d0edfd974a2130f356c4e16433b40ad9.ppt
- Количество слайдов: 118
 The many Wonders of Fe. EDTA: Room Temperature Incineration of Pollutants, and The Detection of Peroxide Based Explosives Frank Cheng Associate Professor of Chemistry University of Idaho Moscow, ID 83844 -2343 Email: ifcheng@uidaho. edu Tel. : 208 -885 -6387 Fax: 208 -885 -6173 Homepage: http: //www. chem. uidaho. edu/faculty/ifcheng/ 11 September 2007 University of Idaho 1
The many Wonders of Fe. EDTA: Room Temperature Incineration of Pollutants, and The Detection of Peroxide Based Explosives Frank Cheng Associate Professor of Chemistry University of Idaho Moscow, ID 83844 -2343 Email: ifcheng@uidaho. edu Tel. : 208 -885 -6387 Fax: 208 -885 -6173 Homepage: http: //www. chem. uidaho. edu/faculty/ifcheng/ 11 September 2007 University of Idaho 1
![Outline n Background and History of EDTA n I] Room Temperature and Pressure Combustion Outline n Background and History of EDTA n I] Room Temperature and Pressure Combustion](https://present5.com/presentation/d0edfd974a2130f356c4e16433b40ad9/image-2.jpg) Outline n Background and History of EDTA n I] Room Temperature and Pressure Combustion of Organic Pollutants and Nerve Agent Surrogates – The Search for a Green Oxidant n II] A Detector for Peroxide-Based Explosives n III] A Model for the Role of Calcium and Zinc in Biological Oxidations 11 September 2007 University of Idaho 2
Outline n Background and History of EDTA n I] Room Temperature and Pressure Combustion of Organic Pollutants and Nerve Agent Surrogates – The Search for a Green Oxidant n II] A Detector for Peroxide-Based Explosives n III] A Model for the Role of Calcium and Zinc in Biological Oxidations 11 September 2007 University of Idaho 2
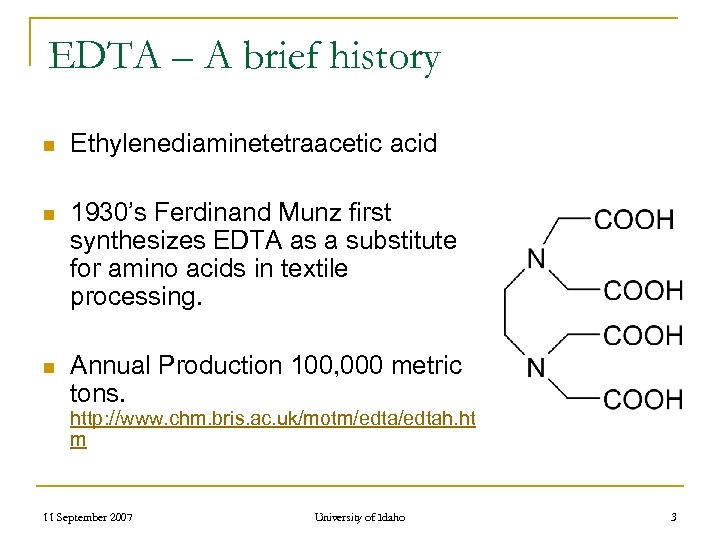 EDTA – A brief history n Ethylenediaminetetraacetic acid n 1930’s Ferdinand Munz first synthesizes EDTA as a substitute for amino acids in textile processing. n Annual Production 100, 000 metric tons. http: //www. chm. bris. ac. uk/motm/edtah. ht m 11 September 2007 University of Idaho 3
EDTA – A brief history n Ethylenediaminetetraacetic acid n 1930’s Ferdinand Munz first synthesizes EDTA as a substitute for amino acids in textile processing. n Annual Production 100, 000 metric tons. http: //www. chm. bris. ac. uk/motm/edtah. ht m 11 September 2007 University of Idaho 3
 EDTA – Modern Uses http: //en. wikipedia. org/wiki/EDTA n n n Industrial cleaning: complexation of Ca and Mg ions, binding of heavy metals. Detergents: complexation of Ca and Mg (reduction of water hardness). Pulp and paper industry: complexation of heavy metals during chlorine-free bleaching, stabilization of hydrogen peroxide. Textile industry: complexation of heavy metals, bleach stabilizer. Food: added as preservative to prevent catalytic oxidation by metal ions or stabilizer and for iron fortification. Personal care: added to cosmetics to work in synergy with preservatives and to improve product stability. 11 September 2007 University of Idaho 4
EDTA – Modern Uses http: //en. wikipedia. org/wiki/EDTA n n n Industrial cleaning: complexation of Ca and Mg ions, binding of heavy metals. Detergents: complexation of Ca and Mg (reduction of water hardness). Pulp and paper industry: complexation of heavy metals during chlorine-free bleaching, stabilization of hydrogen peroxide. Textile industry: complexation of heavy metals, bleach stabilizer. Food: added as preservative to prevent catalytic oxidation by metal ions or stabilizer and for iron fortification. Personal care: added to cosmetics to work in synergy with preservatives and to improve product stability. 11 September 2007 University of Idaho 4
 EDTA – Modern Uses -http: //en. wikipedia. org/wiki/EDTA n n n n Oil production: added into the borehole to inhibit mineral precipitation. Dairy and beverage industry: cleaning of bottles from milk stains. Flue gas cleaning: removal of NOx. Medicine: used in chelation therapy (brand name Endrate®, marketed by Hospira; generic product is also on the market) for acute hypercalcemia and mercury and has been used for lead poisoning. Added to many soft drinks containing ascorbic acid and sodium benzoate, to reduce the formation of benzene (a carcinogen). Can be used in the recovery of used lead acid batteries. Used in dentistry as a root canal irrigant to remove compounds of organic and inorganic debris (smearlayer). 11 September 2007 University of Idaho 5
EDTA – Modern Uses -http: //en. wikipedia. org/wiki/EDTA n n n n Oil production: added into the borehole to inhibit mineral precipitation. Dairy and beverage industry: cleaning of bottles from milk stains. Flue gas cleaning: removal of NOx. Medicine: used in chelation therapy (brand name Endrate®, marketed by Hospira; generic product is also on the market) for acute hypercalcemia and mercury and has been used for lead poisoning. Added to many soft drinks containing ascorbic acid and sodium benzoate, to reduce the formation of benzene (a carcinogen). Can be used in the recovery of used lead acid batteries. Used in dentistry as a root canal irrigant to remove compounds of organic and inorganic debris (smearlayer). 11 September 2007 University of Idaho 5
 Metal Complexes http: //en. wikipedia. org/wiki/EDTA n Agrochemicals: Fe, Zn and Cu fertilizer, especially in calcareous soils. n Photography: use of Fe(III)EDTA as oxidizing agent. n Scavenging metal ions: in biochemistry and molecular biology, ion depletion is commonly used to inactivate enzymes which could damage DNA or proteins 11 September 2007 University of Idaho 6
Metal Complexes http: //en. wikipedia. org/wiki/EDTA n Agrochemicals: Fe, Zn and Cu fertilizer, especially in calcareous soils. n Photography: use of Fe(III)EDTA as oxidizing agent. n Scavenging metal ions: in biochemistry and molecular biology, ion depletion is commonly used to inactivate enzymes which could damage DNA or proteins 11 September 2007 University of Idaho 6
 EDTA – Modern Uses http: //www. dow. com/productsafety/images/edtachart. jpg 11 September 2007 University of Idaho 7
EDTA – Modern Uses http: //www. dow. com/productsafety/images/edtachart. jpg 11 September 2007 University of Idaho 7
 Complexation of Fe(II/III) with EDTA n p. Ka 1 -6 = 0. 0, 1. 5, 2. 0, 2. 66, 6. 16, 10. 24 n Kf(Fe. II) = 1014. 32 n Kf(Fe. III) = 1025. 1 11 September 2007 University of Idaho 8
Complexation of Fe(II/III) with EDTA n p. Ka 1 -6 = 0. 0, 1. 5, 2. 0, 2. 66, 6. 16, 10. 24 n Kf(Fe. II) = 1014. 32 n Kf(Fe. III) = 1025. 1 11 September 2007 University of Idaho 8
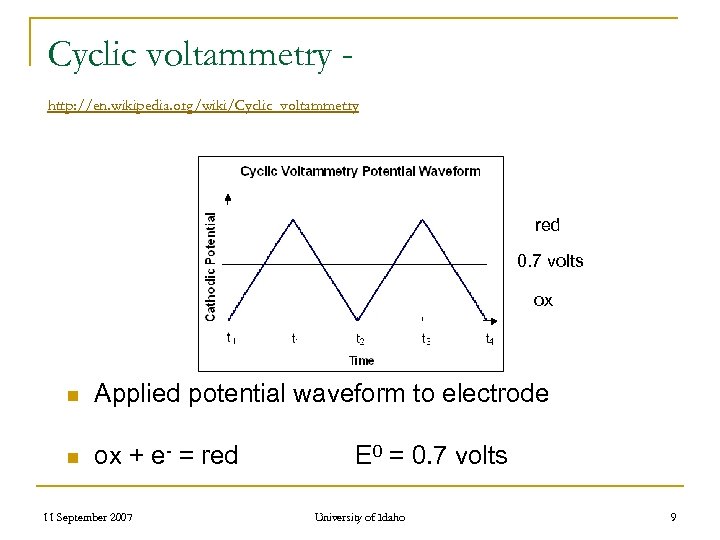 Cyclic voltammetry http: //en. wikipedia. org/wiki/Cyclic_voltammetry red 0. 7 volts ox n Applied potential waveform to electrode n ox + e- = red 11 September 2007 E 0 = 0. 7 volts University of Idaho 9
Cyclic voltammetry http: //en. wikipedia. org/wiki/Cyclic_voltammetry red 0. 7 volts ox n Applied potential waveform to electrode n ox + e- = red 11 September 2007 E 0 = 0. 7 volts University of Idaho 9
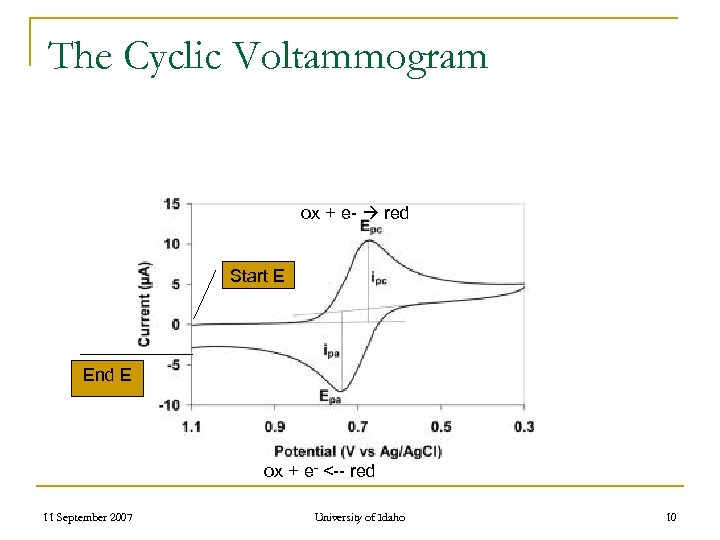 The Cyclic Voltammogram ox + e- red Start E End E ox + e- <-- red 11 September 2007 University of Idaho 10
The Cyclic Voltammogram ox + e- red Start E End E ox + e- <-- red 11 September 2007 University of Idaho 10
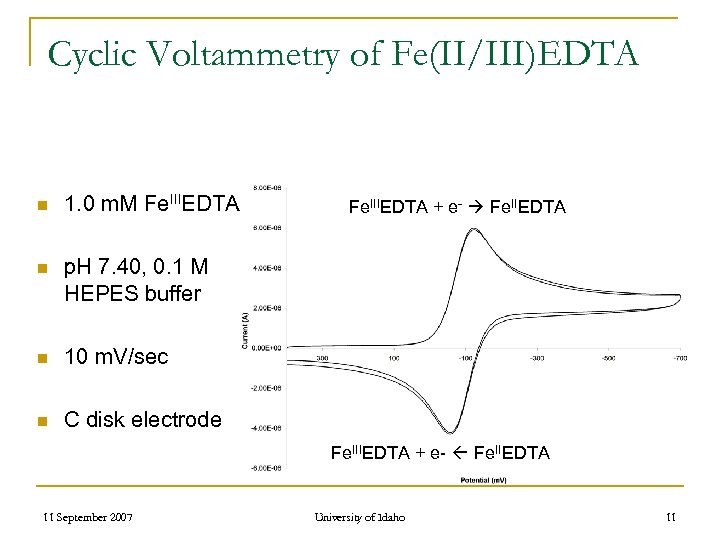 Cyclic Voltammetry of Fe(II/III)EDTA n 1. 0 m. M Fe. IIIEDTA n p. H 7. 40, 0. 1 M HEPES buffer n 10 m. V/sec n C disk electrode Fe. IIIEDTA + e- Fe. IIEDTA 11 September 2007 University of Idaho 11
Cyclic Voltammetry of Fe(II/III)EDTA n 1. 0 m. M Fe. IIIEDTA n p. H 7. 40, 0. 1 M HEPES buffer n 10 m. V/sec n C disk electrode Fe. IIIEDTA + e- Fe. IIEDTA 11 September 2007 University of Idaho 11
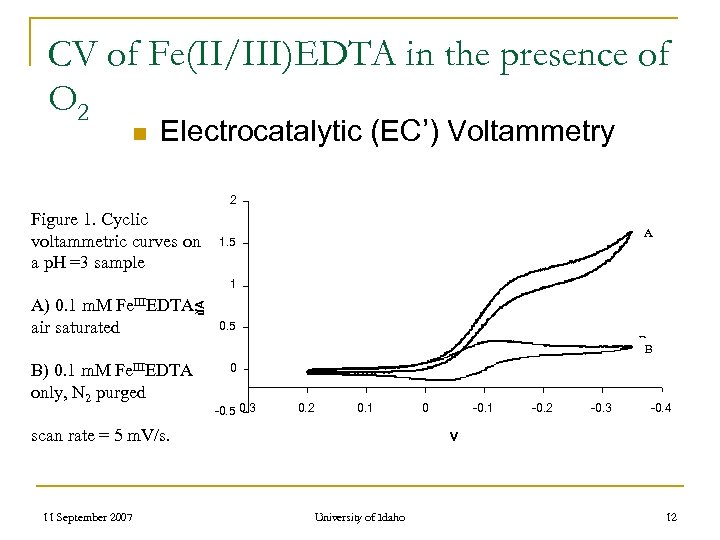 CV of Fe(II/III)EDTA in the presence of O 2 n Electrocatalytic (EC’) Voltammetry 2 Figure 1. Cyclic voltammetric curves on a p. H =3 sample A 1. 5 1 u. A A) 0. 1 m. M Fe. IIIEDTA, air saturated B) 0. 1 m. M Fe. IIIEDTA only, N 2 purged 0. 5 B B 0 -0. 5 0. 3 0. 2 0. 1 scan rate = 5 m. V/s. 11 September 2007 0 -0. 1 -0. 2 -0. 3 -0. 4 V University of Idaho 12
