be accurate and complete, it is critical that vaccines given outside the surgery, eg in antenatal clinics or pharmacies, are reported to the patient’s GP • uptake data for school years 1, 2 and 3 and pilot areas will be manually submitted by providers onto Imm. Form • uptake data for HCWs is manually submitted by trusts and area teams via Imm. Form • data is collected and published monthly on all the groups for whom flu vaccine is indicated at national level and local NHS England team level to enable performance to be reviewed and time to take action if needed The national flu immunisation programme 2016/17
59 Data collection • flu vaccine uptake data is collected via the web-based Imm. Form system (www. immform. dh. gov. uk) where it is managed and published by PHE • over 90% GP practices are able to make automated data returns where the number of their patients vaccinated is directly extracted from their IT system and put into Imm. Form • for data to be accurate and complete, it is critical that vaccines given outside the surgery, eg in antenatal clinics or pharmacies, are reported to the patient’s GP • uptake data for school years 1, 2 and 3 and pilot areas will be manually submitted by providers onto Imm. Form • uptake data for HCWs is manually submitted by trusts and area teams via Imm. Form • data is collected and published monthly on all the groups for whom flu vaccine is indicated at national level and local NHS England team level to enable performance to be reviewed and time to take action if needed The national flu immunisation programme 2016/17
 60 Achieving high uptake (GP Practice checklist) In order to obtain high vaccine uptake, it is recommended that GP practices: 1. Identify a named lead individual within the practice who is responsible for the flu vaccination programme and liaises regularly with all staff involved in the programme 2. Hold a register that can identify all pregnant women and patients in the under 65 years at risk groups, those aged 65 years and over, and those aged two to four years 3. Update the patient register throughout the flu season, paying particular attention to the inclusion of women who become pregnant and patients who enter at-risk groups during the flu season 4. Submit accurate data on the number of its patients eligible to receive flu vaccine and the flu vaccinations given to its patients on Imm. Form (www. immform. dh. gov. uk), ideally using the automated function, and submit data on uptake among healthcare workers in primary care using the Imm. Form data collection tool The national flu immunisation programme 2016/17
60 Achieving high uptake (GP Practice checklist) In order to obtain high vaccine uptake, it is recommended that GP practices: 1. Identify a named lead individual within the practice who is responsible for the flu vaccination programme and liaises regularly with all staff involved in the programme 2. Hold a register that can identify all pregnant women and patients in the under 65 years at risk groups, those aged 65 years and over, and those aged two to four years 3. Update the patient register throughout the flu season, paying particular attention to the inclusion of women who become pregnant and patients who enter at-risk groups during the flu season 4. Submit accurate data on the number of its patients eligible to receive flu vaccine and the flu vaccinations given to its patients on Imm. Form (www. immform. dh. gov. uk), ideally using the automated function, and submit data on uptake among healthcare workers in primary care using the Imm. Form data collection tool The national flu immunisation programme 2016/17
 61 Achieving high uptake (GP Practice checklist cont’d) 5. Order sufficient flu vaccine taking into account past and planned improved performance, expected demographic increase, and to ensure that everyone at risk is offered the flu vaccine. It is recommended that vaccine is ordered from more than one supplier and from PHE central supplies through the Imm. Form website in respect of children 6. It is a requirement of the enhanced service specification that patients recommended to receive the flu vaccine are invited to a flu vaccination clinic or to make an appointment (eg by letter, email, phone call, text) 7. Follow-up patients, especially those in at risk groups, who do not respond or fail to attend scheduled clinics or appointments 8. Start flu vaccination as soon as practicable after receipt of the vaccine. This will help ensure the maximum number of patients are vaccinated as early as possible and protected before flu starts to circulate. Aim to complete immunisation of all eligible patients before flu starts to circulate and ideally by end of December The national flu immunisation programme 2016/17
