fc57f4c68504a6abf4749301db68cbe0.ppt
- Количество слайдов: 78
 The Mole
The Mole
 Unit: The Mole Topic: Atomic Mass Objectives: Day 1 of 4 • To learn how atomic mass is calculated using the average natural abundance of isotopes • To understand the quantity of a mole and Avogadro's number
Unit: The Mole Topic: Atomic Mass Objectives: Day 1 of 4 • To learn how atomic mass is calculated using the average natural abundance of isotopes • To understand the quantity of a mole and Avogadro's number
 Quickwrite Answer one of the questions below 1 -2 sentences: • 1 light year (the distance light travels in a year) is equal to 9. 5 trillion kilometers!!!!! Why do you think scientists use light years to measure distances to nearby stars in light years and not kilometers? ? ? • Your pencil uses graphite (pure carbon) to write with; how many atoms do you think are in 12 grams of graphite or carbon? ? ? ? • How many items make up a dozen? ? ? How many items make up a half dozen? ? How many items are in two dozen? ?
Quickwrite Answer one of the questions below 1 -2 sentences: • 1 light year (the distance light travels in a year) is equal to 9. 5 trillion kilometers!!!!! Why do you think scientists use light years to measure distances to nearby stars in light years and not kilometers? ? ? • Your pencil uses graphite (pure carbon) to write with; how many atoms do you think are in 12 grams of graphite or carbon? ? ? ? • How many items make up a dozen? ? ? How many items make up a half dozen? ? How many items are in two dozen? ?
 The Mole • In 1811, an Italian scientist by the name of Avogadro, Amedeo (1776– 1856) discovered that a mole of atoms is equal to the number 6. 022 x 1023 • In other words: 1 mole = 6. 022 x 1023 • Or, or if you have a mole of carbon atoms, then you have 6. 022 x 1023 atoms
The Mole • In 1811, an Italian scientist by the name of Avogadro, Amedeo (1776– 1856) discovered that a mole of atoms is equal to the number 6. 022 x 1023 • In other words: 1 mole = 6. 022 x 1023 • Or, or if you have a mole of carbon atoms, then you have 6. 022 x 1023 atoms
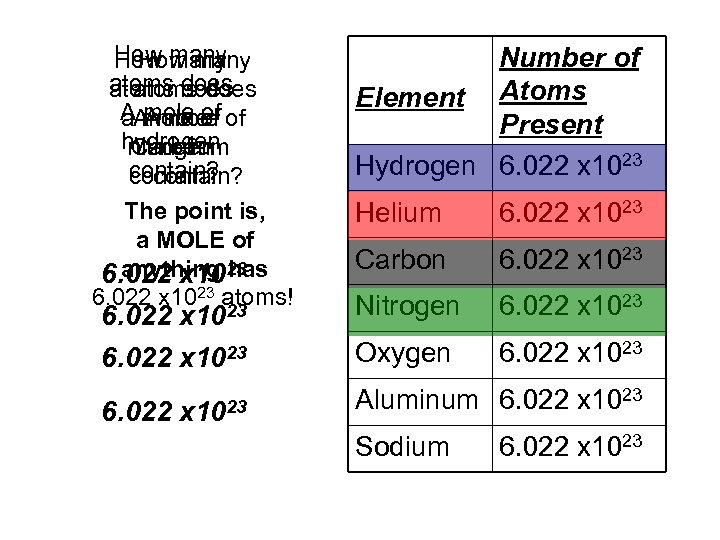 How many atoms does AA mole of a mole of A mole hydrogen nitrogen Carbon helium contain? The point is, a MOLE of anything has 6. 022 x 1023 atoms! 23 6. 022 x 1023 Number of Element Atoms Present Hydrogen 6. 022 x 1023 Helium 6. 022 x 1023 Carbon 6. 022 x 1023 Nitrogen 6. 022 x 1023 Oxygen 6. 022 x 1023 Aluminum 6. 022 x 1023 Sodium 6. 022 x 1023
How many atoms does AA mole of a mole of A mole hydrogen nitrogen Carbon helium contain? The point is, a MOLE of anything has 6. 022 x 1023 atoms! 23 6. 022 x 1023 Number of Element Atoms Present Hydrogen 6. 022 x 1023 Helium 6. 022 x 1023 Carbon 6. 022 x 1023 Nitrogen 6. 022 x 1023 Oxygen 6. 022 x 1023 Aluminum 6. 022 x 1023 Sodium 6. 022 x 1023
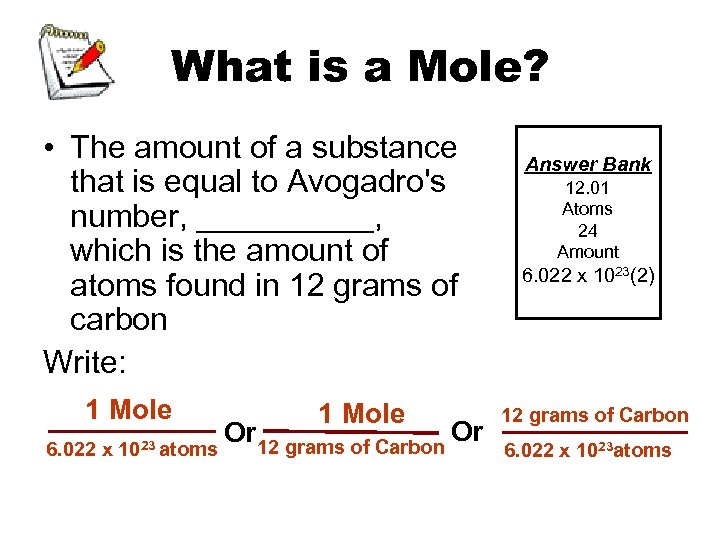 What is a Mole? • The amount of a substance that is equal to Avogadro's number, _____, which is the amount of atoms found in 12 grams of carbon Write: 1 Mole 6. 022 x 1023 atoms 1 Mole Or 12 grams of Carbon Or Answer Bank 12. 01 Atoms 24 Amount 6. 022 x 1023(2) 12 grams of Carbon 6. 022 x 1023 atoms
What is a Mole? • The amount of a substance that is equal to Avogadro's number, _____, which is the amount of atoms found in 12 grams of carbon Write: 1 Mole 6. 022 x 1023 atoms 1 Mole Or 12 grams of Carbon Or Answer Bank 12. 01 Atoms 24 Amount 6. 022 x 1023(2) 12 grams of Carbon 6. 022 x 1023 atoms
 The Mole • As it turns out, one mole of anything contains 6. 022 x 1023 units of that substance • Just as a dozen eggs is 12 eggs, a mole of eggs is 6. 022 x 1023 eggs • The mole is an incredibly large number to imagine 602, 000, 000, 000!!!!!!!!! • We use scientific notation to simply this number • We call this unbelievably large number Avogadro’s number
The Mole • As it turns out, one mole of anything contains 6. 022 x 1023 units of that substance • Just as a dozen eggs is 12 eggs, a mole of eggs is 6. 022 x 1023 eggs • The mole is an incredibly large number to imagine 602, 000, 000, 000!!!!!!!!! • We use scientific notation to simply this number • We call this unbelievably large number Avogadro’s number
 The Mole • If I have a dozen eggs how many eggs do I have? • If I have 2 dozen eggs, how many eggs do I have? • If I have a mole of eggs, how many eggs do I have? • If I have a 2 mole of eggs, how many eggs do I have? (2) x (6. 022 x 1023)
The Mole • If I have a dozen eggs how many eggs do I have? • If I have 2 dozen eggs, how many eggs do I have? • If I have a mole of eggs, how many eggs do I have? • If I have a 2 mole of eggs, how many eggs do I have? (2) x (6. 022 x 1023)
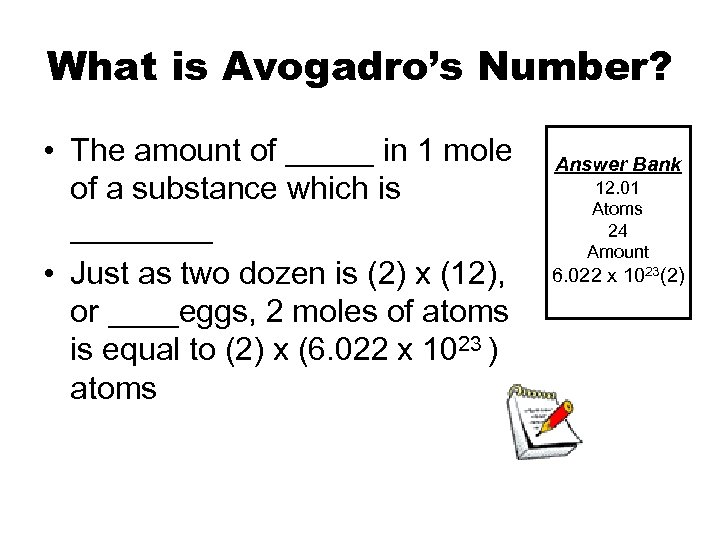 What is Avogadro’s Number? • The amount of _____ in 1 mole of a substance which is ____ • Just as two dozen is (2) x (12), or ____eggs, 2 moles of atoms is equal to (2) x (6. 022 x 1023 ) atoms Answer Bank 12. 01 Atoms 24 Amount 6. 022 x 1023(2)
What is Avogadro’s Number? • The amount of _____ in 1 mole of a substance which is ____ • Just as two dozen is (2) x (12), or ____eggs, 2 moles of atoms is equal to (2) x (6. 022 x 1023 ) atoms Answer Bank 12. 01 Atoms 24 Amount 6. 022 x 1023(2)
 The Mole • • • Take out your periodic table and find Carbon Look Below the Atomic Symbol (C) What number do you see? ? ? That’s right, 12. 01 !!!!!! Below every atomic symbol is number called the ATOMIC MASS • A sample of carbon with a mass of 12. 01 has 6. 022 x 1023 atoms! • So we can say that a mole of hydrogen (H) atoms has a mass of 1. 01 g, a mole of oxygen, (O) has a mass of 16. 00 g, a mole of iron (Fe) is 55. 80 g, and so on
The Mole • • • Take out your periodic table and find Carbon Look Below the Atomic Symbol (C) What number do you see? ? ? That’s right, 12. 01 !!!!!! Below every atomic symbol is number called the ATOMIC MASS • A sample of carbon with a mass of 12. 01 has 6. 022 x 1023 atoms! • So we can say that a mole of hydrogen (H) atoms has a mass of 1. 01 g, a mole of oxygen, (O) has a mass of 16. 00 g, a mole of iron (Fe) is 55. 80 g, and so on
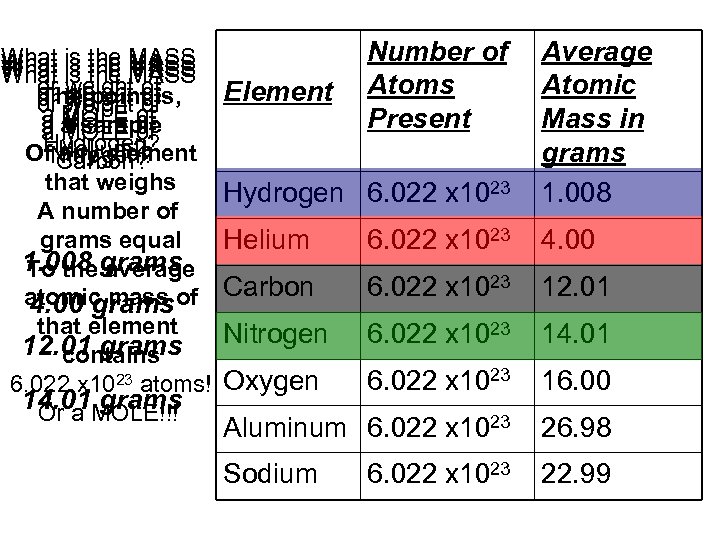 What is the MASS or weight of The point or weight of or MOLE ofis, weight of a a sample a MOLE of Hydrogen? Helium? Of. Nitrogen? any element Carbon? that weighs A number of grams equal 1. 008 grams To the average atomic mass of 4. 00 grams that element 12. 01 grams contains 6. 022 x 1023 atoms! 14. 01 MOLE!!! Or a grams Hydrogen 6. 022 x 1023 Average Atomic Mass in grams 1. 008 Helium 6. 022 x 1023 4. 00 Carbon 6. 022 x 1023 12. 01 Nitrogen 6. 022 x 1023 14. 01 Oxygen 6. 022 x 1023 16. 00 Aluminum 6. 022 x 1023 26. 98 Sodium 22. 99 Element Number of Atoms Present 6. 022 x 1023
What is the MASS or weight of The point or weight of or MOLE ofis, weight of a a sample a MOLE of Hydrogen? Helium? Of. Nitrogen? any element Carbon? that weighs A number of grams equal 1. 008 grams To the average atomic mass of 4. 00 grams that element 12. 01 grams contains 6. 022 x 1023 atoms! 14. 01 MOLE!!! Or a grams Hydrogen 6. 022 x 1023 Average Atomic Mass in grams 1. 008 Helium 6. 022 x 1023 4. 00 Carbon 6. 022 x 1023 12. 01 Nitrogen 6. 022 x 1023 14. 01 Oxygen 6. 022 x 1023 16. 00 Aluminum 6. 022 x 1023 26. 98 Sodium 22. 99 Element Number of Atoms Present 6. 022 x 1023