CV of Fe(II/III)EDTA in the presence of O 2 n Electrocatalytic (EC’) Voltammetry 2 Figure 1. Cyclic voltammetric curves on a p. H =3 sample A 1. 5 1 u. A A) 0. 1 m. M Fe. IIIEDTA, air saturated B) 0. 1 m. M Fe. IIIEDTA only, N 2 purged 0. 5 B B 0 -0. 5 0. 3 0. 2 0. 1 scan rate = 5 m. V/s. 11 September 2007 0 -0. 1 -0. 2 -0. 3 -0. 4 V University of Idaho 12
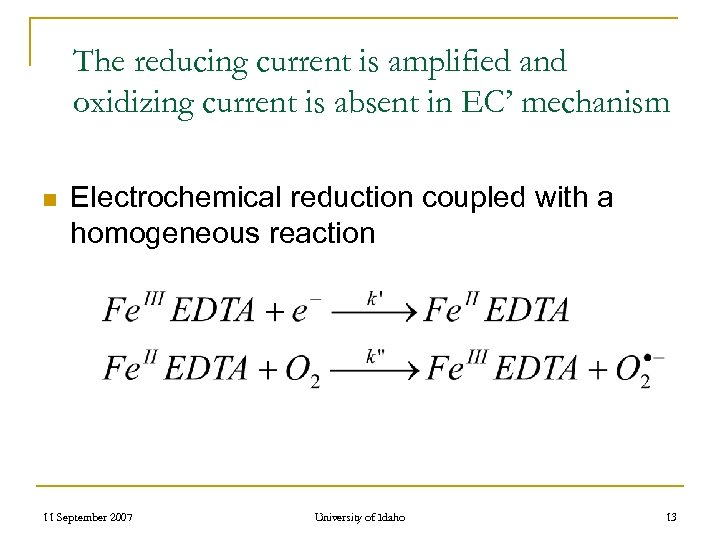 The reducing current is amplified and oxidizing current is absent in EC’ mechanism n Electrochemical reduction coupled with a homogeneous reaction 11 September 2007 University of Idaho 13
The reducing current is amplified and oxidizing current is absent in EC’ mechanism n Electrochemical reduction coupled with a homogeneous reaction 11 September 2007 University of Idaho 13
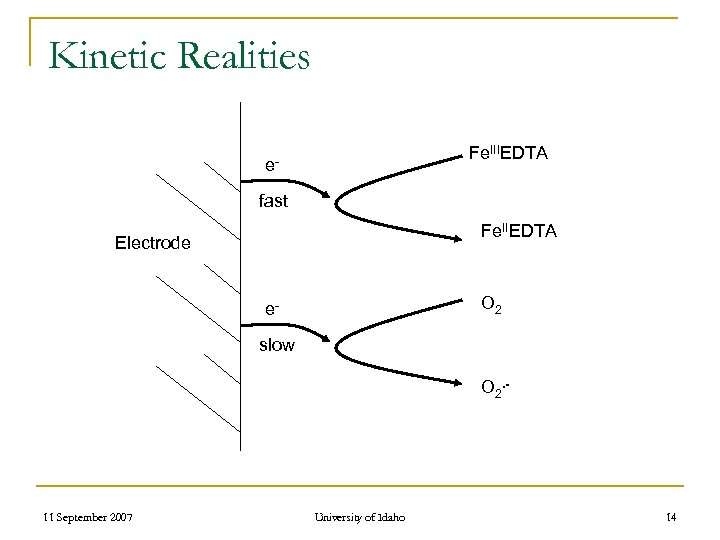 Kinetic Realities Fe. IIIEDTA efast Fe. IIEDTA Electrode O 2 eslow O 2. - 11 September 2007 University of Idaho 14
Kinetic Realities Fe. IIIEDTA efast Fe. IIEDTA Electrode O 2 eslow O 2. - 11 September 2007 University of Idaho 14
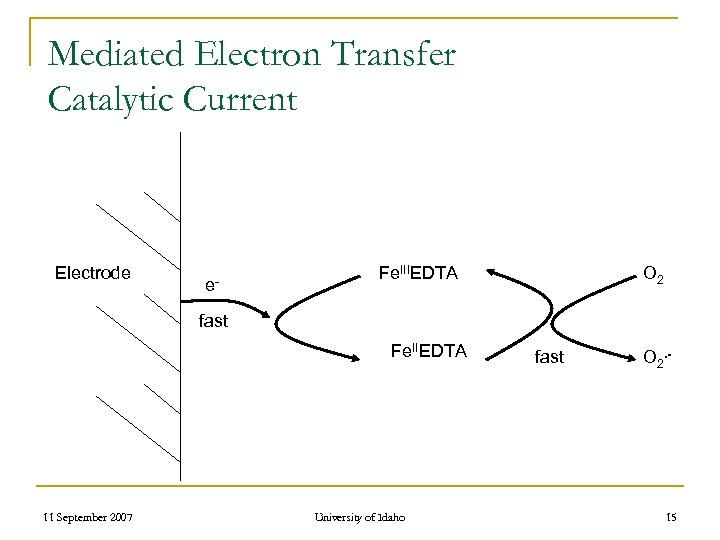 Mediated Electron Transfer Catalytic Current Electrode e- Fe. IIIEDTA O 2 fast Fe. IIEDTA 11 September 2007 University of Idaho fast O 2. - 15
Mediated Electron Transfer Catalytic Current Electrode e- Fe. IIIEDTA O 2 fast Fe. IIEDTA 11 September 2007 University of Idaho fast O 2. - 15
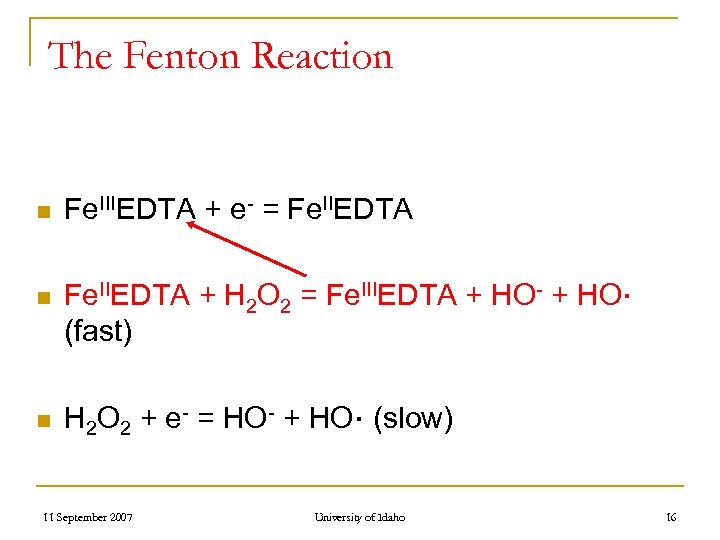 The Fenton Reaction n Fe. IIIEDTA + e- = Fe. IIEDTA n Fe. IIEDTA + H 2 O 2 = Fe. IIIEDTA + HO- + HO∙ (fast) n H 2 O 2 + e- = HO- + HO∙ (slow) 11 September 2007 University of Idaho 16
The Fenton Reaction n Fe. IIIEDTA + e- = Fe. IIEDTA n Fe. IIEDTA + H 2 O 2 = Fe. IIIEDTA + HO- + HO∙ (fast) n H 2 O 2 + e- = HO- + HO∙ (slow) 11 September 2007 University of Idaho 16
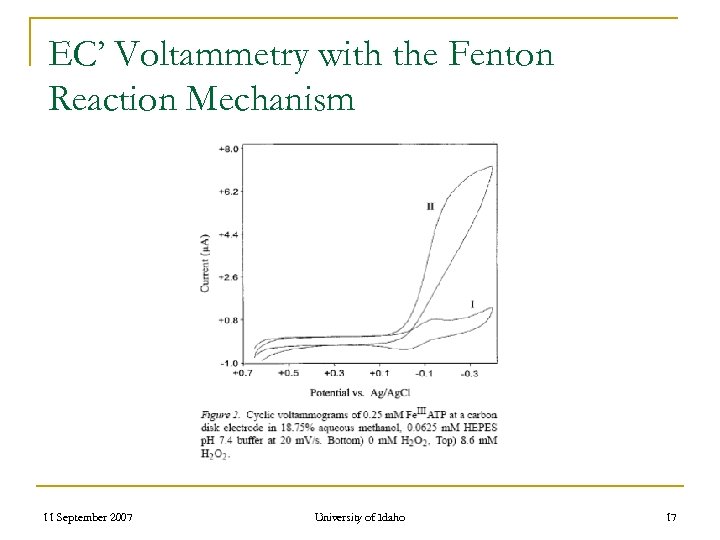 EC’ Voltammetry with the Fenton Reaction Mechanism 11 September 2007 University of Idaho 17
EC’ Voltammetry with the Fenton Reaction Mechanism 11 September 2007 University of Idaho 17
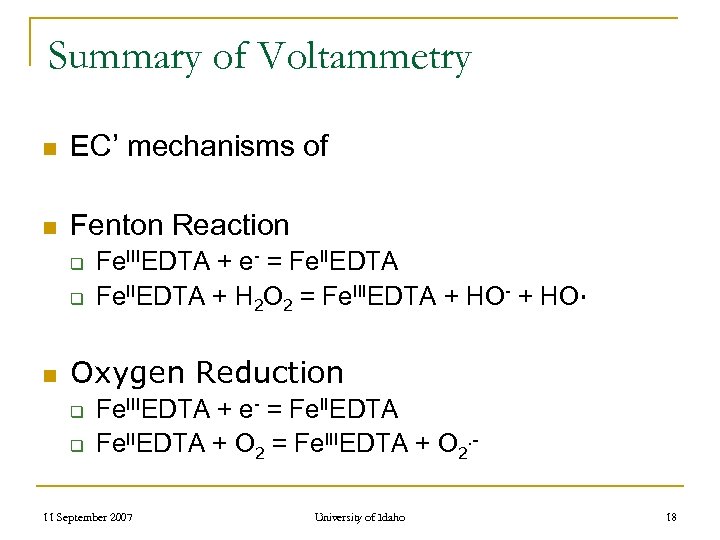 Summary of Voltammetry n EC’ mechanisms of n Fenton Reaction q q n Fe. IIIEDTA + e- = Fe. IIEDTA + H 2 O 2 = Fe. IIIEDTA + HO- + HO∙ Oxygen Reduction q q Fe. IIIEDTA + e- = Fe. IIEDTA + O 2 = Fe. IIIEDTA + O 2. - 11 September 2007 University of Idaho 18
Summary of Voltammetry n EC’ mechanisms of n Fenton Reaction q q n Fe. IIIEDTA + e- = Fe. IIEDTA + H 2 O 2 = Fe. IIIEDTA + HO- + HO∙ Oxygen Reduction q q Fe. IIIEDTA + e- = Fe. IIEDTA + O 2 = Fe. IIIEDTA + O 2. - 11 September 2007 University of Idaho 18
![Outline of Investigation n I] Room Temperature and Pressure Combustion of Organic Pollutants and Outline of Investigation n I] Room Temperature and Pressure Combustion of Organic Pollutants and](https://present5.com/presentation/d0edfd974a2130f356c4e16433b40ad9/image-19.jpg) Outline of Investigation n I] Room Temperature and Pressure Combustion of Organic Pollutants and Nerve Agent Surrogates – The Search for a Green Oxidant n II] A Detector for Peroxide-Based Explosives n III] A Model for the Role of Calcium and Zinc in Biological Oxidations 11 September 2007 University of Idaho 19
Outline of Investigation n I] Room Temperature and Pressure Combustion of Organic Pollutants and Nerve Agent Surrogates – The Search for a Green Oxidant n II] A Detector for Peroxide-Based Explosives n III] A Model for the Role of Calcium and Zinc in Biological Oxidations 11 September 2007 University of Idaho 19
 The search for a “green” oxidant n Problems with chlorine based bleaching methods. n Prefer low temperature and pressures, energy savings n Oxygen is the ultimate green oxidant. n Oxygen is kinetically stable 11 September 2007 University of Idaho 20
The search for a “green” oxidant n Problems with chlorine based bleaching methods. n Prefer low temperature and pressures, energy savings n Oxygen is the ultimate green oxidant. n Oxygen is kinetically stable 11 September 2007 University of Idaho 20
 Overall Goals of Our Green Oxidation Program § The destruction or neutralization of xenobiotics, including nerve agents and chlorinated pesticides using green oxidation chemistry. § Focus on non-biological oxygen activation to eliminate the need for tricky enzyme based systems 11 September 2007 University of Idaho 21
Overall Goals of Our Green Oxidation Program § The destruction or neutralization of xenobiotics, including nerve agents and chlorinated pesticides using green oxidation chemistry. § Focus on non-biological oxygen activation to eliminate the need for tricky enzyme based systems 11 September 2007 University of Idaho 21
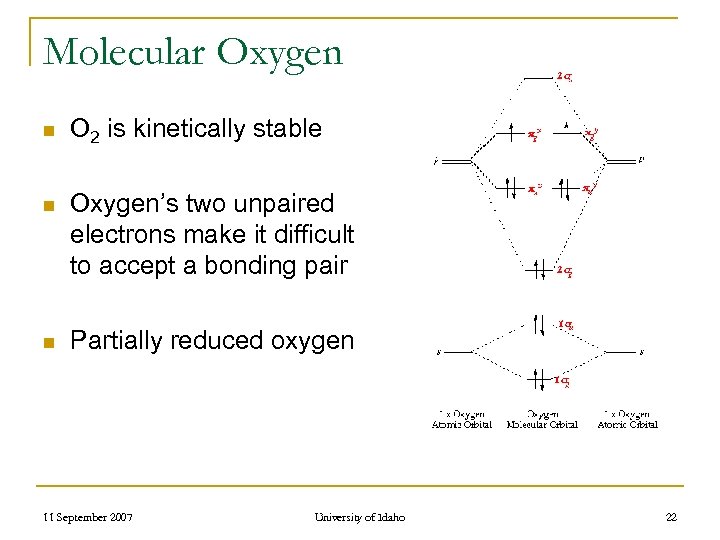 Molecular Oxygen n O 2 is kinetically stable n Oxygen’s two unpaired electrons make it difficult to accept a bonding pair n Partially reduced oxygen 11 September 2007 University of Idaho 22
Molecular Oxygen n O 2 is kinetically stable n Oxygen’s two unpaired electrons make it difficult to accept a bonding pair n Partially reduced oxygen 11 September 2007 University of Idaho 22
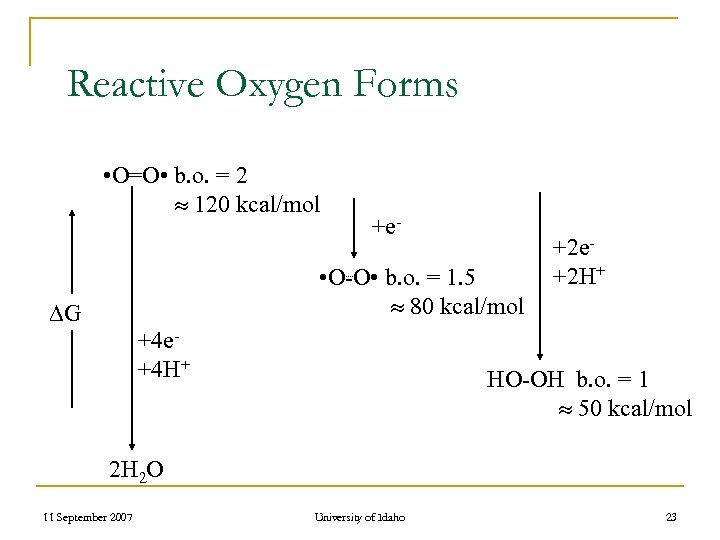 Reactive Oxygen Forms • O=O • b. o. = 2 120 kcal/mol +e- • O-O • b. o. = 1. 5 80 kcal/mol G +4 e+4 H+ +2 e+2 H+ HO-OH b. o. = 1 50 kcal/mol 2 H 2 O 11 September 2007 University of Idaho 23
Reactive Oxygen Forms • O=O • b. o. = 2 120 kcal/mol +e- • O-O • b. o. = 1. 5 80 kcal/mol G +4 e+4 H+ +2 e+2 H+ HO-OH b. o. = 1 50 kcal/mol 2 H 2 O 11 September 2007 University of Idaho 23
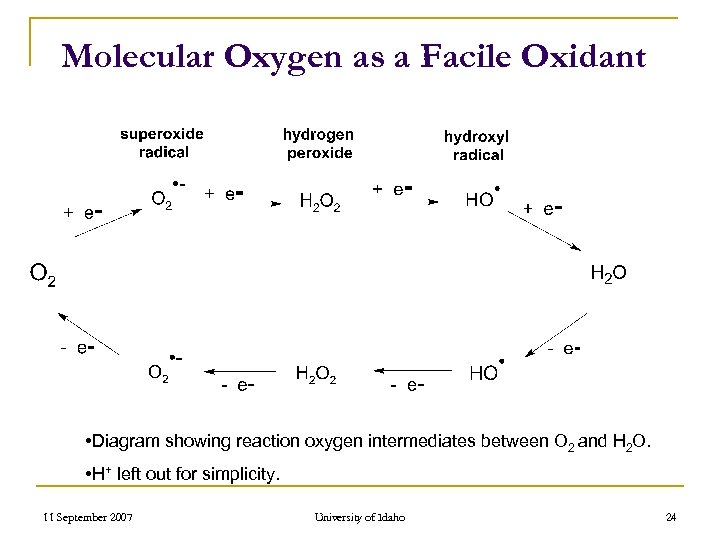 Molecular Oxygen as a Facile Oxidant • Diagram showing reaction oxygen intermediates between O 2 and H 2 O. • H+ left out for simplicity. 11 September 2007 University of Idaho 24
Molecular Oxygen as a Facile Oxidant • Diagram showing reaction oxygen intermediates between O 2 and H 2 O. • H+ left out for simplicity. 11 September 2007 University of Idaho 24
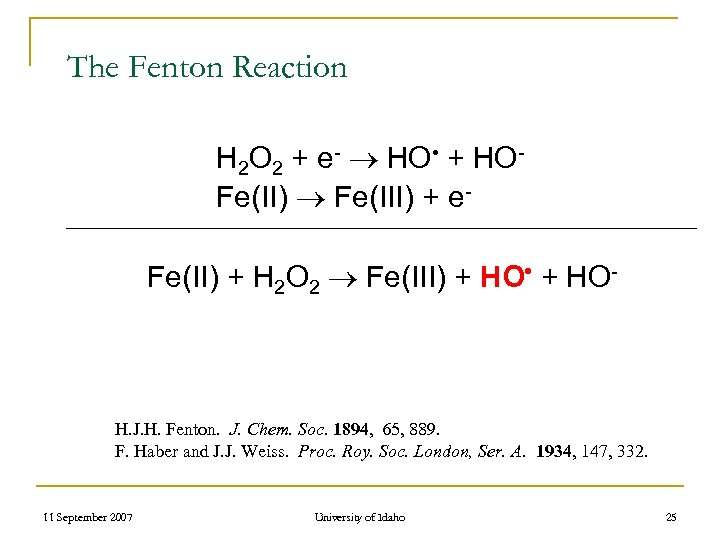 The Fenton Reaction H 2 O 2 + e- HO • + HO- Fe(II) Fe(III) + e- Fe(II) + H 2 O 2 Fe(III) + HO • + HO- H. J. H. Fenton. J. Chem. Soc. 1894, 65, 889. F. Haber and J. J. Weiss. Proc. Roy. Soc. London, Ser. A. 1934, 147, 332. 11 September 2007 University of Idaho 25
The Fenton Reaction H 2 O 2 + e- HO • + HO- Fe(II) Fe(III) + e- Fe(II) + H 2 O 2 Fe(III) + HO • + HO- H. J. H. Fenton. J. Chem. Soc. 1894, 65, 889. F. Haber and J. J. Weiss. Proc. Roy. Soc. London, Ser. A. 1934, 147, 332. 11 September 2007 University of Idaho 25
 Oxygen Activation n Biological q cytochrome P 450 enzymes, monooxygenase 11 September 2007 University of Idaho 26
Oxygen Activation n Biological q cytochrome P 450 enzymes, monooxygenase 11 September 2007 University of Idaho 26
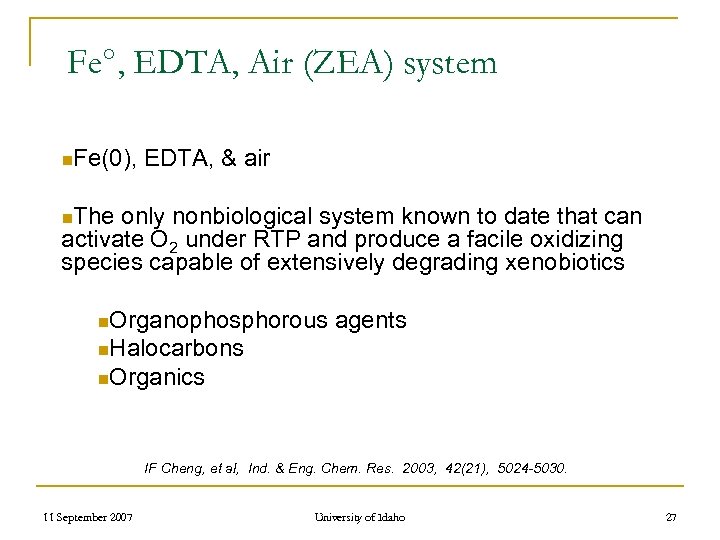 Fe°, EDTA, Air (ZEA) system n. Fe(0), EDTA, & air n. The only nonbiological system known to date that can activate O 2 under RTP and produce a facile oxidizing species capable of extensively degrading xenobiotics n. Organophosphorous agents n. Halocarbons n. Organics IF Cheng, et al, Ind. & Eng. Chem. Res. 2003, 42(21), 5024 -5030. 11 September 2007 University of Idaho 27
Fe°, EDTA, Air (ZEA) system n. Fe(0), EDTA, & air n. The only nonbiological system known to date that can activate O 2 under RTP and produce a facile oxidizing species capable of extensively degrading xenobiotics n. Organophosphorous agents n. Halocarbons n. Organics IF Cheng, et al, Ind. & Eng. Chem. Res. 2003, 42(21), 5024 -5030. 11 September 2007 University of Idaho 27
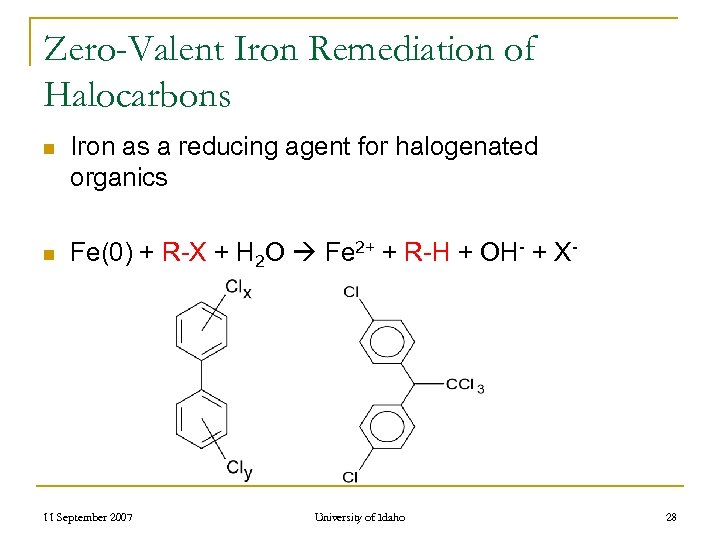 Zero-Valent Iron Remediation of Halocarbons n Iron as a reducing agent for halogenated organics n Fe(0) + R-X + H 2 O Fe 2+ + R-H + OH- + X- 11 September 2007 University of Idaho 28
