 The MOLE An important unit in Chemistry
The MOLE An important unit in Chemistry
 Measuring Matter 12 roses is considered to be what? 2 gloves would be called a ______ of gloves? Do you buy paper individually? We need a way to count atoms/particles too!
Measuring Matter 12 roses is considered to be what? 2 gloves would be called a ______ of gloves? Do you buy paper individually? We need a way to count atoms/particles too!
 The mole The unit used for atoms/molecules that are SO small that numbers like pair, dozen, etc are not enough! Mole is the base unit for measuring the amount of a substance
The mole The unit used for atoms/molecules that are SO small that numbers like pair, dozen, etc are not enough! Mole is the base unit for measuring the amount of a substance
 Avogadro’s Number 6. 022 x 1023 is called Avogadro’s Number 1 mol of anything = 6. 022 x 1023 anythings Example: How many molecules are in 3. 50 mol of sucrose?
Avogadro’s Number 6. 022 x 1023 is called Avogadro’s Number 1 mol of anything = 6. 022 x 1023 anythings Example: How many molecules are in 3. 50 mol of sucrose?
 The Answer sucrose 3. 50 mol sucrose x 6. 022 x 1023 molecule 1 mol sucrose = 2. 11 x 1024 molecules sucrose
The Answer sucrose 3. 50 mol sucrose x 6. 022 x 1023 molecule 1 mol sucrose = 2. 11 x 1024 molecules sucrose
 Mass and the Mole Would a dozen limes have the same mass as a dozen eggs? NO! Therefore molecules/atoms do not have the same mass either!
Mass and the Mole Would a dozen limes have the same mass as a dozen eggs? NO! Therefore molecules/atoms do not have the same mass either!
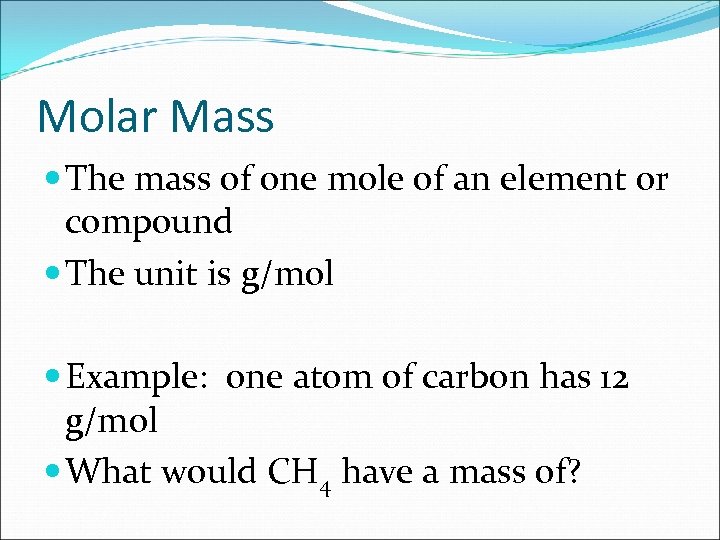 Molar Mass The mass of one mole of an element or compound The unit is g/mol Example: one atom of carbon has 12 g/mol What would CH 4 have a mass of?
Molar Mass The mass of one mole of an element or compound The unit is g/mol Example: one atom of carbon has 12 g/mol What would CH 4 have a mass of?
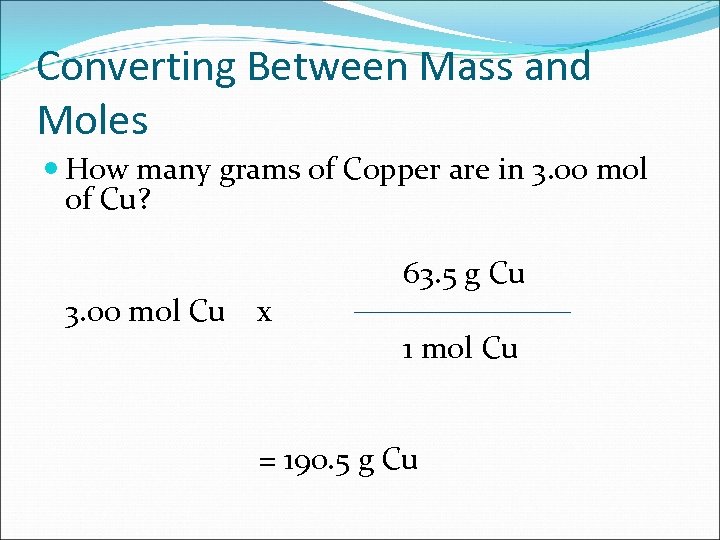 Converting Between Mass and Moles How many grams of Copper are in 3. 00 mol of Cu? 3. 00 mol Cu x 63. 5 g Cu 1 mol Cu = 190. 5 g Cu
Converting Between Mass and Moles How many grams of Copper are in 3. 00 mol of Cu? 3. 00 mol Cu x 63. 5 g Cu 1 mol Cu = 190. 5 g Cu
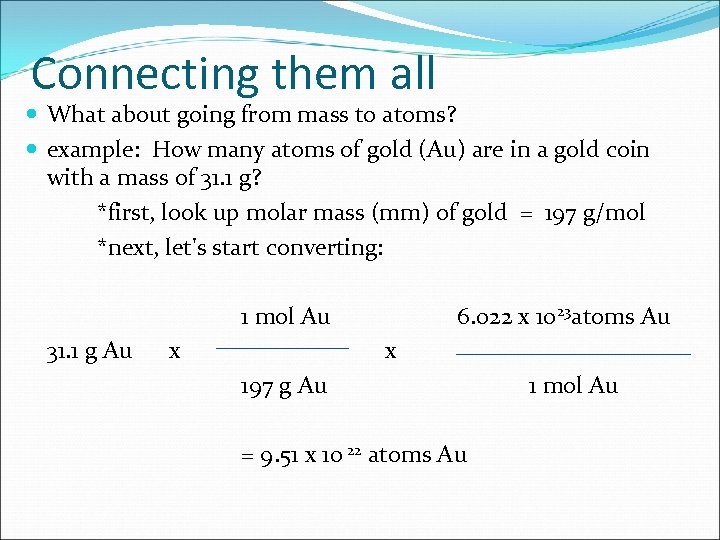 Connecting them all What about going from mass to atoms? example: How many atoms of gold (Au) are in a gold coin with a mass of 31. 1 g? *first, look up molar mass (mm) of gold = 197 g/mol *next, let's start converting: 1 mol Au 31. 1 g Au x 6. 022 x 1023 atoms Au x 197 g Au = 9. 51 x 10 22 atoms Au 1 mol Au
Connecting them all What about going from mass to atoms? example: How many atoms of gold (Au) are in a gold coin with a mass of 31. 1 g? *first, look up molar mass (mm) of gold = 197 g/mol *next, let's start converting: 1 mol Au 31. 1 g Au x 6. 022 x 1023 atoms Au x 197 g Au = 9. 51 x 10 22 atoms Au 1 mol Au