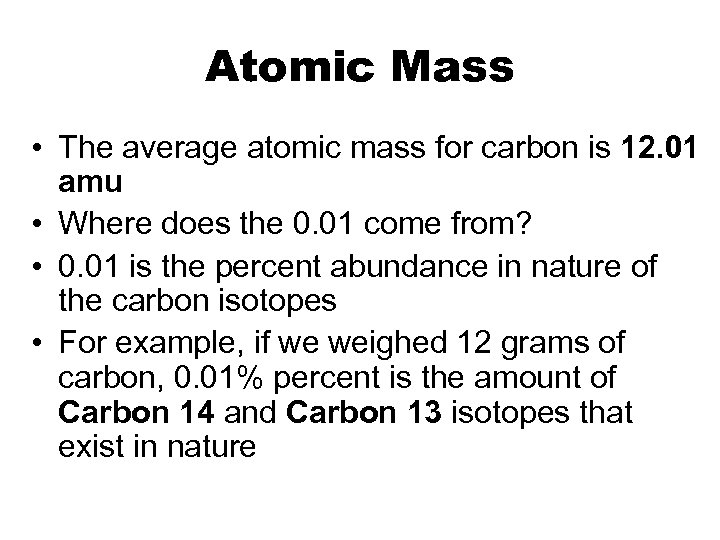 Atomic Mass • The average atomic mass for carbon is 12. 01 amu • Where does the 0. 01 come from? • 0. 01 is the percent abundance in nature of the carbon isotopes • For example, if we weighed 12 grams of carbon, 0. 01% percent is the amount of Carbon 14 and Carbon 13 isotopes that exist in nature
Atomic Mass • The average atomic mass for carbon is 12. 01 amu • Where does the 0. 01 come from? • 0. 01 is the percent abundance in nature of the carbon isotopes • For example, if we weighed 12 grams of carbon, 0. 01% percent is the amount of Carbon 14 and Carbon 13 isotopes that exist in nature
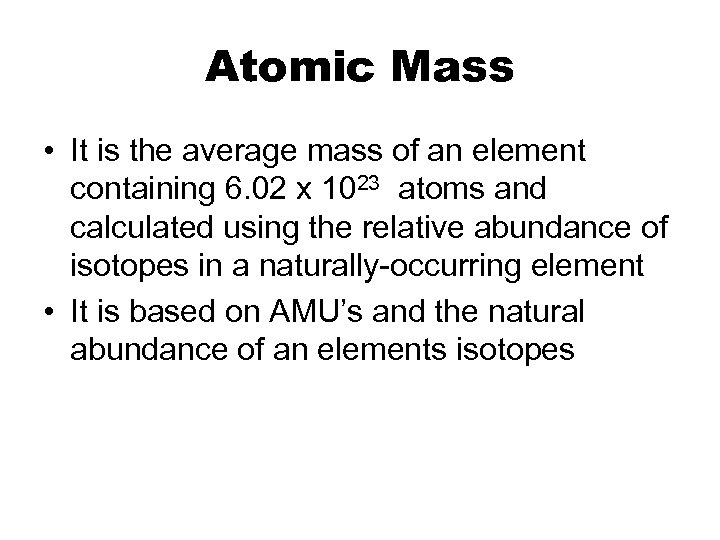 Atomic Mass • It is the average mass of an element containing 6. 02 x 1023 atoms and calculated using the relative abundance of isotopes in a naturally-occurring element • It is based on AMU’s and the natural abundance of an elements isotopes
Atomic Mass • It is the average mass of an element containing 6. 02 x 1023 atoms and calculated using the relative abundance of isotopes in a naturally-occurring element • It is based on AMU’s and the natural abundance of an elements isotopes
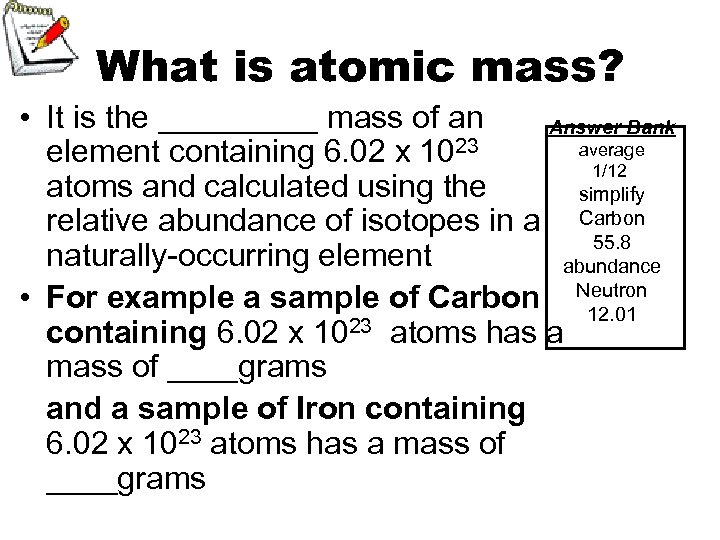 What is atomic mass? • It is the _____ mass of an Answer Bank average element containing 6. 02 x 1023 1/12 atoms and calculated using the simplify relative abundance of isotopes in a Carbon 55. 8 naturally-occurring element abundance • For example a sample of Carbon Neutron 12. 01 23 atoms has a containing 6. 02 x 10 mass of ____grams and a sample of Iron containing 6. 02 x 1023 atoms has a mass of ____grams
What is atomic mass? • It is the _____ mass of an Answer Bank average element containing 6. 02 x 1023 1/12 atoms and calculated using the simplify relative abundance of isotopes in a Carbon 55. 8 naturally-occurring element abundance • For example a sample of Carbon Neutron 12. 01 23 atoms has a containing 6. 02 x 10 mass of ____grams and a sample of Iron containing 6. 02 x 1023 atoms has a mass of ____grams
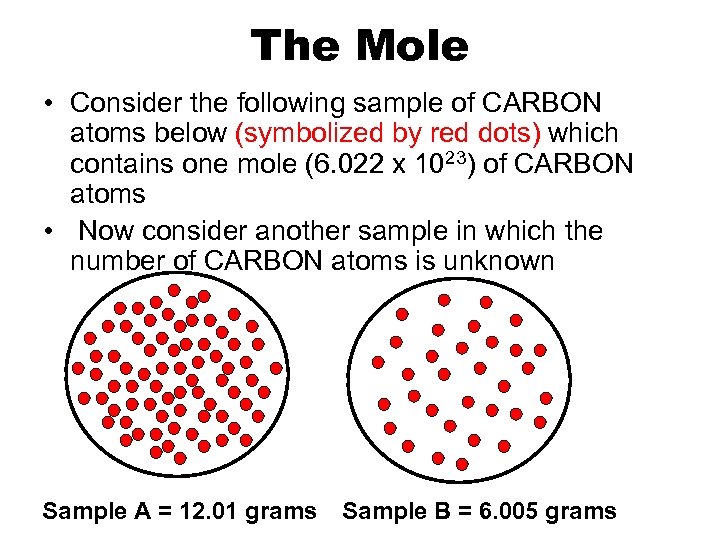 The Mole • Consider the following sample of CARBON atoms below (symbolized by red dots) which contains one mole (6. 022 x 1023) of CARBON atoms • Now consider another sample in which the number of CARBON atoms is unknown Sample A = 12. 01 grams Sample B = 6. 005 grams
The Mole • Consider the following sample of CARBON atoms below (symbolized by red dots) which contains one mole (6. 022 x 1023) of CARBON atoms • Now consider another sample in which the number of CARBON atoms is unknown Sample A = 12. 01 grams Sample B = 6. 005 grams
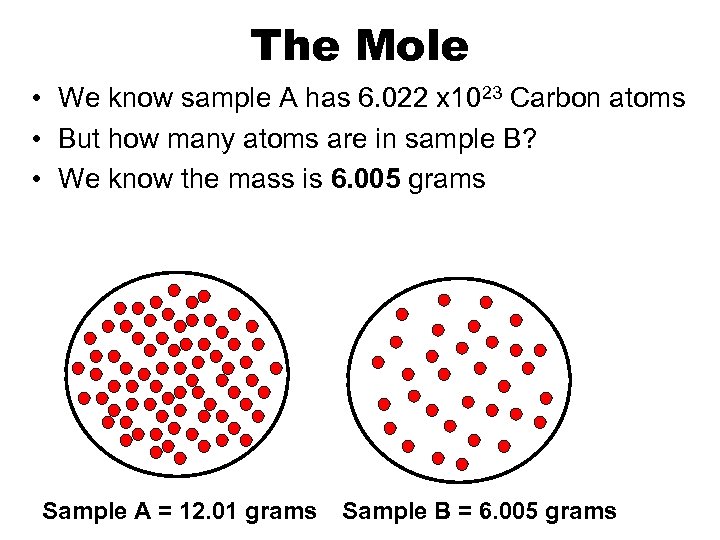 The Mole • We know sample A has 6. 022 x 1023 Carbon atoms • But how many atoms are in sample B? • We know the mass is 6. 005 grams Sample A = 12. 01 grams Sample B = 6. 005 grams
The Mole • We know sample A has 6. 022 x 1023 Carbon atoms • But how many atoms are in sample B? • We know the mass is 6. 005 grams Sample A = 12. 01 grams Sample B = 6. 005 grams
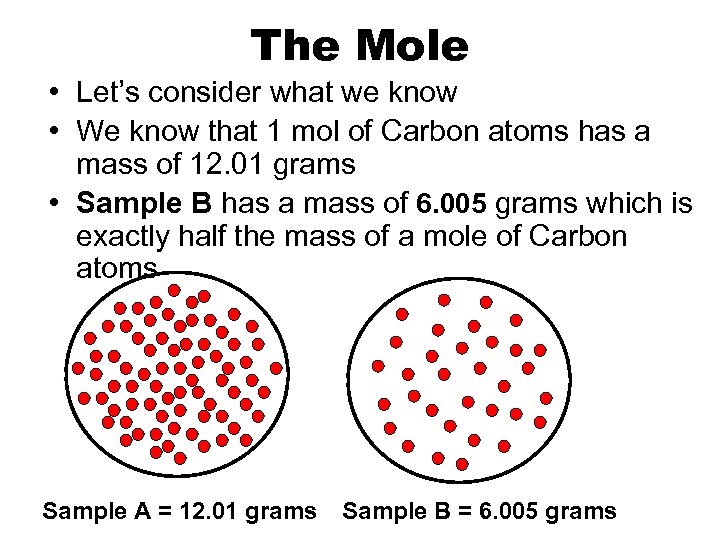 The Mole • Let’s consider what we know • We know that 1 mol of Carbon atoms has a mass of 12. 01 grams • Sample B has a mass of 6. 005 grams which is exactly half the mass of a mole of Carbon atoms Sample A = 12. 01 grams Sample B = 6. 005 grams
The Mole • Let’s consider what we know • We know that 1 mol of Carbon atoms has a mass of 12. 01 grams • Sample B has a mass of 6. 005 grams which is exactly half the mass of a mole of Carbon atoms Sample A = 12. 01 grams Sample B = 6. 005 grams
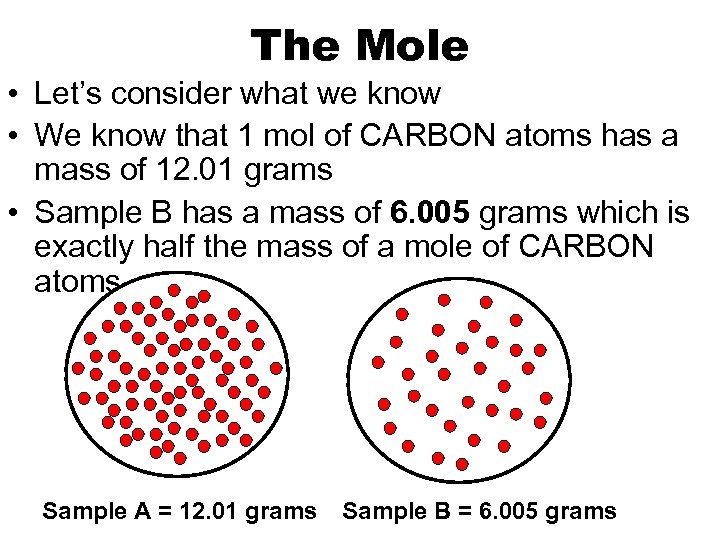 The Mole • Let’s consider what we know • We know that 1 mol of CARBON atoms has a mass of 12. 01 grams • Sample B has a mass of 6. 005 grams which is exactly half the mass of a mole of CARBON atoms Sample A = 12. 01 grams Sample B = 6. 005 grams
The Mole • Let’s consider what we know • We know that 1 mol of CARBON atoms has a mass of 12. 01 grams • Sample B has a mass of 6. 005 grams which is exactly half the mass of a mole of CARBON atoms Sample A = 12. 01 grams Sample B = 6. 005 grams
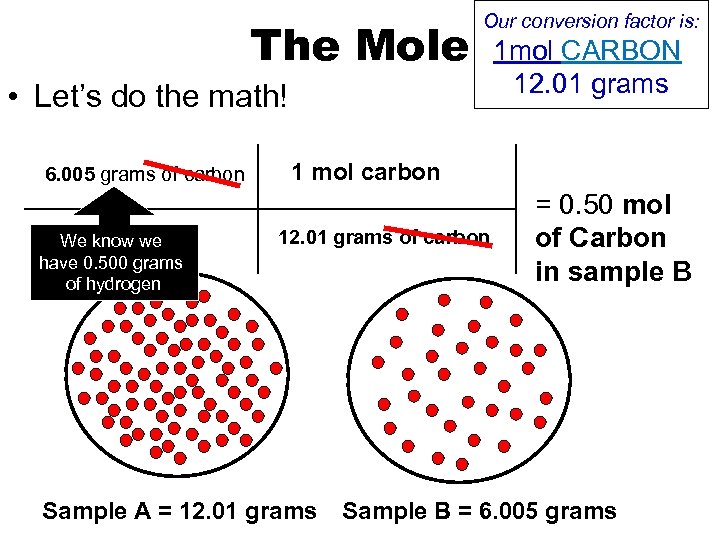 The Mole Our conversion factor is: • Let’s do the math! 6. 005 grams of carbon We know we have 0. 500 grams of hydrogen 1 mol CARBON 12. 01 grams 1 mol carbon 12. 01 grams of carbon Sample A = 12. 01 grams = 0. 50 mol of Carbon in sample B Sample B = 6. 005 grams
The Mole Our conversion factor is: • Let’s do the math! 6. 005 grams of carbon We know we have 0. 500 grams of hydrogen 1 mol CARBON 12. 01 grams 1 mol carbon 12. 01 grams of carbon Sample A = 12. 01 grams = 0. 50 mol of Carbon in sample B Sample B = 6. 005 grams
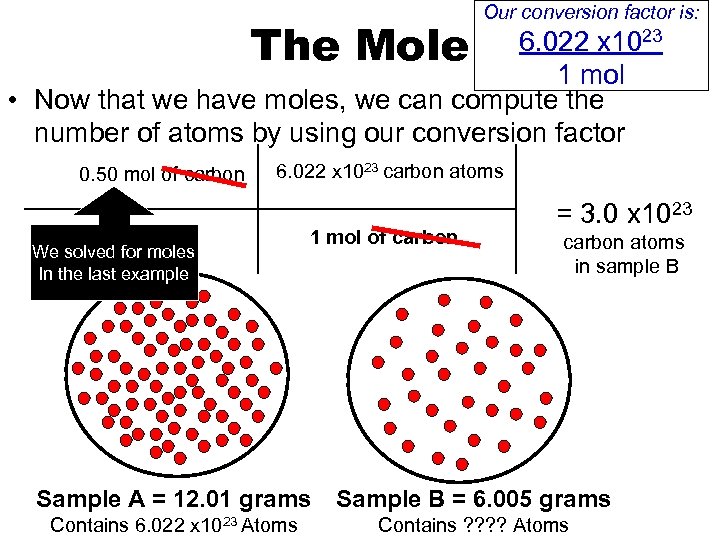 Our conversion factor is: 6. 022 x 1023 The Mole 1 mol • Now that we have moles, we can compute the number of atoms by using our conversion factor 0. 50 mol of carbon 6. 022 x 1023 carbon atoms We solved for moles In the last example 1 mol of carbon = 3. 0 x 1023 carbon atoms in sample B Sample A = 12. 01 grams Sample B = 6. 005 grams Contains 6. 022 x 1023 Atoms Contains ? ? Atoms
Our conversion factor is: 6. 022 x 1023 The Mole 1 mol • Now that we have moles, we can compute the number of atoms by using our conversion factor 0. 50 mol of carbon 6. 022 x 1023 carbon atoms We solved for moles In the last example 1 mol of carbon = 3. 0 x 1023 carbon atoms in sample B Sample A = 12. 01 grams Sample B = 6. 005 grams Contains 6. 022 x 1023 Atoms Contains ? ? Atoms
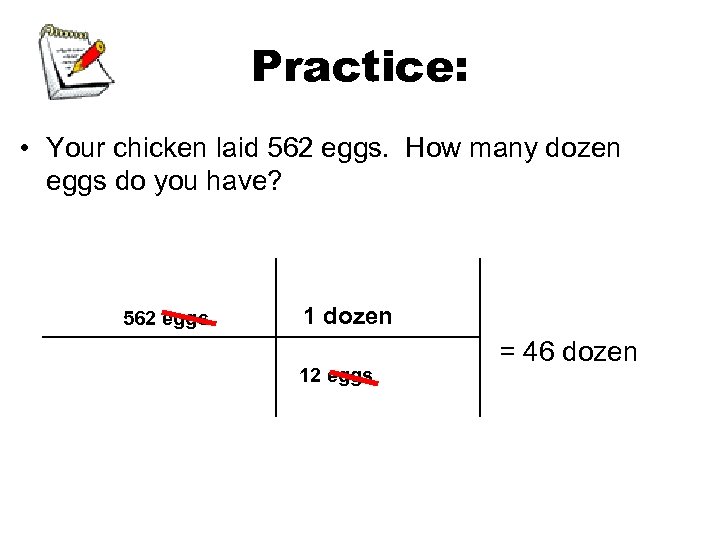 Practice: • Your chicken laid 562 eggs. How many dozen eggs do you have? 562 eggs 1 dozen 12 eggs = 46 dozen
Practice: • Your chicken laid 562 eggs. How many dozen eggs do you have? 562 eggs 1 dozen 12 eggs = 46 dozen
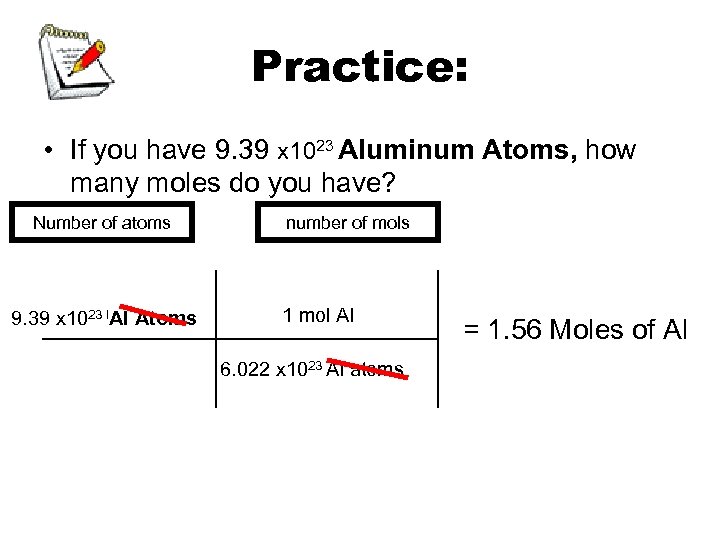 Practice: • If you have 9. 39 x 1023 Aluminum Atoms, how many moles do you have? Number of atoms of aluminum? of mols number • 1. 56 mol 9. 39 x 1023 l. Al Atoms 1 mol Al 6. 022 x 1023 Al atoms = 1. 56 Moles of Al
Practice: • If you have 9. 39 x 1023 Aluminum Atoms, how many moles do you have? Number of atoms of aluminum? of mols number • 1. 56 mol 9. 39 x 1023 l. Al Atoms 1 mol Al 6. 022 x 1023 Al atoms = 1. 56 Moles of Al
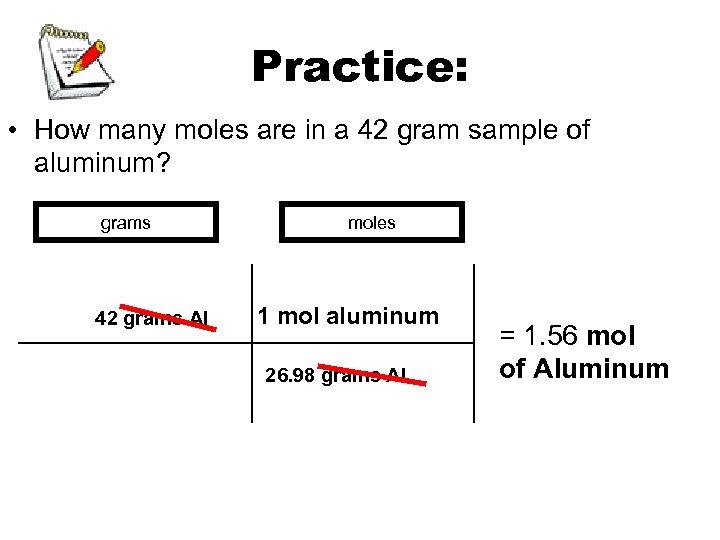 Practice: • How many moles are in a 42 gram sample of aluminum? grams 42 grams Al moles 1 mol aluminum 26. 98 grams Al = 1. 56 mol of Aluminum
Practice: • How many moles are in a 42 gram sample of aluminum? grams 42 grams Al moles 1 mol aluminum 26. 98 grams Al = 1. 56 mol of Aluminum
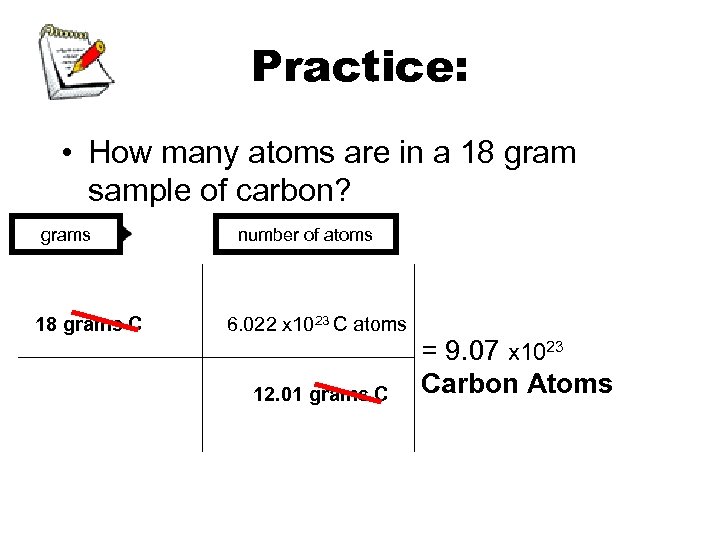 Practice: • How many atoms are in a 18 gram sample of carbon? grams 18 grams C number of atoms 6. 022 x 1023 C atoms 12. 01 grams C = 9. 07 x 1023 Carbon Atoms
Practice: • How many atoms are in a 18 gram sample of carbon? grams 18 grams C number of atoms 6. 022 x 1023 C atoms 12. 01 grams C = 9. 07 x 1023 Carbon Atoms
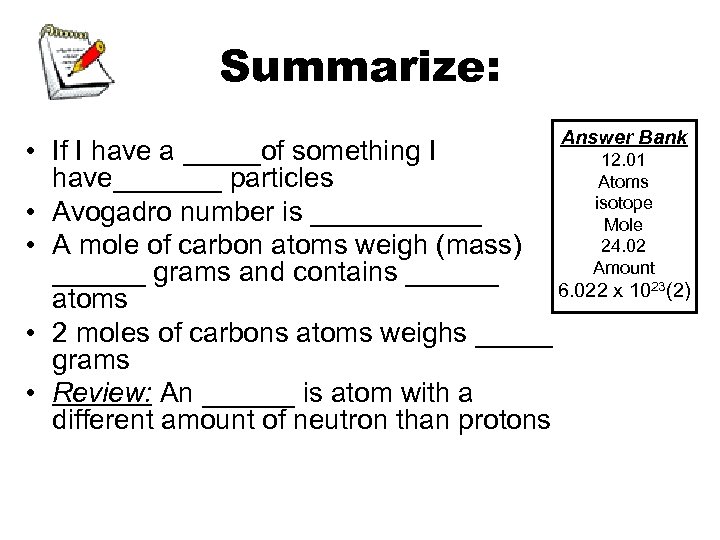 Summarize: Answer Bank • If I have a _____of something I 12. 01 have_______ particles Atoms isotope • Avogadro number is ______ Mole 24. 02 • A mole of carbon atoms weigh (mass) Amount ______ grams and contains ______ 6. 022 x 1023(2) atoms • 2 moles of carbons atoms weighs _____ grams • Review: An ______ is atom with a different amount of neutron than protons