Zero-Valent Iron Remediation of Halocarbons n Iron as a reducing agent for halogenated organics n Fe(0) + R-X + H 2 O Fe 2+ + R-H + OH- + X- 11 September 2007 University of Idaho 28
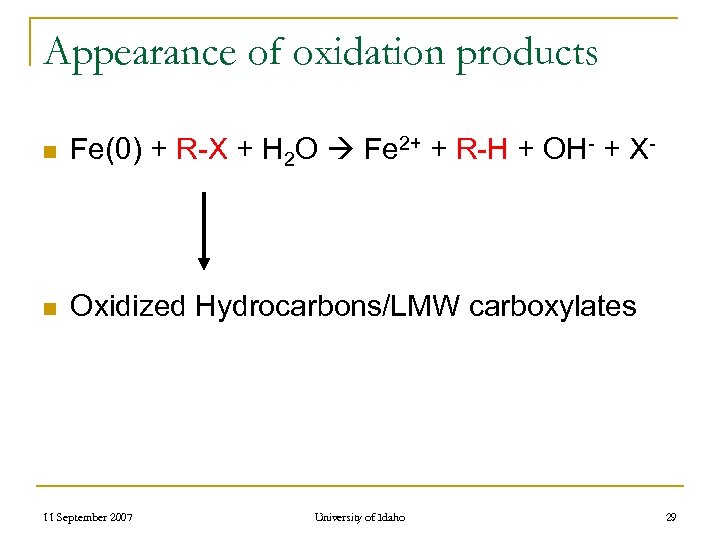 Appearance of oxidation products n Fe(0) + R-X + H 2 O Fe 2+ + R-H + OH- + X- n Oxidized Hydrocarbons/LMW carboxylates 11 September 2007 University of Idaho 29
Appearance of oxidation products n Fe(0) + R-X + H 2 O Fe 2+ + R-H + OH- + X- n Oxidized Hydrocarbons/LMW carboxylates 11 September 2007 University of Idaho 29
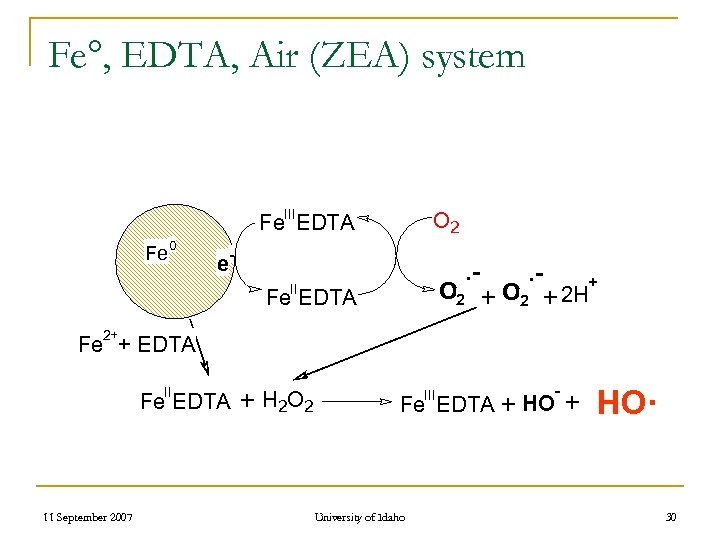 Fe°, EDTA, Air (ZEA) system III O 2 Fe EDTA Fe 0 - e . - O 2 + 2 H+ II Fe EDTA 2+ Fe + EDTA II Fe EDTA 11 September 2007 + H 2 O 2 III - Fe EDTA + HO University of Idaho + HO· 30
Fe°, EDTA, Air (ZEA) system III O 2 Fe EDTA Fe 0 - e . - O 2 + 2 H+ II Fe EDTA 2+ Fe + EDTA II Fe EDTA 11 September 2007 + H 2 O 2 III - Fe EDTA + HO University of Idaho + HO· 30
 Outline of ZEA Research n Introduction q q n General Reaction Scheme q n 11 September 2007 EDTA q Chlorinated phenols q Organophosphorus and UXO compounds Mechanisms q n Zero-valent iron/EDTA/air (ZEA) system Degradation Kinetics and Reaction Products q n Environmental Impact of EDTA The RTP Dioxygen Activation Rate-limiting Step Conclusions University of Idaho 31
Outline of ZEA Research n Introduction q q n General Reaction Scheme q n 11 September 2007 EDTA q Chlorinated phenols q Organophosphorus and UXO compounds Mechanisms q n Zero-valent iron/EDTA/air (ZEA) system Degradation Kinetics and Reaction Products q n Environmental Impact of EDTA The RTP Dioxygen Activation Rate-limiting Step Conclusions University of Idaho 31
 Concerns about EDTA q q q Many industrial chelating agents are not degradable by methods currently found in wastewater treatment facilities Not readily biodegradable Considerable quantities of EDTA pass through wastewater treatment facilities in the form of Fe. IIIEDTA, as high as 18µM. Sillanpaa, Mika; Orma, Marjatt; Ramo, Jaakko; Oikair; “The importance of ligand speciation in environmental research: a case study”; The Science of the Total Environment; 2001; 267, 2331. Sillanpaa, M; Pirkanniemi, K. ; “Recent Developments in Chelate Degradation”; Environmental Technology, 2001, 22, 791. Kari, F. G. ; Giger W; Modeling the Photochemical Degradation of Ethylenediaminetetraacetate in the River Glatt; Environmental Science and Technology; 1995, 29, 2814. Nirel, P. et. al. ; Method for EDTA Speciation Deteremination: Application to Sewage Treatment Plant Effluents; Wat. Res; 1998; 32, 3615. Kari, F. G. and Giger W. ; Speciation and fate of EDTA in municipal wasterwater treatment. Wat. Res. , 1996, 30, 122 -134. 11 September 2007 University of Idaho 32
Concerns about EDTA q q q Many industrial chelating agents are not degradable by methods currently found in wastewater treatment facilities Not readily biodegradable Considerable quantities of EDTA pass through wastewater treatment facilities in the form of Fe. IIIEDTA, as high as 18µM. Sillanpaa, Mika; Orma, Marjatt; Ramo, Jaakko; Oikair; “The importance of ligand speciation in environmental research: a case study”; The Science of the Total Environment; 2001; 267, 2331. Sillanpaa, M; Pirkanniemi, K. ; “Recent Developments in Chelate Degradation”; Environmental Technology, 2001, 22, 791. Kari, F. G. ; Giger W; Modeling the Photochemical Degradation of Ethylenediaminetetraacetate in the River Glatt; Environmental Science and Technology; 1995, 29, 2814. Nirel, P. et. al. ; Method for EDTA Speciation Deteremination: Application to Sewage Treatment Plant Effluents; Wat. Res; 1998; 32, 3615. Kari, F. G. and Giger W. ; Speciation and fate of EDTA in municipal wasterwater treatment. Wat. Res. , 1996, 30, 122 -134. 11 September 2007 University of Idaho 32
 Concerns about EDTA n Questions regarding the ability to mobilize metals in the environment. q q q n Currently not being monitored or treated at waste water treatment facilities Concern for heavy metal mobility and longer bioavailability of metals to aquatic plants and animals Stable in aquatic environment EDTA is anthropogenic and long-lived. 11 September 2007 University of Idaho 33
Concerns about EDTA n Questions regarding the ability to mobilize metals in the environment. q q q n Currently not being monitored or treated at waste water treatment facilities Concern for heavy metal mobility and longer bioavailability of metals to aquatic plants and animals Stable in aquatic environment EDTA is anthropogenic and long-lived. 11 September 2007 University of Idaho 33
 Goals • The destruction or neutralization of EDTA (xenobiotics) • Search for in situ conditions that will aid in the reduction in the release of EDTA in emerging green chemistries. Inexpensive & Safe Processes. • Room Temperature and Pressure Conditions (RTP) • Common Reagents – Long Term Storage • No Specialized Catalysts • System that may be incorporated into existing water treatment systems 11 September 2007 University of Idaho 34
Goals • The destruction or neutralization of EDTA (xenobiotics) • Search for in situ conditions that will aid in the reduction in the release of EDTA in emerging green chemistries. Inexpensive & Safe Processes. • Room Temperature and Pressure Conditions (RTP) • Common Reagents – Long Term Storage • No Specialized Catalysts • System that may be incorporated into existing water treatment systems 11 September 2007 University of Idaho 34
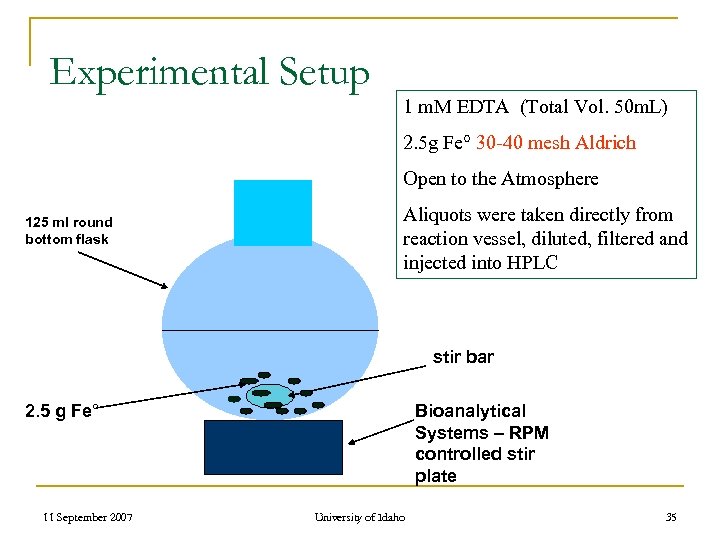 Experimental Setup 1 m. M EDTA (Total Vol. 50 m. L) 2. 5 g Fe° 30 -40 mesh Aldrich Open to the Atmosphere 125 ml round bottom flask Aliquots were taken directly from reaction vessel, diluted, filtered and injected into HPLC stir bar 2. 5 g Fe° 11 September 2007 Bioanalytical Systems – RPM controlled stir plate University of Idaho 35
Experimental Setup 1 m. M EDTA (Total Vol. 50 m. L) 2. 5 g Fe° 30 -40 mesh Aldrich Open to the Atmosphere 125 ml round bottom flask Aliquots were taken directly from reaction vessel, diluted, filtered and injected into HPLC stir bar 2. 5 g Fe° 11 September 2007 Bioanalytical Systems – RPM controlled stir plate University of Idaho 35
![Visual Detection of H 2 O 2 Produced by ZEA System n [HCl] = Visual Detection of H 2 O 2 Produced by ZEA System n [HCl] =](https://present5.com/presentation/d0edfd974a2130f356c4e16433b40ad9/image-36.jpg) Visual Detection of H 2 O 2 Produced by ZEA System n [HCl] = 0. 04 M [ammonium molybdate] = 0. 08 m. M [KI] = 80 m. M [EDTA] = 0 or 1. 2 m. M Agar (Starch) n Proposed n n 2 Fe. IIEDTA + O 2 + 2 H+ 2 Fe. IIIEDTA + H 2 O 2 + 3 I- +2 H+ I 3 - + 2 H 2 O I 3 - + starch blue-violet complex 11 September 2007 University of Idaho 36
Visual Detection of H 2 O 2 Produced by ZEA System n [HCl] = 0. 04 M [ammonium molybdate] = 0. 08 m. M [KI] = 80 m. M [EDTA] = 0 or 1. 2 m. M Agar (Starch) n Proposed n n 2 Fe. IIEDTA + O 2 + 2 H+ 2 Fe. IIIEDTA + H 2 O 2 + 3 I- +2 H+ I 3 - + 2 H 2 O I 3 - + starch blue-violet complex 11 September 2007 University of Idaho 36
 Iron wire: EDTA Absent & starch reagents 11 September 2007 University of Idaho 37
Iron wire: EDTA Absent & starch reagents 11 September 2007 University of Idaho 37
 Iron wire: EDTA Present 11 September 2007 University of Idaho 38
Iron wire: EDTA Present 11 September 2007 University of Idaho 38
 EDTA required for the ZEA reaction Qualitative Results Fe(0) + O 2 + 2 H+ Fe 2+ + H 2 O 2 Slow 2 Fe. IIEDTA + O 2 + 2 H+ 2 Fe. IIIEDTA + H 2 O 2 Fast 11 September 2007 University of Idaho 39
EDTA required for the ZEA reaction Qualitative Results Fe(0) + O 2 + 2 H+ Fe 2+ + H 2 O 2 Slow 2 Fe. IIEDTA + O 2 + 2 H+ 2 Fe. IIIEDTA + H 2 O 2 Fast 11 September 2007 University of Idaho 39
 Evidence for Production of Reactive Oxygen Species n ROS q n O 2 - • , H 2 O 2, HO. , Fe. IV=O, etc. Two Analyses were performed q q Thiobarbituric acid-reactive substances (TBARS) assay Addition of known radical scavenger, 1 -butanol 11 September 2007 University of Idaho 40