61 Achieving high uptake (GP Practice checklist cont’d) 5. Order sufficient flu vaccine taking into account past and planned improved performance, expected demographic increase, and to ensure that everyone at risk is offered the flu vaccine. It is recommended that vaccine is ordered from more than one supplier and from PHE central supplies through the Imm. Form website in respect of children 6. It is a requirement of the enhanced service specification that patients recommended to receive the flu vaccine are invited to a flu vaccination clinic or to make an appointment (eg by letter, email, phone call, text) 7. Follow-up patients, especially those in at risk groups, who do not respond or fail to attend scheduled clinics or appointments 8. Start flu vaccination as soon as practicable after receipt of the vaccine. This will help ensure the maximum number of patients are vaccinated as early as possible and protected before flu starts to circulate. Aim to complete immunisation of all eligible patients before flu starts to circulate and ideally by end of December The national flu immunisation programme 2016/17
 62 Achieving high uptake (GP Practice checklist cont’d ) 9. Collaborate with maternity services to offer and provide flu vaccination to pregnant women and to identify, offer and provide to newly pregnant women as the flu season progresses 10. The GP practice should offer flu vaccination in clinics and opportunistically 11. The GP practice and/ or CCG should collaborate with other providers such as community pharmacies and community or health and social care trusts to identify and offer flu vaccination to residents in care homes, nursing homes and housebound patients and to ensure that mechanisms are in place to update the patient record when flu vaccinations are given by other providers The GP practice checklist highlights good practice. • it is based upon the findings from a study examining the factors associated with higher vaccine uptake in general practice • GP practices are encouraged to review their systems in the light of the checklist • most recommendations will apply to other settings where flu vaccine is given The national flu immunisation programme 2016/17
62 Achieving high uptake (GP Practice checklist cont’d ) 9. Collaborate with maternity services to offer and provide flu vaccination to pregnant women and to identify, offer and provide to newly pregnant women as the flu season progresses 10. The GP practice should offer flu vaccination in clinics and opportunistically 11. The GP practice and/ or CCG should collaborate with other providers such as community pharmacies and community or health and social care trusts to identify and offer flu vaccination to residents in care homes, nursing homes and housebound patients and to ensure that mechanisms are in place to update the patient record when flu vaccinations are given by other providers The GP practice checklist highlights good practice. • it is based upon the findings from a study examining the factors associated with higher vaccine uptake in general practice • GP practices are encouraged to review their systems in the light of the checklist • most recommendations will apply to other settings where flu vaccine is given The national flu immunisation programme 2016/17
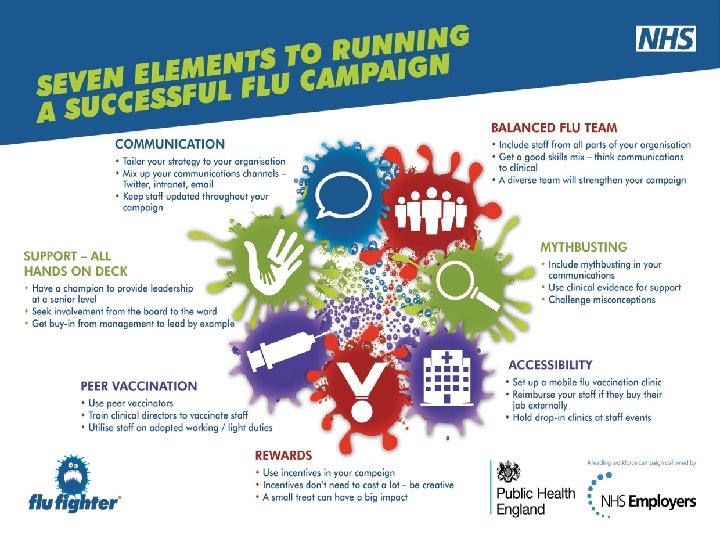 63 Presentation title - edit in Header and Footer
63 Presentation title - edit in Header and Footer
 64 Key messages • flu immunisation is one of the most effective interventions we can provide to reduce harm from flu and pressures on health and social care services during the winter • it is important to increase flu vaccine uptake in clinical risk groups because of increased risk of death and serious illness if people in these groups catch flu • for a number of years, only around half of patients aged six months to under 65 years in clinical risk groups have been vaccinated • influenza during pregnancy may be associated with perinatal mortality, prematurity, smaller neonatal size, lower birth weight and increased risk of complications for mother • vaccination of health and social care workers protects them and reduces risk of spreading flu to their patients, service users, colleagues and family members • by preventing flu infection through vaccination, secondary bacterial infections such as pneumonia are prevented. This reduces the need for antibiotics and helps prevent antibiotic resistance The national flu immunisation programme 2016/17
64 Key messages • flu immunisation is one of the most effective interventions we can provide to reduce harm from flu and pressures on health and social care services during the winter • it is important to increase flu vaccine uptake in clinical risk groups because of increased risk of death and serious illness if people in these groups catch flu • for a number of years, only around half of patients aged six months to under 65 years in clinical risk groups have been vaccinated • influenza during pregnancy may be associated with perinatal mortality, prematurity, smaller neonatal size, lower birth weight and increased risk of complications for mother • vaccination of health and social care workers protects them and reduces risk of spreading flu to their patients, service users, colleagues and family members • by preventing flu infection through vaccination, secondary bacterial infections such as pneumonia are prevented. This reduces the need for antibiotics and helps prevent antibiotic resistance The national flu immunisation programme 2016/17