Summarize: Answer Bank • If I have a _____of something I 12. 01 have_______ particles Atoms isotope • Avogadro number is ______ Mole 24. 02 • A mole of carbon atoms weigh (mass) Amount ______ grams and contains ______ 6. 022 x 1023(2) atoms • 2 moles of carbons atoms weighs _____ grams • Review: An ______ is atom with a different amount of neutron than protons
 Unit: The Mole Topic: Molar Mass (Molecular Weight) Percent Composition & Objectives: Day 2 of 4 • To learn how to calculate Molar Mass • To learn how to convert between moles and grams • To learn how to calculate % composition
Unit: The Mole Topic: Molar Mass (Molecular Weight) Percent Composition & Objectives: Day 2 of 4 • To learn how to calculate Molar Mass • To learn how to convert between moles and grams • To learn how to calculate % composition
 Quickwrite Answer one of the questions below 1 -2 sentences: • Let’s say you want to find the weight of your dog, which is too big to stand on your bathroom scale; how could you find his weight? ? ? • Together, you and your dog weigh 100 kilograms, you know that you weigh 75 kilograms, what percent by weight does your dog weigh? ? • You know that a mole oxygen has a mass of 16 grams and a mole of hydrogen has a mass of 1. 0 grams, but how could you find the mass of 1 mole of water (H 2 O)
Quickwrite Answer one of the questions below 1 -2 sentences: • Let’s say you want to find the weight of your dog, which is too big to stand on your bathroom scale; how could you find his weight? ? ? • Together, you and your dog weigh 100 kilograms, you know that you weigh 75 kilograms, what percent by weight does your dog weigh? ? • You know that a mole oxygen has a mass of 16 grams and a mole of hydrogen has a mass of 1. 0 grams, but how could you find the mass of 1 mole of water (H 2 O)
 Molar Mass of a Compound • Earlier we learned that a mole of any 1 mole of H O = element contains 6. 02 x 1023 atoms 6. 02 x 10 Molecules of H O • But what about a compound or Molecule such as water (H 2 O)? • A chemical compound such as water (H 2 O) is a collection of atoms • Water contains 1 oxygen atom and 2 hydrogen atoms 2 23 2 • But how do we calculate the mass of one mole of water? • In other words, what is the mass of 6. 022 x 1023 H 2 O Water molecules?
Molar Mass of a Compound • Earlier we learned that a mole of any 1 mole of H O = element contains 6. 02 x 1023 atoms 6. 02 x 10 Molecules of H O • But what about a compound or Molecule such as water (H 2 O)? • A chemical compound such as water (H 2 O) is a collection of atoms • Water contains 1 oxygen atom and 2 hydrogen atoms 2 23 2 • But how do we calculate the mass of one mole of water? • In other words, what is the mass of 6. 022 x 1023 H 2 O Water molecules?
 Molar Mass of a Compound • If we put one mole of water (H 2 O) on a scale and mass it, it will read 18. 02 grams • But how did we come up with that number? • Well, we know water is made up of Oxygen and Hydrogen • We also know the mass of one mole of Oxygen by looking on our periodic table 16. 00 grams • We also know the mass of one mole of Hydrogen by looking on our periodic table 1. 01 grams • Finally, we also know that water contains 2 Hydrogen atoms 1 mole of H 2 O = 6. 02 x 1023 Molecules of H 2 O 18. 02 grams
Molar Mass of a Compound • If we put one mole of water (H 2 O) on a scale and mass it, it will read 18. 02 grams • But how did we come up with that number? • Well, we know water is made up of Oxygen and Hydrogen • We also know the mass of one mole of Oxygen by looking on our periodic table 16. 00 grams • We also know the mass of one mole of Hydrogen by looking on our periodic table 1. 01 grams • Finally, we also know that water contains 2 Hydrogen atoms 1 mole of H 2 O = 6. 02 x 1023 Molecules of H 2 O 18. 02 grams
 Molar Mass of a Compound • Because each water molecule (H 2 O) contains 1 Oxygen atom and 2 Hydrogen atoms, 1 mol (H 2 O) molecules consists of 1 mol Oxygen atoms and 2 mol of Hydrogen atoms • So the mass of 1 mol of (H 2 O) is equal to: Mass of 1 mol of Oxygen (O) = 1 x 16. 00 g = 16. 00 g Mass of 2 mol of hydrogen (H) = 2 x 1. 01 = _______ 2. 02 g Mass of 1 mol of (H 2 O) = 18. 02 g 1 mole of H 2 O = 6. 02 x 1023 Molecules of H 2 O And contains 1 mole of Oxygen Atoms And 2 moles of Hydrogen atoms
Molar Mass of a Compound • Because each water molecule (H 2 O) contains 1 Oxygen atom and 2 Hydrogen atoms, 1 mol (H 2 O) molecules consists of 1 mol Oxygen atoms and 2 mol of Hydrogen atoms • So the mass of 1 mol of (H 2 O) is equal to: Mass of 1 mol of Oxygen (O) = 1 x 16. 00 g = 16. 00 g Mass of 2 mol of hydrogen (H) = 2 x 1. 01 = _______ 2. 02 g Mass of 1 mol of (H 2 O) = 18. 02 g 1 mole of H 2 O = 6. 02 x 1023 Molecules of H 2 O And contains 1 mole of Oxygen Atoms And 2 moles of Hydrogen atoms
 Molar Mass of a Compound • Molar Mass is the mass in grams of 1 Mole of a substance or compound • It is calculated by the SUM of the atomic weights for every element that makes up the compound • Ex: H 2 O Mass of 1 mol of Oxygen (O) = 1 x 16. 00 g = Mass of 2 mol of Hydrogen (H) = 2 x 1. 01 = 16. 00 g 2. 02 g _______ Mass of 1 mol of (H 2 O) = 18. 02 g
Molar Mass of a Compound • Molar Mass is the mass in grams of 1 Mole of a substance or compound • It is calculated by the SUM of the atomic weights for every element that makes up the compound • Ex: H 2 O Mass of 1 mol of Oxygen (O) = 1 x 16. 00 g = Mass of 2 mol of Hydrogen (H) = 2 x 1. 01 = 16. 00 g 2. 02 g _______ Mass of 1 mol of (H 2 O) = 18. 02 g
 What is molar mass or molecular weight? Answer Bank Total Percent Element Adding 6. 02 x 1023 mole weight • It is the mass in grams of 1 _____ of a substance or compound containing ______ molecules of that substance • It is calculated by the sum or ______up the atomic mass for every element that makes up the compound • Ex: H 2 O Mass of 1 mol of Oxygen (O) = 1 x 16. 00 g = Mass of 2 mol of Hydrogen (H) = 2 x 1. 01 = 16. 00 g 2. 02 g _______ Mass of 1 mol of (H 2 O) = 18. 02 g
What is molar mass or molecular weight? Answer Bank Total Percent Element Adding 6. 02 x 1023 mole weight • It is the mass in grams of 1 _____ of a substance or compound containing ______ molecules of that substance • It is calculated by the sum or ______up the atomic mass for every element that makes up the compound • Ex: H 2 O Mass of 1 mol of Oxygen (O) = 1 x 16. 00 g = Mass of 2 mol of Hydrogen (H) = 2 x 1. 01 = 16. 00 g 2. 02 g _______ Mass of 1 mol of (H 2 O) = 18. 02 g
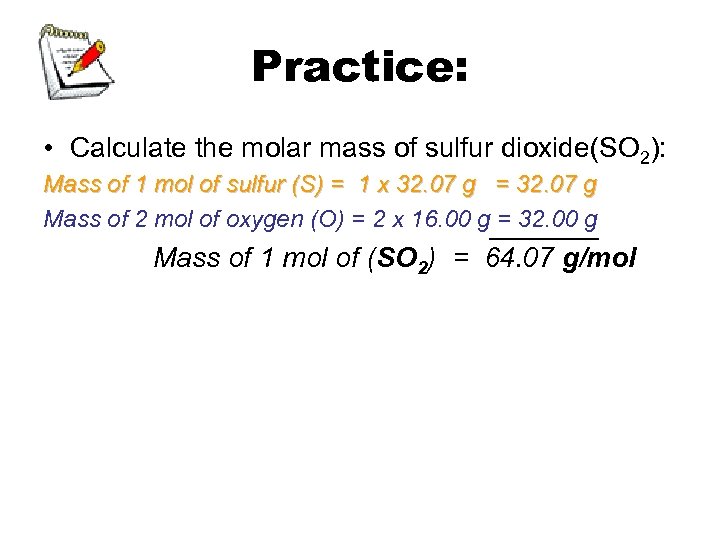 Practice: • Calculate the molar mass of sulfur dioxide(SO 2): Mass of 1 mol of sulfur (S) = 1 x 32. 07 g = 32. 07 g Mass of 2 mol of oxygen (O) = 2 x 16. 00 g_______ = 32. 00 g Mass of 1 mol of (SO 2) = 64. 07 g/mol
Practice: • Calculate the molar mass of sulfur dioxide(SO 2): Mass of 1 mol of sulfur (S) = 1 x 32. 07 g = 32. 07 g Mass of 2 mol of oxygen (O) = 2 x 16. 00 g_______ = 32. 00 g Mass of 1 mol of (SO 2) = 64. 07 g/mol
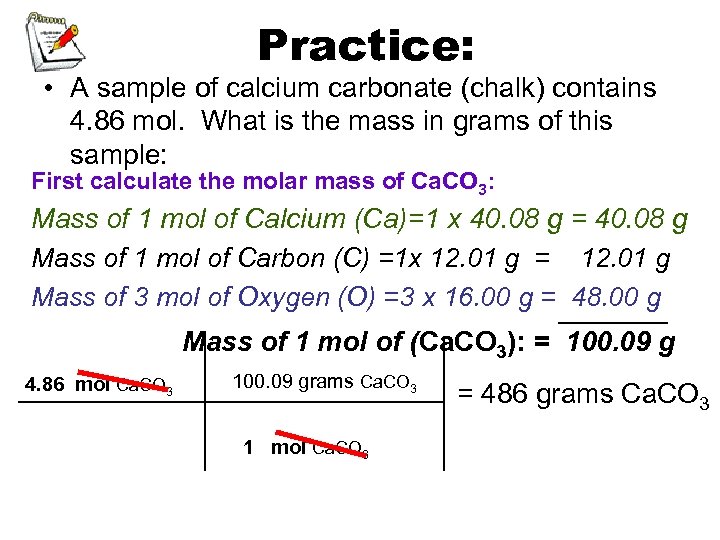 Practice: • A sample of calcium carbonate (chalk) contains 4. 86 mol. What is the mass in grams of this sample: First calculate the molar mass of Ca. CO 3: Mass of 1 mol of Calcium (Ca)=1 x 40. 08 g = 40. 08 g Mass of 1 mol of Carbon (C) =1 x 12. 01 g = 12. 01 g Mass of 3 mol of Oxygen (O) =3 x 16. 00 g = _______ 48. 00 g Mass of 1 mol of (Ca. CO 3): = 100. 09 g 4. 86 mol Ca. CO 3 100. 09 grams Ca. CO 3 1 mol Ca. CO 3 = 486 grams Ca. CO 3