Evidence for Production of Reactive Oxygen Species n ROS q n O 2 - • , H 2 O 2, HO. , Fe. IV=O, etc. Two Analyses were performed q q Thiobarbituric acid-reactive substances (TBARS) assay Addition of known radical scavenger, 1 -butanol 11 September 2007 University of Idaho 40
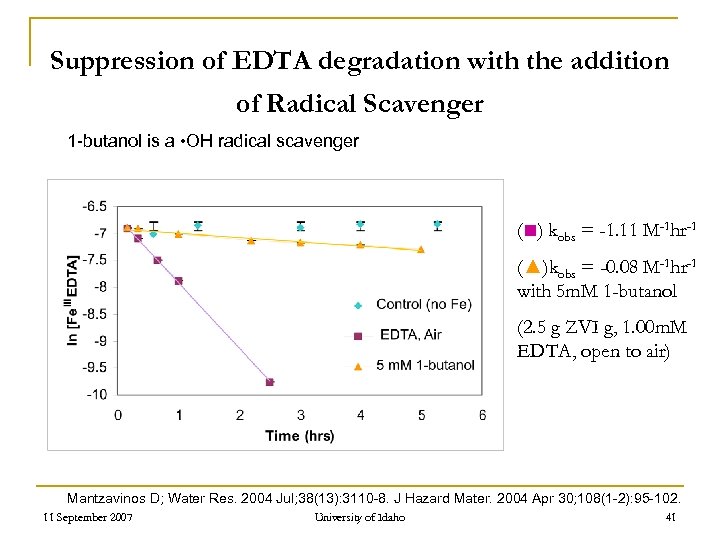 Suppression of EDTA degradation with the addition of Radical Scavenger 1 -butanol is a • OH radical scavenger (■) kobs = -1. 11 M-1 hr-1 (▲)kobs = -0. 08 M-1 hr-1 with 5 m. M 1 -butanol (2. 5 g ZVI g, 1. 00 m. M EDTA, open to air) Mantzavinos D; Water Res. 2004 Jul; 38(13): 3110 -8. J Hazard Mater. 2004 Apr 30; 108(1 -2): 95 -102. 11 September 2007 University of Idaho 41
Suppression of EDTA degradation with the addition of Radical Scavenger 1 -butanol is a • OH radical scavenger (■) kobs = -1. 11 M-1 hr-1 (▲)kobs = -0. 08 M-1 hr-1 with 5 m. M 1 -butanol (2. 5 g ZVI g, 1. 00 m. M EDTA, open to air) Mantzavinos D; Water Res. 2004 Jul; 38(13): 3110 -8. J Hazard Mater. 2004 Apr 30; 108(1 -2): 95 -102. 11 September 2007 University of Idaho 41
 Summary of ZEA system and O 2 n n n Both TBARS and butanol tests indicate that ZEA system is able to produce facile oxidant from air at RTP Rare form of abiotic O 2 activation at RTP Identity of oxidant isn’t clear q q HO· Fe. IV=O 11 September 2007 University of Idaho 42
Summary of ZEA system and O 2 n n n Both TBARS and butanol tests indicate that ZEA system is able to produce facile oxidant from air at RTP Rare form of abiotic O 2 activation at RTP Identity of oxidant isn’t clear q q HO· Fe. IV=O 11 September 2007 University of Idaho 42
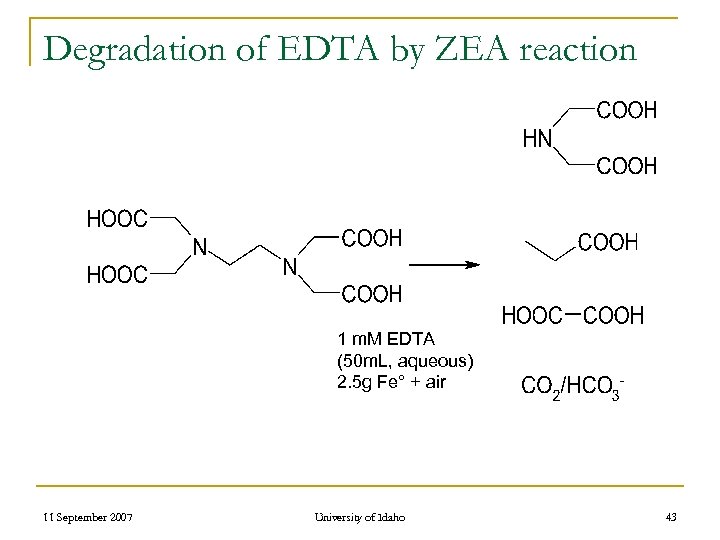 Degradation of EDTA by ZEA reaction 1 m. M EDTA (50 m. L, aqueous) 2. 5 g Fe° + air 11 September 2007 University of Idaho 43
Degradation of EDTA by ZEA reaction 1 m. M EDTA (50 m. L, aqueous) 2. 5 g Fe° + air 11 September 2007 University of Idaho 43
 Carbon Balance - Total Organic Carbon*, ESI-MS**, HPLC# Trapping of the volatile gases using Tenax® showed no volatile organic carbon of molecular weight C 4 and above released from the system during the course of the reaction 11 September 2007 University of Idaho 44
Carbon Balance - Total Organic Carbon*, ESI-MS**, HPLC# Trapping of the volatile gases using Tenax® showed no volatile organic carbon of molecular weight C 4 and above released from the system during the course of the reaction 11 September 2007 University of Idaho 44
 Products of ZEA System n None of the products of EDTA are significant metal chelation agents. n All are more easily biodegraded. n The ZEA system has proven successful at the degradation of other organic xenobiotics. n n n 11 September 2007 Halocarbons Organophosphorus Organics University of Idaho 45
Products of ZEA System n None of the products of EDTA are significant metal chelation agents. n All are more easily biodegraded. n The ZEA system has proven successful at the degradation of other organic xenobiotics. n n n 11 September 2007 Halocarbons Organophosphorus Organics University of Idaho 45
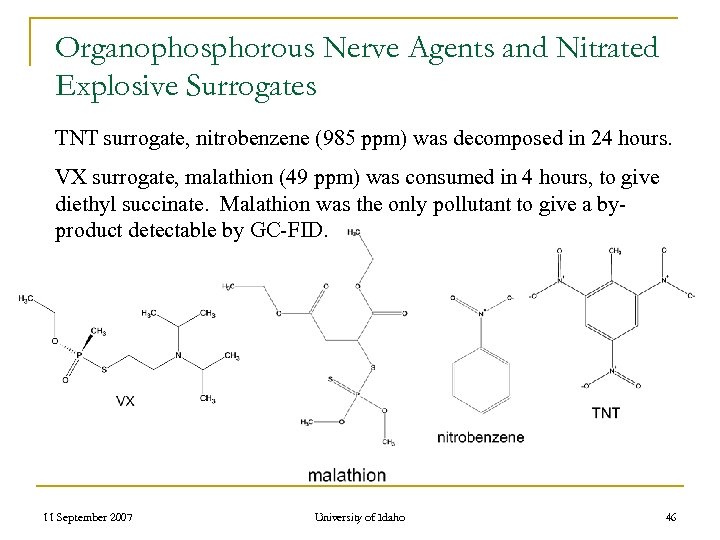 Organophosphorous Nerve Agents and Nitrated Explosive Surrogates TNT surrogate, nitrobenzene (985 ppm) was decomposed in 24 hours. VX surrogate, malathion (49 ppm) was consumed in 4 hours, to give diethyl succinate. Malathion was the only pollutant to give a byproduct detectable by GC-FID. 11 September 2007 University of Idaho 46
Organophosphorous Nerve Agents and Nitrated Explosive Surrogates TNT surrogate, nitrobenzene (985 ppm) was decomposed in 24 hours. VX surrogate, malathion (49 ppm) was consumed in 4 hours, to give diethyl succinate. Malathion was the only pollutant to give a byproduct detectable by GC-FID. 11 September 2007 University of Idaho 46
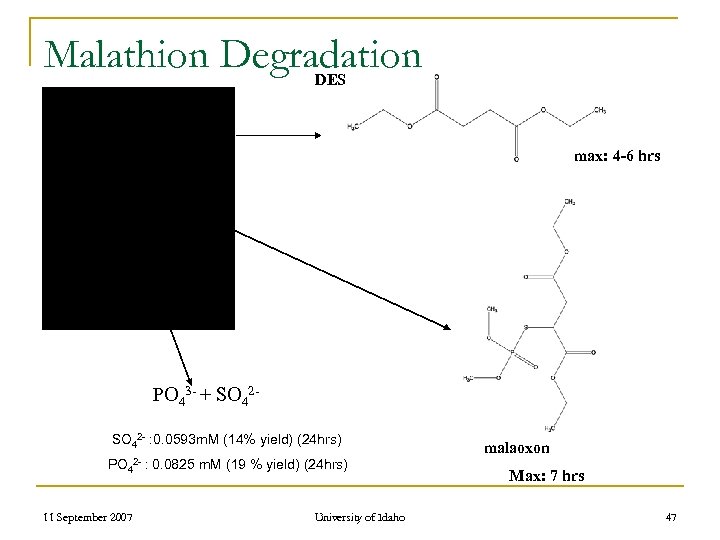 Malathion Degradation DES malathion max: 4 -6 hrs PO 43 - + SO 42 - : 0. 0593 m. M (14% yield) (24 hrs) PO 42 - : 0. 0825 m. M (19 % yield) (24 hrs) 11 September 2007 University of Idaho malaoxon Max: 7 hrs 47
Malathion Degradation DES malathion max: 4 -6 hrs PO 43 - + SO 42 - : 0. 0593 m. M (14% yield) (24 hrs) PO 42 - : 0. 0825 m. M (19 % yield) (24 hrs) 11 September 2007 University of Idaho malaoxon Max: 7 hrs 47
 Kinetics of Malathion Degradation Malathion Diethyl Succinate (DES) GC/FID chromatograph: each data point indicates an individual reaction vial extracted using 50/50 hexane/ethyl acetate, error bars indicate the standard deviation between three measurements of each sample vial. 11 September 2007 University of Idaho 48
Kinetics of Malathion Degradation Malathion Diethyl Succinate (DES) GC/FID chromatograph: each data point indicates an individual reaction vial extracted using 50/50 hexane/ethyl acetate, error bars indicate the standard deviation between three measurements of each sample vial. 11 September 2007 University of Idaho 48
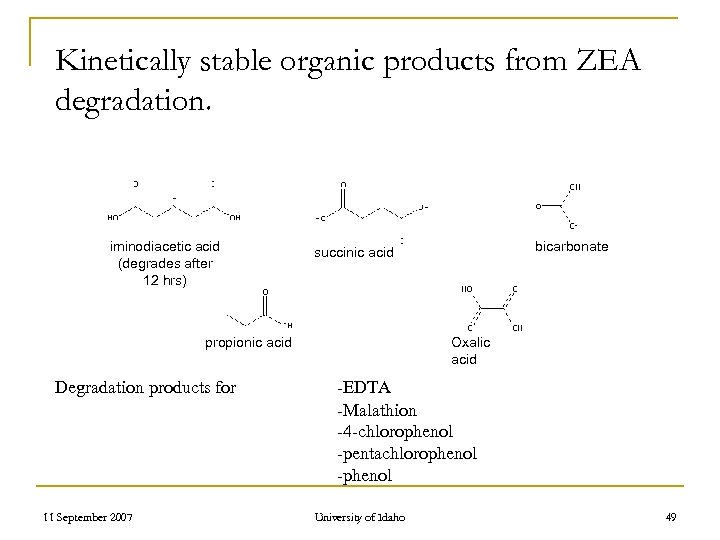 Kinetically stable organic products from ZEA degradation. iminodiacetic acid (degrades after 12 hrs) propionic acid Degradation products for 11 September 2007 bicarbonate succinic acid Oxalic acid -EDTA -Malathion -4 -chlorophenol -pentachlorophenol -phenol University of Idaho 49
Kinetically stable organic products from ZEA degradation. iminodiacetic acid (degrades after 12 hrs) propionic acid Degradation products for 11 September 2007 bicarbonate succinic acid Oxalic acid -EDTA -Malathion -4 -chlorophenol -pentachlorophenol -phenol University of Idaho 49
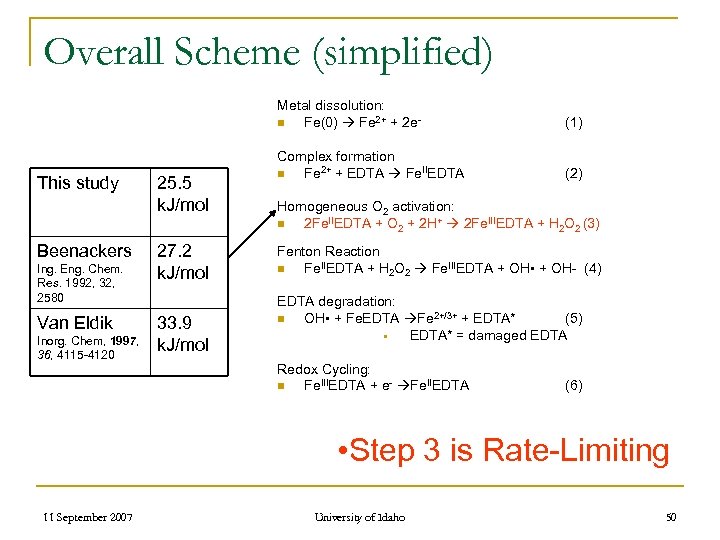 Overall Scheme (simplified) Metal dissolution: n Fe(0) Fe 2+ + 2 e- This study Beenackers Ing. Eng. Chem. Res. 1992, 32, 2580 Van Eldik Inorg. Chem, 1997, 36, 4115 -4120 25. 5 k. J/mol 27. 2 k. J/mol 33. 9 k. J/mol (1) Complex formation n Fe 2+ + EDTA Fe. IIEDTA (2) Homogeneous O 2 activation: n 2 Fe. IIEDTA + O 2 + 2 H+ 2 Fe. IIIEDTA + H 2 O 2 (3) Fenton Reaction n Fe. IIEDTA + H 2 O 2 Fe. IIIEDTA + OH • + OH- (4) EDTA degradation: n OH • + Fe. EDTA Fe 2+/3+ + EDTA* (5) § EDTA* = damaged EDTA Redox Cycling: n Fe. IIIEDTA + e- Fe. IIEDTA (6) • Step 3 is Rate-Limiting 11 September 2007 University of Idaho 50
Overall Scheme (simplified) Metal dissolution: n Fe(0) Fe 2+ + 2 e- This study Beenackers Ing. Eng. Chem. Res. 1992, 32, 2580 Van Eldik Inorg. Chem, 1997, 36, 4115 -4120 25. 5 k. J/mol 27. 2 k. J/mol 33. 9 k. J/mol (1) Complex formation n Fe 2+ + EDTA Fe. IIEDTA (2) Homogeneous O 2 activation: n 2 Fe. IIEDTA + O 2 + 2 H+ 2 Fe. IIIEDTA + H 2 O 2 (3) Fenton Reaction n Fe. IIEDTA + H 2 O 2 Fe. IIIEDTA + OH • + OH- (4) EDTA degradation: n OH • + Fe. EDTA Fe 2+/3+ + EDTA* (5) § EDTA* = damaged EDTA Redox Cycling: n Fe. IIIEDTA + e- Fe. IIEDTA (6) • Step 3 is Rate-Limiting 11 September 2007 University of Idaho 50
 Voltammetric Studies n n ZEA reaction rate is dependent on p. H 2. 5 < p. H < 4. 5 LMW Acids Self Buffers the ZEA reaction at 3. 5 Oxygen Activation Rates Measured By Cyclic Voltammetry 11 September 2007 University of Idaho 51
Voltammetric Studies n n ZEA reaction rate is dependent on p. H 2. 5 < p. H < 4. 5 LMW Acids Self Buffers the ZEA reaction at 3. 5 Oxygen Activation Rates Measured By Cyclic Voltammetry 11 September 2007 University of Idaho 51
 CV of Fe(II/III)EDTA in the presence of O 2 n Electrocatalytic (EC’) Voltammetry 2 Figure 1. Cyclic voltammetric curves on a p. H =3 sample A 1. 5 1 u. A A) 0. 1 m. M Fe. IIIEDTA and O 2 saturated, B) 0. 1 m. M Fe. IIIEDTA only, 0. 5 B B 0 -0. 5 0. 3 0. 2 0. 1 scan rate = 5 m. V/s. 11 September 2007 0 -0. 1 -0. 2 -0. 3 -0. 4 V University of Idaho 52
CV of Fe(II/III)EDTA in the presence of O 2 n Electrocatalytic (EC’) Voltammetry 2 Figure 1. Cyclic voltammetric curves on a p. H =3 sample A 1. 5 1 u. A A) 0. 1 m. M Fe. IIIEDTA and O 2 saturated, B) 0. 1 m. M Fe. IIIEDTA only, 0. 5 B B 0 -0. 5 0. 3 0. 2 0. 1 scan rate = 5 m. V/s. 11 September 2007 0 -0. 1 -0. 2 -0. 3 -0. 4 V University of Idaho 52