 65 Resources • Flu Plan and Supporting Letter detailing 2016/17 flu programme Department of Health, Public Health England, NHS England. Published 26 May 2016. Available at: https: //www. gov. uk/government/publications/flu-plan-winter-2016 -to-2017 • Green Book Influenza chapter Available at: https: //www. gov. uk/government/collections/immunisation-against-infectious-disease-the-green-book • Leaflets, posters, Q&As and other resources to support the annual flu programme Available at: https: //www. gov. uk/government/collections/annual-flu-programme • A video for health professionals on how to administer the LAIV vaccine produced by NHS Education for Scotland is available at http: //www. nes. scot. nhs. uk/education-and-training/by-themeinitiative/public-health/health-protection/seasonal-flu. aspx • Summary of Product Characteristics (SPC) for flu vaccines are available at http: //www. medicines. org. uk/emc/ • PGD templates for flu vaccines https: //www. gov. uk/government/collections/immunisation-patientgroup-direction-pgd • Find out more about antibiotic resistance, other ways to reduce its rise and how you can help through www. antibioticguardian. com The national flu immunisation programme 2016/17
65 Resources • Flu Plan and Supporting Letter detailing 2016/17 flu programme Department of Health, Public Health England, NHS England. Published 26 May 2016. Available at: https: //www. gov. uk/government/publications/flu-plan-winter-2016 -to-2017 • Green Book Influenza chapter Available at: https: //www. gov. uk/government/collections/immunisation-against-infectious-disease-the-green-book • Leaflets, posters, Q&As and other resources to support the annual flu programme Available at: https: //www. gov. uk/government/collections/annual-flu-programme • A video for health professionals on how to administer the LAIV vaccine produced by NHS Education for Scotland is available at http: //www. nes. scot. nhs. uk/education-and-training/by-themeinitiative/public-health/health-protection/seasonal-flu. aspx • Summary of Product Characteristics (SPC) for flu vaccines are available at http: //www. medicines. org. uk/emc/ • PGD templates for flu vaccines https: //www. gov. uk/government/collections/immunisation-patientgroup-direction-pgd • Find out more about antibiotic resistance, other ways to reduce its rise and how you can help through www. antibioticguardian. com The national flu immunisation programme 2016/17
 66 About Public Health England exists to protect and improve the nation's health and wellbeing, and reduce health inequalities. It does this through world-class science, knowledge and intelligence, advocacy, partnerships and the delivery of specialist public health services. PHE is an operationally autonomous executive agency of the Department of Health. Public Health England Wellington House 133 -155 Waterloo Road London SE 1 8 UG Tel: 020 7654 8000 www. gov. uk/phe Twitter: @PHE_uk Facebook: www. facebook. com/Public. Health. England For enquiries relating to this document, please contact: immunisation. lead@phe. gov. uk © Crown copyright 2016 You may re-use this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence v 3. 0. To view this licence, visit OGL or email psi@nationalarchives. gsi. gov. uk. Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned. Published July 2016 PHE publications gateway number: 2016174 The national flu immunisation programme 2016/17
66 About Public Health England exists to protect and improve the nation's health and wellbeing, and reduce health inequalities. It does this through world-class science, knowledge and intelligence, advocacy, partnerships and the delivery of specialist public health services. PHE is an operationally autonomous executive agency of the Department of Health. Public Health England Wellington House 133 -155 Waterloo Road London SE 1 8 UG Tel: 020 7654 8000 www. gov. uk/phe Twitter: @PHE_uk Facebook: www. facebook. com/Public. Health. England For enquiries relating to this document, please contact: immunisation. lead@phe. gov. uk © Crown copyright 2016 You may re-use this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence v 3. 0. To view this licence, visit OGL or email psi@nationalarchives. gsi. gov. uk. Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned. Published July 2016 PHE publications gateway number: 2016174 The national flu immunisation programme 2016/17