Practice: • A sample of calcium carbonate (chalk) contains 4. 86 mol. What is the mass in grams of this sample: First calculate the molar mass of Ca. CO 3: Mass of 1 mol of Calcium (Ca)=1 x 40. 08 g = 40. 08 g Mass of 1 mol of Carbon (C) =1 x 12. 01 g = 12. 01 g Mass of 3 mol of Oxygen (O) =3 x 16. 00 g = _______ 48. 00 g Mass of 1 mol of (Ca. CO 3): = 100. 09 g 4. 86 mol Ca. CO 3 100. 09 grams Ca. CO 3 1 mol Ca. CO 3 = 486 grams Ca. CO 3
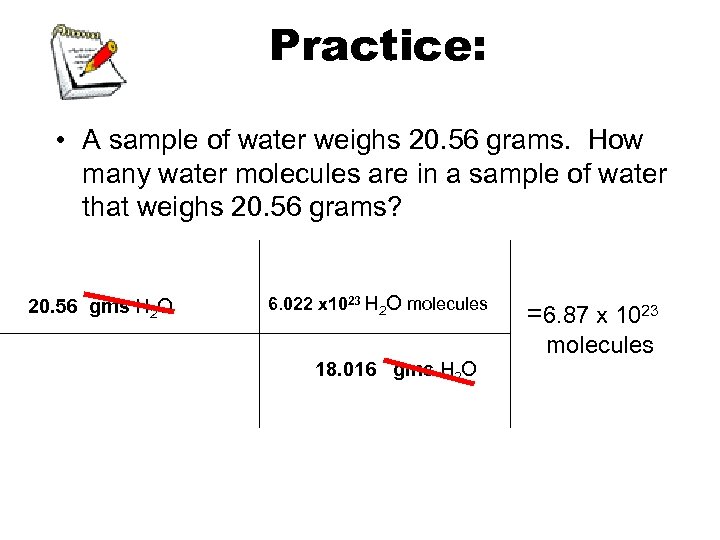 Practice: • A sample of water weighs 20. 56 grams. How many water molecules are in a sample of water that weighs 20. 56 grams? 20. 56 gms H 2 O 6. 022 x 1023 H 2 O molecules =6. 87 x 1023 molecules 18. 016 gms H 2 O
Practice: • A sample of water weighs 20. 56 grams. How many water molecules are in a sample of water that weighs 20. 56 grams? 20. 56 gms H 2 O 6. 022 x 1023 H 2 O molecules =6. 87 x 1023 molecules 18. 016 gms H 2 O
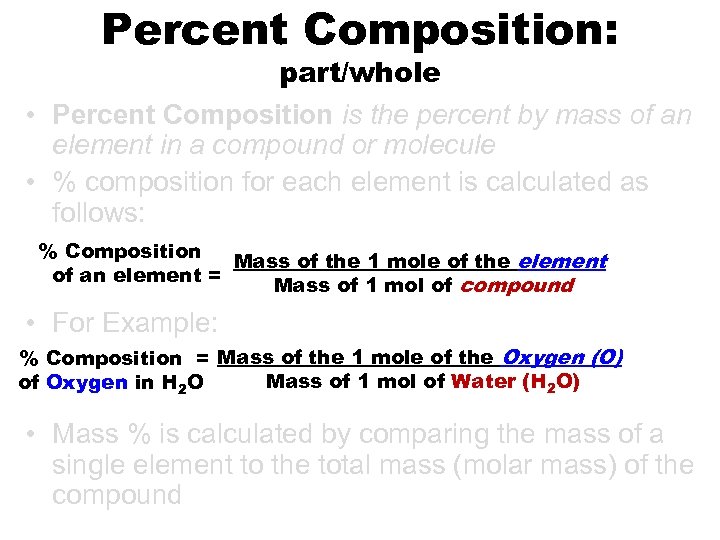 Percent Composition: part/whole • Percent Composition is the percent by mass of an element in a compound or molecule • % composition for each element is calculated as follows: % Composition Mass of the 1 mole of the element of an element = Mass of 1 mol of compound • For Example: % Composition = Mass of the 1 mole of the Oxygen (O) Mass of 1 mol of Water (H 2 O) of Oxygen in H 2 O • Mass % is calculated by comparing the mass of a single element to the total mass (molar mass) of the compound
Percent Composition: part/whole • Percent Composition is the percent by mass of an element in a compound or molecule • % composition for each element is calculated as follows: % Composition Mass of the 1 mole of the element of an element = Mass of 1 mol of compound • For Example: % Composition = Mass of the 1 mole of the Oxygen (O) Mass of 1 mol of Water (H 2 O) of Oxygen in H 2 O • Mass % is calculated by comparing the mass of a single element to the total mass (molar mass) of the compound
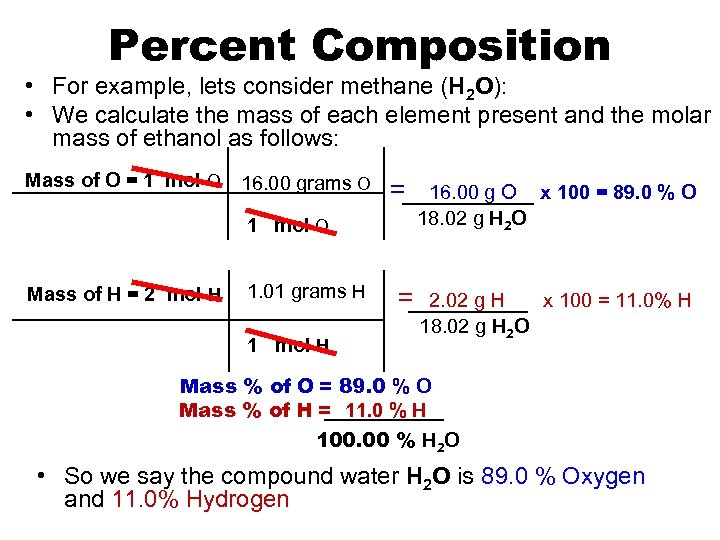 Percent Composition • For example, lets consider methane (H 2 O): • We calculate the mass of each element present and the molar mass of ethanol as follows: Mass of O = 1 mol O 16. 00 grams O = 1 mol O Mass of H = 2 mol H 1. 01 grams H 1 mol H = 16. 00 g O x 100 = 89. 0 % O 18. 02 g H 2 O 2. 02 g H x 100 = 11. 0% H 18. 02 g H 2 O Mass % of O = 89. 0 % O Mass % of H = 11. 0 % H 100. 00 % H 2 O • So we say the compound water H 2 O is 89. 0 % Oxygen and 11. 0% Hydrogen
Percent Composition • For example, lets consider methane (H 2 O): • We calculate the mass of each element present and the molar mass of ethanol as follows: Mass of O = 1 mol O 16. 00 grams O = 1 mol O Mass of H = 2 mol H 1. 01 grams H 1 mol H = 16. 00 g O x 100 = 89. 0 % O 18. 02 g H 2 O 2. 02 g H x 100 = 11. 0% H 18. 02 g H 2 O Mass % of O = 89. 0 % O Mass % of H = 11. 0 % H 100. 00 % H 2 O • So we say the compound water H 2 O is 89. 0 % Oxygen and 11. 0% Hydrogen
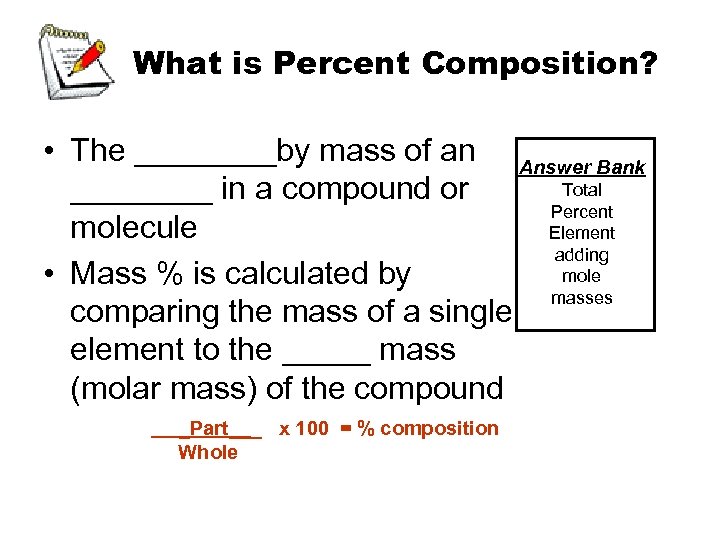 What is Percent Composition? • The ____by mass of an Answer Bank Total ____ in a compound or Percent molecule Element adding mole • Mass % is calculated by masses comparing the mass of a single element to the _____ mass (molar mass) of the compound _Part___ x 100 = % composition Whole
What is Percent Composition? • The ____by mass of an Answer Bank Total ____ in a compound or Percent molecule Element adding mole • Mass % is calculated by masses comparing the mass of a single element to the _____ mass (molar mass) of the compound _Part___ x 100 = % composition Whole
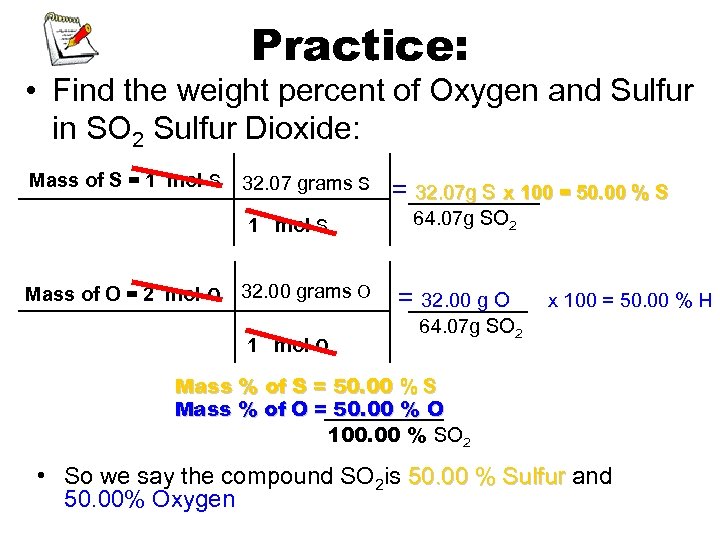 Practice: • Find the weight percent of Oxygen and Sulfur in SO 2 Sulfur Dioxide: Mass of S = 1 mol S 32. 07 grams S 1 mol S Mass of O = 2 mol O 32. 00 grams O 1 mol O = 32. 07 g S x 100 = 50. 00 % S 64. 07 g SO 2 = 32. 00 g O x 100 = 50. 00 % H 64. 07 g SO 2 Mass % of S = 50. 00 % S Mass % of O = 50. 00 % O 100. 00 % SO 2 • So we say the compound SO 2 is 50. 00 % Sulfur and 50. 00% Oxygen
Practice: • Find the weight percent of Oxygen and Sulfur in SO 2 Sulfur Dioxide: Mass of S = 1 mol S 32. 07 grams S 1 mol S Mass of O = 2 mol O 32. 00 grams O 1 mol O = 32. 07 g S x 100 = 50. 00 % S 64. 07 g SO 2 = 32. 00 g O x 100 = 50. 00 % H 64. 07 g SO 2 Mass % of S = 50. 00 % S Mass % of O = 50. 00 % O 100. 00 % SO 2 • So we say the compound SO 2 is 50. 00 % Sulfur and 50. 00% Oxygen
 Summarize: • Explain Molar mass in your own words: • Describe and explain how you would calculate % compostion: • _____is the mass in grams of one mole of a compound which contains 6. 02 x 1023 molcules • To calculate Molar mass, you would add up the ______for each element that make up a molecule • The percent by mass of an element in a compound is called it’s ______ • Calculate the percentage of nitrogen in nitrogen dioxide NO 2
Summarize: • Explain Molar mass in your own words: • Describe and explain how you would calculate % compostion: • _____is the mass in grams of one mole of a compound which contains 6. 02 x 1023 molcules • To calculate Molar mass, you would add up the ______for each element that make up a molecule • The percent by mass of an element in a compound is called it’s ______ • Calculate the percentage of nitrogen in nitrogen dioxide NO 2
 Unit: The Mole Topic: Molecular and Empirical Formulas Objectives: Day 3 of 4 • To understand the difference between molecular and empirical formulas • To learn how to calculate empirical formulas and molecular formulas given percent composition and mass
Unit: The Mole Topic: Molecular and Empirical Formulas Objectives: Day 3 of 4 • To understand the difference between molecular and empirical formulas • To learn how to calculate empirical formulas and molecular formulas given percent composition and mass
 Quickwrite Answer one of the questions below 1 -2 sentences: • Review: In iron (III) oxide, Fe 2 O 3, how many iron atoms are present? How many oxygen atoms are present? • How do think scientists determine the subscripts on chemical formulas such as Fe 2 O 3? In other words how did they come up with the subscripts 2 and 3? • Can hydrogen peroxide (H 2 O 2) be simplified into a more basic chemical formula? If so, how?
Quickwrite Answer one of the questions below 1 -2 sentences: • Review: In iron (III) oxide, Fe 2 O 3, how many iron atoms are present? How many oxygen atoms are present? • How do think scientists determine the subscripts on chemical formulas such as Fe 2 O 3? In other words how did they come up with the subscripts 2 and 3? • Can hydrogen peroxide (H 2 O 2) be simplified into a more basic chemical formula? If so, how?
 Empirical Formulas • The formula for a compound that is determined experimentally. • A formula that represents the Smallest whole-number mole ratio of the different atoms in the compound. • In other words, it is the simplest formula for a compound.
Empirical Formulas • The formula for a compound that is determined experimentally. • A formula that represents the Smallest whole-number mole ratio of the different atoms in the compound. • In other words, it is the simplest formula for a compound.