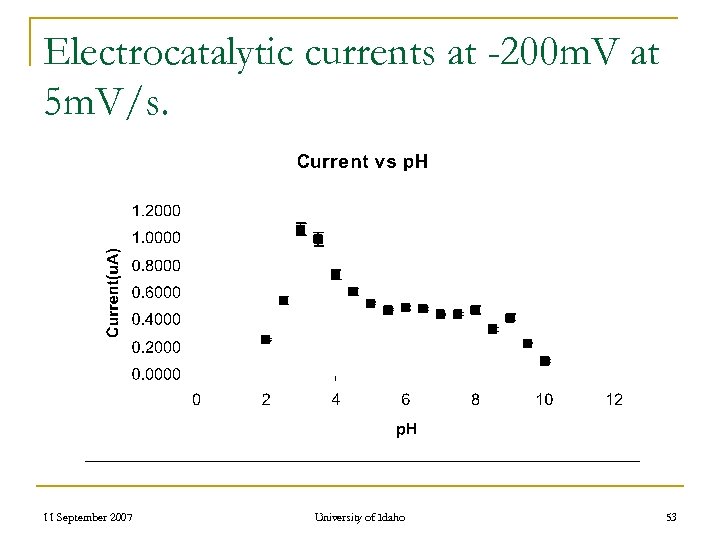 Electrocatalytic currents at -200 m. V at 5 m. V/s. 11 September 2007 University of Idaho 53
Electrocatalytic currents at -200 m. V at 5 m. V/s. 11 September 2007 University of Idaho 53
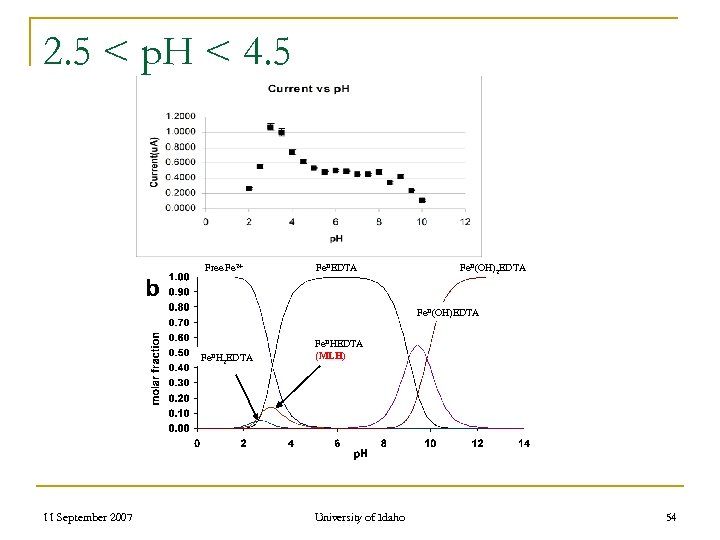 2. 5 < p. H < 4. 5 Free Fe 2+ Fe. IIEDTA Fe. II(OH)2 EDTA Fe. II(OH)EDTA Fe. IIH 11 September 2007 2 EDTA Fe. IIHEDTA (MLH) University of Idaho 54
2. 5 < p. H < 4. 5 Free Fe 2+ Fe. IIEDTA Fe. II(OH)2 EDTA Fe. II(OH)EDTA Fe. IIH 11 September 2007 2 EDTA Fe. IIHEDTA (MLH) University of Idaho 54
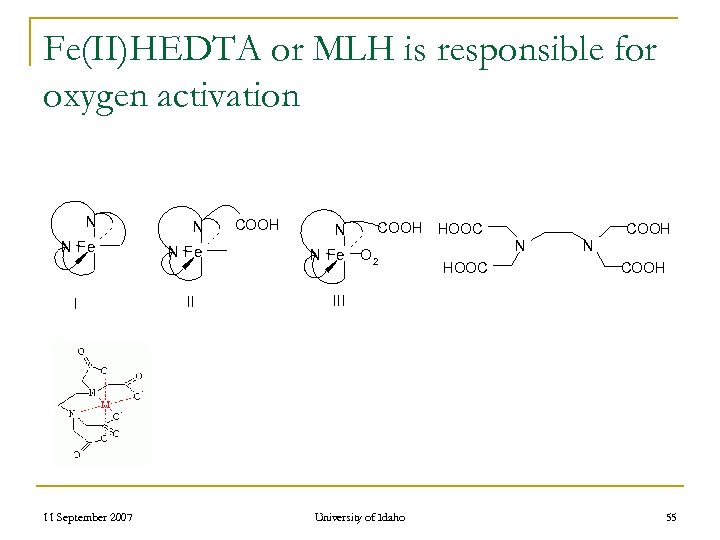 Fe(II)HEDTA or MLH is responsible for oxygen activation N N N Fe I 11 September 2007 II COOH N N Fe COOH HOOC O 2 COOH N HOOC N COOH III University of Idaho 55
Fe(II)HEDTA or MLH is responsible for oxygen activation N N N Fe I 11 September 2007 II COOH N N Fe COOH HOOC O 2 COOH N HOOC N COOH III University of Idaho 55
 ZEA Reaction - Conclusions n This system is a viable option for the destruction of a variety of pollutants and has a strong possibility for scale up. n The only system known to date that can obtain non-biological Oxygen Activation at room temperature and pressure to produce reactive oxygen species that are capable of fully degrading pollutants to LMW carboxylates and inorganic forms n Due to the duality of EDTA acting as both a pro-oxidant and antioxidant, controlling the [EDTA] is imperative to the success of the process. n Rate-limiting steps is (are) oxygen activation n Fe. IIEDTA may provide insights into biological oxygen activation 11 September 2007 University of Idaho 56
ZEA Reaction - Conclusions n This system is a viable option for the destruction of a variety of pollutants and has a strong possibility for scale up. n The only system known to date that can obtain non-biological Oxygen Activation at room temperature and pressure to produce reactive oxygen species that are capable of fully degrading pollutants to LMW carboxylates and inorganic forms n Due to the duality of EDTA acting as both a pro-oxidant and antioxidant, controlling the [EDTA] is imperative to the success of the process. n Rate-limiting steps is (are) oxygen activation n Fe. IIEDTA may provide insights into biological oxygen activation 11 September 2007 University of Idaho 56
 Detector for Triacetone Triperoxide n Wikipedia http: //en. wikipedia. org/wiki/Acetone_peroxide n Acetone peroxide (triacetone triperoxide, peroxyacetone, TATP, TCAP) is an organic peroxide and a primary high explosive. 11 September 2007 University of Idaho 57
Detector for Triacetone Triperoxide n Wikipedia http: //en. wikipedia. org/wiki/Acetone_peroxide n Acetone peroxide (triacetone triperoxide, peroxyacetone, TATP, TCAP) is an organic peroxide and a primary high explosive. 11 September 2007 University of Idaho 57
 TATP Detector Outline n Background q q n Need for Detection Systems q q q n Dangers Recent News Fast Field Portable (handheld) Selective and LOD Electrochemical Detection Via Fenton Rxn 11 September 2007 University of Idaho 58
TATP Detector Outline n Background q q n Need for Detection Systems q q q n Dangers Recent News Fast Field Portable (handheld) Selective and LOD Electrochemical Detection Via Fenton Rxn 11 September 2007 University of Idaho 58
 TATP – the threat • Due to the cost and ease with which the precursors can be obtained, acetone peroxide is commonly manufactured by those without the resources needed to manufacture or buy more sophisticated explosives. When the reaction is carried out without proper equipment the risk of an accident is significant. • http: //en. wikipedia. org/wiki/Acetone_peroxide 11 September 2007 University of Idaho 59
TATP – the threat • Due to the cost and ease with which the precursors can be obtained, acetone peroxide is commonly manufactured by those without the resources needed to manufacture or buy more sophisticated explosives. When the reaction is carried out without proper equipment the risk of an accident is significant. • http: //en. wikipedia. org/wiki/Acetone_peroxide 11 September 2007 University of Idaho 59
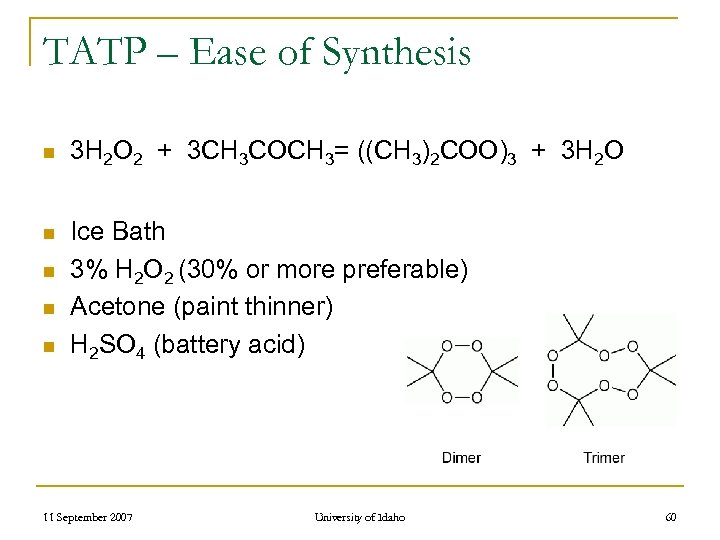 TATP – Ease of Synthesis n n n 3 H 2 O 2 + 3 CH 3 COCH 3= ((CH 3)2 COO)3 + 3 H 2 O Ice Bath 3% H 2 O 2 (30% or more preferable) Acetone (paint thinner) H 2 SO 4 (battery acid) 11 September 2007 University of Idaho 60
TATP – Ease of Synthesis n n n 3 H 2 O 2 + 3 CH 3 COCH 3= ((CH 3)2 COO)3 + 3 H 2 O Ice Bath 3% H 2 O 2 (30% or more preferable) Acetone (paint thinner) H 2 SO 4 (battery acid) 11 September 2007 University of Idaho 60
 TATP – physiochemical characteristics n n n Shock Sensitive Heat Sensitive High V. P. 7 Pa @ 300 K* q n n n 66% weight loss within 2 weeks at room temperature** No Possible Commercial or Military Applications *Propellants, Explosives, Pyrotechnics 30 (2005)127 **J. Am. Chem. Soc. 2005, 127, 1146 -1159 11 September 2007 University of Idaho 61
TATP – physiochemical characteristics n n n Shock Sensitive Heat Sensitive High V. P. 7 Pa @ 300 K* q n n n 66% weight loss within 2 weeks at room temperature** No Possible Commercial or Military Applications *Propellants, Explosives, Pyrotechnics 30 (2005)127 **J. Am. Chem. Soc. 2005, 127, 1146 -1159 11 September 2007 University of Idaho 61
 TATP is suicide bombers' weapon of choice n Times (London) July 15, 2005 By Philippe Naughton n Rediscovered in the West Bank (Israel) in the early 1980 s and soon became an extremists' staple. n Suicide Bombers – “Mother of Satan” n But as the Palestinian bomb-makers will attest - 40 Palestinians are thought to have been killed making or handling the explosive - it is highly unstable and sensitive to heat and friction. 11 September 2007 University of Idaho 62
TATP is suicide bombers' weapon of choice n Times (London) July 15, 2005 By Philippe Naughton n Rediscovered in the West Bank (Israel) in the early 1980 s and soon became an extremists' staple. n Suicide Bombers – “Mother of Satan” n But as the Palestinian bomb-makers will attest - 40 Palestinians are thought to have been killed making or handling the explosive - it is highly unstable and sensitive to heat and friction. 11 September 2007 University of Idaho 62
 TATP – Most Recent News n NY Times Sept. 5, 2007 n FRANKFURT, Sept. 5 — The German police have arrested three Islamic militants suspected of planning large-scale terrorist attacks against several sites frequented by Americans, including discos, bars, airports, and military installations. n She said the suspects had amassed large amounts of hydrogen peroxide, the main chemical used to manufacture the explosives used in the suicide bombings in London in July 2005. 11 September 2007 University of Idaho 63
TATP – Most Recent News n NY Times Sept. 5, 2007 n FRANKFURT, Sept. 5 — The German police have arrested three Islamic militants suspected of planning large-scale terrorist attacks against several sites frequented by Americans, including discos, bars, airports, and military installations. n She said the suspects had amassed large amounts of hydrogen peroxide, the main chemical used to manufacture the explosives used in the suicide bombings in London in July 2005. 11 September 2007 University of Idaho 63
 TATP – London Subway Bombings n July 7, 2005 http: //news. bbc. co. uk/nol/shared/spl/hi/pop_ups/05/uk_enl_1121567244/img/1. jpg 11 September 2007 University of Idaho 64
TATP – London Subway Bombings n July 7, 2005 http: //news. bbc. co. uk/nol/shared/spl/hi/pop_ups/05/uk_enl_1121567244/img/1. jpg 11 September 2007 University of Idaho 64
 TATP – Shoe Bomber n http: //www. univie. ac. at/cga/art/shoe_bomb 3. gif 11 September 2007 University of Idaho 65
TATP – Shoe Bomber n http: //www. univie. ac. at/cga/art/shoe_bomb 3. gif 11 September 2007 University of Idaho 65