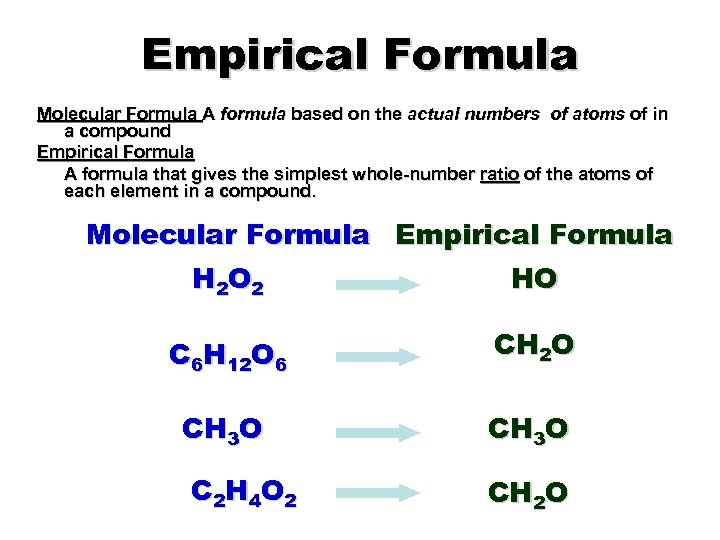 Empirical Formula Molecular Formula A formula based on the actual numbers of atoms of in a compound Empirical Formula A formula that gives the simplest whole-number ratio of the atoms of each element in a compound. Molecular Formula Empirical Formula H 2 O 2 HO C 6 H 12 O 6 CH 2 O CH 3 O C 2 H 4 O 2 CH 2 O
Empirical Formula Molecular Formula A formula based on the actual numbers of atoms of in a compound Empirical Formula A formula that gives the simplest whole-number ratio of the atoms of each element in a compound. Molecular Formula Empirical Formula H 2 O 2 HO C 6 H 12 O 6 CH 2 O CH 3 O C 2 H 4 O 2 CH 2 O
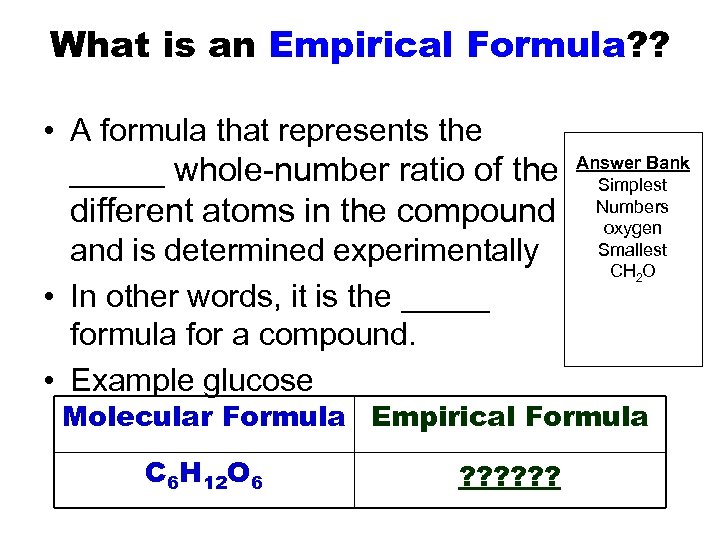 What is an Empirical Formula? ? • A formula that represents the _____ whole-number ratio of the different atoms in the compound and is determined experimentally • In other words, it is the _____ formula for a compound. • Example glucose Answer Bank Simplest Numbers oxygen Smallest CH 2 O Molecular Formula Empirical Formula C 6 H 12 O 6 ? ? ?
What is an Empirical Formula? ? • A formula that represents the _____ whole-number ratio of the different atoms in the compound and is determined experimentally • In other words, it is the _____ formula for a compound. • Example glucose Answer Bank Simplest Numbers oxygen Smallest CH 2 O Molecular Formula Empirical Formula C 6 H 12 O 6 ? ? ?
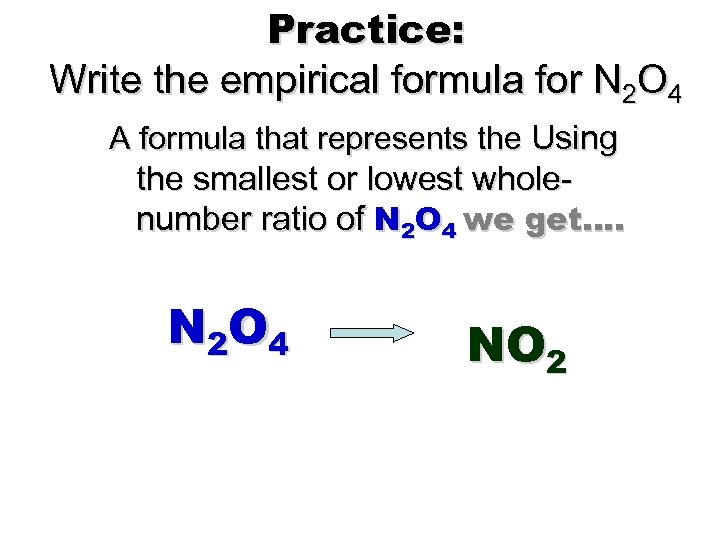 Practice: Write the empirical formula for N 2 O 4 A formula that represents the Using the smallest or lowest wholenumber ratio of N 2 O 4 we get…. N 2 O 4 NO 2
Practice: Write the empirical formula for N 2 O 4 A formula that represents the Using the smallest or lowest wholenumber ratio of N 2 O 4 we get…. N 2 O 4 NO 2
 Steps for determining Empirical Formulas 1) Assume a 100 g sample when given percents. This makes 10. 3 % = 10. 3 g 2) Convert grams into moles for each element. 3) Divide all the moles by the smallest number of moles to get the lowest whole number ratio. 4) Write the empirical formula.
Steps for determining Empirical Formulas 1) Assume a 100 g sample when given percents. This makes 10. 3 % = 10. 3 g 2) Convert grams into moles for each element. 3) Divide all the moles by the smallest number of moles to get the lowest whole number ratio. 4) Write the empirical formula.
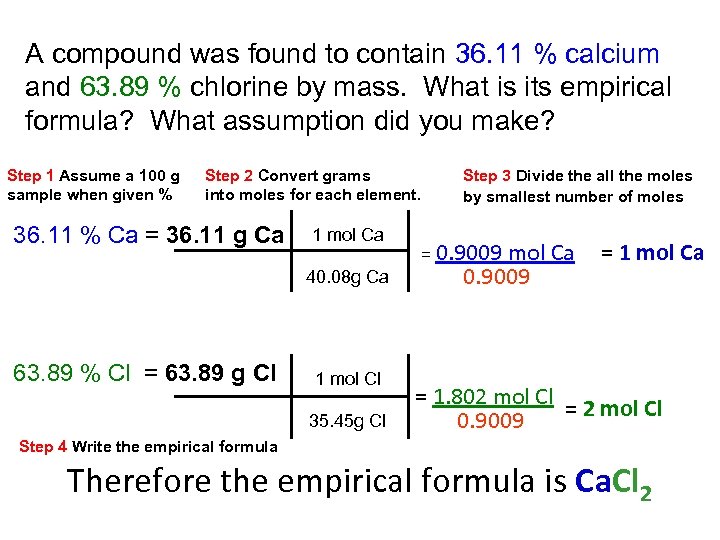 A compound was found to contain 36. 11 % calcium and 63. 89 % chlorine by mass. What is its empirical formula? What assumption did you make? Step 1 Assume a 100 g sample when given % Step 2 Convert grams into moles for each element. 36. 11 % Ca = 36. 11 g Ca 1 mol Ca 40. 08 g Ca 63. 89 % Cl = 63. 89 g Cl 1 mol Cl 35. 45 g Cl Step 3 Divide the all the moles by smallest number of moles = 0. 9009 mol Ca 0. 9009 = 1 mol Ca = 1. 802 mol Cl = 2 mol Cl 0. 9009 Step 4 Write the empirical formula Therefore the empirical formula is Ca. Cl 2
A compound was found to contain 36. 11 % calcium and 63. 89 % chlorine by mass. What is its empirical formula? What assumption did you make? Step 1 Assume a 100 g sample when given % Step 2 Convert grams into moles for each element. 36. 11 % Ca = 36. 11 g Ca 1 mol Ca 40. 08 g Ca 63. 89 % Cl = 63. 89 g Cl 1 mol Cl 35. 45 g Cl Step 3 Divide the all the moles by smallest number of moles = 0. 9009 mol Ca 0. 9009 = 1 mol Ca = 1. 802 mol Cl = 2 mol Cl 0. 9009 Step 4 Write the empirical formula Therefore the empirical formula is Ca. Cl 2
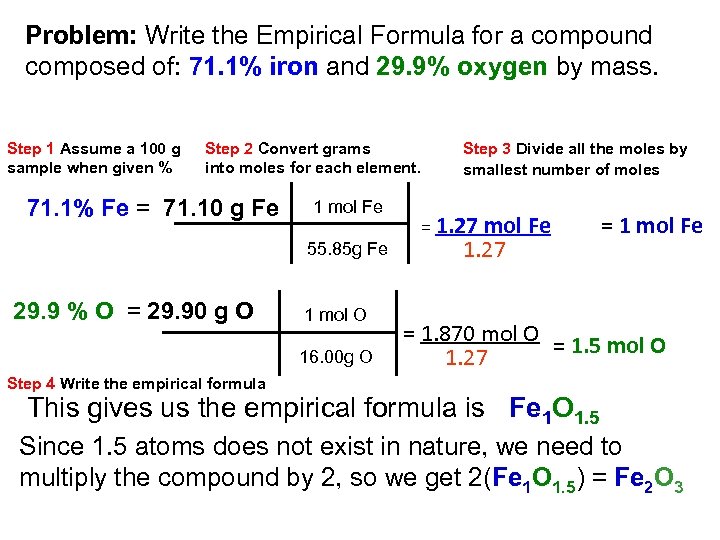 Problem: Write the Empirical Formula for a compound composed of: 71. 1% iron and 29. 9% oxygen by mass. Step 1 Assume a 100 g sample when given % Step 2 Convert grams into moles for each element. 71. 1% Fe = 71. 10 g Fe 1 mol Fe 55. 85 g Fe 29. 9 % O = 29. 90 g O 1 mol O 16. 00 g O Step 3 Divide all the moles by smallest number of moles = 1. 27 mol Fe 1. 27 = 1 mol Fe = 1. 870 mol O = 1. 5 mol O 1. 27 Step 4 Write the empirical formula This gives us the empirical formula is Fe 1 O 1. 5 Since 1. 5 atoms does not exist in nature, we need to multiply the compound by 2, so we get 2(Fe 1 O 1. 5) = Fe 2 O 3
Problem: Write the Empirical Formula for a compound composed of: 71. 1% iron and 29. 9% oxygen by mass. Step 1 Assume a 100 g sample when given % Step 2 Convert grams into moles for each element. 71. 1% Fe = 71. 10 g Fe 1 mol Fe 55. 85 g Fe 29. 9 % O = 29. 90 g O 1 mol O 16. 00 g O Step 3 Divide all the moles by smallest number of moles = 1. 27 mol Fe 1. 27 = 1 mol Fe = 1. 870 mol O = 1. 5 mol O 1. 27 Step 4 Write the empirical formula This gives us the empirical formula is Fe 1 O 1. 5 Since 1. 5 atoms does not exist in nature, we need to multiply the compound by 2, so we get 2(Fe 1 O 1. 5) = Fe 2 O 3
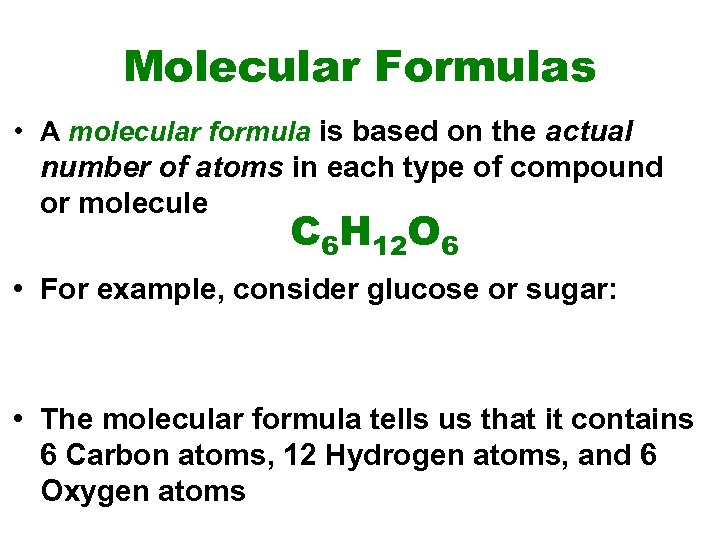 Molecular Formulas • A molecular formula is based on the actual number of atoms in each type of compound or molecule C 6 H 12 O 6 • For example, consider glucose or sugar: • The molecular formula tells us that it contains 6 Carbon atoms, 12 Hydrogen atoms, and 6 Oxygen atoms
Molecular Formulas • A molecular formula is based on the actual number of atoms in each type of compound or molecule C 6 H 12 O 6 • For example, consider glucose or sugar: • The molecular formula tells us that it contains 6 Carbon atoms, 12 Hydrogen atoms, and 6 Oxygen atoms
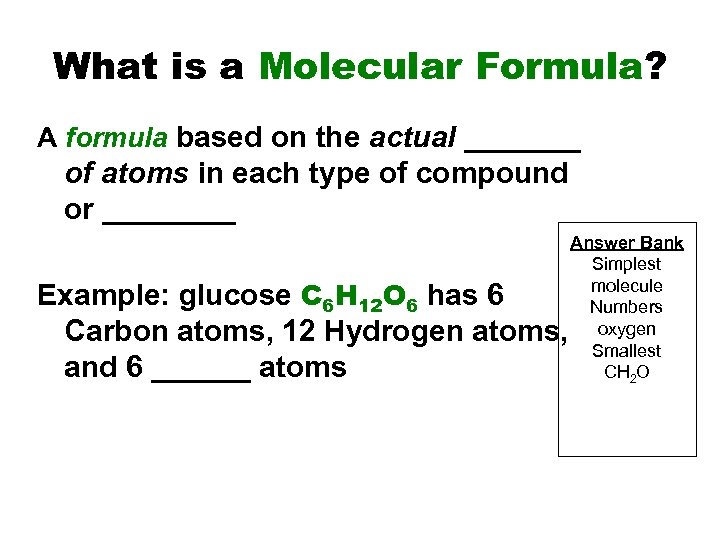 What is a Molecular Formula? A formula based on the actual _______ of atoms in each type of compound or ____ Example: glucose C 6 H 12 O 6 has 6 Carbon atoms, 12 Hydrogen atoms, and 6 ______ atoms Answer Bank Simplest molecule Numbers oxygen Smallest CH 2 O
What is a Molecular Formula? A formula based on the actual _______ of atoms in each type of compound or ____ Example: glucose C 6 H 12 O 6 has 6 Carbon atoms, 12 Hydrogen atoms, and 6 ______ atoms Answer Bank Simplest molecule Numbers oxygen Smallest CH 2 O
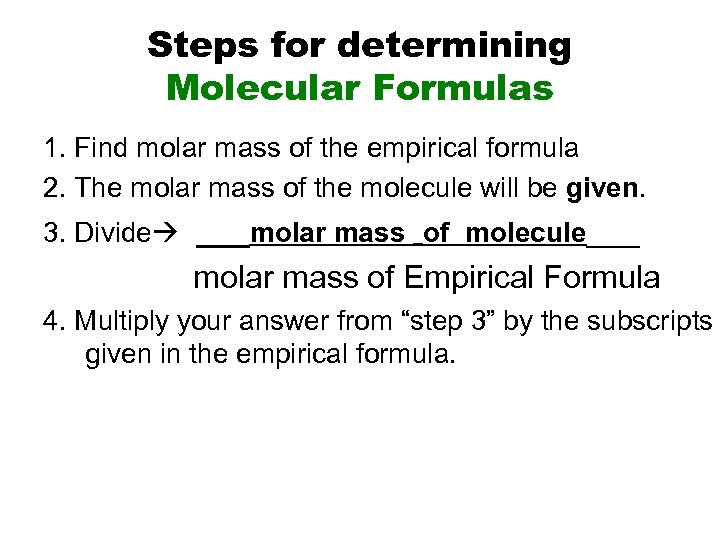 Steps for determining Molecular Formulas 1. Find molar mass of the empirical formula 2. The molar mass of the molecule will be given. 3. Divide ___molar mass _of molecule___ molar mass of Empirical Formula 4. Multiply your answer from “step 3” by the subscripts given in the empirical formula.
Steps for determining Molecular Formulas 1. Find molar mass of the empirical formula 2. The molar mass of the molecule will be given. 3. Divide ___molar mass _of molecule___ molar mass of Empirical Formula 4. Multiply your answer from “step 3” by the subscripts given in the empirical formula.
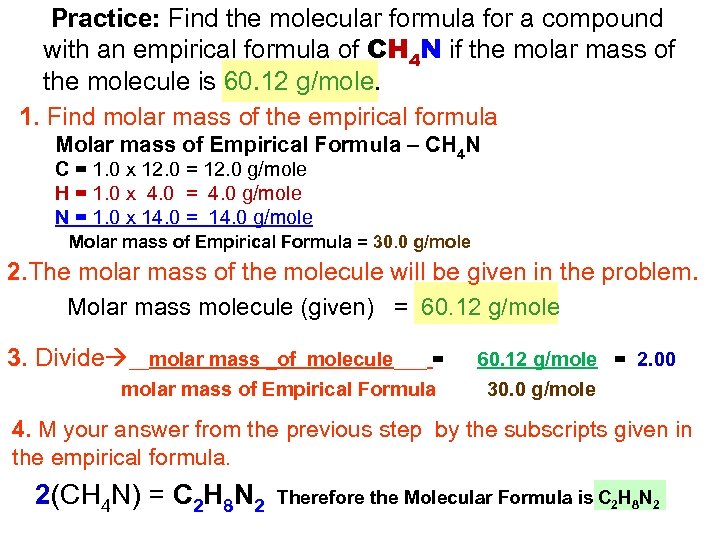 Practice: Find the molecular formula for a compound with an empirical formula of CH 4 N if the molar mass of the molecule is 60. 12 g/mole. 1. Find molar mass of the empirical formula Molar mass of Empirical Formula – CH 4 N C = 1. 0 x 12. 0 = 12. 0 g/mole H = 1. 0 x 4. 0 = 4. 0 g/mole N = 1. 0 x 14. 0 = 14. 0 g/mole Molar mass of Empirical Formula = 30. 0 g/mole 2. The molar mass of the molecule will be given in the problem. Molar mass molecule (given) = 60. 12 g/mole 3. Divide __molar mass _of molecule___ = molar mass of Empirical Formula 60. 12 g/mole = 2. 00 30. 0 g/mole 4. M your answer from the previous step by the subscripts given in the empirical formula. 2(CH 4 N) = C 2 H 8 N 2 Therefore the Molecular Formula is C 2 H 8 N 2
Practice: Find the molecular formula for a compound with an empirical formula of CH 4 N if the molar mass of the molecule is 60. 12 g/mole. 1. Find molar mass of the empirical formula Molar mass of Empirical Formula – CH 4 N C = 1. 0 x 12. 0 = 12. 0 g/mole H = 1. 0 x 4. 0 = 4. 0 g/mole N = 1. 0 x 14. 0 = 14. 0 g/mole Molar mass of Empirical Formula = 30. 0 g/mole 2. The molar mass of the molecule will be given in the problem. Molar mass molecule (given) = 60. 12 g/mole 3. Divide __molar mass _of molecule___ = molar mass of Empirical Formula 60. 12 g/mole = 2. 00 30. 0 g/mole 4. M your answer from the previous step by the subscripts given in the empirical formula. 2(CH 4 N) = C 2 H 8 N 2 Therefore the Molecular Formula is C 2 H 8 N 2
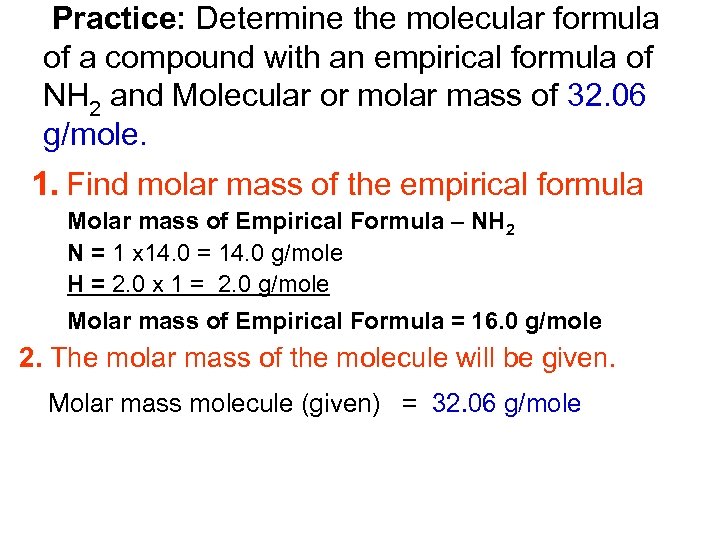 Practice: Determine the molecular formula of a compound with an empirical formula of NH 2 and Molecular or molar mass of 32. 06 g/mole. 1. Find molar mass of the empirical formula Molar mass of Empirical Formula – NH 2 N = 1 x 14. 0 = 14. 0 g/mole H = 2. 0 x 1 = 2. 0 g/mole Molar mass of Empirical Formula = 16. 0 g/mole 2. The molar mass of the molecule will be given. Molar mass molecule (given) = 32. 06 g/mole
Practice: Determine the molecular formula of a compound with an empirical formula of NH 2 and Molecular or molar mass of 32. 06 g/mole. 1. Find molar mass of the empirical formula Molar mass of Empirical Formula – NH 2 N = 1 x 14. 0 = 14. 0 g/mole H = 2. 0 x 1 = 2. 0 g/mole Molar mass of Empirical Formula = 16. 0 g/mole 2. The molar mass of the molecule will be given. Molar mass molecule (given) = 32. 06 g/mole
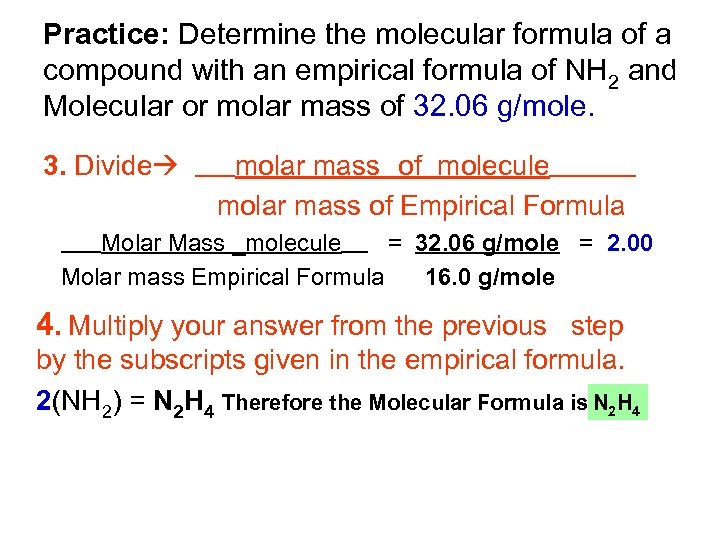 Practice: Determine the molecular formula of a compound with an empirical formula of NH 2 and Molecular or molar mass of 32. 06 g/mole. 3. Divide molar mass _of molecule_______ molar mass of Empirical Formula ______ Molar Mass _molecule____ = 32. 06 g/mole = 2. 00 Molar mass Empirical Formula 16. 0 g/mole __ __ 4. Multiply your answer from the previous step by the subscripts given in the empirical formula. 2(NH 2) = N 2 H 4 Therefore the Molecular Formula is N 2 H 4
Practice: Determine the molecular formula of a compound with an empirical formula of NH 2 and Molecular or molar mass of 32. 06 g/mole. 3. Divide molar mass _of molecule_______ molar mass of Empirical Formula ______ Molar Mass _molecule____ = 32. 06 g/mole = 2. 00 Molar mass Empirical Formula 16. 0 g/mole __ __ 4. Multiply your answer from the previous step by the subscripts given in the empirical formula. 2(NH 2) = N 2 H 4 Therefore the Molecular Formula is N 2 H 4
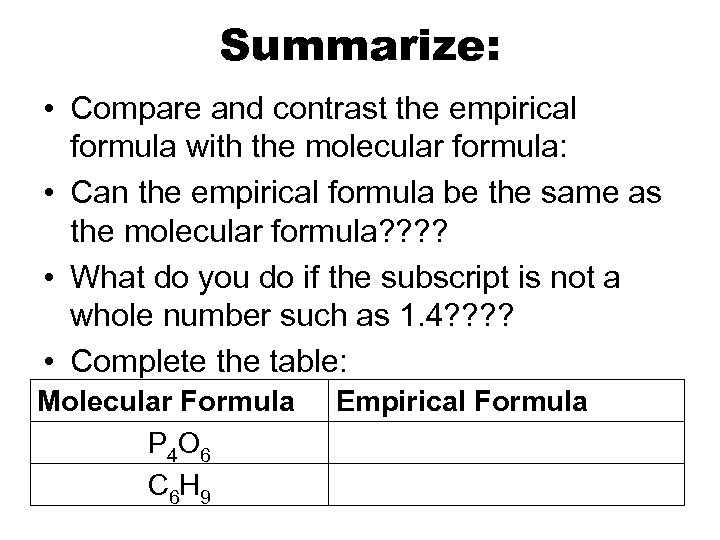 Summarize: • Compare and contrast the empirical formula with the molecular formula: • Can the empirical formula be the same as the molecular formula? ? • What do you do if the subscript is not a whole number such as 1. 4? ? • Complete the table: Molecular Formula P 4 O 6 C 6 H 9 Empirical Formula
Summarize: • Compare and contrast the empirical formula with the molecular formula: • Can the empirical formula be the same as the molecular formula? ? • What do you do if the subscript is not a whole number such as 1. 4? ? • Complete the table: Molecular Formula P 4 O 6 C 6 H 9 Empirical Formula
 Unit: Topic: The Moles in Chemical Reactions Objectives: Day 4 of 4 • To learn how we go from moles of a reactant to moles of a product • To learn how to calculate between moles of reactants to moles of products
Unit: Topic: The Moles in Chemical Reactions Objectives: Day 4 of 4 • To learn how we go from moles of a reactant to moles of a product • To learn how to calculate between moles of reactants to moles of products
 Quickwrite Answer one of the questions below 1 -2 sentences: • A cake recipe requires 2 eggs, 2 cups of flour and 1 cup of sugar; you need to make 6 cakes for a friends birthday party, how many eggs, cups of flour and sugar should you buy? ? • Using the recipe below: 2 eggs + 2 cups of flour + 1 cup of sugar → 1 cake To make one cake, a recipe requires how many cups of flour? How could you write this as a ratio?
Quickwrite Answer one of the questions below 1 -2 sentences: • A cake recipe requires 2 eggs, 2 cups of flour and 1 cup of sugar; you need to make 6 cakes for a friends birthday party, how many eggs, cups of flour and sugar should you buy? ? • Using the recipe below: 2 eggs + 2 cups of flour + 1 cup of sugar → 1 cake To make one cake, a recipe requires how many cups of flour? How could you write this as a ratio?
 Moles in Reactions • Chemistry is really all about reactions • Reactions involve the rearrangement of atoms • The calculation of the quantities of chemical elements or compounds involved in chemical reactions is called Stoichiometry (our next unit) • It is the coefficients in the balanced chemical equation that enables us to determine just how much product forms • What we once called coefficients are now called moles!!!!
Moles in Reactions • Chemistry is really all about reactions • Reactions involve the rearrangement of atoms • The calculation of the quantities of chemical elements or compounds involved in chemical reactions is called Stoichiometry (our next unit) • It is the coefficients in the balanced chemical equation that enables us to determine just how much product forms • What we once called coefficients are now called moles!!!!