 TATP – Domestic Terrorists n http: //www. koco. com/news/ 5058347/detail. html n Sources Identify TATP As Component Of Bomb TATP Same Component Used By Infamous Shoe Bomber POSTED: 9: 56 pm CDT October 4, 2005 UPDATED: 10: 11 pm CDT October 4, 2005 n NORMAN, Okla. -- Sources confirmed Tuesday night that at least one of the components in the bomb used by Joel Henry Hinrichs III Saturday night was a product called TATP. 11 September 2007 University of Idaho 66
TATP – Domestic Terrorists n http: //www. koco. com/news/ 5058347/detail. html n Sources Identify TATP As Component Of Bomb TATP Same Component Used By Infamous Shoe Bomber POSTED: 9: 56 pm CDT October 4, 2005 UPDATED: 10: 11 pm CDT October 4, 2005 n NORMAN, Okla. -- Sources confirmed Tuesday night that at least one of the components in the bomb used by Joel Henry Hinrichs III Saturday night was a product called TATP. 11 September 2007 University of Idaho 66
 TATP – TSA Fluid Ban n Effective November 10, 2006, the TSA has advised that travelers may now carry through security checkpoints travel-size toiletries (3. 4 ounces/100 ml or less) that fit comfortably in ONE, QUARTSIZE, clear plastic re-sealable bag. q q q The 3 -1 -1 Kit contains six 2 -1/2 oz and four 1 -1/2 oz flexible squeeze tubes, plus one 1 -3/4 oz Envirosprayer. Kit is also compliant with the new International Security Measures Accord. http: //www. easytravelerinc. com/ 11 September 2007 University of Idaho 67
TATP – TSA Fluid Ban n Effective November 10, 2006, the TSA has advised that travelers may now carry through security checkpoints travel-size toiletries (3. 4 ounces/100 ml or less) that fit comfortably in ONE, QUARTSIZE, clear plastic re-sealable bag. q q q The 3 -1 -1 Kit contains six 2 -1/2 oz and four 1 -1/2 oz flexible squeeze tubes, plus one 1 -3/4 oz Envirosprayer. Kit is also compliant with the new International Security Measures Accord. http: //www. easytravelerinc. com/ 11 September 2007 University of Idaho 67
 TATP Detection the Challenge n The Need for a Fast Portable Detector n Innocuous Appearing White Powder n Despite a high VP Cannot be Sensed by Dogs n Lacks Chromophoric Groups (not detectable by UV-vis absorbance) 11 September 2007 University of Idaho 68
TATP Detection the Challenge n The Need for a Fast Portable Detector n Innocuous Appearing White Powder n Despite a high VP Cannot be Sensed by Dogs n Lacks Chromophoric Groups (not detectable by UV-vis absorbance) 11 September 2007 University of Idaho 68
 TATP – Detector Requirements n Unknown Materials – Public Safety, e. g. Airports q n Air Samples, e. g. Airports q n Moderate Selectivity– Low Limits of Detection Required Debris at Post-Explosion Sites q n High Selectivity – Low Limits of Detection not Required High Selectivity– Low Detection Limits Field Portability Schulte-Ladbeck, R. ; Vogel, M. ; Karst, U Recent methods for the determination of peroxide-based explosives Anal. Bioanal. Chem. 386 559 -565 (2006) 11 September 2007 University of Idaho 69
TATP – Detector Requirements n Unknown Materials – Public Safety, e. g. Airports q n Air Samples, e. g. Airports q n Moderate Selectivity– Low Limits of Detection Required Debris at Post-Explosion Sites q n High Selectivity – Low Limits of Detection not Required High Selectivity– Low Detection Limits Field Portability Schulte-Ladbeck, R. ; Vogel, M. ; Karst, U Recent methods for the determination of peroxide-based explosives Anal. Bioanal. Chem. 386 559 -565 (2006) 11 September 2007 University of Idaho 69
 TATP - Detector n IR-Raman q n Fluorescence/UV-vis Absorbance q n Low LOD requires tagging Ion Mobility q n High Selectivity – Relatively High LOD Good Selectivity, moderate LOD HPLC or GC q Excellent Selectivity and LOD 11 September 2007 University of Idaho 70
TATP - Detector n IR-Raman q n Fluorescence/UV-vis Absorbance q n Low LOD requires tagging Ion Mobility q n High Selectivity – Relatively High LOD Good Selectivity, moderate LOD HPLC or GC q Excellent Selectivity and LOD 11 September 2007 University of Idaho 70
 TATP – State of Detectors n Costs n Lack of Field Portability q n Ideal – Handheld Sensor May Require Knowledgeable User q e. g. Commercial Glucose Sensors 11 September 2007 University of Idaho 71
TATP – State of Detectors n Costs n Lack of Field Portability q n Ideal – Handheld Sensor May Require Knowledgeable User q e. g. Commercial Glucose Sensors 11 September 2007 University of Idaho 71
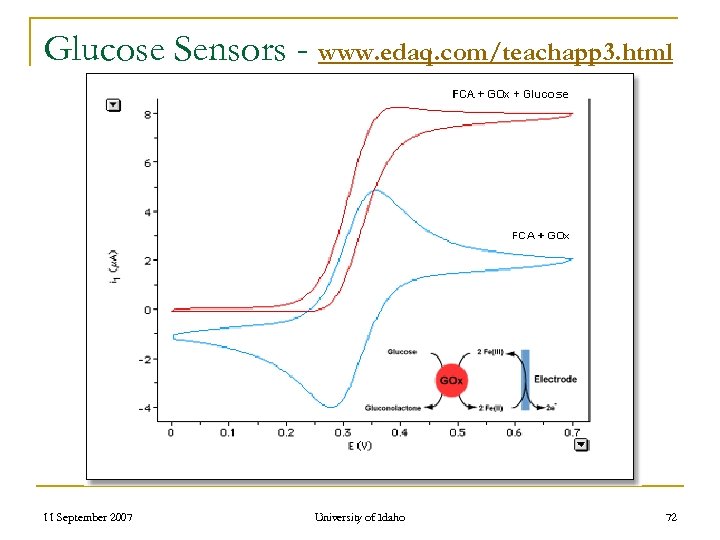 Glucose Sensors - www. edaq. com/teachapp 3. html 11 September 2007 University of Idaho 72
Glucose Sensors - www. edaq. com/teachapp 3. html 11 September 2007 University of Idaho 72
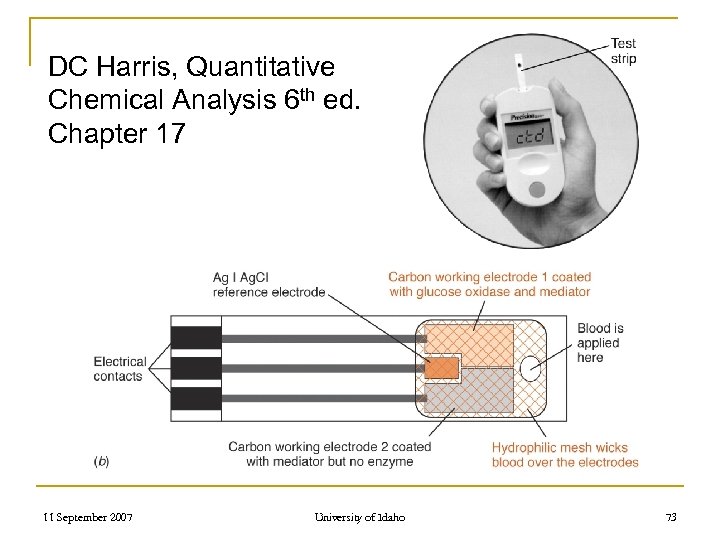 DC Harris, Quantitative Chemical Analysis 6 th ed. Chapter 17 11 September 2007 University of Idaho 73
DC Harris, Quantitative Chemical Analysis 6 th ed. Chapter 17 11 September 2007 University of Idaho 73
 TATP – Detection by Electrochemical Means n n Proposed Basis For Detection Fenton Reaction for Organic Peroxides RO-OR + Fe. IIEDTA RO- + RO· + Fe. IIIEDTA 11 September 2007 University of Idaho 74
TATP – Detection by Electrochemical Means n n Proposed Basis For Detection Fenton Reaction for Organic Peroxides RO-OR + Fe. IIEDTA RO- + RO· + Fe. IIIEDTA 11 September 2007 University of Idaho 74
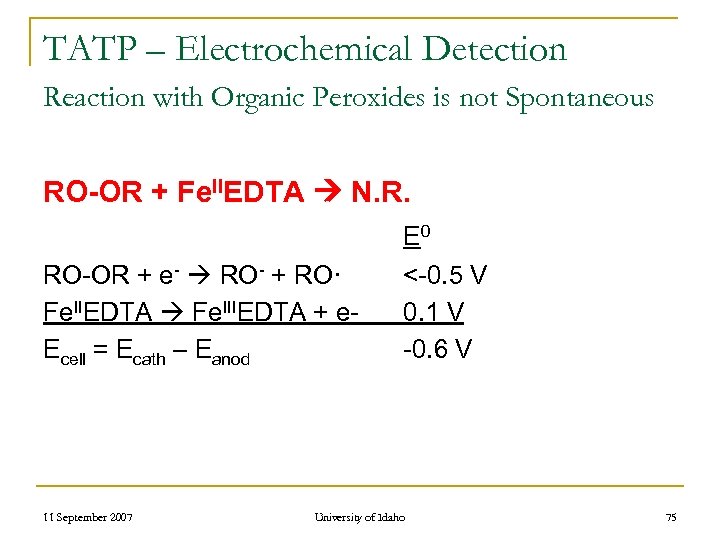 TATP – Electrochemical Detection Reaction with Organic Peroxides is not Spontaneous RO-OR + Fe. IIEDTA N. R. RO-OR + e- RO- + RO· Fe. IIEDTA Fe. IIIEDTA + e. Ecell = Ecath – Eanod 11 September 2007 E 0 <-0. 5 V 0. 1 V -0. 6 V University of Idaho 75
TATP – Electrochemical Detection Reaction with Organic Peroxides is not Spontaneous RO-OR + Fe. IIEDTA N. R. RO-OR + e- RO- + RO· Fe. IIEDTA Fe. IIIEDTA + e. Ecell = Ecath – Eanod 11 September 2007 E 0 <-0. 5 V 0. 1 V -0. 6 V University of Idaho 75
 TATP – Electrochemical Detection Reaction with Peroxides and Hydroperoxides is Spontaneous RO-OH + e- RO- + HO· HO-OH + e- HO- + HO· E 0 ≈0. 4 V 0. 8 V Fe. IIEDTA + RO-OH/HO-OH Fe. IIIEDTA + RO∙/H+ n Requires that TATP be degraded 11 September 2007 University of Idaho 76
TATP – Electrochemical Detection Reaction with Peroxides and Hydroperoxides is Spontaneous RO-OH + e- RO- + HO· HO-OH + e- HO- + HO· E 0 ≈0. 4 V 0. 8 V Fe. IIEDTA + RO-OH/HO-OH Fe. IIIEDTA + RO∙/H+ n Requires that TATP be degraded 11 September 2007 University of Idaho 76
 TATP – Degradation to HOOH/ROOH n Acid degradation n TATP + H+ H 2 O 2 + Products n Conc. [HCl] 10 minutes n 11 September 2007 University of Idaho 77
TATP – Degradation to HOOH/ROOH n Acid degradation n TATP + H+ H 2 O 2 + Products n Conc. [HCl] 10 minutes n 11 September 2007 University of Idaho 77
 TATP – Cyclic Voltammograms after Acid Digestion A B Figure 1. Cyclic voltammograms of two solutions both containing 10 m. M TATP and 1 m. M Fe. IIIEDTA under dearated conditions, 30 m. V/s. A) Acid treated TATP. B) Non-acid treated TATP. 11 September 2007 University of Idaho 78
TATP – Cyclic Voltammograms after Acid Digestion A B Figure 1. Cyclic voltammograms of two solutions both containing 10 m. M TATP and 1 m. M Fe. IIIEDTA under dearated conditions, 30 m. V/s. A) Acid treated TATP. B) Non-acid treated TATP. 11 September 2007 University of Idaho 78
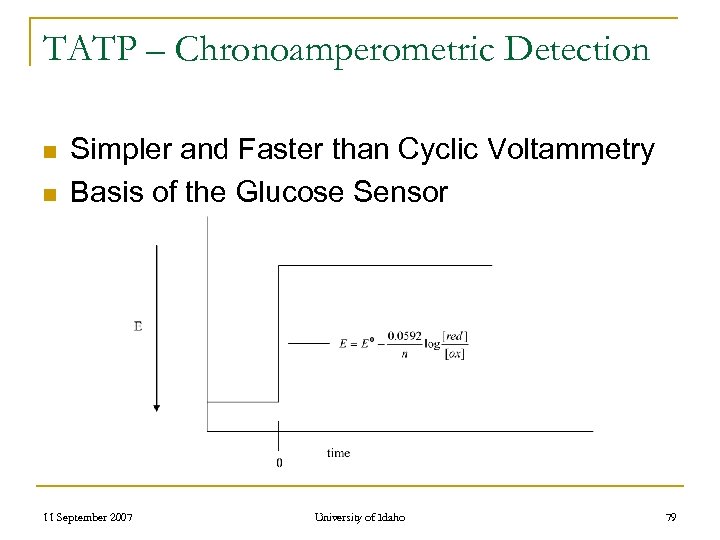 TATP – Chronoamperometric Detection n n Simpler and Faster than Cyclic Voltammetry Basis of the Glucose Sensor 11 September 2007 University of Idaho 79
TATP – Chronoamperometric Detection n n Simpler and Faster than Cyclic Voltammetry Basis of the Glucose Sensor 11 September 2007 University of Idaho 79
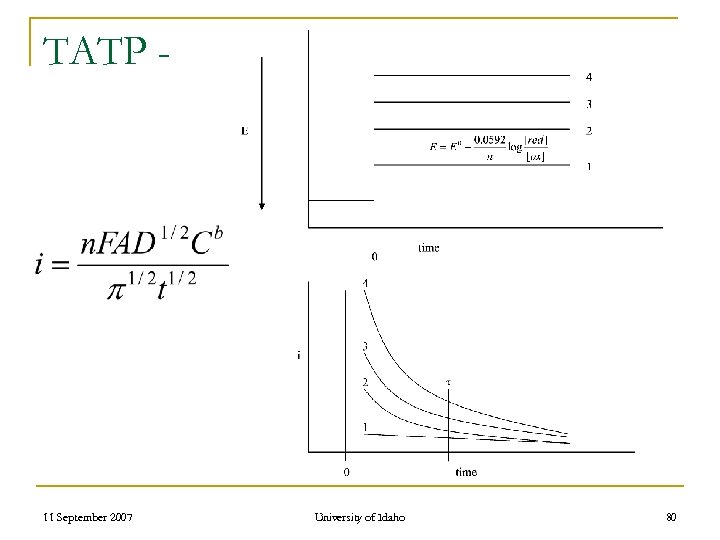 TATP - 11 September 2007 University of Idaho 80
TATP - 11 September 2007 University of Idaho 80
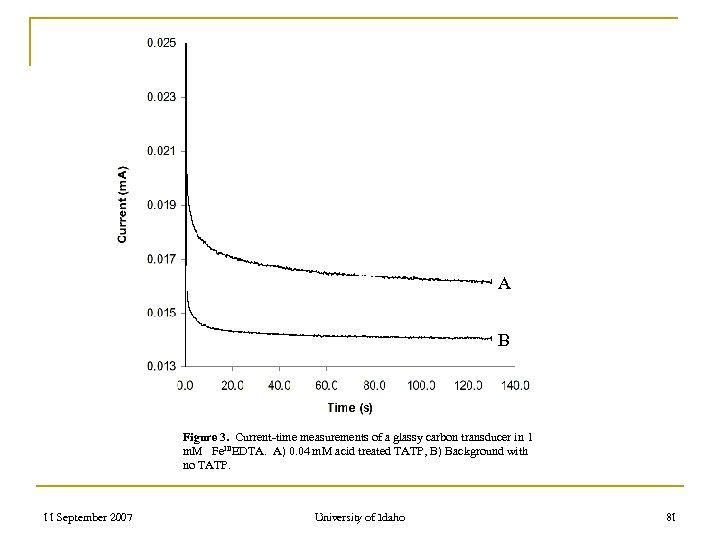 A B Figure 3. Current-time measurements of a glassy carbon transducer in 1 m. M Fe. IIIEDTA. A) 0. 04 m. M acid treated TATP, B) Background with no TATP. 11 September 2007 University of Idaho 81