 Moles in Reactions • To explore this idea, consider a nonchemical analogy • A particular cake recipe requires 2 eggs, 2 cups of flour, and 1 cup of sugar • Or, you might represent this by: 2 eggs + 2 cups of flour + 1 cup of sugar → 1 cake
Moles in Reactions • To explore this idea, consider a nonchemical analogy • A particular cake recipe requires 2 eggs, 2 cups of flour, and 1 cup of sugar • Or, you might represent this by: 2 eggs + 2 cups of flour + 1 cup of sugar → 1 cake
 Moles in Reactions • Now, lets say you need to make 50 cakes for a large party • You will need ingredients to make 50 cakes • How do you figure out how much of each ingredient you need to buy? • You could multiply the previous equation by 50: 50(2 eggs) +50 (2 cups of flour) + 50(1 cup of sugar) → 50 (1 cake) 100 eggs+ 100 cups of flour + 50 cups of sugar → 50 cakes
Moles in Reactions • Now, lets say you need to make 50 cakes for a large party • You will need ingredients to make 50 cakes • How do you figure out how much of each ingredient you need to buy? • You could multiply the previous equation by 50: 50(2 eggs) +50 (2 cups of flour) + 50(1 cup of sugar) → 50 (1 cake) 100 eggs+ 100 cups of flour + 50 cups of sugar → 50 cakes
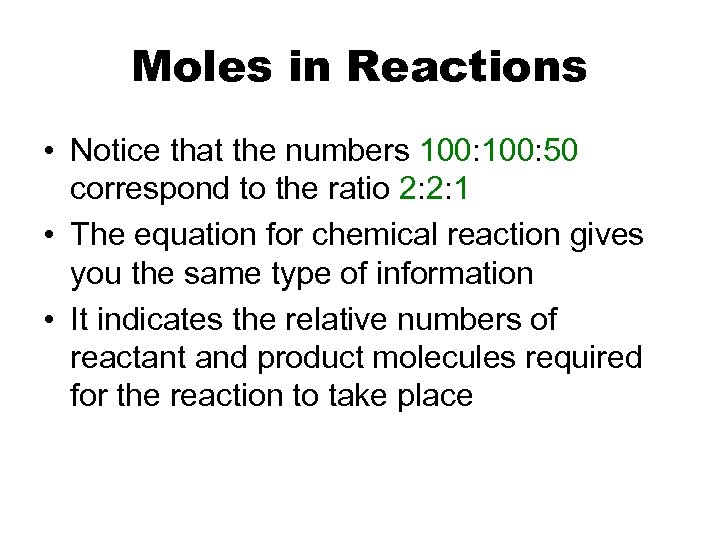 Moles in Reactions • Notice that the numbers 100: 50 correspond to the ratio 2: 2: 1 • The equation for chemical reaction gives you the same type of information • It indicates the relative numbers of reactant and product molecules required for the reaction to take place
Moles in Reactions • Notice that the numbers 100: 50 correspond to the ratio 2: 2: 1 • The equation for chemical reaction gives you the same type of information • It indicates the relative numbers of reactant and product molecules required for the reaction to take place
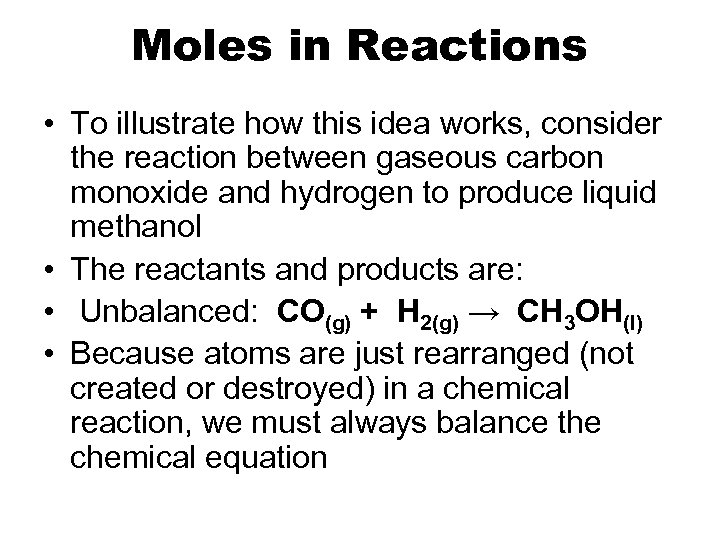 Moles in Reactions • To illustrate how this idea works, consider the reaction between gaseous carbon monoxide and hydrogen to produce liquid methanol • The reactants and products are: • Unbalanced: CO(g) + H 2(g) → CH 3 OH(l) • Because atoms are just rearranged (not created or destroyed) in a chemical reaction, we must always balance the chemical equation
Moles in Reactions • To illustrate how this idea works, consider the reaction between gaseous carbon monoxide and hydrogen to produce liquid methanol • The reactants and products are: • Unbalanced: CO(g) + H 2(g) → CH 3 OH(l) • Because atoms are just rearranged (not created or destroyed) in a chemical reaction, we must always balance the chemical equation
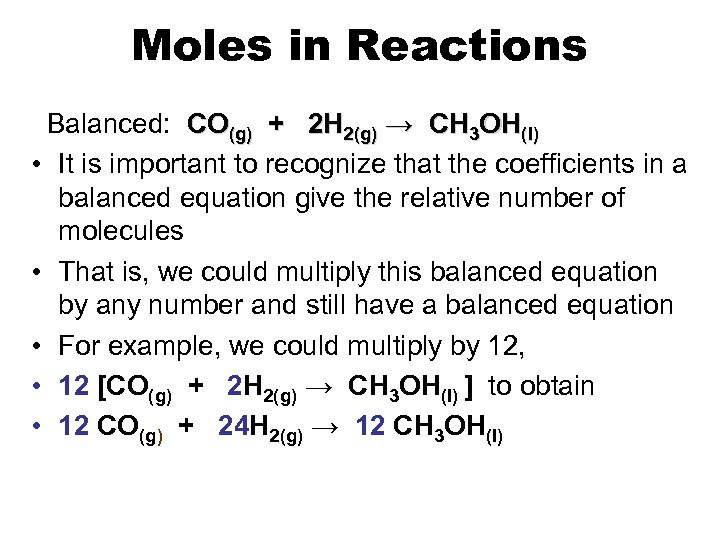 Moles in Reactions Balanced: CO(g) + 2 H 2(g) → CH 3 OH(l) • It is important to recognize that the coefficients in a balanced equation give the relative number of molecules • That is, we could multiply this balanced equation by any number and still have a balanced equation • For example, we could multiply by 12, • 12 [CO(g) + 2 H 2(g) → CH 3 OH(l) ] to obtain • 12 CO(g) + 24 H 2(g) → 12 CH 3 OH(l)
Moles in Reactions Balanced: CO(g) + 2 H 2(g) → CH 3 OH(l) • It is important to recognize that the coefficients in a balanced equation give the relative number of molecules • That is, we could multiply this balanced equation by any number and still have a balanced equation • For example, we could multiply by 12, • 12 [CO(g) + 2 H 2(g) → CH 3 OH(l) ] to obtain • 12 CO(g) + 24 H 2(g) → 12 CH 3 OH(l)
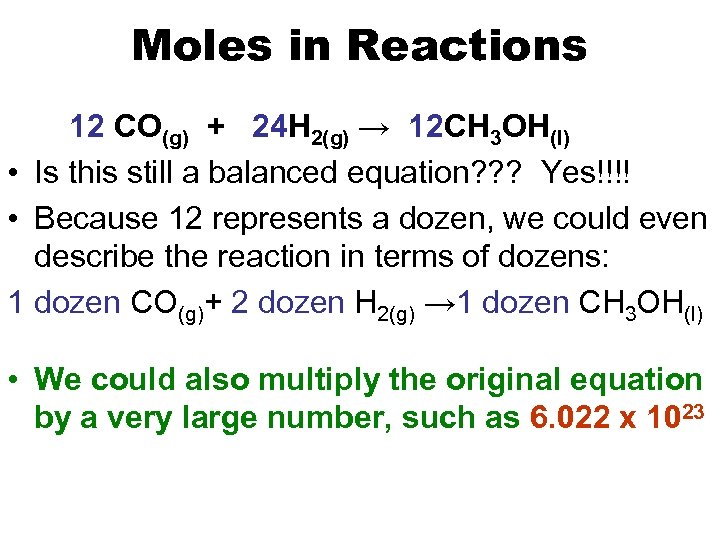 Moles in Reactions 12 CO(g) + 24 H 2(g) → 12 CH 3 OH(l) • Is this still a balanced equation? ? ? Yes!!!! • Because 12 represents a dozen, we could even describe the reaction in terms of dozens: 1 dozen CO(g)+ 2 dozen H 2(g) → 1 dozen CH 3 OH(l) • We could also multiply the original equation by a very large number, such as 6. 022 x 1023
Moles in Reactions 12 CO(g) + 24 H 2(g) → 12 CH 3 OH(l) • Is this still a balanced equation? ? ? Yes!!!! • Because 12 represents a dozen, we could even describe the reaction in terms of dozens: 1 dozen CO(g)+ 2 dozen H 2(g) → 1 dozen CH 3 OH(l) • We could also multiply the original equation by a very large number, such as 6. 022 x 1023
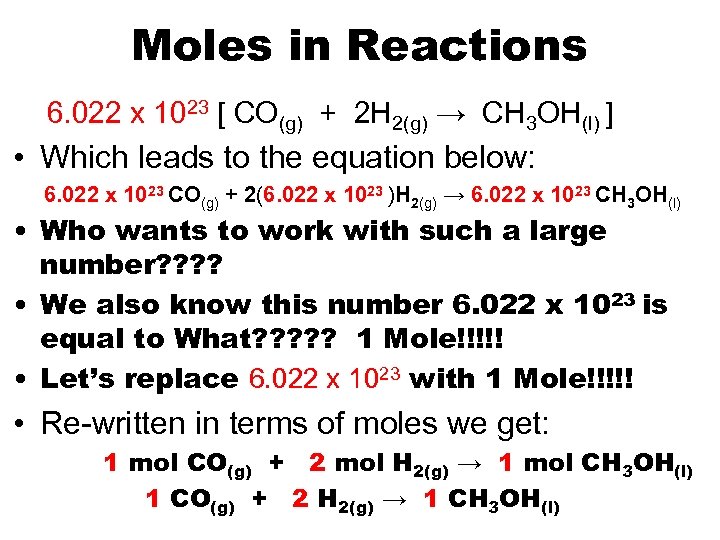 Moles in Reactions 6. 022 x 1023 [ CO(g) + 2 H 2(g) → CH 3 OH(l) ] • Which leads to the equation below: 6. 022 x 1023 CO(g) + 2(6. 022 x 1023 )H 2(g) → 6. 022 x 1023 CH 3 OH(l) • Who wants to work with such a large number? ? • We also know this number 6. 022 x 1023 is equal to What? ? ? 1 Mole!!!!! • Let’s replace 6. 022 x 1023 with 1 Mole!!!!! • Re-written in terms of moles we get: 1 mol CO(g) + 2 mol H 2(g) → 1 mol CH 3 OH(l) 1 CO(g) + 2 H 2(g) → 1 CH 3 OH(l)
Moles in Reactions 6. 022 x 1023 [ CO(g) + 2 H 2(g) → CH 3 OH(l) ] • Which leads to the equation below: 6. 022 x 1023 CO(g) + 2(6. 022 x 1023 )H 2(g) → 6. 022 x 1023 CH 3 OH(l) • Who wants to work with such a large number? ? • We also know this number 6. 022 x 1023 is equal to What? ? ? 1 Mole!!!!! • Let’s replace 6. 022 x 1023 with 1 Mole!!!!! • Re-written in terms of moles we get: 1 mol CO(g) + 2 mol H 2(g) → 1 mol CH 3 OH(l) 1 CO(g) + 2 H 2(g) → 1 CH 3 OH(l)
 Moles in Reactions • Think of the number of moles in a chemical reaction as the amount of ingredients necessary for the reaction to take place • Just like you need a certain amount ingredients to make a cake, you also need a certain amount of ingredients, or in this case molecules for a chemical reaction • We can use an equation to predict the moles of products that a given number of moles of reactants will produce • For example, consider the combination oxygen and hydrogen gas to synthesize water: 2 H 2(g) + O 2(g) → 2 H 2 O(l) • The equation tells us that 2 mol of H 2 reacts and requires 1 mol of O 2 to create or produce 2 mol of H 2 O
Moles in Reactions • Think of the number of moles in a chemical reaction as the amount of ingredients necessary for the reaction to take place • Just like you need a certain amount ingredients to make a cake, you also need a certain amount of ingredients, or in this case molecules for a chemical reaction • We can use an equation to predict the moles of products that a given number of moles of reactants will produce • For example, consider the combination oxygen and hydrogen gas to synthesize water: 2 H 2(g) + O 2(g) → 2 H 2 O(l) • The equation tells us that 2 mol of H 2 reacts and requires 1 mol of O 2 to create or produce 2 mol of H 2 O
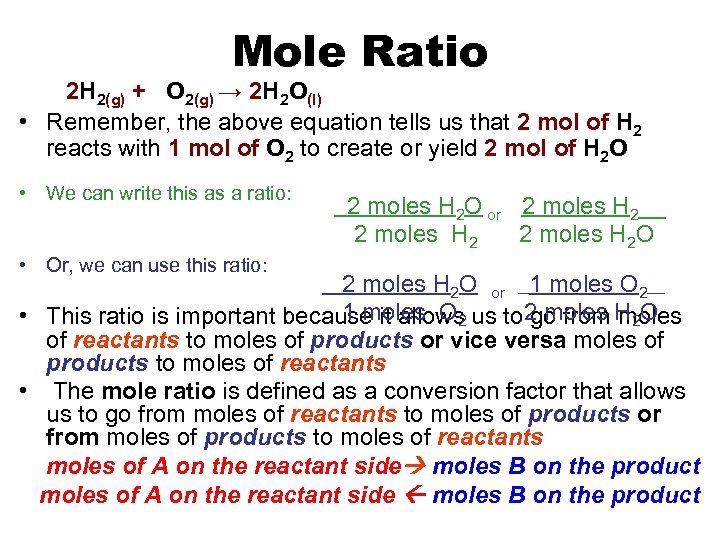 Mole Ratio 2 H 2(g) + O 2(g) → 2 H 2 O(l) • Remember, the above equation tells us that 2 mol of H 2 reacts with 1 mol of O 2 to create or yield 2 mol of H 2 O • We can write this as a ratio: • Or, we can use this ratio: 2 moles H 2 O or 2 moles H 2__ 2 moles H 2 O or __1 moles O 2___ 1 moles O 2 • This ratio is important because it allows us to 2 moles H 2 O go from moles of reactants to moles of products or vice versa moles of products to moles of reactants • The mole ratio is defined as a conversion factor that allows us to go from moles of reactants to moles of products or from moles of products to moles of reactants moles of A on the reactant side moles B on the product moles of A on the reactant side moles B on the product
Mole Ratio 2 H 2(g) + O 2(g) → 2 H 2 O(l) • Remember, the above equation tells us that 2 mol of H 2 reacts with 1 mol of O 2 to create or yield 2 mol of H 2 O • We can write this as a ratio: • Or, we can use this ratio: 2 moles H 2 O or 2 moles H 2__ 2 moles H 2 O or __1 moles O 2___ 1 moles O 2 • This ratio is important because it allows us to 2 moles H 2 O go from moles of reactants to moles of products or vice versa moles of products to moles of reactants • The mole ratio is defined as a conversion factor that allows us to go from moles of reactants to moles of products or from moles of products to moles of reactants moles of A on the reactant side moles B on the product moles of A on the reactant side moles B on the product