A B Figure 3. Current-time measurements of a glassy carbon transducer in 1 m. M Fe. IIIEDTA. A) 0. 04 m. M acid treated TATP, B) Background with no TATP. 11 September 2007 University of Idaho 81
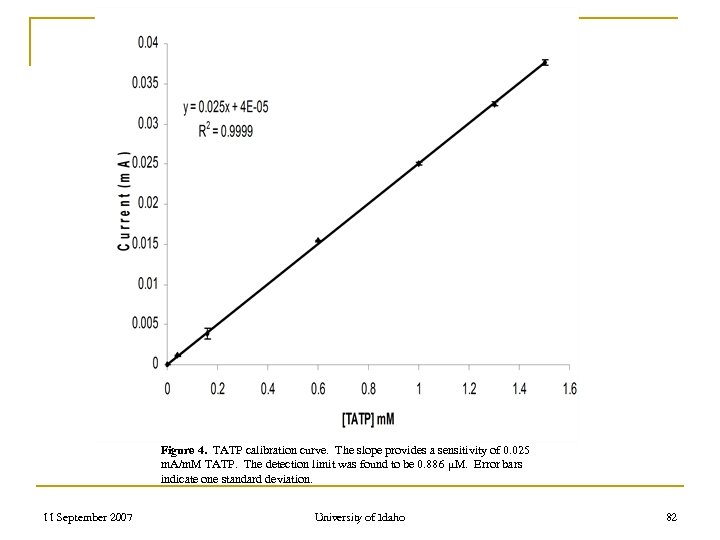 Figure 4. TATP calibration curve. The slope provides a sensitivity of 0. 025 m. A/m. M TATP. The detection limit was found to be 0. 886 μM. Error bars indicate one standard deviation. 11 September 2007 University of Idaho 82
Figure 4. TATP calibration curve. The slope provides a sensitivity of 0. 025 m. A/m. M TATP. The detection limit was found to be 0. 886 μM. Error bars indicate one standard deviation. 11 September 2007 University of Idaho 82
 Conclusions – Chronoamperometric TATP Detection n 0. 886 µM LOD n O 2 interference q q n Fe. IIEDTA + O 2 Fe. IIIEDTA + O 2. Fe. IIEDTA + HO-OH Fe. IIIEDTA + HO∙ + HO- Requires Acid Pretreatment q 10 min. Sample Pretreatment 11 September 2007 University of Idaho 83
Conclusions – Chronoamperometric TATP Detection n 0. 886 µM LOD n O 2 interference q q n Fe. IIEDTA + O 2 Fe. IIIEDTA + O 2. Fe. IIEDTA + HO-OH Fe. IIIEDTA + HO∙ + HO- Requires Acid Pretreatment q 10 min. Sample Pretreatment 11 September 2007 University of Idaho 83
 Future Work n Elimination of O 2 interference q q n n Metal Complex Reduction Potential Kinetics of H 2 O 2 vs. O 2 reduction Optimal Hydrolysis Design of probes q q Air Samples Liquid Sample 11 September 2007 University of Idaho 84
Future Work n Elimination of O 2 interference q q n n Metal Complex Reduction Potential Kinetics of H 2 O 2 vs. O 2 reduction Optimal Hydrolysis Design of probes q q Air Samples Liquid Sample 11 September 2007 University of Idaho 84
 Acknowledgements Derek Laine Funding NIH Kenichi Shimizu NSF Simon Mc. Allister NSF-SGER Ruhba Ponraj Mark D. Engelmann, Ph. D. 2003 Tina Noradoun, Ph. D. 2005 Ryan Hutcheson, B. S. 2004 NASA EPRI BLM UI Rob Bobier, B. S. 2002 Terry Hiatt, B. S. 2000 11 September 2007 University of Idaho 85
Acknowledgements Derek Laine Funding NIH Kenichi Shimizu NSF Simon Mc. Allister NSF-SGER Ruhba Ponraj Mark D. Engelmann, Ph. D. 2003 Tina Noradoun, Ph. D. 2005 Ryan Hutcheson, B. S. 2004 NASA EPRI BLM UI Rob Bobier, B. S. 2002 Terry Hiatt, B. S. 2000 11 September 2007 University of Idaho 85
 11 September 2007 University of Idaho 86
11 September 2007 University of Idaho 86
 Reactive Oxygen Species (ROS) in Biology 11 September 2007 University of Idaho 87
Reactive Oxygen Species (ROS) in Biology 11 September 2007 University of Idaho 87
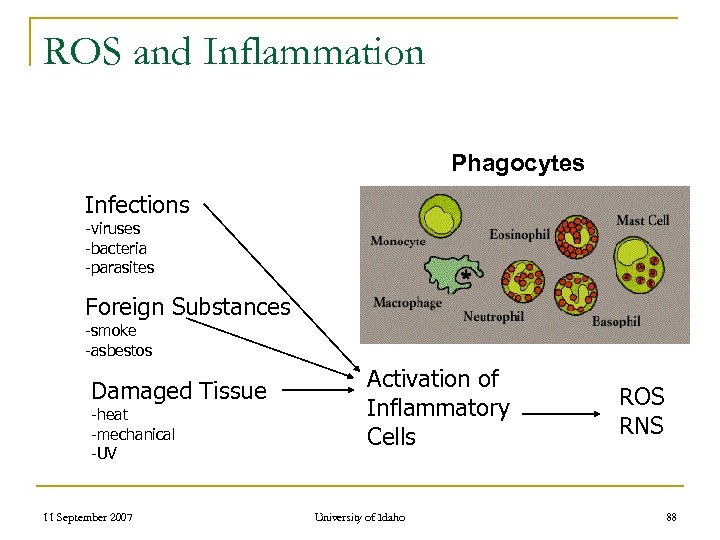 ROS and Inflammation Phagocytes Infections -viruses -bacteria -parasites Foreign Substances -smoke -asbestos Damaged Tissue -heat -mechanical -UV 11 September 2007 Activation of Inflammatory Cells University of Idaho ROS RNS 88
ROS and Inflammation Phagocytes Infections -viruses -bacteria -parasites Foreign Substances -smoke -asbestos Damaged Tissue -heat -mechanical -UV 11 September 2007 Activation of Inflammatory Cells University of Idaho ROS RNS 88
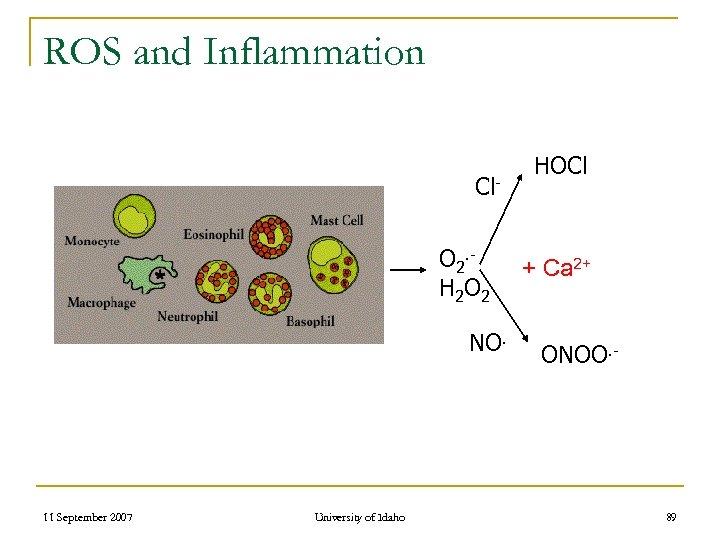 ROS and Inflammation Cl. O 2. H 2 O 2 NO. 11 September 2007 University of Idaho HOCl + Ca 2+ ONOO. - 89
ROS and Inflammation Cl. O 2. H 2 O 2 NO. 11 September 2007 University of Idaho HOCl + Ca 2+ ONOO. - 89
 Iron Enzymes and the Fenton Reaction Hemes/Cytochromes Oxygenases Oxidases H 2 O 2 + reducing agent HO. • All Fe-containing enzymes are quite good Fenton Reaction agents. • Oxidative Stress 11 September 2007 University of Idaho 90
Iron Enzymes and the Fenton Reaction Hemes/Cytochromes Oxygenases Oxidases H 2 O 2 + reducing agent HO. • All Fe-containing enzymes are quite good Fenton Reaction agents. • Oxidative Stress 11 September 2007 University of Idaho 90
 11 September 2007 University of Idaho 91
11 September 2007 University of Idaho 91
 Inflammation The Good Inflammation protects the body • Destroys invading pathogens • Dissolves damaged tissue The Bad Chronic or prolong inflammation Allergies and Autoimmune Diseases • All the many types of allergies • Many of the autoimmune diseases 11 September 2007 University of Idaho 92
Inflammation The Good Inflammation protects the body • Destroys invading pathogens • Dissolves damaged tissue The Bad Chronic or prolong inflammation Allergies and Autoimmune Diseases • All the many types of allergies • Many of the autoimmune diseases 11 September 2007 University of Idaho 92
 Role of Calcium in Inflammation 11 September 2007 University of Idaho 93
Role of Calcium in Inflammation 11 September 2007 University of Idaho 93
 Role of Calcium in Inflammation n Enhances Oxidative Processes in Inflammation ROS n n Paradoxically Redox Inactive, i. e. always Ca 2+ Inflammation { H 2 O 2. - Ca 2+ Role in Biological Oxidations Not Well Understood 11 September 2007 University of Idaho 94
Role of Calcium in Inflammation n Enhances Oxidative Processes in Inflammation ROS n n Paradoxically Redox Inactive, i. e. always Ca 2+ Inflammation { H 2 O 2. - Ca 2+ Role in Biological Oxidations Not Well Understood 11 September 2007 University of Idaho 94
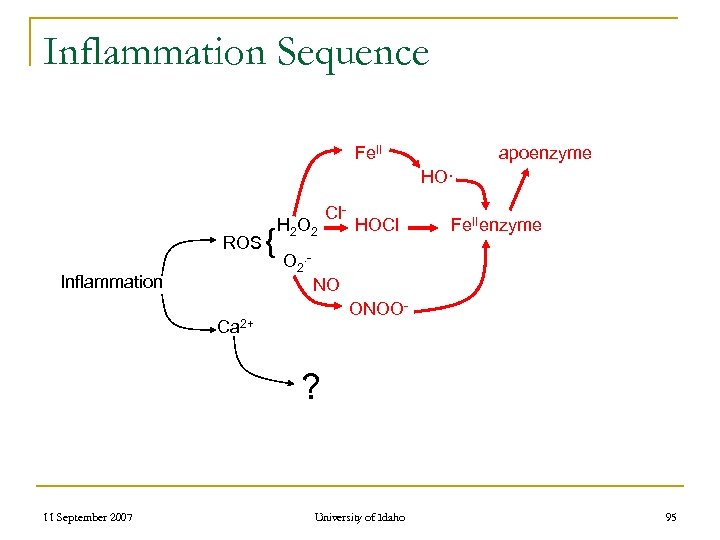 Inflammation Sequence Fe. II apoenzyme HO· ROS Inflammation { H 2 O 2 Cl- HOCl Fe. IIenzyme O 2. NO ONOO- Ca 2+ ? 11 September 2007 University of Idaho 95
Inflammation Sequence Fe. II apoenzyme HO· ROS Inflammation { H 2 O 2 Cl- HOCl Fe. IIenzyme O 2. NO ONOO- Ca 2+ ? 11 September 2007 University of Idaho 95
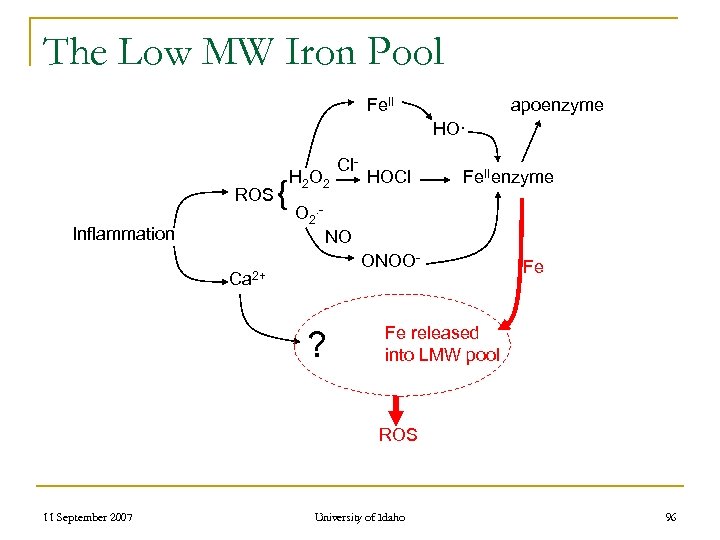 The Low MW Iron Pool Fe. II apoenzyme HO· ROS Inflammation { H 2 O 2 Cl- HOCl Fe. IIenzyme O 2. NO ONOO- Ca 2+ ? Fe Fe released into LMW pool ROS 11 September 2007 University of Idaho 96
The Low MW Iron Pool Fe. II apoenzyme HO· ROS Inflammation { H 2 O 2 Cl- HOCl Fe. IIenzyme O 2. NO ONOO- Ca 2+ ? Fe Fe released into LMW pool ROS 11 September 2007 University of Idaho 96
 Ligands for the LMW Fe pool n n n Glutamate Citrate Phosphates, e. g. ATP, ADP, GTP, etc. Amino Acids Peptides Proteins (HMW) 11 September 2007 University of Idaho 97
Ligands for the LMW Fe pool n n n Glutamate Citrate Phosphates, e. g. ATP, ADP, GTP, etc. Amino Acids Peptides Proteins (HMW) 11 September 2007 University of Idaho 97
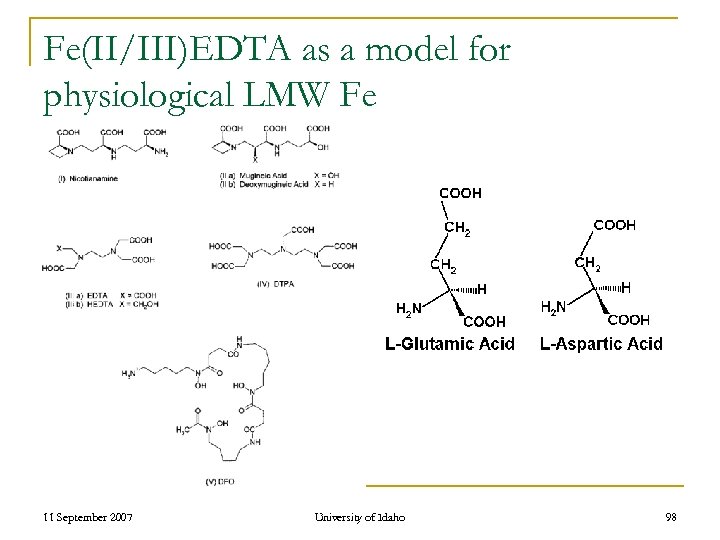 Fe(II/III)EDTA as a model for physiological LMW Fe 11 September 2007 University of Idaho 98
Fe(II/III)EDTA as a model for physiological LMW Fe 11 September 2007 University of Idaho 98
![The concentration ratio of [Chelate/Ligand]: [Fe] is very high n n n Physiological Concentration The concentration ratio of [Chelate/Ligand]: [Fe] is very high n n n Physiological Concentration](https://present5.com/presentation/d0edfd974a2130f356c4e16433b40ad9/image-99.jpg) The concentration ratio of [Chelate/Ligand]: [Fe] is very high n n n Physiological Concentration Mobile (Free) Iron ≈ 10 -9 M [Chelates/Ligand] ≈ 10 -3 M Does this have any effect of the reactivity of LMW-Fe in the Fenton Rxn? 11 September 2007 University of Idaho 99
The concentration ratio of [Chelate/Ligand]: [Fe] is very high n n n Physiological Concentration Mobile (Free) Iron ≈ 10 -9 M [Chelates/Ligand] ≈ 10 -3 M Does this have any effect of the reactivity of LMW-Fe in the Fenton Rxn? 11 September 2007 University of Idaho 99
 11 September 2007 University of Idaho 100
11 September 2007 University of Idaho 100
![Fe. II/IIIEDTA CV in [Fe]: [EDTA] at 1: 10 and 1: 1 11 September Fe. II/IIIEDTA CV in [Fe]: [EDTA] at 1: 10 and 1: 1 11 September](https://present5.com/presentation/d0edfd974a2130f356c4e16433b40ad9/image-101.jpg) Fe. II/IIIEDTA CV in [Fe]: [EDTA] at 1: 10 and 1: 1 11 September 2007 University of Idaho 101
Fe. II/IIIEDTA CV in [Fe]: [EDTA] at 1: 10 and 1: 1 11 September 2007 University of Idaho 101
 EC’ CV of Fe. II/IIIEDTA (1: 1) with H 2 O 2 n n n Fe. IIIEDTA + e- = Fe. IIEDTA + H 2 O 2 = Fe. IIIEDTA + HO- + HO∙ (fast) H 2 O 2 + e- = HO- + HO∙ (slow) 11 September 2007 University of Idaho 102
EC’ CV of Fe. II/IIIEDTA (1: 1) with H 2 O 2 n n n Fe. IIIEDTA + e- = Fe. IIEDTA + H 2 O 2 = Fe. IIIEDTA + HO- + HO∙ (fast) H 2 O 2 + e- = HO- + HO∙ (slow) 11 September 2007 University of Idaho 102
![EC’ current measured at -700 m. V various ratios of [Fe]: [Ligand] 11 September EC’ current measured at -700 m. V various ratios of [Fe]: [Ligand] 11 September](https://present5.com/presentation/d0edfd974a2130f356c4e16433b40ad9/image-103.jpg) EC’ current measured at -700 m. V various ratios of [Fe]: [Ligand] 11 September 2007 University of Idaho 103
EC’ current measured at -700 m. V various ratios of [Fe]: [Ligand] 11 September 2007 University of Idaho 103
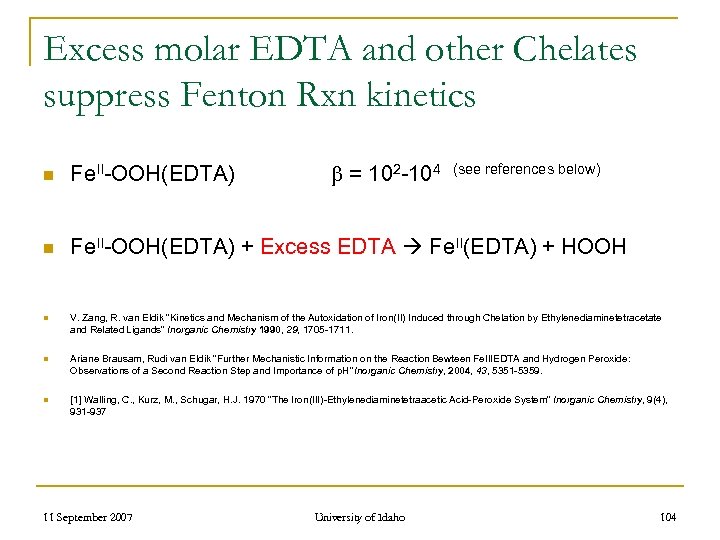 Excess molar EDTA and other Chelates suppress Fenton Rxn kinetics = 102 -104 (see references below) n Fe. II-OOH(EDTA) + Excess EDTA Fe. II(EDTA) + HOOH n V. Zang, R. van Eldik “Kinetics and Mechanism of the Autoxidation of Iron(II) Induced through Chelation by Ethylenediaminetetracetate and Related Ligands” Inorganic Chemistry 1990, 29, 1705 -1711. n Ariane Brausam, Rudi van Eldik “Further Mechanistic Information on the Reaction Bewteen Fe. IIIEDTA and Hydrogen Peroxide: Observations of a Second Reaction Step and Importance of p. H” Inorganic Chemistry, 2004, 43, 5351 -5359. n [1] Walling, C. , Kurz, M. , Schugar, H. J. 1970 “The Iron(III)-Ethylenediaminetetraacetic Acid-Peroxide System” Inorganic Chemistry, 9(4), 931 -937 11 September 2007 University of Idaho 104
Excess molar EDTA and other Chelates suppress Fenton Rxn kinetics = 102 -104 (see references below) n Fe. II-OOH(EDTA) + Excess EDTA Fe. II(EDTA) + HOOH n V. Zang, R. van Eldik “Kinetics and Mechanism of the Autoxidation of Iron(II) Induced through Chelation by Ethylenediaminetetracetate and Related Ligands” Inorganic Chemistry 1990, 29, 1705 -1711. n Ariane Brausam, Rudi van Eldik “Further Mechanistic Information on the Reaction Bewteen Fe. IIIEDTA and Hydrogen Peroxide: Observations of a Second Reaction Step and Importance of p. H” Inorganic Chemistry, 2004, 43, 5351 -5359. n [1] Walling, C. , Kurz, M. , Schugar, H. J. 1970 “The Iron(III)-Ethylenediaminetetraacetic Acid-Peroxide System” Inorganic Chemistry, 9(4), 931 -937 11 September 2007 University of Idaho 104
 Hypothesized Role of Calcium in Inflammation 11 September 2007 University of Idaho 105
Hypothesized Role of Calcium in Inflammation 11 September 2007 University of Idaho 105
![Ca 2+ increases Fenton Rxn Kinetics in high [EDTA]: [Fe] ratios n Recovery of Ca 2+ increases Fenton Rxn Kinetics in high [EDTA]: [Fe] ratios n Recovery of](https://present5.com/presentation/d0edfd974a2130f356c4e16433b40ad9/image-106.jpg) Ca 2+ increases Fenton Rxn Kinetics in high [EDTA]: [Fe] ratios n Recovery of Fenton reactivity of Fe complexes by addition of Ca 2+. n 1: 50 [Fe. III]: [citrate] n 1: 20 [Fe. III]: [NTA] 1: 10 [Fe. III]: [EDTA] n n Other common conditions: 0. 1 m. M Fe 3+, 22. 8 m. M H 2 O 2, and p. H 7. 4 HEPES, carbon disk electrode, 10 m. V/s sweep rate 11 September 2007 University of Idaho 106
Ca 2+ increases Fenton Rxn Kinetics in high [EDTA]: [Fe] ratios n Recovery of Fenton reactivity of Fe complexes by addition of Ca 2+. n 1: 50 [Fe. III]: [citrate] n 1: 20 [Fe. III]: [NTA] 1: 10 [Fe. III]: [EDTA] n n Other common conditions: 0. 1 m. M Fe 3+, 22. 8 m. M H 2 O 2, and p. H 7. 4 HEPES, carbon disk electrode, 10 m. V/s sweep rate 11 September 2007 University of Idaho 106
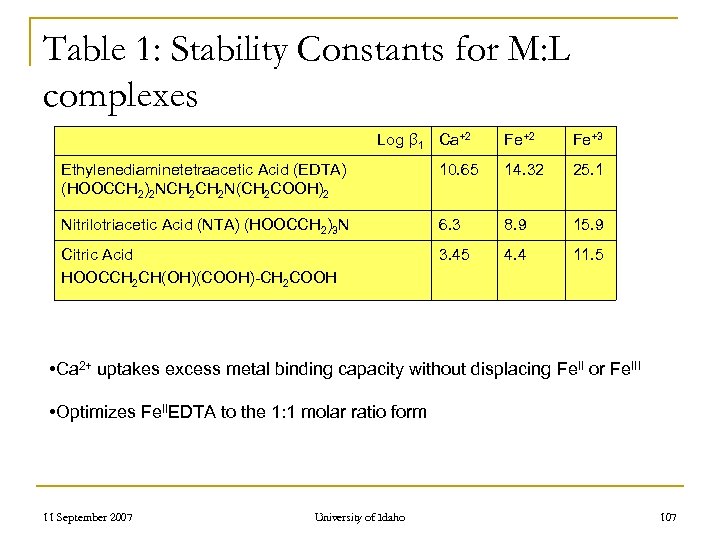 Table 1: Stability Constants for M: L complexes Log β 1 Ca+2 Fe+3 Ethylenediaminetetraacetic Acid (EDTA) (HOOCCH 2)2 NCH 2 N(CH 2 COOH)2 10. 65 14. 32 25. 1 Nitrilotriacetic Acid (NTA) (HOOCCH 2)3 N 6. 3 8. 9 15. 9 Citric Acid HOOCCH 2 CH(OH)(COOH)-CH 2 COOH 3. 45 4. 4 11. 5 • Ca 2+ uptakes excess metal binding capacity without displacing Fe. II or Fe. III • Optimizes Fe. IIEDTA to the 1: 1 molar ratio form 11 September 2007 University of Idaho 107
Table 1: Stability Constants for M: L complexes Log β 1 Ca+2 Fe+3 Ethylenediaminetetraacetic Acid (EDTA) (HOOCCH 2)2 NCH 2 N(CH 2 COOH)2 10. 65 14. 32 25. 1 Nitrilotriacetic Acid (NTA) (HOOCCH 2)3 N 6. 3 8. 9 15. 9 Citric Acid HOOCCH 2 CH(OH)(COOH)-CH 2 COOH 3. 45 4. 4 11. 5 • Ca 2+ uptakes excess metal binding capacity without displacing Fe. II or Fe. III • Optimizes Fe. IIEDTA to the 1: 1 molar ratio form 11 September 2007 University of Idaho 107
 Fe. II apoenzyme HO· ROS Inflammation { H 2 O 2 Cl- HOCl Fe. IIenzyme O 2. NO ONOO- Ca 2+ High [L]: [Fe. II] Oxidatively inactive Fe released into LMW pool Ca 2+ optimized [L]: [Fe. II] Oxidatively active 11 September 2007 University of Idaho 108
Fe. II apoenzyme HO· ROS Inflammation { H 2 O 2 Cl- HOCl Fe. IIenzyme O 2. NO ONOO- Ca 2+ High [L]: [Fe. II] Oxidatively inactive Fe released into LMW pool Ca 2+ optimized [L]: [Fe. II] Oxidatively active 11 September 2007 University of Idaho 108
 Fe. II apoenzyme HO· ROS { Inflammation H 2 O 2 Cl- HOCl Fe. IIenzyme O 2. - Fe released into LMW pool NO ONOOHigh [L]: [Fe. II] inactive Ca 2+ redox cycling H 2 O 2 HO· Damage to DNA Lipids Proteins optimized [L]: [Fe. II] active 11 September 2007 University of Idaho 109
Fe. II apoenzyme HO· ROS { Inflammation H 2 O 2 Cl- HOCl Fe. IIenzyme O 2. - Fe released into LMW pool NO ONOOHigh [L]: [Fe. II] inactive Ca 2+ redox cycling H 2 O 2 HO· Damage to DNA Lipids Proteins optimized [L]: [Fe. II] active 11 September 2007 University of Idaho 109
 Conclusions II n Fe. II/IIIEDTA is a good model for LMW physiological iron n Ca 2+ part of the inflammation cascade to optimize Fe chelation sphere for the Fenton Rxn. 11 September 2007 University of Idaho 110
Conclusions II n Fe. II/IIIEDTA is a good model for LMW physiological iron n Ca 2+ part of the inflammation cascade to optimize Fe chelation sphere for the Fenton Rxn. 11 September 2007 University of Idaho 110
 Other studies using Fe. EDTA n Sensor for Peroxide Based Explosives n O 2 reduction catalysts for fuel cells 11 September 2007 University of Idaho 111
Other studies using Fe. EDTA n Sensor for Peroxide Based Explosives n O 2 reduction catalysts for fuel cells 11 September 2007 University of Idaho 111
 Acknowledgements Derek Laine Funding NIH Kenichi Shimizu NSF Simon Mc. Allister NASA Ruhba Ponraj Mark D. Engelmann, Ph. D. 2003 Tina Noradoun, Ph. D. 2005 Ryan Hutcheson, B. S. 2004 EPRI BLM UI Rob Bobier, B. S. 2002 Terry Hiatt, B. S. 2000 11 September 2007 University of Idaho 112
Acknowledgements Derek Laine Funding NIH Kenichi Shimizu NSF Simon Mc. Allister NASA Ruhba Ponraj Mark D. Engelmann, Ph. D. 2003 Tina Noradoun, Ph. D. 2005 Ryan Hutcheson, B. S. 2004 EPRI BLM UI Rob Bobier, B. S. 2002 Terry Hiatt, B. S. 2000 11 September 2007 University of Idaho 112
 http: //www. moscow. com/ 11 September 2007 University of Idaho 113
http: //www. moscow. com/ 11 September 2007 University of Idaho 113
 11 September 2007 University of Idaho 114
11 September 2007 University of Idaho 114
 www. latahrealty. com/ 11 September 2007 University of Idaho 115
www. latahrealty. com/ 11 September 2007 University of Idaho 115
 11 September 2007 University of Idaho 116
11 September 2007 University of Idaho 116
 Visit Our Web Site! www. chem. uidaho. edu 11 September 2007 University of Idaho 117
Visit Our Web Site! www. chem. uidaho. edu 11 September 2007 University of Idaho 117
 UI Stipend goes along way in Moscow, Idaho TA+Renfrew Scholarship 12 months $18, 800 Effective Fees -$1, 940 Health Insurance -$1, 200 Average One Bedroom Apt $350 -550 Average Two Bedroom Apt $450 -650 11 September 2007 University of Idaho 118
UI Stipend goes along way in Moscow, Idaho TA+Renfrew Scholarship 12 months $18, 800 Effective Fees -$1, 940 Health Insurance -$1, 200 Average One Bedroom Apt $350 -550 Average Two Bedroom Apt $450 -650 11 September 2007 University of Idaho 118