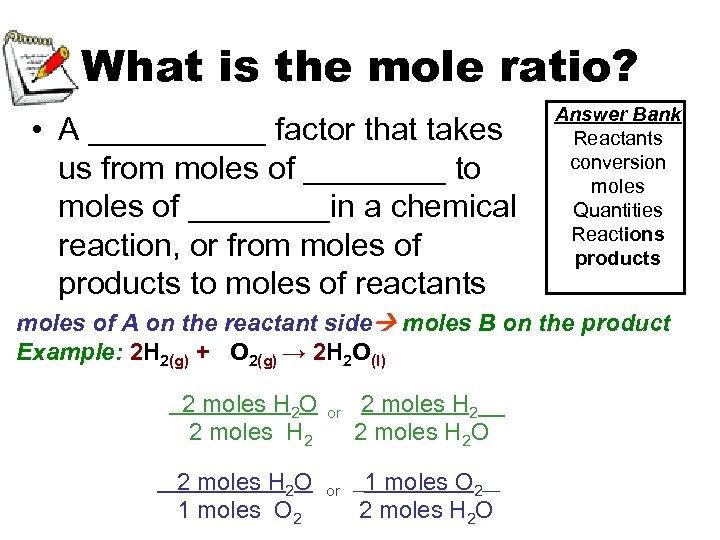 What is the mole ratio? • A _____ factor that takes us from moles of ____ to moles of ____in a chemical reaction, or from moles of products to moles of reactants Answer Bank Reactants conversion moles Quantities Reactions products moles of A on the reactant side moles B on the product Example: 2 H 2(g) + O 2(g) → 2 H 2 O(l) 2 moles H 2 O 1 moles O 2 or or 2 moles H 2__ 2 moles H 2 O 1 moles O 2___ 2 moles H 2 O __
What is the mole ratio? • A _____ factor that takes us from moles of ____ to moles of ____in a chemical reaction, or from moles of products to moles of reactants Answer Bank Reactants conversion moles Quantities Reactions products moles of A on the reactant side moles B on the product Example: 2 H 2(g) + O 2(g) → 2 H 2 O(l) 2 moles H 2 O 1 moles O 2 or or 2 moles H 2__ 2 moles H 2 O 1 moles O 2___ 2 moles H 2 O __
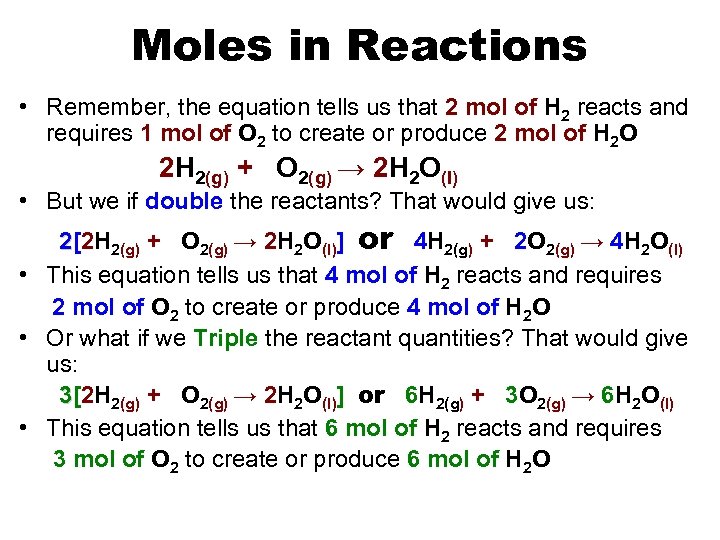 Moles in Reactions • Remember, the equation tells us that 2 mol of H 2 reacts and requires 1 mol of O 2 to create or produce 2 mol of H 2 O 2 H 2(g) + O 2(g) → 2 H 2 O(l) • But we if double the reactants? That would give us: 2[2 H 2(g) + O 2(g) → 2 H 2 O(l)] or 4 H 2(g) + 2 O 2(g) → 4 H 2 O(l) • This equation tells us that 4 mol of H 2 reacts and requires 2 mol of O 2 to create or produce 4 mol of H 2 O • Or what if we Triple the reactant quantities? That would give us: 3[2 H 2(g) + O 2(g) → 2 H 2 O(l)] or 6 H 2(g) + 3 O 2(g) → 6 H 2 O(l) • This equation tells us that 6 mol of H 2 reacts and requires 3 mol of O 2 to create or produce 6 mol of H 2 O
Moles in Reactions • Remember, the equation tells us that 2 mol of H 2 reacts and requires 1 mol of O 2 to create or produce 2 mol of H 2 O 2 H 2(g) + O 2(g) → 2 H 2 O(l) • But we if double the reactants? That would give us: 2[2 H 2(g) + O 2(g) → 2 H 2 O(l)] or 4 H 2(g) + 2 O 2(g) → 4 H 2 O(l) • This equation tells us that 4 mol of H 2 reacts and requires 2 mol of O 2 to create or produce 4 mol of H 2 O • Or what if we Triple the reactant quantities? That would give us: 3[2 H 2(g) + O 2(g) → 2 H 2 O(l)] or 6 H 2(g) + 3 O 2(g) → 6 H 2 O(l) • This equation tells us that 6 mol of H 2 reacts and requires 3 mol of O 2 to create or produce 6 mol of H 2 O
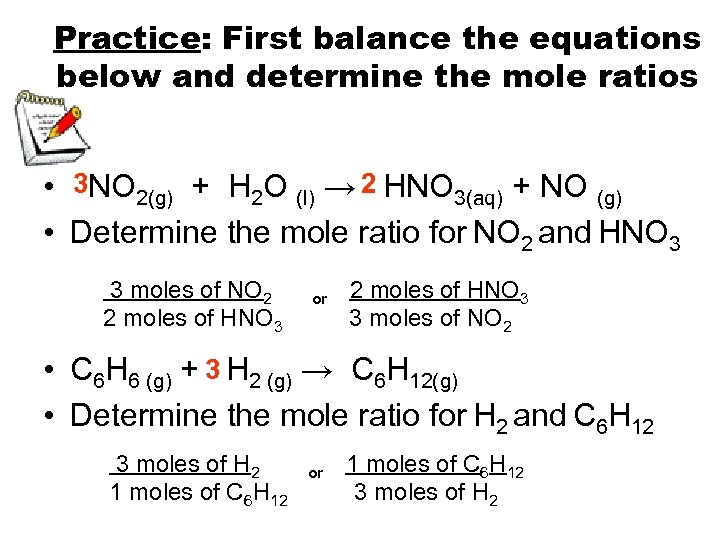 Practice: First balance the equations below and determine the mole ratios • 3 NO 2(g) + H 2 O (l) → 2 HNO 3(aq) + NO (g) • Determine the mole ratio for NO 2 and HNO 3 3 moles of NO 2 2 moles of HNO 3 or 2 moles of HNO 3 3 moles of NO 2 • C 6 H 6 (g) + 3 H 2 (g) → C 6 H 12(g) • Determine the mole ratio for H 2 and C 6 H 12 3 moles of H 2 1 moles of C 6 H 12 or 1 moles of C 6 H 12 3 moles of H 2
Practice: First balance the equations below and determine the mole ratios • 3 NO 2(g) + H 2 O (l) → 2 HNO 3(aq) + NO (g) • Determine the mole ratio for NO 2 and HNO 3 3 moles of NO 2 2 moles of HNO 3 or 2 moles of HNO 3 3 moles of NO 2 • C 6 H 6 (g) + 3 H 2 (g) → C 6 H 12(g) • Determine the mole ratio for H 2 and C 6 H 12 3 moles of H 2 1 moles of C 6 H 12 or 1 moles of C 6 H 12 3 moles of H 2
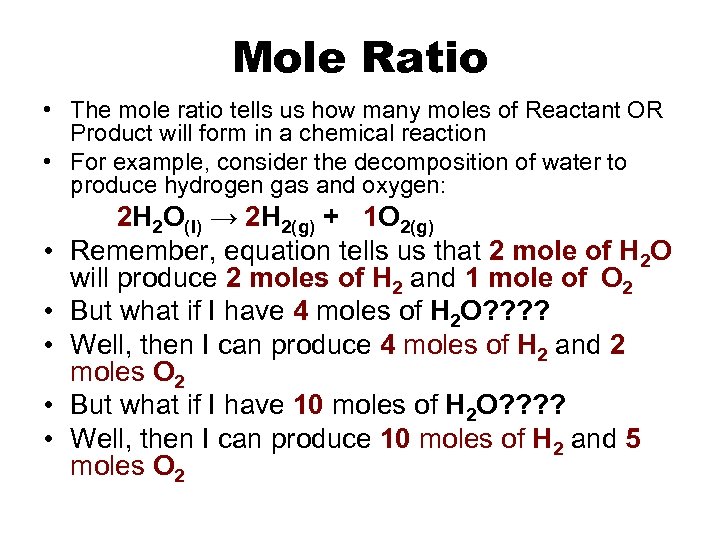 Mole Ratio • The mole ratio tells us how many moles of Reactant OR Product will form in a chemical reaction • For example, consider the decomposition of water to produce hydrogen gas and oxygen: • • • 2 H 2 O(l) → 2 H 2(g) + 1 O 2(g) Remember, equation tells us that 2 mole of H 2 O will produce 2 moles of H 2 and 1 mole of O 2 But what if I have 4 moles of H 2 O? ? Well, then I can produce 4 moles of H 2 and 2 moles O 2 But what if I have 10 moles of H 2 O? ? Well, then I can produce 10 moles of H 2 and 5 moles O 2
Mole Ratio • The mole ratio tells us how many moles of Reactant OR Product will form in a chemical reaction • For example, consider the decomposition of water to produce hydrogen gas and oxygen: • • • 2 H 2 O(l) → 2 H 2(g) + 1 O 2(g) Remember, equation tells us that 2 mole of H 2 O will produce 2 moles of H 2 and 1 mole of O 2 But what if I have 4 moles of H 2 O? ? Well, then I can produce 4 moles of H 2 and 2 moles O 2 But what if I have 10 moles of H 2 O? ? Well, then I can produce 10 moles of H 2 and 5 moles O 2
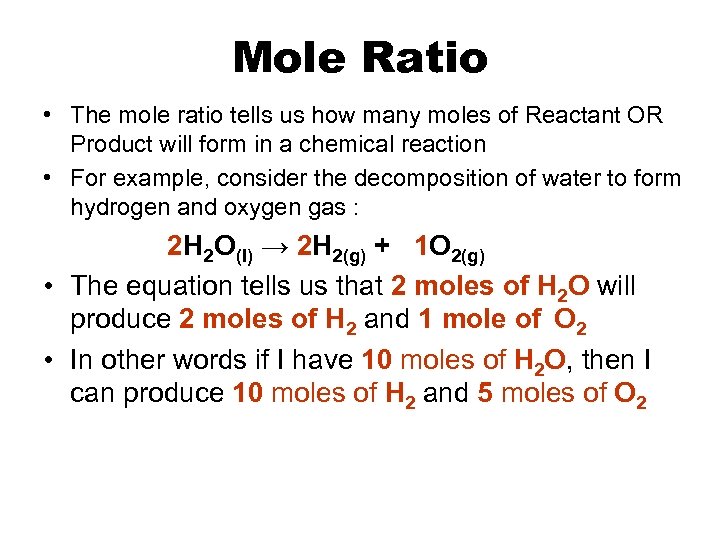 Mole Ratio • The mole ratio tells us how many moles of Reactant OR Product will form in a chemical reaction • For example, consider the decomposition of water to form hydrogen and oxygen gas : 2 H 2 O(l) → 2 H 2(g) + 1 O 2(g) • The equation tells us that 2 moles of H 2 O will produce 2 moles of H 2 and 1 mole of O 2 • In other words if I have 10 moles of H 2 O, then I can produce 10 moles of H 2 and 5 moles of O 2
Mole Ratio • The mole ratio tells us how many moles of Reactant OR Product will form in a chemical reaction • For example, consider the decomposition of water to form hydrogen and oxygen gas : 2 H 2 O(l) → 2 H 2(g) + 1 O 2(g) • The equation tells us that 2 moles of H 2 O will produce 2 moles of H 2 and 1 mole of O 2 • In other words if I have 10 moles of H 2 O, then I can produce 10 moles of H 2 and 5 moles of O 2
 Practice: • Using the equation: 2 H 2 O(l) → 2 H 2(g)+ O 2(g) How many moles of O 2 are produced by 4 moles of water? ? ? • Using the equation: 2 H 2 O(l) → 2 H 2(g)+ O 2(g) How many moles of O 2 are produced by 1 mole of water? ? ?
Practice: • Using the equation: 2 H 2 O(l) → 2 H 2(g)+ O 2(g) How many moles of O 2 are produced by 4 moles of water? ? ? • Using the equation: 2 H 2 O(l) → 2 H 2(g)+ O 2(g) How many moles of O 2 are produced by 1 mole of water? ? ?
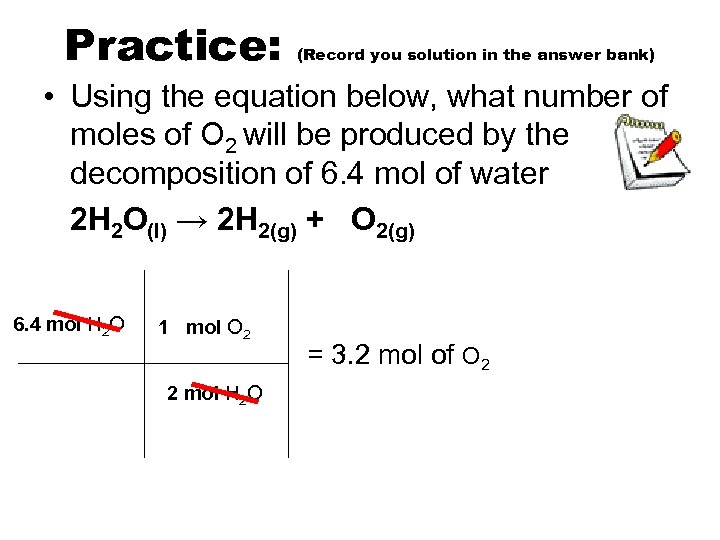 Practice: (Record you solution in the answer bank) • Using the equation below, what number of moles of O 2 will be produced by the decomposition of 6. 4 mol of water 2 H 2 O(l) → 2 H 2(g) + O 2(g) 6. 4 mol H 2 O 1 mol O 2 2 mol H 2 O = 3. 2 mol of O 2
Practice: (Record you solution in the answer bank) • Using the equation below, what number of moles of O 2 will be produced by the decomposition of 6. 4 mol of water 2 H 2 O(l) → 2 H 2(g) + O 2(g) 6. 4 mol H 2 O 1 mol O 2 2 mol H 2 O = 3. 2 mol of O 2
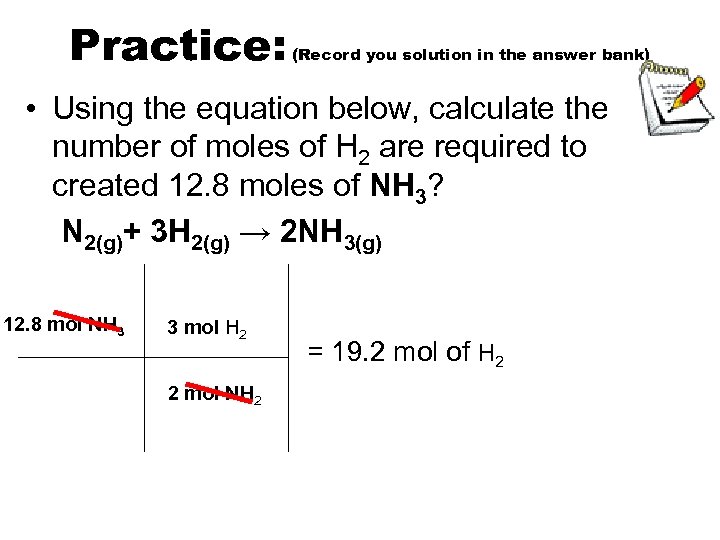 Practice: (Record you solution in the answer bank) • Using the equation below, calculate the number of moles of H 2 are required to created 12. 8 moles of NH 3? N 2(g)+ 3 H 2(g) → 2 NH 3(g) 12. 8 mol NH 3 3 mol H 2 2 mol NH 2 = 19. 2 mol of H 2
Practice: (Record you solution in the answer bank) • Using the equation below, calculate the number of moles of H 2 are required to created 12. 8 moles of NH 3? N 2(g)+ 3 H 2(g) → 2 NH 3(g) 12. 8 mol NH 3 3 mol H 2 2 mol NH 2 = 19. 2 mol of H 2
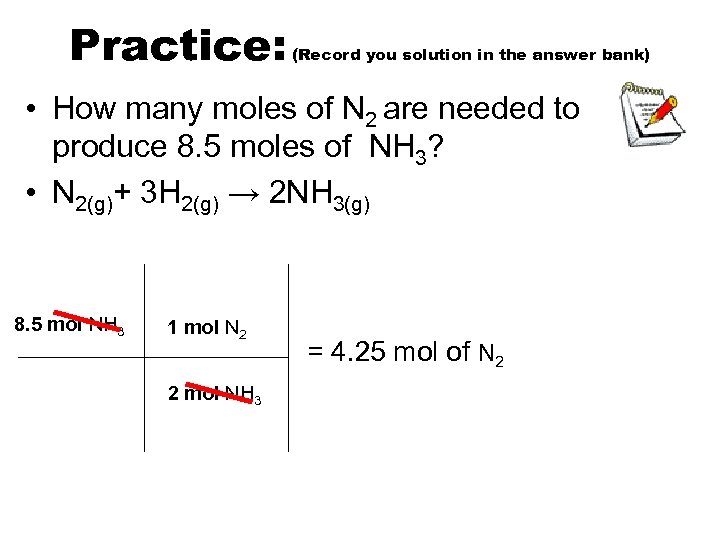 Practice: (Record you solution in the answer bank) • How many moles of N 2 are needed to produce 8. 5 moles of NH 3? • N 2(g)+ 3 H 2(g) → 2 NH 3(g) 8. 5 mol NH 3 1 mol N 2 2 mol NH 3 = 4. 25 mol of N 2
Practice: (Record you solution in the answer bank) • How many moles of N 2 are needed to produce 8. 5 moles of NH 3? • N 2(g)+ 3 H 2(g) → 2 NH 3(g) 8. 5 mol NH 3 1 mol N 2 2 mol NH 3 = 4. 25 mol of N 2
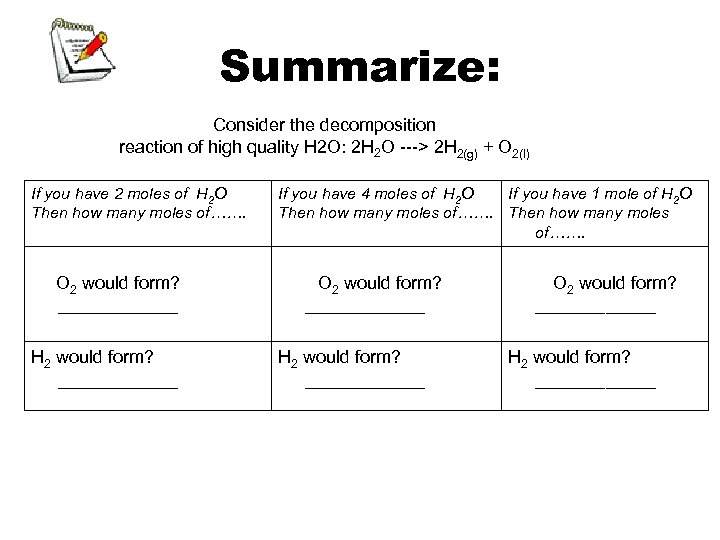 Summarize: Consider the decomposition reaction of high quality H 2 O: 2 H 2 O ---> 2 H 2(g) + O 2(l) If you have 2 moles of H 2 O Then how many moles of……. O 2 would form? ______ H 2 would form? ______ If you have 4 moles of H 2 O If you have 1 mole of H 2 O Then how many moles of……. O 2 would form? ____________ H 2 would form? ____________
Summarize: Consider the decomposition reaction of high quality H 2 O: 2 H 2 O ---> 2 H 2(g) + O 2(l) If you have 2 moles of H 2 O Then how many moles of……. O 2 would form? ______ H 2 would form? ______ If you have 4 moles of H 2 O If you have 1 mole of H 2 O Then how many moles of……. O 2 would form? ____________ H 2 would form? ____________


